Abstract
Introduction
TXA has been evaluated in THA in several randomized controlled trials for the past 16 years. We attempted to evaluate the trends in the evidence using recursive cumulative meta-analysis and publication bias using Rosenthal and ‘Trim and Fill’ methods
Methods
Electronic databases were searched for randomized controlled clinical trials comparing TXA with either placebo (or no TXA administration) or TXA, administered through different routes in patients with osteoarthritis or osteonecrosis of the hip who underwent THA. We considered the total number of patients requiring blood transfusion as the clinical outcome for both the analyses and used quality effects model for assessing the changes in the pooled estimates with addition of new clinical trial data. We also assessed the publication bias by plotting individual study estimates with the standard errors using Rosenthal and ‘Trim and Fill’methods.
Results
A total of 20 studies were included. The pooled cumulative meta-analysis indicates that from 2014 the addition of estimate from new studies in the following years is only narrowing the confidence interval without any significant change in the point estimate. Rosenthal fail-safe-N for the comparisons of intravenous bolus TXA, intra-operative and post-operative intravenous TXA and topical with control groups were 54, 6 and 16 respectively. Fail-safe-N for the combined intravenous and topical TXA with intravenous TXA alone was 13.
Conclusion
Adequate evidence exists supporting the use of intravenous TXA in reducing the need for blood transfusion in THA. There is possible existence of small studies with null effects evaluating the use of TXA in THA.
Keywords: Trim and fill method, Rosenthal method, Fisher method
1. Introduction
Total hip arthroplasty [THA] is associated with a blood loss ranging between 1000 and 2000 ml and nearly 37% of patients undergoing THA require blood transfusion.1 Tranexamic acid (TXA) to reduce the bleeding and the need for blood transfusion in THA as well as in total knee arthroplasty is proven in randomized controlled trials and meta-analyses. 2, 3 The earliest evidence evaluating the efficacy of TXA was in the 1987 in thyroid surgeries and in 2000 for THA.4 However, till date nearly 30 randomized clinical trials and several observational studies are being carried out on TXA in THA. A common limitation observed in the meta-analyses evaluating TXA in THA is publication bias. Most of the studies did not assess publication bias and its impact on the effect estimates. 5, 6 Presence of unpublished literature may obscure the real findings and so it is vital to identify the extent or guesstimate the risk. 7 TXA has been evaluated in several routes and time of administration and there are no consensus as to which is the best route and time.8 Despite many randomized controlled trials and meta-analyses from 2000, recent clinical trials evaluating TXA is continuing.9
Cumulative meta-analysis is used to calculate the summary effect estimates from the results of studies that have accrued till date. 10 This helps us identify the trends and robustness of the accumulated evidence. This identifies the usefulness of studies conducted with indirect effect on the utility of the resources in a better way. Cumulative meta-analysis by precision also can be used to identify the presence of any publication bias. 11 The Rosenthal method also known as ‘file drawer analysis’ calculates the number of studies required to be added to the given set, in order to average the null results for a given level of confidence. 12 Additionally, ‘Trim and Fill’ method estimates the number of missing studies by using the effect estimates of the included studies in a meta-analysis. 13 With this background, we tried to assess the changes in the summary estimates with the addition of new randomized clinical trials using recursive cumulative meta-analysis. Additionally, we also assessed the publication bias using Rosenthal and, Trim and Fill methods.
2. Methods
2.1. Information sources and search strategy
The protocol for this review was registered with PROSPERO with the registration number CRD42017058116. A thorough literature search was completed on 01 March 2017 with the search strategy: Tranexamic acid [tiab] AND arthroplasty [tiab]. Databases searched for potential articles include Medline (through PubMed), Cochrane CENTRAL and Google Scholar. No limits were placed with respect to either language or year of publication. The references from the screened literature were also searched for eligible studies.
2.2. Eligibility criteria
Only randomized controlled clinical trials comparing TXA with either placebo (or no TXA administration) or TXA administered through any route in patients with osteoarthritis or osteonecrosis of the hip who underwent THA were included for the assessment of trend analysis. We attempted to assess the trends and publication bias for only those comparisons that had a minimum of three studies. We considered the total number of patients requiring blood transfusion as the clinical outcome for both the analyses.
2.3. Study procedure and statistical considerations
Two authors independently performed literature search in the above mentioned databases. Both the authors independently extracted the following data from each eligible study: trial site, year, trial methods, participants, interventions, and outcomes. Disagreement between the authors was resolved through discussion. The present review and network meta-analysis has been reported as per the preferred reporting items for systematic reviews and meta-analysis guidelines (PRISMA). 14 We analysed the risk of bias of the included studies using Cochrane risk of bias tool under seven domains. 15 Odds ratio [95% confidence interval] was used as the effect estimate. MetaXL 16 was used to generate pooled estimates for the cumulative meta-analysis wherein the result of each trial is added to the previous pooled estimates in a repetitive fashion using quality effects model. The quality effects model 16 links the quality of each randomized controlled trial to the corresponding effect estimate. The quality of each trial was assessed based on the risk of bias tool wherein a score of 3 was given low risk, 2 for intermediate and 1 for high risk in each domain. We assessed the publication bias by ‘Rosenthal’ and ‘Trim and Fill’ methods using Meta-Essentials. 17
3. Results
3.1. Search results
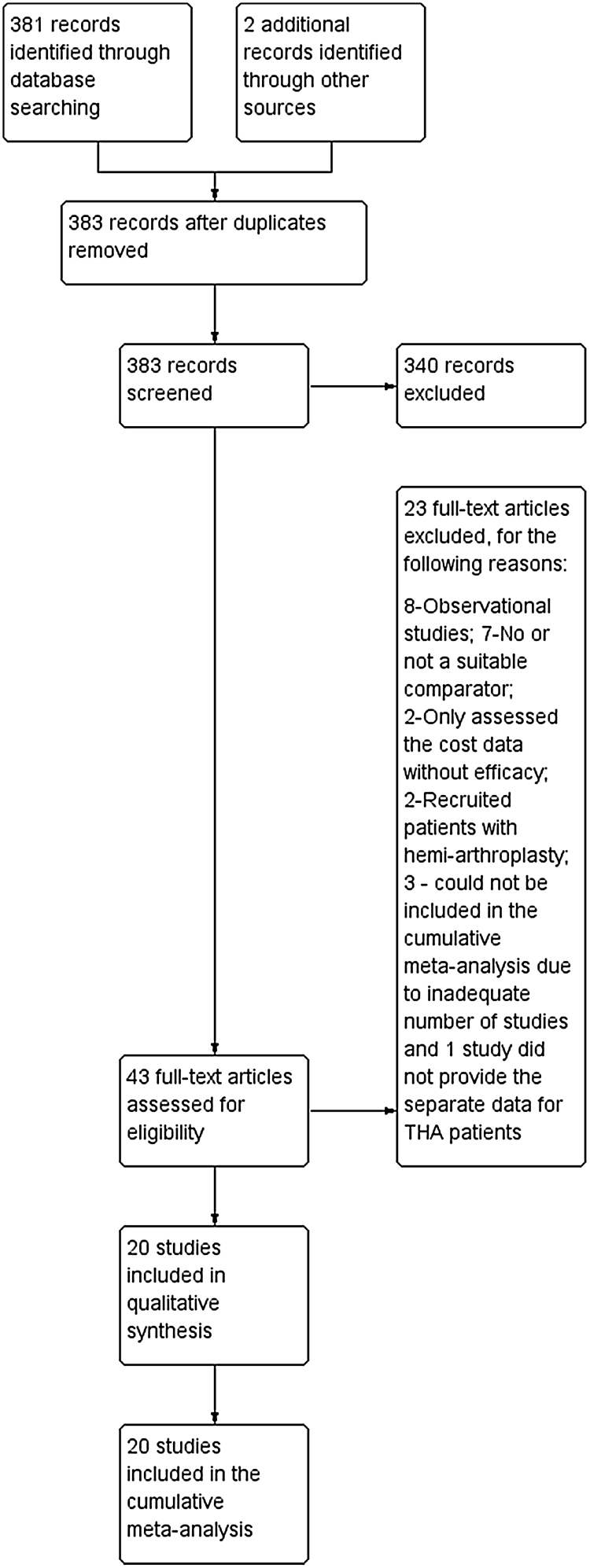
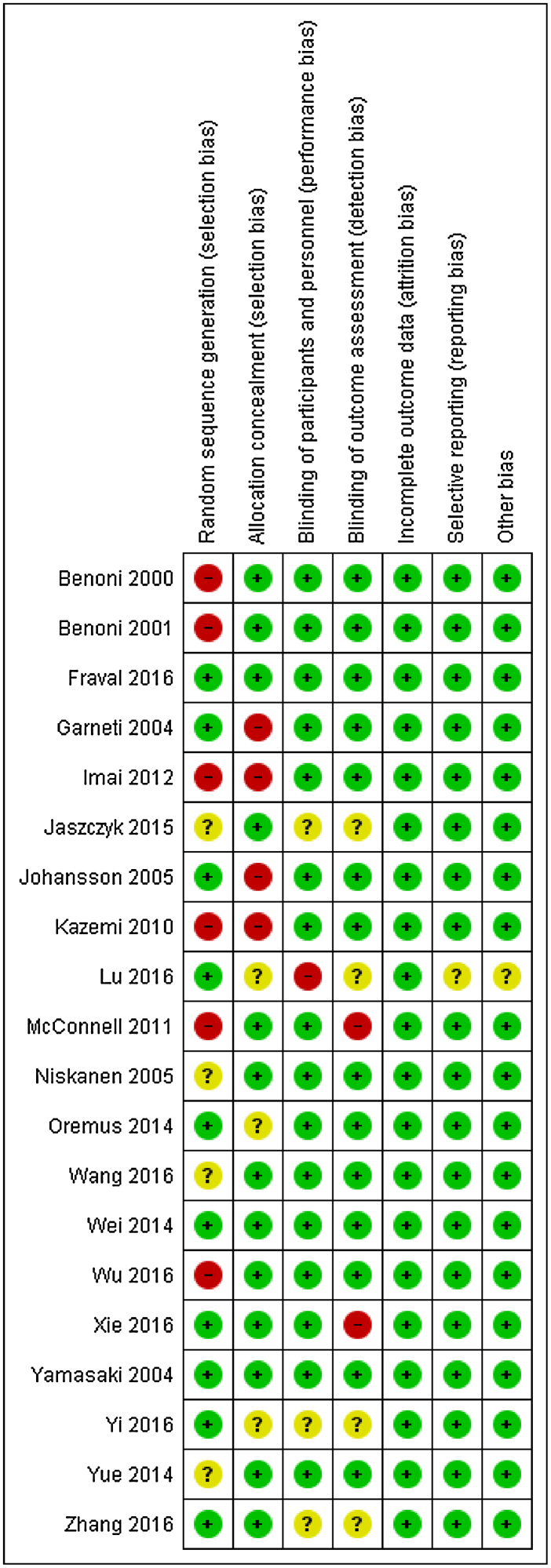
A total of 383 articles were obtained with the above mentioned search strategy and references from the screened literature. Finally 20 studies 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 were included (Fig. 1). Eight studies compared the intravenous bolus administration of TXA either at the time of induction of anaesthesia or just before surgery, four compared TXA during surgery and post-operatively, and one study compared topical TXA with the control group. Four studies compared topical combined with intravenous bolus TXA to intravenous bolus TXA. One study compared intravenous bolus TXA at the time of induction with both intra and post-operative administration of TXA to placebo and two studies compared topical and intravenous bolus TXA to placebo. We also obtained four studies that compared TXA administration through both topical and intravenous route with intravenous TXA. Risk of bias of the included studies is depicted in Fig. 2.
Fig. 1.
PRSIMA flow chart.
A total of 383 articles were identified of which finally 20 studies were included in this cumulative meta-analysis.
Fig. 2.
Risk of bias of the included studies.
Red circle with minus symbol indicate high risk of bias; Yellow circle with question symbol indicate unclear risk and green circle with plus symbol indicate low risk of bias.
3.2. Pooled results of the cumulative meta-analysis
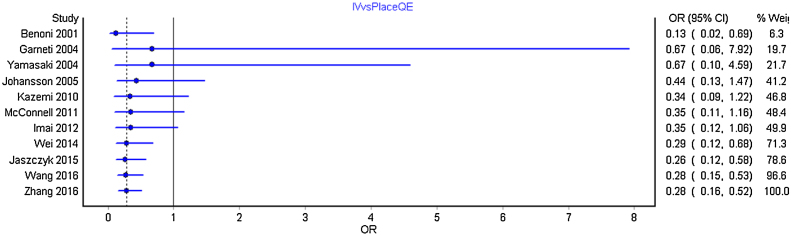
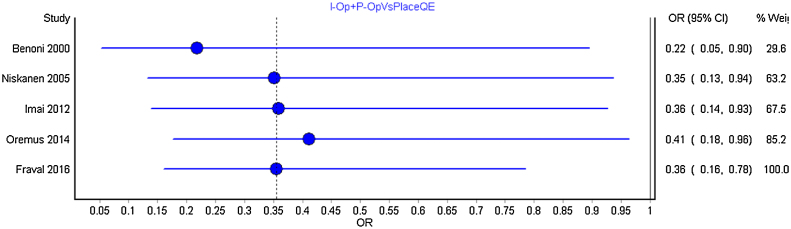
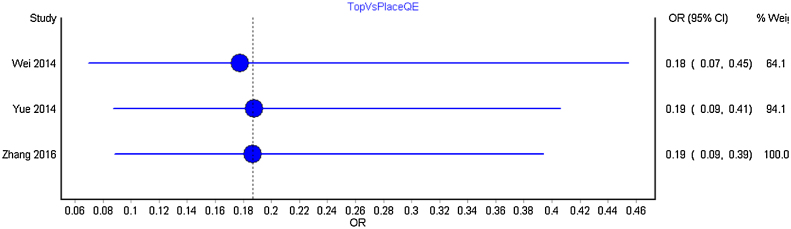
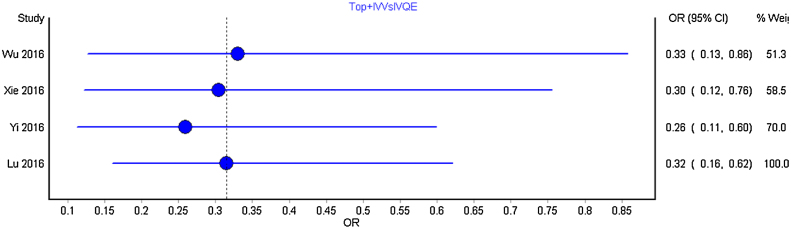
We carried out separate cumulative meta-analysis for the following comparisons: Intravenous bolus TXA with placebo; TXA intra and post-operatively with placebo; TXA topical with placebo and TXA topical with intravenous and intravenous TXA alone. Fig. 3 represent the Forest plot of the cumulative meta-analysis for the comparison of TXA intravenous bolus dose with placebo. As can be observed, from the year 2014, with the addition of estimate from new studies, there is just shortening of the confidence interval without any significant change. Similar observations were noted for all other comparisons (Fig. 4, Fig. 5, Fig. 6 ).
Fig. 3.
Forest plot of the cumulative meta-analysis by year-wise for intravenous TXA with control group.
From the year 2014, the effect sizes when added to the pooled estimates of the previous studies in a repetitive fashion did not change the pooled estimate much with just shortening of the confidence interval.
Fig. 4.
Forest plot of year-wise cumulative meta-analysis for TXA administration both intra and post-operatively with control group.
No significant differences were observed between the pooled estimates and the overall interpretation.
Fig. 5.
Forest plot of year-wise cumulative meta-analysis for topical TXA with the control group.
No significant changes were observed between the pooled estimates with addition of new studies.
Fig. 6.
Forest plot of year-wise cumulative meta-analysis for combined topical and intravenous TXA with intravenous TXA.
No significant changes were observed between the pooled estimates with addition of new studies.
3.3. Publication bias of the included randomized controlled trials by Rosenthal and Trim and Fill methods
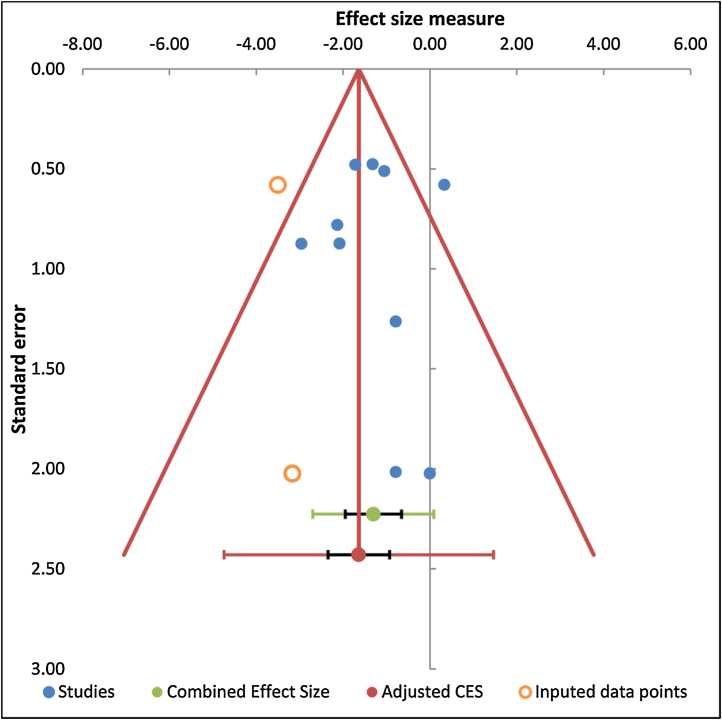
Funnel plot was possible only for the comparison of intravenous bolus TXA with the control group and is depicted in Fig. 7. Trim and Fill method of analysis for this comparison inferred that three studies were missing. Due to limited number of studies with other comparisons, Trim and Fill method of analysis could not be performed. Rosenthal fail-safe-N for the comparisons of intravenous bolus TXA, intra-operative and post-operative intravenous TXA and topical with control groups were 54, 6 and 16 respectively. Fail-safe-N for the combined intravenous and topical TXA with intravenous TXA alone was 13.
Fig. 7.
Funnel plots for the comparisons of intravenous TXA bolus with control group.
One study is missing in the middle and two at the bottom on the left side of the central line thus suggestive of publication bias.
4. Discussion
We conducted the present recursive cumulative meta-analysis to assess the trends in the pooled estimates for various comparisons of TXA in THA. We also intended to assess potential publication bias in each of the possible comparisons. We included 20 randomized controlled trials with the following comparisons either with placebo or no TXA groups: Intravenous bolus TXA, intra and post-operative TXA and topical. Additionally, we also compared the estimates of combined topical and intravenous TXA with intravenous TXA alone. The cumulative meta-analysis did not identify any significant changes with addition of new clinical trials recently for all the comparisons except for narrowing of the confidence interval surrounding the pooled estimates. This infers that there is no need for further randomized controlled trials required with the above mentioned comparisons. However, we also assessed publication bias by two independent methods for all the above mentioned comparisons. We observed low Fail-safe-N values for all the comparisons thus suggestive of existence of publication bias.
The first meta-analysis evaluating the efficacy of TXA in patients undergoing elective surgery was published in the year 2001. 38 Additionally, Ker et al. 39 in 2012 did a cumulative meta-analysis for the use of TXA in surgical patients and had shown that the evidence for the reduction of need for blood transfusion with TXA has been available for the past 10 years. However, the authors in that study did not perform a sub-group analysis for individual indications for TXA. Our present recursive cumulativeanalysis was done specific to the use of TXA in THA and confirmed that the evidence supporting the use of TXA is available from the year 2014. However, it has to be noted that this evidence is mainly available for the intravenous TXA use as a bolus either at the induction of anaesthesia or just before surgery. Despite this evidence, trials evaluating the use of TXA by intravenous bolus route are continuing thus suggestive of improper use of resources. Future researchers should attempt in exploring the most appropriate dose of TXA through various routes of administration. Also, the assessment of publication bias indicates the possibility of studies with negative effects remaining in the drawer. Researchers should aim at publishing studies even when the results are not favourable to the experimental intervention. Similarly, journal editors should encourage such studies and attempt in publishing provided the scientific content are accurate and credible.
The strength of this meta-analysis is that this is the first cumulative meta-analysis evaluating the use of TXA in THA and we have used quality effects model that links quality of each included study to the effect estimate which is more robust than random effects model. However, the study is limited in having analyzed adequate studies only pertaining to intravenous route, combined intra-operative and post-operative dose and topical TXA administrations. From this cumulative meta-analysis, we conclude that adequate evidence supporting the intravenous use of TXA for reducing the need of blood transfusion in patients with THA exists. There is a possible existence of small studies with null effects evaluating the use of TXA in THA.
Funding
No fund was obtained to carry out this study.
Conflict of interest
The authors have none to declare.
Acknowledgements
We thank PROSPERO for registering the protocol of this review. We are grateful to Epigear for utilising MetaXL software for generating the pooled estimates and Erasmus Research Institute of Management for utilising Meta-Essential software. We also thank Cochrane for utilizing RevMan 5.3 to evaluate the risk of bias of the included studies.
References
- 1.Kim C., Park S.S.-H., Davey J.R. Tranexamic acid for the prevention and management of orthopaedic surgical haemorrhage: current evidence. J Blood Med. 2015;6:239–244. doi: 10.2147/JBM.S61915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang P., Liang Y., Chen P., Fang Y., He J., Wang J. Combined application versus topical and intravenous application of tranexamic acid following primary total hip arthroplasty: A meta-analysis. BMC Musculoskelet Disord. 2017;18:90. doi: 10.1186/s12891-017-1429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin C., Qi Y., Jie L. Is combined topical with intravenous tranexamic acid superior than topical, intravenous tranexamic acid alone and control groups for blood loss controlling after total knee arthroplasty A meta-analysis. Medicine (Baltimore) 2016;95:51. doi: 10.1097/MD.0000000000005344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auvinen O., Baer G.A., Nordback I., Saaristo J. Antifibrinolytic therapy for prevention of hemorrhage during surgery of the thyroid gland. Klin Wochenschr. 1987;65:253–255. doi: 10.1007/BF01773442. [DOI] [PubMed] [Google Scholar]
- 5.Chen S., Wu K., Kong G., Feng W., Deng Z., Wang H. The efficacy of topical tranexamic acid in total hip arthroplasty: a meta-analysis. BMC Musculoskelet Disord. 2016;17:81. doi: 10.1186/s12891-016-0923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sukeik M., Alshryda S., Haddad F.S., Mason J.M. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93:39–46. doi: 10.1302/0301-620X.93B1.24984. [DOI] [PubMed] [Google Scholar]
- 7.Winters M., Weir A. Grey matters; on the importance of publication bias in systematic reviews. Br J Sports Med. 2017;51:488–489. doi: 10.1136/bjsports-2016-096679. [DOI] [PubMed] [Google Scholar]
- 8.Fu Y., Shi Z., Han B. Comparing efficacy and safety of 2 methods of tranexamic acid administration in reducing blood loss following total knee arthroplasty A meta-analysis. Medicine (Baltimore) 2016;95:50. doi: 10.1097/MD.0000000000005583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Zhang L., Ma X. What is the optimal approach for tranexamic acid application in patients with unilateral total hip arthroplasty? Orthopade. 2016;45:616–621. doi: 10.1007/s00132-016-3252-y. [DOI] [PubMed] [Google Scholar]
- 10.Clarke M., Brice A., Chalmers I. Accumulating research: A systematic account of how cumulative meta-analyses would have provided knowledge, improved health, reduced harm and saved resources. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0102670. e102670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atakpo P., Vassar M. Cumulative meta-analysis by precision as a method to evaluate publication bias. J Dermatol Sci. 2016;83:240–253. doi: 10.1016/j.jdermsci.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal R. The File Drawer Problem and tolerance for null results. Psychol Bull. 1979;86:638–641. [Google Scholar]
- 13.Duval S., Tweedie R. Trim and Fill A simple Funnel-Plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D.G. The PRISMA Group: preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 edition. Available from www.cochrane-handbook.org. Accessed on 03 Mar 2017).
- 16.Doi S.A., Barendregt J.J., Khan S., Thalib L., Williams G.M. Advances in the meta-analysis of heterogeneous clinical trials II: The quality effects model. Contemp Clin Trials. 2015;45:123–129. doi: 10.1016/j.cct.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Van Rhee H.J., Suurmond Hak R.T. Rotterdam, The Netherlands: Erasmus Research Institute of Management.; 2017. User Manual for Meta-Essentials: Workbooks for Meta-analysis (Version 1.0) Available from www.erim.eur.nl/research-support/meta-essentials =. Accessed on 12 Mar 17] [Google Scholar]
- 18.Benoni G., Fredin H., Knebel R., Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72:442–448. doi: 10.1080/000164701753532754. [DOI] [PubMed] [Google Scholar]
- 19.Johansson T., Pettersson L.G., Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76:314–319. [PubMed] [Google Scholar]
- 20.Wei W., Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29:2113–2116. doi: 10.1016/j.arth.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Niskanen R.O., Korkala O.L. Tranexamic acid reduces blood loss in cemented hip arthroplasty: a randomized, double-blind study of 39 patients with osteoarthritis. Acta Orthop. 2005;76:829–832. doi: 10.1080/17453670510045444. [DOI] [PubMed] [Google Scholar]
- 22.Yi Z., Bin S., Jing Y., Zongke Z., Pengde K., Fuxing P. Tranexamic acid administration in primary total hip arthroplasty: a randomized controlled trial of intravenous combined with topical versus single-dose intravenous administration. J Bone Joint Surg Am. 2016;98:983–991. doi: 10.2106/JBJS.15.00638. [DOI] [PubMed] [Google Scholar]
- 23.Xie J., Ma J., Yue C., Kang P., Pei F. Combined use of intravenous and topical tranexamic acid following cementless total hip arthroplasty: a randomised clinical trial. Hip Int. 2016;26:36–42. doi: 10.5301/hipint.5000291. [DOI] [PubMed] [Google Scholar]
- 24.Wang C., Kang P., Ma J., Yue C., Xie J., Pei F. Single-dose tranexamic acid for reducing bleeding and transfusions in total hip arthroplasty: a double-blind, randomized controlled trial of different doses. Thromb Res. 2016;141:119–123. doi: 10.1016/j.thromres.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 25.Yue C., Kang P., Yang P., Xie J., Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29:2452–2456. doi: 10.1016/j.arth.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y.G., Zeng Y., Yang T.M., Si H.B., Cao F., Shen B. The efficacy and safety of combination of intravenous and topical tranexamic acid in revision hip arthroplasty: a randomized, controlled trial. J Arthroplasty. 2016;31:2548–2553. doi: 10.1016/j.arth.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 27.Fraval A., Effeney P., Fiddelaers L., Smith B., Towell B., Tran P. OBTAIN A: Outcome benefits of tranexamic acid in hip arthroplasty. A randomized double-blinded controlled trial. J Arthroplasty. 2016 doi: 10.1016/j.arth.2016.11.045. S0883-5403(16)30845-2. [DOI] [PubMed] [Google Scholar]
- 28.Oremus K., Sostaric S., Trkulja V., Haspl M. Influence of tranexamic acid on postoperative autologous blood retransfusion in primary total hip and knee arthroplasty: a randomized controlled trial. Transfusion. 2014;54:31–41. doi: 10.1111/trf.12224. [DOI] [PubMed] [Google Scholar]
- 29.McConnell J.S., Shewale S., Munro N.A., Shah K., Deakin A.H., Kinninmonth A.W. Reduction of blood loss in primary hip arthroplasty with tranexamic acid or fibrin spray. Acta Orthop. 2011;82:660–663. doi: 10.3109/17453674.2011.623568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benoni G., Lethagen S., Nilsson P., Fredin H. Tranexamic acid, given at the end of the operation, does not reduce postoperative blood loss in hip arthroplasty. Acta Orthop Scand. 2000;71:250–254. doi: 10.1080/000164700317411834. [DOI] [PubMed] [Google Scholar]
- 31.Kazemi S.M., Mosaffa F., Eajazi A. The effect of tranexamic acid on reducing blood loss in cementless total hip arthroplasty under epidural anesthesia. Orthopedics. 2010;33:17. doi: 10.3928/01477447-20091124-30. [DOI] [PubMed] [Google Scholar]
- 32.Imai N., Dohmae Y., Suda K., Miyasaka D., Ito T., Endo N. Tranexamic acid for reduction of blood loss during total hip arthroplasty. J Arthroplasty. 2012;27:1838–1843. doi: 10.1016/j.arth.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Jaszczyk M., Kozerawski D., Kołodziej Ł., Kazimierczak A., Sarnecki P., Sieczka Ł. Effect of single preoperative dose of tranexamic acid on blood loss and transfusion in hip arthroplasty. Ortop Traumatol Rehab. 2015;17:265–273. doi: 10.5604/15093492.1162426. [DOI] [PubMed] [Google Scholar]
- 34.Garneti N., Field J. Bone bleeding during total hip arthroplasty after administration of tranexamic acid. J Arthroplasty. 2004;19:488–492. doi: 10.1016/j.arth.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y., Zhang L., Ma X. What is the optimal approach for tranexamic acid application in patients with unilateral total hip arthroplasty? Orthopade. 2016;45:616–621. doi: 10.1007/s00132-016-3252-y. [DOI] [PubMed] [Google Scholar]
- 36.Yamasaki S., Masuhara K., Fuji T. Tranexamic acid reduces blood loss after cementless total hip arthroplasty-prospective randomized study in 40 cases. Int Orthop. 2004;28:69–73. doi: 10.1007/s00264-003-0511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu C., Guo H., Hao Y.Q., Chen X.S., Cai Y.Z., Xu P. A prospective randomized controlled study of tranexamic acid used in different ways to reduce blood loss in total hip arthroplasty. CJBJS. 2016;9:140–144. [Google Scholar]
- 38.Henry D.A., Moxey A.J., Carless P.A. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2001;1 doi: 10.1002/14651858.CD001886. CD001886. [DOI] [PubMed] [Google Scholar]
- 39.Ker K., Perel P., Shakur H., Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054. doi: 10.1136/bmj.e3054. [DOI] [PMC free article] [PubMed] [Google Scholar]