ABSTRACT
The reduction of microbial load in food and water systems is critical for their safety and shelf life. Conventionally, physical processes such as heat or light are used for the rapid inactivation of microbes, while natural compounds such as lactic acid may be used as preservatives after the initial physical process. This study demonstrates the enhanced and rapid inactivation of bacteria based on a synergistic combination of sublethal levels of stresses induced by UV-A light and two food-grade organic acids. A reduction of 4.7 ± 0.5 log CFU/ml in Escherichia coli O157:H7 was observed using a synergistic combination of UV-A light, gallic acid (GA), and lactic acid (LA), while the individual treatments and the combination of individual organic acids with UV-A light resulted in a reduction of less than 1 log CFU/ml. Enhanced inactivation of bacteria on the surfaces of lettuce and spinach leaves was also observed based on the synergistic combination. Mechanistic investigations suggested that the treatment with a synergistic combination of GA plus LA plus UV-A (GA+LA+UV-A) resulted in significant increases in membrane permeability and intracellular thiol oxidation and affected the metabolic machinery of E. coli. In addition, the antimicrobial activity of the synergistic combination of GA+LA+UV-A was effective only against metabolically active E. coli O157:H7. In summary, this study illustrates the potential of simultaneously using a combination of sublethal concentrations of natural antimicrobials and a low level of physical stress in the form of UV-A light to inactivate bacteria in water and food systems.
IMPORTANCE There is a critical unmet need to improve the microbial safety of the food supply, while retaining optimal nutritional and sensory properties of food. Furthermore, there is a need to develop novel technologies that can reduce the impact of food processing operations on energy and water resources. Conventionally, physical processes such as heat and light are used for inactivating microbes in food products, but these processes often significantly reduce the sensory and nutritional properties of food and are highly energy intensive. This study demonstrates that the combination of two natural food-grade antimicrobial agents with a sublethal level of physical stress in the form of UV-A light can greatly increase microbial load inactivation. In addition, this report elucidates the potential mechanisms for this synergistic interaction among physical and chemical stresses. Overall, these results provide a novel approach to develop antimicrobial solutions for food and water systems.
KEYWORDS: food-grade antimicrobial, gallic acid, lactic acid, microbial inactivation, sublethal stress, UV-A light, synergism
INTRODUCTION
The reduction of microbial contamination is critical for the safety and quality of food and water systems. The most commonly used methods to reduce microbial load can be broadly divided into physical and chemical approaches. Physical approaches, such as nonionizing light irradiation, pulsed electric field, heat, and high pressure, are widely used to control the microbial load in food systems where diverse stresses induced by these methods cause rapid inactivation of microbes (1–3). However, some of these methods can have significant negative effects on the sensory and nutritional properties of food (4, 5). Chemical agents, such as chlorine, peptides, and organic acids, are also used for the preservation of food and water systems, as well as for sanitation (6–8). One of the key advantages of chemical antimicrobials, in contrast to physical methods, is the reduced impact on the quality of the final food product (9). However, chemical agents are often less efficient than physical methods (e.g., heat and pressure) in achieving microbial reduction. This limitation is, in part, due to regulatory limits on the allowable concentrations of these chemical agents in food and water systems and to the dependence of their biological activity on factors such as pH and temperature (10, 11). Consequently, extended exposure times are often required to achieve an effective microbial load reduction using chemical agents. To overcome these limitations, traditionally some chemical agents have been used following an initial physical treatment, mainly with the aims of extending the shelf life of food and preserving food and water systems (10, 12).
Diverse classes of antimicrobial compounds, such as salts, acids, oxides, and antibiotics, can be used as chemical agents in food and water systems (10, 13, 14). Among a large variety of chemical compounds, food-grade natural antimicrobials are a highly attractive class of compounds with potential for food applications (15, 16). The major advantages of using food-grade antimicrobials include an adherence to the existing regulatory requirements, an increased consumer acceptance, and a lower probability of developing antimicrobial resistance against these food-grade chemicals than with standard antibiotics (7, 15, 17). Despite their significant potential, many of these food-grade compounds have limited efficacies compared with those of conventional antimicrobials, such as antibiotics, chlorine, and nitrates (18). Therefore, synergistic combinations of food-grade compounds and low levels of physical stresses have been suggested as promising methodologies to enhance antimicrobial activity in many applications (16).
There has been an increasing interest in evaluating the synergistic action of various combinations of chemical agents or combinations of chemical agents and physical stresses (19–24). The motivating factor is to reduce the impact of physical stresses on the quality of food while also reducing the use of chemical agents. This research direction is based on an improved understanding of the mechanisms involved in microbial inactivation. Thus, complementary sites of action are targeted for enhanced microbial inactivation using a combination of chemical and physical agents. In a recent study, we evaluated the potential of combining the activity of a food-grade chemical compound, gallic acid (GA), and a sublethal level of physical stress in the form of UV-A light to increase the reduction of bacterial cells in simulated wash water (25). The results of that study illustrated the advantage of combining physical stresses and chemical agents to achieve an up to 6-log CFU/ml reduction in bacterial load in 30 min. In addition, a few other studies have also reported synergistic activity of organic acids, including GA, with light for the inactivation of bacteria, but there is still a limited understanding of the biological impacts of these treatments on cells and the synergy between physical stress and chemical agents (22, 26, 27). Furthermore, other studies have evaluated the synergy among chemical agents, such as a combination of lactic acid (LA) with other natural antimicrobials (28–30). The overall motivations for these studies are to enhance the antimicrobial activity and reduce the concentrations of individual antimicrobial agents.
In this study, we evaluated a synergistic combination of sublethal concentrations of two food-grade organic acids—GA and LA—with sublethal levels of UV-A light to enhance bacterial inactivation. This study also evaluated the potential mechanisms for the synergistic antimicrobial interactions between chemical and physical stresses. The choice of sublethal levels of physical stress and chemical agents is ideal for potential applications in food, as these levels of physical stress and concentrations of chemical agents are expected to present a limited impact on the nutritional quality and sensory properties of food (31). To assess the biological damage induced by the physical stress of UV-A light, the selected chemical agents, and their synergistic combination, we measured the changes in permeability of the cellular membrane, the metabolic activity of cells, and the oxidation of intracellular thiol content. To investigate the potential mechanism of action, the synergistic interaction between GA plus LA plus UV-A (GA+LA+UV-A) light and Escherichia coli O157:H7 was evaluated under different physical and chemical treatments, including the incubation of bacteria at a low temperature and the addition of sodium azide to suppress the metabolic activity of cells. Lastly, the applicability of this approach to inactivate E. coli O157:H7 on produce surfaces was assessed. Overall, this study illustrates the synergistic activities of food-grade compounds in the presence of light to inactivate target bacteria in aqueous environments and on surfaces of fresh produce.
RESULTS
UV-A light-mediated antimicrobial activity.
The results of the inactivation of E. coli cells treated with GA, LA, or GA+LA and exposed to UV-A light are shown in Fig. 1a. After 30 min of UV-A light exposure, the cells incubated with 1 mM GA plus 5 mM LA showed a 4.7 ± 0.5-log CFU/ml reduction in the bacterial plate count in contrast to reductions of 1 ± 0.3 and 1.3 ± 0.2 log CFU/ml in bacterial plate counts for samples separately incubated with 1 mM GA and 5 mM LA, respectively. UV-A light by itself did not cause inactivation of E. coli. In the absence of UV-A light exposure (Fig. 1b), cells incubated with GA, LA, or GA+LA showed no significant reduction in the bacterial count (<1 log CFU/ml). Therefore, it can be inferred that a combination of these two sublethal level stresses (i.e., UV-A light exposure and GA+LA treatment) generated a synergistic antimicrobial activity which resulted in a lower bacterial plate count than that produced by individual treatments (gallic or lactic acid) with and without UV-A light.
FIG 1.
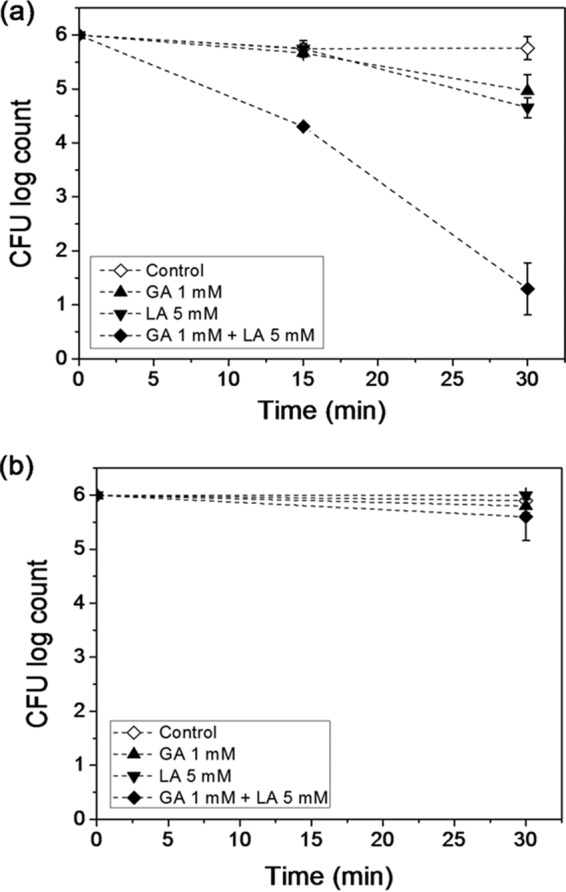
Inactivation of E. coli O157:H7 treated with gallic acid (GA), lactic acid (LA), or GA+LA and exposed (a) or not exposed (b) to UV-A light as measured using the standard plate counting method. Control includes E. coli cells suspended in sterile water without GA and LA. The limit of detection of the plate counting method was 1 log CFU/ml.
To further characterize the UV-A-enhanced antimicrobial activity of GA+LA, its efficacy for the inactivation of E. coli was evaluated at different initial bacterial loads, namely, at 4 log, 6 log, and 8 log CFU/ml. Figure 2 shows a more effective bacterial inactivation at higher initial bacterial concentrations. After 30 min of UV-A light radiation, a maximum of 5- to 6-log CFU/ml microbial reduction was observed from an initial bacterial load of 8 log CFU/ml, while a 4- to 5-log CFU/ml reduction was achieved from an initial concentration of 6 log CFU/ml. In the case of bacterial samples with an initial load of 4 log CFU/ml, a reduction of between 1.6 and 2.8 log CFU/ml of bacteria was observed after 30 min of UV-A treatment (detection limit, 1 log CFU/ml). In addition, there was no significant difference (P > 0.05) in the numbers of residual bacterial cells among the samples with different initial loads of bacteria after 30 min of treatment. This suggests that the UV-A-enhanced antimicrobial activity of GA+LA is not limited by the initial amount of bacteria, and a more than 4-log CFU/ml bacterial reduction can be achieved for higher initial bacterial concentrations (6 or 8 log CFU/ml).
FIG 2.

Influence of various initial bacterial concentrations on inactivation of E. coli O157:H7 cells upon treatment with the combination of 1 mM gallic acid (GA), 5 mM lactic acid (LA), and UV-A light. The initial bacterial concentration levels evaluated in this measurement were 8 log (■), 6 log (▲), and 4 log (●) CFU · ml−1. The final microbial counts after 30 min of treatment for the various initial levels of bacterial concentration were not statistically significant (P > 0.05).
The influence of temperature on the UV-A-mediated antimicrobial activity of GA+LA was also evaluated. Figure 3a shows the inactivation of E. coli cells treated with 1 mM GA plus 5 mM LA and exposed to UV-A light at 4°C and 25°C. After 30 min, a microbial inactivation of 4.7 ± 0.5 log CFU/ml was achieved at 25°C, while at 4°C, there was no significant bacterial inactivation compared with that of the control (P > 0.05). The metabolic activities of E. coli at 4°C and 25°C were measured using the resazurin assay (see Fig. S1 in the supplemental material). The results in Fig. S1 show that the metabolic activity of E. coli cells was significantly suppressed at 4°C compared with that at 25°C. Together, these results indicate that metabolic changes experienced by E. coli cells incubated at 4°C made them resistant to the antimicrobial activity of GA+LA in the presence of UV-A light.
FIG 3.
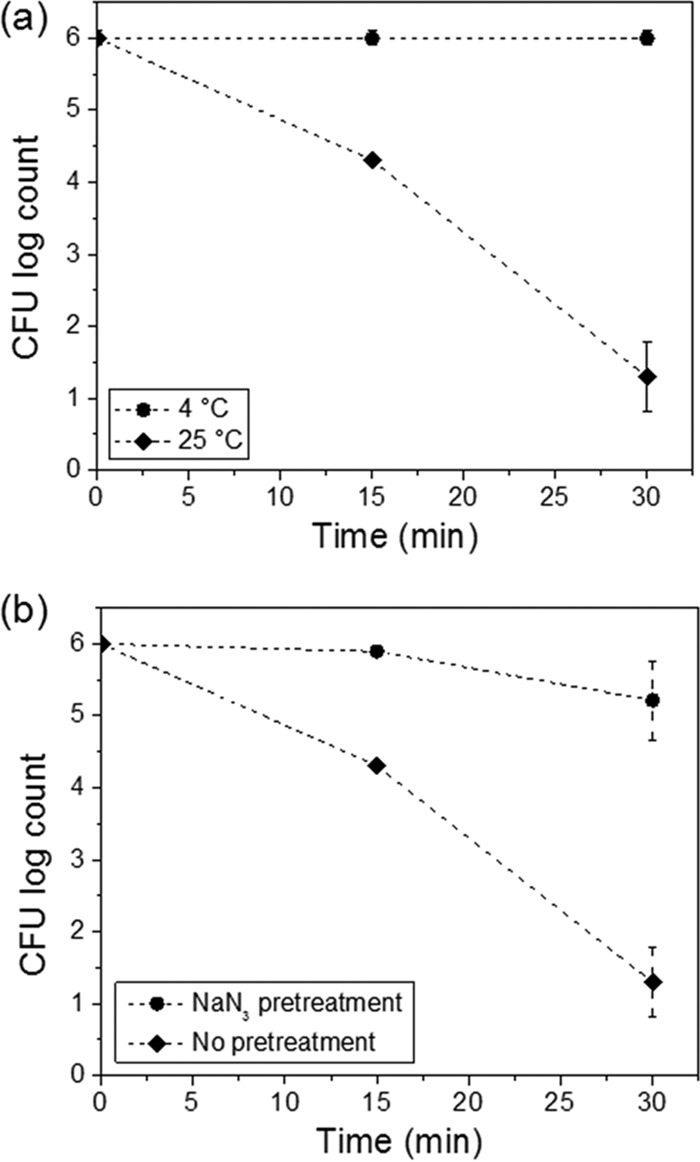
(a) Impact of incubation temperature conditions (4°C and 25°C) on inactivation of E. coli O157:H7 upon treatment with the combination of 1 mM gallic acid (GA), 5 mM lactic acid (LA), and UV-A light. (b) Influence of sodium azide pretreatment (0.5% level) on inactivation of E. coli O157:H7 treated with the combination of GA, LA, and UV-A light. The controls included cells not pretreated with sodium azide.
To further investigate the influence of cellular metabolic activity on the UV-A-mediated antimicrobial activity of GA+LA, E. coli cells were pretreated with sodium azide prior to GA+LA treatment under UV-A exposure. Sodium azide is a known bacteriostatic compound that inhibits the oxidation of cytochrome c, affecting cellular respiration and leading to an overall reduction in the metabolic activity (32, 33). As shown in Fig. 3b, pretreatment with sodium azide followed by GA+LA treatment in the presence of UV-A light resulted in an only 1-log CFU/ml reduction in bacterial load. This is in contrast to a 4.7-log CFU/ml reduction in bacterial load that was achieved without sodium azide pretreatment. The combined results shown in Fig. 3 suggest that an active intracellular metabolism is required for GA and LA to act as antimicrobials in the presence of UV-A light.
Metabolic intracellular resazurin reduction.
After E. coli cells were treated with GA, LA, and GA+LA in the absence or presence of UV-A light, the cells were incubated with resazurin to measure their metabolic activity. The changes in metabolic activity of treated cells with respect to the controls was assessed based on the reduction of resazurin to its fluorescent form, resorufin (Fig. 4). The fluorescence signal intensity increases with the reduction of resazurin to resorufin, while further reduction of resorufin to a colorless compound, hydroresorufin, decreases the fluorescence intensity. In this study, the times required to achieve maximum fluorescence intensity were compared among the different treatments to evaluate the influence of these treatments on the metabolic activity of bacterial cells. In the absence of light, control cells achieved maximum signal intensity in approximately 4.6 h, while the cells previously treated with organic acids (GA, LA, and GA+LA) needed between 5 and 5.5 h to reach maximum intensity. A considerably longer delay in the reduction of resazurin was observed for cells treated with a combination of organic acids and UV-A light. For these cells previously treated with GA, LA, and GA+LA in the presence of UV-A light, the maximum fluorescence intensities were observed only after 6.0, 7.4, and 9.1 h, respectively. The longest delay in resazurin reduction was observed when LA, GA, and UV-A light were combined, suggesting that bacterial damages caused by this treatment dramatically impair the bacterial metabolic activity.
FIG 4.

Metabolic activities of E. coli O157:H7 cells upon incubation with gallic acid (GA), lactic acid (LA), and GA+LA with (a) and without (b) UV-A light exposure. The time required to achieve maximum fluorescence intensity indicates the relative level of metabolic activity of bacterial cells under different incubation conditions. Controls include E. coli cells exposed and not exposed to UV-A light without treatment with GA and LA.
A possible limitation of this assay is the strong dependence of the rate of resazurin reduction on the initial number of bacterial cells. Based on the results shown in Fig. 1, it is anticipated that 15 min of UV-A exposure in the presence of GA+LA would reduce the initial bacterial load and thus contribute to the observed delay in achieving maximum fluorescence intensity. To address the influence of the initial bacterial load on the time required to achieve maximum fluorescence intensity, resazurin was incubated with various levels of bacterial loads, ranging from 2 log CFU/ml to 6 log CFU/ml (see Fig. S2). It was observed that the delay in reaching maximum intensity caused by the combined treatment of organic acids and UV-A (Fig. 4b) could not be completely described by the reduction in the initial number of bacterial cells. For example, a reduction in bacterial count from 6 to 4.4 log CFU/ml without the treatment increased the time to reach maximum fluorescence intensity by 2.2 h, while the synergistic treatment for the same reduction in bacterial count increased the time to reach maximum fluorescence intensity by 4.5 h. The same trend was observed for GA- and LA-treated cells under UV-A light, where the times required for reaching maximum fluorescence intensity increased by 1.4 and 2.8 h, respectively, compared with that of the control. Thus, it can be inferred that the observed changes in metabolic activity are due to a combination of the influence of organic acids and UV-A treatment on the metabolic activity of cells in addition to a reduction in bacterial count.
Cell permeability.
The increase in permeation of SYTOX orange (SO) into E. coli cells was evaluated to monitor the changes in cell membrane permeability as a function of GA/LA treatment and UV-A light exposure (Fig. 5). The permeation of SO in control and various treatment sets was normalized with respect to the fluorescence signal observed for mechanically lysed cells, which represent the maximum SO permeation (100%). The results show a low level of SO dye permeation in the control cells without any treatment (10%). Exposure of control cells to UV-A did not significantly (P > 0.05) increase the permeability of cells to the SO dye. Organic acid treatment without UV-A irradiation increased SO permeation to approximately 30% of the maximum level, regardless of the acid used (i.e., GA and/or LA). Upon exposure to UV-A light, further changes in cell permeability were dependent on the type of organic acid or their combination. GA in combination with UV-A light did not significantly increase (P > 0.05) permeability compared with that of GA treatment without UV-A light. By contrast, when LA was present, either by itself or in combination with GA, the permeability increased significantly (P < 0.05) to more than 50% of the maximum level. Thus, interactions of LA with UV-A light increased the extent of membrane damage.
FIG 5.

Permeation of SYTOX orange into E. coli O157:H7 cells treated with gallic acid (GA), lactic acid (LA), and GA+LA with (L+) and without (L−) exposure to UV-A light. To assess the relative increase in cell permeability, the results were normalized with respect to the mechanically lysed cells, representing 100% permeation of the dye. Control consists of E. coli cells suspended in sterile water. The results marked with different lowercase letters were statistically significant (P < 0.05).
Intracellular thiol content.
Intracellular thiol is a comprehensive biomarker that indicates the redox balance in cells and is often affected by oxidative stress. Changes in the total intracellular thiol content of E. coli cells treated with GA/LA and exposed or not exposed to UV-A light were characterized, and the results are represented in Fig. 6. Control cells not exposed to any stress (i.e., light or acid) had the highest level of total thiol content—approximately 80 μM—compared with that of treated cells. Once cells were exposed to UV-A light alone for 30 min, some oxidative stress was generated, thereby reducing the intracellular thiol content. A similar behavior was observed when the E. coli cells were treated—without exposure to UV-A light—with GA, LA, or GA+LA, where the total thiol content was reduced compared with that of the control (the total thiol content ranged between 50 and 60 μM). In addition, when E. coli cells were treated with GA or LA in the presence of UV-A light, no significant increase in thiol oxidation was observed (P > 0.05). In fact, the individual effect of UV-A light, GA, LA, or GA+LA or the combined effect of GA+UV-A light or LA+UV-A light presented the same outcome (P > 0.05) on the oxidation of intracellular thiols compared with that of the control bacteria. On the other hand, the only treatment that showed a significantly increased reduction of thiol content (P < 0.05) was GA+LA under UV-A light, highlighting the synergistic interaction between them. The investigated synergistic antimicrobial activity seems to be correlated with a decrease in intracellular thiol content, suggesting that the highest level of microbial inactivation observed in Fig. 1 may be due to a stronger oxidative stress induced by the combination of GA+LA+UV-A light.
FIG 6.

Changes in the total intracellular thiol content of E. coli O157:H7 cells upon treatment with gallic acid (GA), lactic acid (LA), and GA+LA with (L+) and without (L−) exposure to UV-A light. Control consists of E. coli cells suspended in sterile water. The results marked with different lowercase letters were statistically significant (P < 0.05).
Inactivation of E. coli O157:H7 on produce surfaces.
The results of the inactivation of E. coli O157:H7 inoculated on lettuce and spinach surfaces and exposed to UV-A light are shown in Fig. 7. As expected, there was no reduction in bacterial count for E. coli cells spotted on model produce surfaces and treated with UV-A light in the absence of GA and LA. On the other hand, the presence of GA+LA during UV-A light exposure led to a reduction in bacterial count from 4.0 ± 0.1 log CFU/leaf to 2.2 ± 0.2 log CFU/leaf on spinach surfaces and from 4.0 ± 0.1 log CFU/leaf to 1.1 ± 0.3 log CFU/leaf on lettuce surfaces. These results are in agreement with the observations reported in Fig. 2, where UV-A light irradiation with GA+LA was able to reduce bacterial counts in solution from 4 log CFU/ml to levels between 1.2 and 2.4 CFU/ml. These results suggest that the synergistic antimicrobial activity among GA, LA, and UV-A light was effective in reducing levels of inoculated bacteria on fresh produce surfaces.
FIG 7.
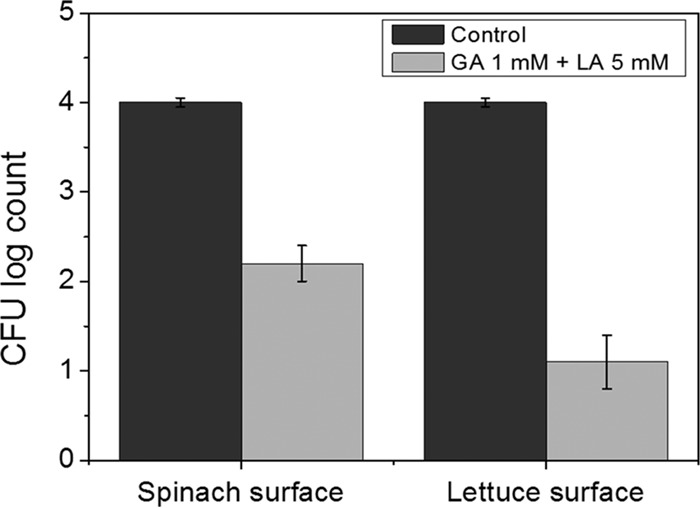
Inactivation of E. coli O157:H7 inoculated on the surfaces of spinach and lettuce leaves treated with 1 mM gallic acid (GA) plus 5 mM lactic acid (LA) and exposed to UV-A light. Control consists of E. coli cells treated with sterile water without GA and LA and exposed to UV-A light. The initial concentration of inoculated bacteria was 4 log CFU/leaf. The limit of detection of the plate counting method was 1 log CFU/leaf.
DISCUSSION
UV-A light-mediated bacterial damage.
UV light irradiation has been used extensively as a process technology for reducing microbial load. Although the biological effects of UV-B and UV-C irradiation on bacterial cells are well known, UV-A irradiation effects are not yet fully understood (34, 35). It has been suggested that exposure to a high UV-A fluence rate (100 to 2,000 kJ · m−2) reduces the activities of several key enzymes, such as catalase, ATPase, and glutathione reductase, as well as causes an increase in membrane depolarization and permeabilization (36).
In this study, 30 min of UV-A exposure corresponds to a fluence rate of approximately 57 kJ · m−2. Under this condition, there was no change in membrane permeability, as indicated by SO permeation (Fig. 5). Prior studies suggest that UV-A irradiation can cause membrane permeabilization, but only at a high fluence rate (>1,000 kJ · m−2) (37, 38). By contrast, a significant change in the intracellular thiol content (an approximately 25% decrease in the initial thiol content) was observed for E. coli cells exposed to UV-A light. This reduction in the thiol content level corresponds to the UVA-induced sublethal oxidative stress that may reduce the total thiol content from biomolecules such as proteins and glutathiones (36, 39). On the basis of the results of the resazurin assay, a small increase in the time to achieve maximum fluorescence intensity was observed compared with that in the controls. This increase in time indicates reduced cellular metabolic activity. Together with the observed reduction in thiol content, the results from the resazurin assay indicate that some key metabolic components are affected during UV-A light exposure. This observation is supported by the literature (36, 40). The results from one of these studies have suggested that the respiratory chain may be the cell's Achilles heel during UV-A inactivation (36). It was observed that UV-A exposure led to a reduction in the activity of several key respiratory-chain enzymes, including NADH oxidase, which is directly correlated with resazurin metabolism (41). In addition, a significant inhibition of the activities of these enzymes is reported with fluence rates as low as 50 kJ · m−2 (36), which corresponds to the fluence rate experienced by the E. coli cells in this study. It is important to note that the culturability of the UV-A-treated E. coli cells was not affected, meaning that the damages caused by the UV-A light itself were sufficiently low enough for the cells to recover from it during plating. In fact, significant changes in culturability (>1 log CFU/ml) have been reported only for fluence rates higher than 500 kJ · m−2 (37, 42).
GA/LA-mediated bacterial damage without UV-A light.
Similar to UV-A light irradiation alone, the individual effect of GA, LA, or GA and LA combined did not present strong antimicrobial activity, where less than 1 log CFU/ml of bacteria was inactivated. The limited antimicrobial activity of these organic acids is likely due to the short exposure time, up to 30 min, and the low concentrations of GA and LA, 1 mM and 5 mM, respectively. In fact, most studies reporting GA and LA as antimicrobial agents are based on a longer incubation time (1 to 24 h) and/or usually at higher concentration levels (30 to 300 mM) (27, 43–46).
Despite the low levels of antimicrobial activity, the selected organic acids or their combination caused significant biological damages. One of the observed damages was an increase in membrane permeability. Bacterial cells exposed to GA, LA, or GA and LA combined experienced 3 times more SO permeation than the control cells. In general, organic acids are reported to increase permeabilization of bacterial cells (20, 21, 43, 47). A recent study showed that the treatment of E. coli with acetic acid, lactic acid, and citric acid led to a higher level of nucleic acid staining, where fluorescent dyes similar to SO were used (20). Lactic acid has also been reported to cause damage to the cellular membrane of E. coli, which in turn may contribute to an increase in permeabilization (43).
In addition to affecting membrane permeabilization, GA, LA, and GA+LA treatments led to a decrease in the intracellular thiol content. In fact, treatment with these organic acids lowered the intracellular thiol content to levels similar to that with UV-A light itself. The most likely reason for the decrease in intracellular thiol content is the oxidation of free thiols to disulfides. Organic acids, such as GA and LA, can dissociate upon entering the cell, thereby lowering the cytoplasmic pH and disturbing the intracellular redox balance (48). When the intracellular pH is lowered, complex stress response systems are induced. These stress responses can change the metabolic activity of cells to reduce the impact of oxidative stress (49). One of the primary defense systems in cells against oxidative stress, including acid stress, involves low-molecular-mass thiols such as glutathiones and cysteines. These thiol molecules often react faster with the oxidizing agents, getting reversibly oxidized and, consequently, lowering the total intracellular thiol content (39, 50). The results from the resazurin metabolic activity assay suggest that, similar to that with the UV-A treatment, the metabolic activity of bacterial cells is reduced upon treatment with GA, LA, and GA+LA. These results are supported by those from prior studies that indicate changes in intracellular redox activity as well as the depletion of ATP levels in some Gram-negative bacteria strains upon exposure to organic acids (48, 51).
Synergistic combination of UV-A light with GA and LA.
Individual treatments of GA or LA with UV-A light showed limited microbial reduction (<1.5 log CFU/ml), while the combination treatment of both acids with UV-A light led to a significantly higher microbial reduction (4.7 log CFU/ml). In addition, the intensities of biochemical damages (thiol oxidation and metabolic activity) in bacteria increased upon exposure to GA+LA with UV-A light compared with that from individual treatments of GA or LA with UV-A.
Some studies have reported that certain organic acids in combination with light exposure can increase microbial inactivation (25, 26, 52–54). Most of these studies were performed using a blue light-emitting diode (LED) light (380 to 420 nm) in contrast to the UV-A light (320 to 400 nm) used in this report. GA was able to reduce E. coli populations by 5 log CFU/ml when exposed to blue LED light for 10 min (26). Citric acid, caffeic acid, and chlorogenic acid have also been reported to inactivate 5 log CFU/ml of E. coli cells after 10- to 20-min light exposure (52, 53). In addition, other microbes, such as Staphylococcus aureus and Listeria monocytogenes, were also susceptible to the combined exposure of LED light and GA (52, 53). However, it is important to highlight that the light source used in these previous studies presented higher light intensities (between 80 and 260 mW · cm−2) than the light source used in this report (3.2 mW · cm−2). Consequently, the total light fluence experienced by the GA and the microbes in those prior studies was also considerably higher (between 720 and 1,560 kJ · m−2). Our research group has previously reported the antimicrobial activity of UV-A and GA exposure, showing that 5-log CFU/ml inactivation of E. coli can be achieved with a combination of 10 mM GA and a low light fluence of approximately 25 kJ · m−2 (25). In this report, a 4- to 5-log CFU/ml reduction in microbial load was achieved by the sublethal concentrations of GA+LA after exposure to a light fluence of 57.6 kJ · m−2. Therefore, the combination of GA and UV-A light, instead of blue LED light, presented considerable improvement in microbial inactivation efficiency.
The mechanism of action for the antimicrobial activity of organic acids under light radiation is still not well understood. Previous studies suggested that the generation of reactive oxygen species (ROS) was the main mechanism for the antimicrobial activity of LED-excited GA (26, 52). It was reported that GA could generate hydroxyl radicals (·OH) and hydrogen peroxide (H2O2) upon light exposure, which in turn could cause lipid peroxidation on the cellular membrane (26). In contrast to these previous studies, the experimental evidence in this study indicates that ROS generation may not be the only mechanism for the enhanced activity of GA+LA in the presence of UV-A light. Based on the experimental measurements from this study, the results illustrated that antimicrobial activity was strongly influenced by the metabolic state of bacterial cells (i.e., the lack of antimicrobial response at 4°C and also in the presence of sodium azide). The results of this study also indicate that antimicrobial efficacy was not influenced by the initial bacterial concentration. Together, these observations suggest that the metabolic state of the cell has a significant impact on the antimicrobial activity of GA+LA in the presence of UV-A light and that the classical ROS activity may not be the leading mechanism for microbial inactivation.
The intracellular metabolism of microbes, such as Gram-negative bacteria, is very complex. There are several possible explanations for the increase in UV-A-mediated antimicrobial activity when GA and LA are combined. One possibility could be that each component has a different intracellular target. For instance, LA can increase the permeability of cell membrane as well as reduce the intracellular pH. On the other hand, GA could be interacting with specific enzymes, such as glutathione S-transferase and glutathione reductase, considerably reducing their activity as previously suggested (55). Furthermore, UV-A light treatment can reduce the activity of respiration-chain enzymes (36). Another possibility is that LA or GA could be metabolized by the bacterial cells and their metabolites could play an important role on microbial inactivation. For example, GA and LA can both be metabolized by some Gram-negative bacteria to form pyruvic acid and further metabolized to form acetic acid, which in turn has been linked with reducing ATP levels in Gram-negative bacteria (51, 56). It is also possible that the cumulative stress induced by UV-A and the combination of organic acids is lethal to cells based on their broad impacts on metabolic activity, ATP depletion, and the oxidation of intracellular thiols.
The findings in this work highlight that the combination of two different organic acids (GA+LA) can act synergistically under UV-A light, causing intracellular damages (i.e., membrane permeabilization and thiol oxidation) and leading to microbial inactivation. In addition, synergistic antimicrobial activity was observed at sublethal organic acids concentrations and low light fluence compared with those previously reported (25, 26, 54). This work also helps to advance the mechanistic understanding of light-mediated bacterial inactivation, where the metabolic state of cells is a key factor influencing bacterial inactivation. Although, more detailed molecular analysis using discovery tools, such as proteomic and metabolomic analyses, are needed to completely understand the antimicrobial mechanisms of organic acids under UV-A light. Lastly, the reported ability to inactivate bacteria on the surfaces of lettuce and spinach leaves demonstrates that this antimicrobial approach can be translated to food applications. Further studies are needed to determine the optimal process conditions, including light intensity and concentrations of selected sanitizers, to reduce the time required for inactivation of bacteria on diverse food substrates.
MATERIALS AND METHODS
Reagents.
Gallic acid (GA), lactic acid (LA), reduced l-glutathione (GSH), sodium chloride (NaCl), hydrochloric acid (HCl), resazurin sodium salt, sodium azide (NaN3), sodium dodecyl sulfate (SDS), EDTA, Triton X-100, and carbonate/bicarbonate buffer were obtained from Sigma-Aldrich (St. Louis, MO, USA). SYTOX orange (SO), a dye that stains nucleic acids, was purchased from Molecular Probes (Eugene, OR, USA). Zirconia-silica beads (500-μm diameter) were acquired from Biospec Products (Bartlesville, OK, USA). Lysogeny broth (LB), tryptic soy broth (TSB), tryptic soy agar (TSA), phosphate-buffered saline (PBS), Tris-hydrochloride ([Tris-HCl] 1 M), and 5,5-dithiobis-(2-nitrobenzoic acid) ([DTNB] Ellman's reagent) were purchased from Fisher BioReagents (Pittsburgh, PA, USA). Ultrapure water was obtained using a Milli-Q filtration system (EMD Millipore, Billerica, MA, USA).
Microbial culture.
Escherichia coli O157:H7 (ATCC 700728; Manassas, VA, USA) was provided by Linda Harris from the Department of Food Science and Technology at the University of California, Davis. This strain is depleted of Shiga toxin-like genes and has been modified with a rifampin resistance plasmid. Bacteria were grown overnight in LB containing rifampin (50 μg · ml−1) at 37°C with shaking at 150 rpm until an absorbance of 1.5 units, reflecting a stationary phase, was reached (approximately 7 × 108 CFU · ml−1).
Fresh bacterial suspensions were prepared before each experiment and centrifuged at 10,000 × g for 10 min, and bacterial pellets were washed before being resuspended in sterile water to a final concentration of 2.0 × 104, 2.0 × 106, or 2.0 × 108 CFU · ml−1.
UV-A light-mediated antimicrobial activity.
The antimicrobial activities of GA, LA, and a combination of GA+LA were evaluated in the presence and absence of UV-A light. Samples containing 106 CFU · ml−1 bacteria were prepared by mixing one milliliter of the organic acids solution with one milliliter of the E. coli suspension to achieve a final concentration of 1 mM GA, 5 mM LA, or 1 mM GA plus 5 mM LA. Experiments with the addition of sterile water instead of GA or LA were also carried out as a control. To evaluate the influence of the initial bacterial load during the UV-A light-mediated inactivation of bacteria, experiments were also conducted using 104 and 108 CFU · ml−1 bacteria. The effect of pretreatment with NaN3 was also assessed. A bacterial suspension (2.0 × 106 CFU · ml−1) was treated with 0.5% (wt/vol) NaN3 for 1 h at room temperature. One milliliter of the NaN3-pretreated bacterial suspension was centrifuged (16,000 × g for 2 min) and the cells were resuspended in 2 ml of a 1 mM GA plus 5 mM LA solution and treated with UV-A light.
UV-A light exposures of the samples were performed using a benchtop UV-A chamber with four UV-A lamps (320 to 400 nm, 18 W; Actinic BL, Philips, Holland) on the underside of the lid of a closed plastic box (Suncast Corporation, Batavia, IL, USA) as previously reported (57). Two milliliters of each sample was poured in individual wells of a sterile 12-well flat-bottom polystyrene plate. The plate was placed at a distance of 8 cm from the UV-A lamps at the center of the chamber. The average light intensity of the UV-A light was 3.2 ± 0.2 mW · cm−2. Aliquots were withdrawn after 0, 15, and 30 min of exposure, were serially diluted in sterile PBS, and were spread on TSA plates containing 50 μg · ml−1 rifampin. The plates were incubated for up to 48 h at 37°C before bacteria were counted by the standard plate counting method. E. coli populations were determined and expressed as CFU log counts. Antimicrobial experiments in the absence of UV-A light were also performed.
The UV-A-mediated antimicrobial activity of GA+LA was also evaluated at 4°C. For this experiment, the UV-A light system described above was set up in a cold room (4°C), where all solutions, including the bacterial suspensions, were preincubated for at least 1 h before sample preparation and UV-A light exposure. The bacterial samples were prepared and treated as described above.
Fluorescence assay of metabolic resazurin reduction.
Metabolic activities of E. coli O157:H7 cells treated with GA, LA, and GA+LA with and without exposure to UV-A light were assessed by monitoring the reduction of resazurin to its fluorescent form, resorufin, by metabolically active cells (41). Bacterial cells (106 CFU · ml−1) were incubated with GA, LA, or GA+LA following exposure to UV-A light for 15 min, as described in the previous section. After treatment, 1 ml of each sample was transferred into a 1.5-ml sterile tube and centrifuged (16,000 × g for 2 min). The supernatants were discarded and the recovered bacterial cells were resuspended in 1 ml of TSB containing 50 μM resazurin and transferred to individual wells of a 24-well transparent flat-bottom polystyrene plate. The plate was incubated at 37°C for 16 h, and resazurin reduction was monitored with a fluorescence plate reader (Tecan SpectraFluor Plus) using a 530-nm excitation filter and a 580-nm emission filter. Control measurements with untreated cells were also carried out using the same set of experimental conditions.
Cell permeability assessment.
Changes in the cell permeability after exposure to GA, LA, and GA+LA in the presence or absence of UV-A light were monitored using the SO dye, a cell-impermeable dye that can permeate only membrane-compromised cells (58). SYTOX orange has weak fluorescence in its native form, but intense fluorescence is observed when it binds to nucleic acids. The samples containing 108 CFU · ml−1 cells were prepared and treated as described previously. After 30 min of treatment (exposure or no exposure to UV-A light), 1 ml of each sample was transferred into a 1.5-ml sterile tube and centrifuged (16,000 × g for 2 min). The supernatants were discarded and the pellets were resuspended in 1 ml of sterile PBS. After complete resuspension of the pellet, 1 μl of a 5 mM stock solution of SO was added to each sample (final concentration, 5 μM), and the samples were gently vortexed and incubated at room temperature for 15 min. An aliquot of 150 μl of each sample was placed in a 96-well flat-bottom black polystyrene plate and fluorescence signals were recorded with a fluorescence plate reader (Tecan SpectraFluor Plus) using a 530-nm excitation filter and a 580-nm emission filter. The results were normalized with respect to the positive-control sample. The positive-control sample was prepared by mechanical lysis of the bacterial cells using zirconia-silica beads to mimic extensive membrane damage. To prepare the positive-control sample, one milliliter of the E. coli suspension was diluted with one milliliter of PBS, and the diluted bacterial suspension was vortexed in the presence of zirconia-silica beads at high speed for 10 min. After lysing, SO was added to the bacterial solution and incubated for 15 min, and the fluorescence intensity was recorded. The fluorescence intensity value of SO for the lysed cells simulated the maximum permeability, and the cell permeability data for each sample were expressed as a percentage relative to the positive control.
Intracellular bacterial thiol content quantification.
Results of the reduction in intracellular thiol contents of E. coli cells treated with GA and/or LA with and without exposure to UV-A light were evaluated. The intracellular thiol-containing compounds were extracted by lysing the bacterial cells through bead beating. First, 10 ml of each sample containing 108 CFU · ml−1 cells was treated in the presence or absence of UV-A light, as previously described, for 30 min. The 10-ml volume was placed in a 6-well flat-bottom polystyrene plate. After the treatment, samples were centrifuged (10,000 × g for 10 min) and the bacterial pellets were resuspended in 500 μl of a sterile lysis buffer (50 mM Tris-HCl, 25 mM NaCl, 25 mM EDTA, 2% SDS, and 1% Triton X-100) and transferred into a sterile 1.5-ml tube containing 400 μl of zirconia-silica beads. The bacterial suspensions were vortexed for 10 min and then centrifuged (16,000 × g for 10 min) before the supernatants to be used for the total thiol content measurements were recovered. The total thiol content was quantified using the well-established Ellman's assay with some modifications (59). Briefly, 25 μl of the obtained lysates was added to 100 μl of 0.05 M carbonate/bicarbonate buffer (pH 9.6) and 10 μl of 3 mM DTNB (Ellman's reagent) in the wells of a 96-well transparent flat-bottom polystyrene plate. The plate was incubated at room temperature in the dark for 30 min and the absorbance at 412 nm was measured using a plate reader (Spectramax 340). The total thiol content (μM) was determined using a standard curve based on known concentration levels of reduced glutathione (GSH).
Inactivation of E. coli O157:H7 on produce surfaces.
Fresh romaine lettuce hearts (Lactuca sativa var. longifolia) and fresh baby spinach leaves (Spinacia oleracea) were purchased from a local retailer and stored at 4°C until use. The lettuce and spinach leaves were rinsed with sterile water and then cut in disks with diameters of 2 cm. Ten microliters of a suspension of 106 CFU · ml−1 E. coli O157:H7 was spotted on the center of the leaf disks (104 CFU/leaf) and incubated at room temperature for 10 min. One hundred microliters of a solution containing 1 mM GA and 5 mM LA was added to the inoculated leaf surfaces. The disks were then transferred to the light chamber and exposed to UV-A light for 30 min as previously described. After light exposure, each disk was transferred to a 15-ml tube containing 10 ml of PBS. Silica carbide particles of 1-mm size were added to the tubes that were then vortexed for 2 min to fully detach the bacteria from the surfaces of the leaf disks. Aliquots were withdrawn, were serially diluted in sterile PBS, and were spread on TSA plates containing 50 μg · ml−1 of rifampin. The plates were incubated for up to 48 h at 37°C before plate counting. E. coli O157:H7 populations were determined and expressed as CFU log counts. Experiments with sterile water applied to the inoculated leaf surfaces and exposed to UV-A light were also carried out as a control.
Statistical analysis.
Statistical analysis was conducted using one-way analyses of variance (ANOVAs), and the pairwise differences were evaluated using Tukey's range test to identify significant differences between each sample group (P < 0.05).
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by the Agriculture and Food Research Initiative by grant no. 2014-67017-21642 from the USDA National Institute of Food and Agriculture (USDA-NIFA) Program in Improving Food Quality (A1361) and by grant no. 2015-68003-23411 from the USDA-NIFA Program Enhancing Food Safety through Improved Processing Technologies (A4131). CAPES/BR supported this work through a “Science without Borders” scholarship to Erick F. de Oliveira (recipient no. 11897-13-9).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00383-17.
REFERENCES
- 1.Olivier SA, Bull MK, Stone G, van Diepenbeek RJ, Kormelink F, Jacops L, Chapman B. 2011. Strong and consistently synergistic inactivation of spores of spoilage-associated Bacillus and Geobacillus spp. by high pressure and heat compared with inactivation by heat alone. Appl Environ Microbiol 77:2317–2324. doi: 10.1128/AEM.01957-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim S-J, Kim D-K, Kang D-H. 2015. Using UVC light-emitting diodes at wavelengths of 266 to 279 nanometers to inactivate foodborne pathogens and pasteurize sliced cheese. Appl Environ Microbiol 82:11–17. doi: 10.1128/AEM.02092-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guionet A, Joubert-Durigneux V, Packan D, Cheype C, Garnier J-P, David F, Zaepffel C, Leroux R-M, Teissié J, Blanckaert V. 2014. Effect of nanosecond pulsed electric field on Escherichia coli in water: inactivation and impact on protein changes. J Appl Microbiol 117:721–728. doi: 10.1111/jam.12558. [DOI] [PubMed] [Google Scholar]
- 4.Polydera AC, Stoforos NG, Taoukis PS. 2005. Quality degradation kinetics of pasteurised and high pressure processed fresh navel orange juice: Nutritional parameters and shelf life. Innov Food Sci Emerg Technol 6:1–9. doi: 10.1016/j.ifset.2004.10.004. [DOI] [Google Scholar]
- 5.Terefe NS, Kleintschek T, Gamage T, Fanning KJ, Netzel G, Versteeg C, Netzel M. 2013. Comparative effects of thermal and high pressure processing on phenolic phytochemicals in different strawberry cultivars. Innov Food Sci Emerg Technol 19:57–65. doi: 10.1016/j.ifset.2013.05.003. [DOI] [Google Scholar]
- 6.Van Haute S, Sampers I, Holvoet K, Uyttendaele M. 2013. Physicochemical quality and chemical safety of chlorine as a reconditioning agent and wash water disinfectant for fresh-cut lettuce washing. Appl Environ Microbiol 79:2850–2861. doi: 10.1128/AEM.03283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan M, Kjos M, Nes IF, Diep DB, Lotfipour F. 2012. Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. J Appl Microbiol 113:723–736. doi: 10.1111/j.1365-2672.2012.05338.x. [DOI] [PubMed] [Google Scholar]
- 8.Park S-H, Choi M-R, Park J-W, Park K-H, Chung M-S, Ryu S, Kang D-H. 2011. Use of organic acids to inactivate Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh apples and lettuce. J Food Sci 76:M293–M298. doi: 10.1111/j.1750-3841.2011.02205.x. [DOI] [PubMed] [Google Scholar]
- 9.Siroli L, Patrignani F, Serrazanetti DI, Tabanelli G, Montanari C, Gardini F, Lanciotti R. 2015. Lactic acid bacteria and natural antimicrobials to improve the safety and shelf-life of minimally processed sliced apples and lamb's lettuce. Food Microbiol 47:74–84. doi: 10.1016/j.fm.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Pan X, Nakano H. 2014. Effects of chlorine-based antimicrobial treatments on the microbiological qualities of selected leafy vegetables and wash water. Food Sci Technol Res 20:765–774. doi: 10.3136/fstr.20.765. [DOI] [Google Scholar]
- 11.Martínez-Graciá C, González-Bermúdez CA, Cabellero-Valcárcel AM, Santaella-Pascual M, Frontela-Saseta C. 2015. Use of herbs and spices for food preservation: advantages and limitations. Curr Opin Food Sci 6:38–43. doi: 10.1016/j.cofs.2015.11.011. [DOI] [Google Scholar]
- 12.Lavieri NA, Sebranek JG, Brehm-Stecher BF, Cordray JC, Dickson JS, Horsch AM, Jung S, Larson EM, Manu DK, Mendonça AF. 2014. Investigating the control of Listeria monocytogenes on a ready-to-eat ham product using natural antimicrobial ingredients and postlethality interventions. Foodborne Pathog Dis 11:462–467. doi: 10.1089/fpd.2013.1702. [DOI] [PubMed] [Google Scholar]
- 13.Bae Y-M, Lee S-Y. 2015. Combined effects of organic acids and salt depending on type of acids and pathogens in laboratory media and acidified pickle. J Appl Microbiol 119:455–464. doi: 10.1111/jam.12845. [DOI] [PubMed] [Google Scholar]
- 14.Xin B, Zheng J, Xu Z, Li C, Ruan L, Peng D, Sun M. 2015. Three novel lantibiotics, ticins A1, A3, and A4, have extremely stable properties and are promising food biopreservatives. Appl Environ Microbiol 81:6964–6972. doi: 10.1128/AEM.01851-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung Y, Matthews KR. 2015. Chapter 17. Development and application of novel antimicrobials in food and food processing, p 347–364. In Chen CY, Yan X, Jackson CR (ed), Antimicrobial resistance and food safety: methods and techniques. Academic Press, San Diego, CA. [Google Scholar]
- 16.Juneja VK, Dwivedi HP, Yan X. 2012. Novel natural food antimicrobials. Annu Rev Food Sci Technol 3:381–403. doi: 10.1146/annurev-food-022811-101241. [DOI] [PubMed] [Google Scholar]
- 17.Gharsallaoui A, Oulahal N, Joly C, Degraeve P. 2016. Nisin as a food preservative: part 1: physicochemical properties, antimicrobial activity, and main uses. Crit Rev Food Sci Nutr 56:1262–1274. doi: 10.1080/10408398.2013.763765. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan GA, Jackson-Davis AL, Schrader KD, Xi Y, Kulchaiyawat C, Sebranek JG, Dickson JS. 2012. Survey of naturally and conventionally cured commercial frankfurters, ham, and bacon for physio-chemical characteristics that affect bacterial growth. Meat Sci 92:808–815. doi: 10.1016/j.meatsci.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Tirawat D, Phongpaichit S, Benjakul S, Sumpavapol P. 2016. Microbial load reduction of sweet basil using acidic electrolyzed water and lactic acid in combination with mild heat. Food Control 64:29–36. doi: 10.1016/j.foodcont.2015.12.015. [DOI] [Google Scholar]
- 20.Kim SA, Rhee MS. 2013. Marked synergistic bactericidal effects and mode of action of medium-chain fatty acids in combination with organic acids against Escherichia coli O157:H7. Appl Environ Microbiol 79:6552–6560. doi: 10.1128/AEM.02164-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosein AM, Breidt F, Smith CE. 2011. Modeling the effects of sodium chloride, acetic acid, and intracellular pH on survival of Escherichia coli O157:H7. Appl Environ Microbiol 77:889–895. doi: 10.1128/AEM.02136-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirai A, Kajiura M, Omasa T. 2015. Synergistic photobactericidal activity based on ultraviolet-A irradiation and ferulic acid derivatives. Photochem Photobiol 91:1422–1428. doi: 10.1111/php.12507. [DOI] [PubMed] [Google Scholar]
- 23.Ait-Ouazzou A, Espina L, García-Gonzalo D, Pagán R. 2013. Synergistic combination of physical treatments and carvacrol for Escherichia coli O157:H7 inactivation in apple, mango, orange, and tomato juices. Food Control 32:159–167. doi: 10.1016/j.foodcont.2012.11.036. [DOI] [Google Scholar]
- 24.Feyaerts J, Rogiers G, Corthouts J, Michiels CW. 2015. Thiol-reactive natural antimicrobials and high pressure treatment synergistically enhance bacterial inactivation. Innov Food Sci Emerg Technol 27:26–34. doi: 10.1016/j.ifset.2014.12.005. [DOI] [Google Scholar]
- 25.Cossu A, Ercan D, Wang Q, Peer WA, Nitin N, Tikekar RV. 2016. Antimicrobial effect of synergistic interaction between UV-A light and gallic acid against Escherichia coli O157:H7 in fresh produce wash water and biofilm. Innov Food Sci Emerg Technol 37 Part A:44–52. doi: 10.1016/j.ifset.2016.07.020. [DOI] [Google Scholar]
- 26.Nakamura K, Yamada Y, Ikai H, Kanno T, Sasaki K, Niwano Y. 2012. Bactericidal action of photoirradiated gallic acid via reactive oxygen species formation. J Agric Food Chem 60:10048–10054. doi: 10.1021/jf303177p. [DOI] [PubMed] [Google Scholar]
- 27.Borges A, Ferreira C, Saavedra MJ, Simões M. 2013. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb Drug Resist 19:256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- 28.Elramady MG, Aly SS, Rossitto PV, Crook JA, Cullor JS. 2013. Synergistic effects of lactic acid and sodium dodecyl sulfate to decontaminate Escherichia coli O157:H7 on cattle hide sections. Foodborne Pathog Dis 10:661–663. doi: 10.1089/fpd.2012.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tajkarimi M, Ibrahim SA. 2011. Antimicrobial activity of ascorbic acid alone or in combination with lactic acid on Escherichia coli O157:H7 in laboratory medium and carrot juice. Food Control 22:801–804. doi: 10.1016/j.foodcont.2010.11.030. [DOI] [Google Scholar]
- 30.Ibrahim SA, Yang H, Seo CW. 2008. Antimicrobial activity of lactic acid and copper on growth of Salmonella and Escherichia coli O157:H7 in laboratory medium and carrot juice. Food Chem 109:137–143. doi: 10.1016/j.foodchem.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 31.Mosqueda-Melgar J, Raybaudi-Massilia RM, Martín-Belloso O. 2008. Combination of high-intensity pulsed electric fields with natural antimicrobials to inactivate pathogenic microorganisms and extend the shelf-life of melon and watermelon juices. Food Microbiol 25:479–491. doi: 10.1016/j.fm.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Stannard JN, Horecker BL. 1948. The in vitro inhibition of cytochrome oxidase by azide and cyanide. J Biol Chem 172:599–608. [PubMed] [Google Scholar]
- 33.Lichstein HC, Soule MH. 1944. Studies of the effect of sodium azide on microbic growth and respiration. J Bacteriol 47:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santos AL, Oliveira V, Baptista I, Henriques I, Gomes NCM, Almeida A, Correia A, Cunha Â. 2013. Wavelength dependence of biological damage induced by UV radiation on bacteria. Arch Microbiol 195:63–74. doi: 10.1007/s00203-012-0847-5. [DOI] [PubMed] [Google Scholar]
- 35.Coohill TP, Sagripanti J-L. 2008. Overview of the inactivation by 254 nm ultraviolet radiation of bacteria with particular relevance to biodefense. Photochem Photobiol 84:1084–1090. [DOI] [PubMed] [Google Scholar]
- 36.Bosshard F, Bucheli M, Meur Y, Egli T. 2010. The respiratory chain is the cell's Achilles' heel during UVA inactivation in Escherichia coli. Microbiology 156:2006–2015. doi: 10.1099/mic.0.038471-0. [DOI] [PubMed] [Google Scholar]
- 37.Berney M, Weilenmann H-U, Egli T. 2006. Flow-cytometric study of vital cellular functions in Escherichia coli during solar disinfection (SODIS). Microbiology 152:1719–1729. doi: 10.1099/mic.0.28617-0. [DOI] [PubMed] [Google Scholar]
- 38.Pigeot-Rémy S, Simonet F, Atlan D, Lazzaroni JC, Guillard C. 2012. Bactericidal efficiency and mode of action: a comparative study of photochemistry and photocatalysis. Water Res 46:3208–3218. doi: 10.1016/j.watres.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 39.Bindoli A, Fukuto JM, Forman HJ. 2008. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid Redox Signal 10:1549–1564. doi: 10.1089/ars.2008.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sassoubre LM, Nelson KL, Boehm AB. 2012. Mechanisms for photoinactivation of Enterococcus faecalis in seawater. Appl Environ Microbiol 78:7776–7785. doi: 10.1128/AEM.02375-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Brien J, Wilson I, Orton T, Pognan F. 2000. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 42.Berney M, Weilenmann H-U, Ihssen J, Bassin C, Egli T. 2006. Specific growth rate determines the sensitivity of Escherichia coli to thermal, UVA, and solar disinfection. Appl Environ Microbiol 72:2586–2593. doi: 10.1128/AEM.72.4.2586-2593.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C, Chang T, Yang H, Cui M. 2015. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 47:231–236. doi: 10.1016/j.foodcont.2014.06.034. [DOI] [Google Scholar]
- 44.Byelashov OA, Daskalov H, Geornaras I, Kendall PA, Belk KE, Scanga JA, Smith GC, Sofos JN. 2010. Reduction of Listeria monocytogenes on frankfurters treated with lactic acid solutions of various temperatures. Food Microbiol 27:783–790. doi: 10.1016/j.fm.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Stanojević-Nikolić S, Dimić G, Mojović L, Pejin J, Djukić-Vuković A, Kocić-Tanackov S. 2016. Antimicrobial activity of lactic acid against pathogen and spoilage microorganisms. J Food Process Preserv 40:990–998. doi: 10.1111/jfpp.12679. [DOI] [Google Scholar]
- 46.Scott BR, Yang X, Geornaras I, Delmore RJ, Woerner DR, Adler JM, Belk KE. 2015. Antimicrobial efficacy of a lactic acid and citric acid blend against Shiga toxin-producing Escherichia coli, Salmonella, and nonpathogenic Escherichia coli biotype I on inoculated prerigor beef carcass surface tissue. J Food Prot 78:2136–2142. doi: 10.4315/0362-028X.JFP-15-194. [DOI] [PubMed] [Google Scholar]
- 47.Warnecke T, Gill RT. 2005. Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microb Cell Fact 4:25. doi: 10.1186/1475-2859-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lund P, Tramonti A, Biase DD. 2014. Coping with low pH: molecular strategies in neutralophilic bacteria. FEMS Microbiol Rev 38:1091–1125. doi: 10.1111/1574-6976.12076. [DOI] [PubMed] [Google Scholar]
- 49.Kanjee U, Houry WA. 2013. Mechanisms of acid resistance in Escherichia coli. Annu Rev Microbiol 67:65–81. doi: 10.1146/annurev-micro-092412-155708. [DOI] [PubMed] [Google Scholar]
- 50.Murphy MP. 2012. Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Antioxid Redox Signal 16:476–495. doi: 10.1089/ars.2011.4289. [DOI] [PubMed] [Google Scholar]
- 51.Tan SM, Lee SM, Dykes GA. 2015. Acetic acid induces pH-independent cellular energy depletion in Salmonella enterica. Foodborne Pathog Dis 12:183–189. doi: 10.1089/fpd.2014.1853. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura K, Ishiyama K, Sheng H, Ikai H, Kanno T, Niwano Y. 2015. Bactericidal activity and mechanism of photoirradiated polyphenols against Gram-positive and -negative bacteria. J Agric Food Chem 63:7707–7713. doi: 10.1021/jf5058588. [DOI] [PubMed] [Google Scholar]
- 53.McKenzie K, Maclean M, Timoshkin IV, MacGregor SJ, Anderson JG. 2014. Enhanced inactivation of Escherichia coli and Listeria monocytogenes by exposure to 405 nm light under sub-lethal temperature, salt and acid stress conditions. Int J Food Microbiol 170:91–98. doi: 10.1016/j.ijfoodmicro.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Ghate V, Kumar A, Zhou W, Yuk H-G. 2015. Effect of organic acids on the photodynamic inactivation of selected foodborne pathogens using 461 nm LEDs. Food Control 57:333–340. doi: 10.1016/j.foodcont.2015.04.029. [DOI] [Google Scholar]
- 55.Iio M, Kawaguchi H, Sakota Y, Otonari J, Nitahara H. 1993. Effects of polyphenols, including flavonoids, on glutathione S-transferase and glutathione reductase. Biosci Biotechnol Biochem 57:1678–1680. doi: 10.1271/bbb.57.1678. [DOI] [Google Scholar]
- 56.Nogales J, Canales Á Jiménez-Barbero J, Serra B, Pingarrón JM, García JL, Díaz E. 2011. Unravelling the gallic acid degradation pathway in bacteria: the gal cluster from Pseudomonas putida. Mol Microbiol 79:359–374. doi: 10.1111/j.1365-2958.2010.07448.x. [DOI] [PubMed] [Google Scholar]
- 57.Ercan D, Cossu A, Nitin N, Tikekar RV. 2016. Synergistic interaction of ultraviolet light and zinc oxide photosensitizer for enhanced microbial inactivation in simulated wash-water. Innov Food Sci Emerg Technol 33:240–250. doi: 10.1016/j.ifset.2015.11.015. [DOI] [Google Scholar]
- 58.Biggerstaff JP, Le Puil M, Weidow BL, Prater J, Glass K, Radosevich M, White DC. 2006. New methodology for viability testing in environmental samples. Mol Cell Probes 20:141–146. doi: 10.1016/j.mcp.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Ellman GL. 1959. Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


