Abstract
Mullerian Inhibiting Substance (MIS), a 140-kDa homodimer glycoprotein member of the TGF-β superfamily of biological-response modifiers, causes regression of the Mullerian ducts in developing male embryos. MIS also can induce growth arrest and apoptosis in ovarian and cervical cancer cell lines. The embryonic progenitor of the ovarian and cervical epithelium is the coelomic epithelium, the same tissue that regresses under the direction of MIS in the male. The endometrium and uterus also arise from the coelomic epithelium and the Mullerian ducts. Here, we show that both normal human endometrium and endometrial cancers express the receptor for MIS and that MIS can inhibit the proliferation of a number of human endometrial cancer cell lines that express the MIS type II receptor. In the representative endometrial cancer cell line AN3CA, MIS affects the expression of key cell-cycle regulatory proteins. This work broadens the scope of tumors that MIS can potentially control and, by elucidating the MIS signaling pathway, identifies other potential avenues for intervention.
Keywords: uterus, p130, E2F1, AN3CA, KLE
The Mullerian duct, which forms from coelomic epithelium (1, 2, 3), develops into the fallopian tubes, uterus, cervix, proximal vagina, and surface epithelium of the ovary in the female. These structures regress in the male embryo. The existence of Mullerian Inhibiting Substance (MIS) was predicted by in vivo experiments in which testicular fragments, but not testosterone, caused Mullerian duct regression in female rabbit embryos (4, 5). We speculated that tumors derived from Mullerian tissues or those expressing MIS type II receptors (MISRIIs) could respond to MIS in growth-inhibition assays (6-10). MIS signals through a two-receptor system. The MISRII, a transmembrane serine-threonine kinase, controls ligand-binding specificity. The gene for this receptor contains 11 exons and encodes a 63-kDa protein (11, 12, 13). MIS-bound MISRII phosphorylates the type I receptor, which is responsible for signal transduction; potential candidates for this receptor include ALK3 (14), ALK2 (15, 16), and ALK6 (17). The activated type I receptor phosphorylates receptor-specific Smads, initiating a cascade of not yet fully elucidated events leading to the morphologic changes characteristic of Mullerian duct regression (14, 15, 16). Mutations in MIS or MISRII in human receptors cause persistent Mullerian duct syndrome, in which males retain Mullerian ducts in the presence of testes and the Y chromosome (18, 19).
The fetal and postnatal Sertoli cells of the testis produce MIS; MIS levels decrease at the onset of puberty. In the female, ovarian granulosa cells synthesize MIS; hormone levels slowly increase after birth until adolescence, after which a steady-state low serum level persists throughout reproductive life. MIS levels then decrease and become negligible at menopause (20, 21). Although the role of MIS in the postgestational human is unclear, mouse MIS-knockout models provide evidence of its role in controlling growth. MIS-knockout mice demonstrate hyperandrogenism in males and follicular hyperstimulation in females. Loss-of-function mutations created in the mouse MISRII produce Leydig cell tumors in males and early follicular depletion in females (22, 23, 24). Therefore, lack of proper MIS signaling seems to predispose mice to uncontrolled division of MIS-responsive cells. MIS may play a similar homeostatic role in the adult human.
MIS can inhibit the growth of both ovarian and cervical neoplasms. Malignant cells contained in ascites collected from ovarian-cancer patients express MISRII, and colony formation is inhibited in vitro by MIS (6). Similarly, human ovarian cancer cell lines express the MISRII, and their proliferation is inhibited in vitro and in vivo by recombinant MIS (7, 25, 26). In addition, cervical cancer cell lines, which are also derived from the coelomic epithelium, express the MISRII, and treatment with MIS suppresses human cervical cancer cell line proliferation in vitro (9). Such results further support a role for MIS in postembryonic regulation of growth.
Investigation of the signal transduction pathway initiated by MIS in ovarian and cervical cancer cell lines revealed alterations in the protein levels of the cyclin-dependent kinase inhibitor (CDKI) p16 and of the retinoblastoma gene-product-related pocket proteins p130 and p107 (7, 9). These pocket proteins play an important role in progression through the cell cycle, acting as G1 checkpoints (27, 28). In the hypophosphorylated state, p107 and p130 bind and inhibit the activity of the E2F proteins, transcription factors that promote cell division. When p107 and p130 are phosphorylated by the cyclin-dependent kinase (CDK) complexes, they dissociate from the E2F proteins, and cell division can occur. The CDKIs, such as p16, p15, p21, p27, and p57, inhibit the kinase activity of the CDK complexes and allow p107 and p130 to remain in a hypophosphorylated state, preventing E2F activity and cell-cycle progression (28, 29, 30).
MIS treatment causes an increase in protein levels of the CDKI p16 in the ovarian cancer cell line OVCAR 8. In cervical cancer cell lines, MIS induces p16, p107, and p130 (7, 9). In contrast, here we investigated endometrial cancer cell lines whose proliferation is inhibited by MIS and that express the MISRII but do not express p16. MIS treatment induces cell-cycle arrest and apoptosis in these lines. One cell line, AN3CA, was selected as a representative example because it was the most responsive to MIS in vitro. These effects appear to be mediated through inductions of p107 and p130. Because MISRII is expressed in endometrial cancer, MIS holds potential as a treatment for this disease.
Materials and Methods
Purification of Recombinant Human MIS. Human MIS was transfected into CHO cells, which were maintained in a dedicated facility for use in this study. The CHO cells were alternately maintained in 5% female FBS (FFBS)-containing media and a serum-free defined media. MIS levels in MIS gene-transfected cells were measured in conditioned media by using a human MIS-specific ELISA (20).
MIS was purified from the serum-containing media by immunoaffinity chromatography described in detail in ref. 31, and it was purified from the serum-free media by a combination of ammonium sulfate precipitation or lectin affinity followed by FPLC anion-exchange chromatography, described by Lorenzo et al. in ref. 32, as a method to improve yield. The MIS purified by these methods had equivalent biological activity (31, 32).
Cell Lines and Cultures. All cell lines were obtained from the American Type Culture Collection in Rockville, MD. Four of the six commercially available human endometrial cancer cell lines were used in this study. The two lines not used were HEC-1B (a substrain of HEC-1A) and Sk-ut 1B, a line from a patient similar to the patient from which the AN3CA cells were obtained. AN3CA cells were maintained in Eagle's minimal essential medium (EMEM)/10% MIS-free FFBS. KLE cells were maintained in F-12/Ham's medium/10% FFBS. HEC-1A cells were maintained in McCoy's medium/10% FFBS, and RL95-2 cells were maintained in EMEM/10% FFBS. COS-7 cells, used as a negative control, were maintained in DMEM/10% FFBS. All cells were grown at 37°C in an atmosphere of 5% CO2. Cells were passed (1:4) upon reaching 80% confluency and were retired before 30 passages.
Human and Animal Tissue. Discarded human endometrium and endometrial cancer tissue samples were obtained through the Massachusetts General Hospital tissue bank and the hospital's Department of Pathology by using a protocol approved by the Institutional Review Board-Human Research Committee at the hospital. Rat adult tissues were harvested with the approval of the Massachusetts General Hospital institutional animal use committee.
Methylthiazoletetrazolium (MTT) Growth-Inhibition Assay. Cells were harvested at 80% confluency and plated in 96-well plates at 2,000 cells per well in 200 μl of media per well. At 24 h after plating, each lane of 10 wells was treated with MIS at 10 μg/ml (71 nM) (a dose twice that required for regression of the Mullerian ducts in organ culture) or with the PBS (pH 7.4) vehicle control. MIS and buffer control were added again after 4 days. At day 6 or day 8, depending on the growth characteristics of each cell line, the MTT assay was used to quantify the number of viable cells (33). Plates were read on an ELISA plate reader, and absorbance at 550 nm was recorded; this absorbance is proportional to the relative abundance of live cells in a given well. MIS-treated and untreated samples were compared; statistical analysis of results was performed by using the statview statistics program ANOVA, with P ≤ 0.05 conferring statistical significance.
Colony Assays. Cells were stably transfected at 80% confluence in 10-cm plates with 0.6 μg of a hygromycin-resistance plasmid and 5.4 μg of expression constructs consisting of empty vector, full-length MIS, leaderless (nonsecreted) MIS (34), p107, p130, E2F1, and a small interfering RNA vector for E2F1 by using FuGENE 6 transfection reagent (Roche Diagnostics, Indianapolis) and its accompanying protocol. Cells were maintained in media containing 25-100 μg/ml hygromycin for 3-4 weeks. MIS secreted into the conditioned media of these clones was measured by the MIS ELISA (20). The cells were then stained with crystal violet, and the number of colonies >50 cells in size was counted for each transfection and empty-vector control. Each experiment was done in triplicate, and the quantification of colonies was performed in a blinded fashion. Results were then analyzed as described above.
PCR Analyses. Normal and malignant endometrial tissues were obtained from the Tumor Bank and through the Department of Pathology at Massachusetts General Hospital in accordance with an approved Institutional Review Board protocol. RNA was extracted by using Concert Cytoplasmic RNA Reagent (Invitrogen) in accordance with the manufacturer's specifications. Three micrograms of RNA was reverse-transcribed with SuperScript II (Invitrogen) by using 50 μg/ml random primers. PCR was performed by using Platinum Taq (Invitrogen) and PCR enhancer (Invitrogen) in 0.5-1.5× concentration. Primers were used at 1 μM final concentration; the forward primer was GCTGGCTTATGCTCTTCTCCTTC, and the reverse primer was ACCTCGCACTCTGTAGTTCTTTCG. These primers produced a 582-bp product spanning exons 1-6 in the presence of cDNA for the MISRII. PCR was performed at 94°C for 2 min, then 95°C melting, 57°C annealing, and 65°C elongation for 45 cycles. Products were run on a 1% agarose gel. Bands of the appropriate size were extracted from gel by using a gel extraction kit (Qiagen, Valencia, CA) and were cloned by using the TA cloning system (Invitrogen); resulting clones were sequenced by the core DNA facility at Massachusetts General Hospital to confirm product identity.
Antibodies and Western Blot Analyses. Proteins from tissues and cells were harvested in RIPA buffer (50 mM Hepes, pH 7.0/150 nM NaCl/0.1% Triton X-100/0.1% sodium deoxycholate/0.1% SDS) with added protease inhibitors (Complete Midi Protease Inhibitors, Roche Diagnostics). Protein concentrations were determined by Bradford analysis. Proteins were reduced with 2-mercaptoethanol and separated by SDS/PAGE, with equal loading of protein in lanes that were to be compared, for expression varying between 40 and 100 μg per well, depending on the experiment. Proteins separated by electrophoresis were transferred to an Immobilon-P membrane (Millipore). Blots for antibodies were blocked in TBST (Tris-buffered saline and Tween 20: 25 mM Tris, pH 7.4/136 mM NaCl/5 mM KCl/0.1% Tween 20) containing milk (10% for p130 and 5% for other antibodies) for 1 h at room temperature. The p107 and p21 antibodies were incubated in 1% milk/TBST for 2 h at room temperature; the p130 antibody was incubated in 5% milk/TBST for 2 h at room temperature; the antibodies for E2F1, MISRII (153P), and Rb were incubated in 1% milk/TBST overnight at 4°C. After primary antibody incubation, blots were washed three times with 1% milk/TBST (5% milk for p130) and then incubated with the appropriate horseradish peroxidase-conjugated antibodies. Bound antibodies were detected by using ECL Detection Reagent (Amersham Biosciences). Experiments were performed in triplicate.
The MISRII rabbit polyclonal antibody (designated 153P) was generated by injecting animals with the kinase domain of the receptor expressed in Eschericia coli cells and was purified by histidine-affinity chromatography (M. Lorenzen, J. Lorenzen, P.K.D., and D.T.M., unpublished data). The antibody was ligand-affinity purified before use. The specificity of this antibody for the MISRII is documented in ref. 7. Antibodies for p107, p130, E2F1, Rb, and p21 were obtained from Santa Cruz Biotechnology. Antibody for p16 was purchased from BD Biosciences; antibody for cleaved caspase 3 was purchased from Cell Signaling Technology (Beverly, MA). Antibody for MISRII was diluted 1:500; antibodies for p107, p130, E2F1, Rb, p21, and cleaved caspase 3 were used at 1:1,000 dilution.
Immunohistochemistry of MISRII. Paraffin sections were deparaffinized for 60 min in a 60°C oven and then washed as follows: three times with 100% xylene for 5 min each, two times each with 100% ethanol and 95% ethanol, one time with 70% ethanol, and one time with 50% ethanol. Sections were hydrated to buffer and unmasked by microwaving at 85°C for 10 min in 0.01 M sodium citrate. Tissues were blocked by washing in 1% normal goat serum (in 0.1% BSA in PBS). The antigen-affinity purified 153P was incubated at 1:100 dilution overnight at 4°C. After washing in TBST (Tween 20 0.01%), the tissues were incubated in biotinylated goat anti-rabbit IgG at 1:200 dilution in TBS for 60 min. Tissues were colorized by using alkaline phosphatase blue (Vector Laboratories) for 10 min, washed in deionized water, and coverslipped with glycerol-gelatin medium.
FACS Analysis of the Cell Cycle. Cells were treated for 4 days with 71 nM MIS. They were then harvested with ice-cold PBS/EDTA with 1% serum and fixed in 95% ethanol. Cells were stained with 5 μg of propidium iodide in a 1% RNase A solution with 3.8 × 10-4 M sodium citrate for 30 min, after which they were immediately analyzed.
Results
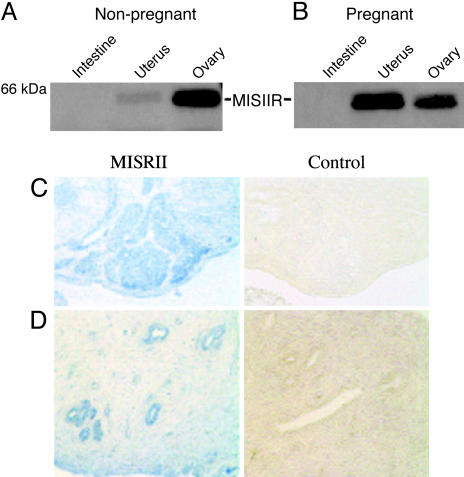
MISRII Expression in Normal and Malignant Endometrium. Western blot using 153P detected the MISRII protein in both pregnant and nonpregnant rat uterine extracts with equal protein loading, showing an increase in the expression of the receptor in the gravid uterus (see Fig. 1 A and B). The 63-kDa MISRII protein band also is seen in the ovary, which serves as a positive control, but not in the negative control tissue, the intestine (Fig. 1 A and B). Immunohistochemistry performed with this same antibody also detected MISRII reproducibly in the endometrium of the rat uterus; staining in the endometrium compares well with the signal seen in studies of adult rat ovary granulosa cells (Fig. 1 C and D).
Fig. 1.
MISRII protein is expressed in the rat uterus, ovary, and endometrium. (A and B) Samples of intestine, uterus, and ovary were harvested from nonpregnant (A) and pregnant (B) female rats. Western blots with the 153P antibody specific for the MISRII detected the MISRII protein in nonpregnant and pregnant uteri; expression of the MISRII protein appeared to increase in the uterus during pregnancy. All lanes were loaded with 75 μg of protein lysate. Rat ovary served as a positive control, and intestine served as a negative control. MISRII protein migrates as a 63-kDa band (n = 2in A and B). (C)(Left) Antibody stained granulosa cells in the adult rat ovary. (Right) This staining is not present when ovary is incubated with preimmune serum. (D) (Left) The receptor is also present in the endometrial portion of the rat uterus. (Right) Again, this staining is not present when the tissue is incubated with control rabbit serum (200×, n = 3).
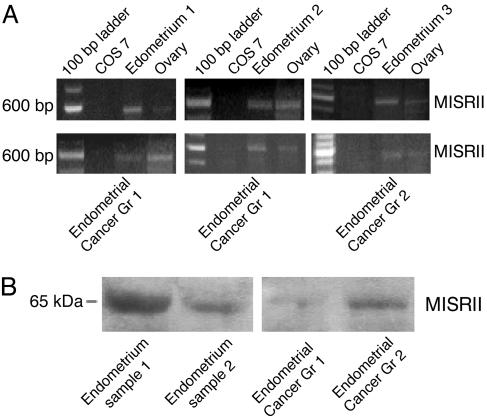
In the human, RT-PCR with primers specific for exons 1-5 (of 11) of the MISRII detected MISRII mRNA in normal endometrium from multiple samples of banked tissue harvested from discarded surgical specimens. These primers generated a PCR product of 582 bp for the spliced mRNA form of the receptor gene, and three representative examples are shown in Fig. 2A. The product identity was confirmed by direct sequencing (data not shown). Human endometrial cancers also express the MISRII. RT-PCR demonstrated the presence of the MISRII mRNA in 6 of 10 samples of grade 1 and in 1 of 2 samples of grade 2 endometriod carcinomas (Fig. 2 A).
Fig. 2.
MISRII is expressed in normal and cancerous human endometrium. (A) PCR products spanning exons 1-5 of the MISRII gene are evident as 582-bp bands in three representative samples of normal tissue, two representative grade 1 endometrial cancers, and one grade 2 lesion. Samples of ovary (positive control) and COS 7 cells (negative control derived from green monkey kidney cells) are shown for comparison. (B) Western analysis using the MISRII antibody 153P confirms the presence of the receptor in the normal (Left) and cancerous (Right) endometrium. Data are representative of three replicate experiments.
Western blots with the 153P antibody detected MISRII protein in normal endometrial tissues (Fig. 2B Left, which is representative of three replicate experiments), and similar analyses also detected MISRII protein in both the grade 1 and 2 carcinomas (Fig. 2B Right).
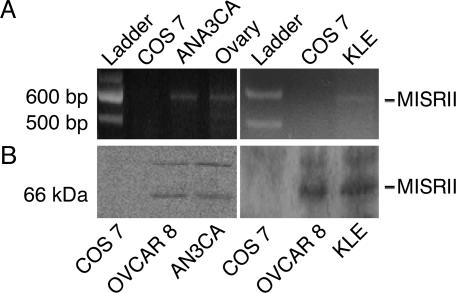
MIS Inhibits the Proliferation of Endometrial Cancer Cell Lines That Express the MISRII Receptor. The ability of MIS to inhibit the proliferation of endometrial cancer cells was tested in vitro. First, potential targets for MIS were identified by evaluating cell lines for expression of the MISRII. Of the four commercially available cell lines that were used in this study, two lines, AN3CA and KLE, express the MISRII and are growth-inhibited by MIS. RT-PCR analysis with the primers mentioned above detected the presence of the MISRII mRNA (Fig. 3A), and Western blots detected the MISRII protein in these two cell lines (Fig. 3B).
Fig. 3.
Human endometrial cancer cell lines AN3CA and KLE express the MISRII mRNA. (A) RNA harvested from each cell line was reverse-transcribed; PCR performed with primers specific for exons 1-5 detected a 582-bp PCR band corresponding to MISRII cDNA for AN3CA (Left) and KLE (Right)(n = 3 for each panel). The COS 7 cell line served as a negative control; ovary served as a positive control for the MISRII mRNA. (B) The human endometrial cancer cell lines AN3CA and KLE express the MISRII protein. Western blot with the 153P antibody for the MISRII detected the protein in cell lysates of AN3CA (Left) and KLE (Right). The COS 7 cell line is MISRII-negative and was used as a negative control. The human ovarian cancer cell line OVCAR8 was used as a positive control (n = 3 for each panel).
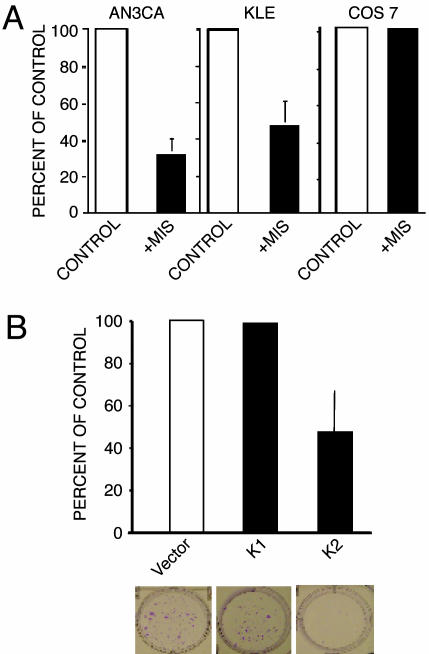
MIS inhibited the proliferation of both AN3CA and KLE. MIS at 71 nM (10 μg/ml) (7) for 8 days inhibited AN3CA cell growth by 67.3% when compared with buffer-treated cells (P < 0.0001, n = 4) as analyzed by methylthiazoletetrazolium assays of monolayer cell cultures, whereas KLE demonstrated 52.5% growth inhibition (P = 0.0041, n = 3; Fig. 4A). HEC-1A and RL95-2 did not respond to MIS and were not used further in these studies (data not shown). To test for specificity, the effect of MIS on COS 7 cells, which are derived from green monkey kidney cells and do not express the MISRII mRNA or protein, was examined. MIS did not inhibit these cells (Fig. 4A; n = 3).
Fig. 4.
MIS inhibits the growth of human endometrial cancer cell lines AN3CA and KLE. (A) MIS at 10 μg/ml inhibited the growth of the endometrial cancer cell lines AN3CA and KLE but not the growth of the COS 7 cell line. Treatment with recombinant human MIS inhibited the growth of AN3CA cells in monolayer culture by 67.3% compared with cells treated with buffer control (P < 0.0001, n = 4). KLE was likewise inhibited by MIS treatment (52.5%, P = 0.0041, n = 3). MIS did not inhibit the growth of COS 7 cells, which do not express the MISRII (n = 3). (B) Stable transfection of AN3CA cells with cDNA for bioactive MIS (K2) or inactive MIS (K1) confirms the MIS-growth inhibitory effect on these endometrial cells. MIS secreted from the transfected cells significantly inhibits AN3CA colony formation (K2; see photograph of colonies in Lower) to a similar degree as the monolayer cell inhibition shown in A. Nonsecreted MIS (K1; see photograph in Lower) had no effect compared with the empty vector control.
To show that the inhibitory effect on treated AN3CA cells was due to MIS and not to contaminants in the MIS preparation, these cells were stably transfected with constructs containing functional MIS (K2); a leaderless, nonsecretable, and therefore inactive form of MIS (K1); or empty vector as a control. AN3CA cells were also transfected with a hygromycin-resistance vector and maintained in cell culture under antibiotic selective pressure. At the end of 3 weeks, the medium from the K2-transfected cells contained a significantly higher concentration of MIS compared with media from both the empty vector and K1-transfected cells (K2 = 1.83 ng/ml; empty vector and K1 = 0 ng/ml). Cultures of AN3CA cells transfected with K2 showed a 52.4% reduction in colony counts compared with K1-transfected cells and a 53.3% reduction in colony counts compared with empty-vector-transfected cells (P = 0.0481 and 0.0381, respectively, n = 3; Fig. 4B), thus confirming MIS inhibition of AN3CA cell proliferation.
MIS caused cell-cycle arrest of the AN3CA cell line. As seen in Table 1, in five of five replicate experiments, an average 9.34% increase in the number of cells in G1 phase (range = 3.52- 21.86%) and a concomitant 5.64% decrease in the fraction of cells in S phase (range = 1.67-16.28%) was observed after 96 h of MIS treatment. Furthermore, treatment of AN3CA cells with MIS for up to 144 h also induced apoptosis, as evidenced by an increase in caspase 3 cleavage products through Western analysis without an increase in total levels of caspase 3 (data not shown).
Table 1. MIS increases G1 in AN3CA cells.
| Cell fraction
|
||||
|---|---|---|---|---|
| Trial | Treatment | G1, % | S, % | G2/M, % |
| 1 | Vehicle plus MIS | 52.98 | 24.84 | 19.28 |
| 74.84 | 8.56 | 15.42 | ||
| 2 | Vehicle plus MIS | 56.81 | 15.72 | 23.51 |
| 62.93 | 14.05 | 19.69 | ||
| 3 | Vehicle plus MIS | 56.48 | 17.87 | 21.93 |
| 60.00 | 15.85 | 21.56 | ||
| 4 | Vehicle plus MIS | 77.64 | 9.45 | 9.01 |
| 83.78 | 5.58 | 5.96 | ||
| 5 | Vehicle plus MIS | 76.21 | 12.20 | 8.87 |
| 83.76 | 7.82 | 5.68 | ||
In five replicate experiments, an average of 9.34% more cells was in G1 phase after treatment for 4 days compared with cells in control samples. In addition, MIS treatment caused a concomitant 5.64% decrease in the fraction of cells in S phase. M, mitotic phase.
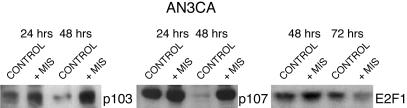
MIS Treatment of AN3CA Alters the Expression of Proteins Responsible for Cell Division. AN3CA cells did express Rb, a pocket protein which regulates of the activity of transcriptional factors controlling cell division, but MIS treatment did not alter Rb levels or phosphorylation in AN3CA cells (data not shown). However, MIS treatment for 48 h induced two retinoblastoma gene-product-related pocket proteins, p130 and p107. Treatment with MIS at 71 nM increased p130 and p107 protein levels at 24 and 48 h when compared with vehicle buffer-treated controls (Fig. 5). These events preceded the significant antiproliferation effects. In contrast, Western blots of AN3CA cells showed that this cell line did not express p16 even after MIS treatment, nor did they express CDKI p21, and MIS treatment did not affect levels of the CDKI p27 (data not shown).
Fig. 5.
MIS alters the expression of cell-cycle regulatory proteins as seen by Western analysis. Treatment of AN3CA endometrial cancer cells with 71 nM (10 μg/ml) MIS increases expression of the Rb pocket proteins p130 and p107 at 24 and 48 h of incubation in vitro. In contrast, MIS decreases E2F1 expression at 72 h of treatment compared with vehicle controls (n = 3 for each condition).
The pocket proteins p107 and p130 affect cell division by regulating the E2F family of transcription factors. Treatment with MIS for 72 h decreased the level of the transcription factor E2F1 in AN3CA cells (Fig. 5) but not the levels of E2F2, E2F3, or E2F4 protein in AN3CA cells (data not shown).
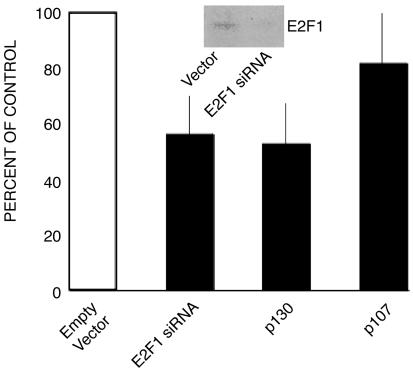
Colony-inhibition experiments were conducted to determine whether increased expression of p107 and p130 can inhibit the growth of AN3CA cells. AN3CA cells stably transfected with a p130 overexpression plasmid and maintained in culture for 3 weeks under antibiotic selective pressure had 43.36% fewer colonies than cultures of cells transfected with empty carrier control vector (P = 0.0050, n = 6; Fig. 6). Transfection of AN3CA cells with a p107 overexpression plasmid also caused an 18.46% reduction in colony counts, although this effect was not statistically significant (P = 0.1987, n = 3; Fig. 6). These results support the hypothesis that up-regulation of p130 plays an important role in growth inhibition of the AN3CA cell line. To test the effect of a decrease in E2F1 protein level on cell growth, AN3CA cells were transfected with an E2F1 small interfering RNA plasmid, which decreases the protein expression of E2F1 (see Fig. 6 Inset for Western blot data). This transfection caused a significant decrease in colony numbers in culture compared with the control vector (35.97%, P = 0.0023, n = 3; Fig. 6).
Fig. 6.
The functional significance of changes in p130, p107, and E2F1 levels due to MIS treatment. Transfection experiments show the consequence of increased expression of p130 (n = 6) and p107 (n = 3) and decreased E2F1 expression (E2F1 small interfering RNA) on AN3CA colony counts in vitro (n = 6) normalized to controls in each case. Black line indicates SD. Western analysis of E2F1 confirms the decreased expression secondary to transfection with interfering RNA (Inset).
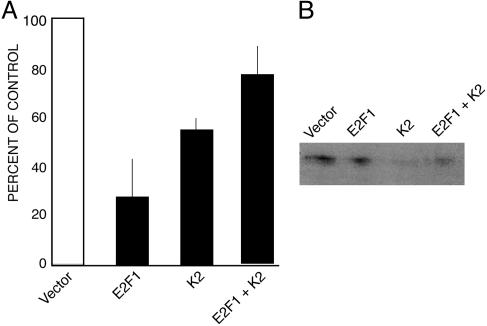
To show that a decrease in E2F1 is responsible for MIS-induced growth inhibition, we performed colony-inhibition experiments in which we cotransfected an expression plasmid for E2F1 with the K2 (MIS-expression) plasmid (Fig. 7). Although the MIS produced by the K2 plasmid normally decreases E2F1 levels in AN3CA, the E2F1 expression vector should counteract this effect. In this experiment, transfection of K2 alone again caused a decrease in colony counts of 47.3% compared with cells transfected with empty vector. Cotransfection with E2F1 rescued the cells from MIS-mediated growth inhibition (P = 0.0034, n = 6). The number of colonies in cultures transfected with both E2F1 and K2 did not differ significantly from the number in cultures of cells transfected with empty control vector (77.28% vs. 100%, P = 0.0872, n = 6).
Fig. 7.
Overexpression of E2F1 partially rescues the inhibitory effects of MIS inhibition of AN3CA colony growth. (A) Stable transfection of these cells with bioactive MIS transcripts (K2) inhibits colony formation relative to empty vector controls (Vector, n = 6) as expected. Increasing E2F1 expression counteracts this effect (E2F1 + K2) as expected, because MIS normally decreases E2F1 expression. This result is seen despite the fact that E2F1 overexpression alone inhibits colony formation, presumably by a non-MIS-mediated pathway (E2F1, n = 6). (B) The level of E2F1 expression in the MIS-treated groups (K2) as detected by Western analysis.
Discussion
In both ovarian- and cervical cancer cell lines, MIS causes cell-cycle arrest and apoptosis through a p16-mediated mechanism that is independent of Rb. This study demonstrated that MIS also inhibits the growth of two endometrial cancer cell lines that express the MISRII: AN3CA and KLE. The ability of MIS to inhibit the growth of endometrial cancer cell lines bodes well for its eventual application in the treatment of human endometrial cancers. However, in the AN3CA cell line, the mechanism of MIS signaling is independent of p16. In the past, histological studies demonstrated that, similar to AN3CA, many endometrial tumors do not express p16 (35). Therefore, it is likely that in vivo MIS-induced growth inhibition of endometrial cancer also would occur through a p16-independent mechanism. AN3CA cells do express Rb, but MIS treatment does not alter either protein level or phosphorylation of this molecule. Instead, MIS signaling in these cells depends upon modifications in the protein levels of two other members of the Rb family, p107 and p130. Both molecules are important modulators of the cell cycle: p130 mediates cell-cycle exit, and p107 is associated with G1-to-S-phase transition (36). These proteins also are important for MIS-induced inhibition of proliferation in cervical cancer cell lines (9). In AN3CA cells, MIS increases both p107 and p130 after 48 h of treatment. The role of p130 in proliferation inhibition of AN3CA cells is further supported by the results of transfection experiments, in which overexpression of p130 caused a statically significant decrease in growth in colony assays that was not observed when an empty vector was transfected. There was not a significant decrease in the number of colonies in the p107 overexpression assay in this experimental protocol.
MIS treatment also altered the level of E2F1 protein in endometrial cancer cells. Unlike its effect on OVCAR 8 and C33A cells (7, 9), MIS treatment of AN3CA caused a decrease in E2F1 protein levels. In this manner, the mechanism of MIS in AN3CA cells is similar to that of TGF-β, which causes a decrease in E2F1 during growth inhibition in mink lung epithelial cells (37, 38). The decrease in E2F1 in AN3CA cells was not seen until 72 h of treatment, after the point at which p130 and p107 levels increase. The ability of a decrease in E2F1 to inhibit AN3CA cell growth was confirmed by transfection of an E2F1 small interfering RNA plasmid, which caused both a reduction in protein expression of E2F1 and a decrease in colony numbers in cell-growth assay. Conversely, overexpression of E2F1 partially rescued cells from the inhibitory effect of MIS; this result supports the idea that a decrease in E2F1 is important to the MIS-inhibitory effect in AN3CA cells. The E2F1 effect was not responsible for increased growth, because E2F1 overexpression had a significant inhibitory effect on cell growth when transfected alone. E2F1 is known to act via multiple pathways to cause both apoptosis and cell arrest; in some pathways, levels decrease, whereas in others, levels increase. For example, the synthetic flavonoid flavopiridol causes apoptosis in lung cancer cell lines through stabilization and up-regulation of E2F1 protein (39). The results of the colony-inhibition experiments in this study support the findings of the Western blot analyses and indicate that although endometrial cancer cell lines are inhibited by MIS, the mechanism of action of MIS in these cells differs from the molecular events found in ovarian and cervical cancer cell lines treated with MIS. ANC3A MIS induces pocket proteins while down-regulating E2F1, the expression of which is known to be enhanced in aneuploid cells (40).
Changes in proteins important to cell-cycle progression were observed at or before 72 h, and evidence of cell-cycle arrest was present after 96 h of treatment. Apoptosis was first observed at 96 h by assay for caspase 3 cleavage but was more evident after 144 h. The sequence of these events is consistent with the effect of MIS observed in OVCAR 8 cells (7). These results suggest that in the human endometrial cancer cell line AN3CA, MIS causes inhibition of growth through a combination of both cell-cycle arrest and apoptosis.
In this study, we also have shown that both normal and human endometrial carcinomas express the protein and mRNA for the MISRII, which is responsible for ligand binding and cellular recognition of MIS. The presence of the MISRII in these tissues identifies them as potential targets for MIS-mediated growth inhibition.
In conclusion, normal and malignant endometrium express both the mRNA and the protein for the MISRII. MIS causes growth inhibition of the cell lines KLE and AN3CA, both of which express MISRII mRNA and protein. In the AN3CA cell line, MIS effects are mediated through changes in p107, p130, and E2F1 and are accompanied by an increase in both cell-cycle arrest and apoptosis. Therefore, in the future, MIS may be added to the armamentarium of treatment modalities currently in use for endometrial cancers.
Acknowledgments
We thank Jose Teixeira, Ph.D., and Shyamala Maheswaran, Ph.D., for helpful suggestions. This work was funded by National Institutes of Health Grants CA17393 and HD31122 (to P.K.D. and D.T.M), an American College of Surgeons Research Fellowship, and a Department of Defense Training Grant (to E.J.R.).
Author contributions: E.J.R. and D.T.M. performed research; E.J.R., D.T.M., E.O., B.R.R., and P.K.D. analyzed data; and E.J.R., D.T.M., and P.K.D. wrote the paper.
Abbreviations: CDKI, cyclin-dependent kinase inhibitor; FFBS, female FBS; MIS, Mullerian Inhibiting Substance; MISRII, MIS type II receptor; TBST, Tris-buffered saline and Tween 20.
References
- 1.MacLaughlin, D. T. & Donahoe, P. K. (2004) N. Engl. J. Med. 350, 367-378. [DOI] [PubMed] [Google Scholar]
- 2.Teixeira, J., Maheswaran, S. & Donahoe, P. K. (2001) Endocr. Rev. 22950, 657-674. [DOI] [PubMed] [Google Scholar]
- 3.Josso, N., di Clemente, N. & Gouedard, L. (2001) Mol. Cell. Endocrinol. 179, 25-32. [DOI] [PubMed] [Google Scholar]
- 4.Jost, A. (1972) Johns Hopkins Med. J. 130, 38-53. [PubMed] [Google Scholar]
- 5.Jost, A. (1947) Arch. Anat. Microsc. Morphol. Exp. 36, 271-315. [Google Scholar]
- 6.Masiakos, P. T., MacLaughlin, D. T., Maheswaran, S., Teixeira, J., Fuller, A. F., Shah, P., Kehas, D. J., Kenneally, M. K., Dombkowski, D. M., Ha, T. U., et al. (1999) Clin. Cancer Res. 5, 488-499. [PubMed] [Google Scholar]
- 7.Ha, T. U., Segev, D., Barbie, D., Masiakos, P., Tran, T. T., Dombkowski, D., Glander, M., Clarke, T. R., Lorenzo, H. K., Donahoe, P. K. & Maheswaran, S. (2002) J. Biol. Chem. 47, 37101-37109. [DOI] [PubMed] [Google Scholar]
- 8.Segev, D. L., Hoshiya, Y., Stephen, A. E., Hoshiya, M., Tran, T. T., MacLaughlin, D. T., Donahoe, P. K. & Maheswaran, S. (2001) J. Biol. Chem. 276, 26799-26806. [DOI] [PubMed] [Google Scholar]
- 9.Barbie, T. U., Barbie, D. A., MacLaughlin, D. T., Maheswaran, S. & Donahoe, P. K. (2003) Proc. Natl. Acad. Sci. USA 100, 15601-15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scully, R. (1970) Hum. Pathol. 1, 73-98. [DOI] [PubMed] [Google Scholar]
- 11.Baarends, W. M., van Helmond, M. J., Post, M., van der Schoot, P., Hoogerbrugge, J. W., de Winter, J., Uilenbroek, J. T., Karels, B., Wilming, L. G., Carel Meijers, J. H., et al. (1994) Development (Cambridge, U.K.) 120, 189-197. [DOI] [PubMed] [Google Scholar]
- 12.Di Clemente, N., Wilson, C., Faure, E., Boussin, L., Carmillo, P., Tizard, R., Picard, J. Y., Vigier, B., Josso, N. & Cate, R. (1994) Mol. Endocrinol. 8, 1006-1020. [DOI] [PubMed] [Google Scholar]
- 13.Teixeira, J., He, W. W., Shah, P. C., Morikawa, N., Lee, M. M., Catlin, E. A., Hudson, P. L., Wing, J., MacLaughlin, D. T. & Donahoe, P. K. (1996) Endocrinology 137, 160-165. [DOI] [PubMed] [Google Scholar]
- 14.Jamin, S. O., Arango, N. A., Mishina, Y., Hanks, M. C. & Behringer, R. R. (2003) Mol. Cell. Endocrinol. 211, 15-19. [DOI] [PubMed] [Google Scholar]
- 15.Visser, J. A., Olaso, R., Verhoef-Post, M., Kramer, P., Themmer, A. P. & Ingraham, H. A. (2001) Mol. Endocrinol. 15, 936-945. [DOI] [PubMed] [Google Scholar]
- 16.Clarke, T. R., Hoshiya, Y., Yi, S. E., Liu, X., Lyons, K. M. & Donahoe, P. K. (2001) Mol. Endocrinol. 15, 946-959. [DOI] [PubMed] [Google Scholar]
- 17.Gouedard, L., Chen, Y. G., Thevenet, L., Racine, C., Borie, S., Lamarre, I., Josso, N., Massague, J., di Clemente, N. (2000) J. Biol. Chem. 275, 27973-27978. [DOI] [PubMed] [Google Scholar]
- 18.Belville, C., Josso, N. & Picard, J. Y. (1999) Am. J. Med. Genet. 89, 218-223. [DOI] [PubMed] [Google Scholar]
- 19.Hoshiya, M., Christian, B. P., Cromie, W. J., Kim, H., Zhan, Y., MacLaughlin, D. T. & Donahoe, P. K. (2003) Birth Defects Res. Part A Clin. Mol. Teratol. 67, 868-874. [DOI] [PubMed] [Google Scholar]
- 20.Hudson, P. L., Dougas, I., Donahoe, P. K., Cate, R. L., Epstein, J., Pepinsky, P. B. & MacLaughlin, D. T. (1990) J. Clin. Endocrinol. Metab. 70, 16-22. [DOI] [PubMed] [Google Scholar]
- 21.Lee, M. M., Donahoe, P. K., Hasegawa, T., Silverman, B., Crist, G. B., Best, S., Hasegawa, Y., Noto, R. A., Schoenfeld, D. & MacLaughlin, D. T. (1996) J. Clin. Endocrinol. Metab. 81, 571-576. [DOI] [PubMed] [Google Scholar]
- 22.Mishina, Y., Rey, R., Finegold, M. J., Matzuk, M. M., Josso, N., Cate, R. L. & Behringer, R. L. (1996) Genes Dev. 10, 2577-2587. [DOI] [PubMed] [Google Scholar]
- 23.Behringer, R. R., Finegold, M. J. & Cate, R. L. (1994) Cell 79, 415-425. [DOI] [PubMed] [Google Scholar]
- 24.Durlinger, A. L., Kramer, P., Karels, B., de Jong, F. H., Uilenbroek, F. H., Grootengoed, J. A. & Themmen, A. P. (1999) Endocrinology 140, 5789-5796. [DOI] [PubMed] [Google Scholar]
- 25.Stephen, A. E., Masiakos, P. T., Segev, D. L., Vacanti, J. P., Donahoe, P. K. & MacLaughlin, D. T. (2001) Proc. Natl. Acad. Sci. USA 98, 3214-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephen, A. E., Pearsall, L. A., Christian, B. P., Donahoe, P. K., Vacanti, J. P. & MacLaughlin, D. T. (2002) Clin. Cancer Res. 8, 2640-2646. [PubMed] [Google Scholar]
- 27.Sherr, C. J. (1993) Cell 73, 1059-1065. [DOI] [PubMed] [Google Scholar]
- 28.Sherr, C. J. & Roberts, J. M. (1995) Genes Dev. 9, 1149-1163. [DOI] [PubMed] [Google Scholar]
- 29.Okuda, T., Hirai, H., Valentine, V. A., Shurtless, S. A., Kidd, V. J., Lahti, J. M., Sherr, C. J. & Doning, J. R. (1995) Genomics 29, 623-630. [DOI] [PubMed] [Google Scholar]
- 30.Hirai, H., Roussel, M. F., Kato, J. Y., Ashmun, R. A. & Sherr, C. J. (1995) Mol. Cell. Biol. 15, 2672-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ragin, R. C., Donahoe, P. K., Kenneally, M. K., Ahmad, M. F. & MacLaughlin, D. T. (1992) Protein Expression Purif. 3, 236-245. [DOI] [PubMed] [Google Scholar]
- 32.Lorenzo, H. K., Teixeira, J., Pahlavan, N., Laurich, V. M., Donahoe, P. K. & MacLaughlin, D. T. (2002) J Chromatogr. B Anal. Technol. Biomed. Life Sci. 766, 89-98. [DOI] [PubMed] [Google Scholar]
- 33.Denizon, F. & Lang, R. (1986) J. Immunol. Methods 89, 271-277. [DOI] [PubMed] [Google Scholar]
- 34.Kurian, M. S., de la Cuesta, R. S., Waneck, G. L., MacLaughlin, D. T., Manganaro, T. F. & Donahoe, P. K. (1995) Clin. Cancer Res. 1, 343-349. [PubMed] [Google Scholar]
- 35.Milde-Langosch, K., Riethdord, L., Bamberger, A. M. & Loning, T. (1999) Virchows Arch. 434, 23-28. [DOI] [PubMed] [Google Scholar]
- 36.Lipinski, M. M. & Jacks, T. (1990) Oncogene 18, 7873-7882. [DOI] [PubMed] [Google Scholar]
- 37.Schwarz, J. K., Bassing, C. H., Kovesdi, I., Datto, M. B., Blazing, M., George, S., Wang, X. F. & Nevins, J. R. (1995) Proc. Natl. Acad. Sci USA 92, 483-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun, P., Dong, P., Dai, K., Hannon, G. J. & Beach, D. (1998) Science 282, 2270-2272. [DOI] [PubMed] [Google Scholar]
- 39.Ma, Y., Cress, W. D. & Haura, E. B. (2001) Mol. Cancer Ther. 2, 73-81. [PubMed] [Google Scholar]
- 40.Hernando, E., Nahle, Z., Juan, G., Diaz-Rodriguez, E., Alaminos, M., Hemann, M., Michel, L., Mittal, V., Gerald, W., Benezra, R., et al. (2004) Nature 430, 797-802. [DOI] [PubMed] [Google Scholar]