ABSTRACT
Erysipelothrix rhusiopathiae causes swine erysipelas, an important infectious disease in the swine industry. In Japan, the incidence of acute swine erysipelas due to E. rhusiopathiae serovar 1a has recently increased markedly. To study the genetic relatedness of the strains from the recent cases, we analyzed 34 E. rhusiopathiae serovar 1a swine isolates collected between 1990 and 2011 and further investigated the possible association of the live Koganei 65-0.15 vaccine strain (serovar 1a) with the increase in cases. Pulsed-field gel electrophoresis analysis revealed no marked variation among the isolates; however, sequencing analysis of a hypervariable region in the surface-protective antigen A gene (spaA) revealed that the strains isolated after 2007 exhibited the same spaA genotype and could be differentiated from older strains. Phylogenetic analysis based on genome-wide single-nucleotide polymorphisms (SNPs) revealed that the Japanese strains examined were closely related, showing a relatively small number of SNPs among them. The strains were classified into four major lineages, with Koganei 65-0.15 (lineage III) being phylogenetically separated from the other three lineages. The strains isolated after 2007 and the two older strains constituted one major lineage (lineage IV) with a specific spaA genotype (M203/I257-SpaA), while the recent isolates were further divided into two geographic groups. The remaining older isolates belonged to either lineage I, with the I203/L257-SpaA type, or lineage II, with the I203/I257-SpaA type. These results indicate that the recent increased incidence of acute swine erysipelas in Japan is associated with two sublineages of lineage IV, which have independently evolved in two different geographic regions.
IMPORTANCE Using large-scale whole-genome sequence data from Erysipelothrix rhusiopathiae isolates from a wide range of hosts and geographic origins, a recent study clarified the existence of three distinct clades (clades 1, 2, and 3) that are found across multiple continents and host species, representing both livestock and wildlife, and an “intermediate” clade between clade 2 and the dominant clade 3 within the species. In this study, we found that the E. rhusiopathiae Japanese strains examined exhibited remarkably low levels of genetic diversity and confirmed that all of the Japanese and Chinese swine isolates examined in this study belong to clonal lineages within the intermediate clade. We report that spaA genotyping of E. rhusiopathiae strains is a practical alternative to whole-genome sequencing analysis of the E. rhusiopathiae isolates from eastern Asian countries.
KEYWORDS: genome-wide SNPs, phylogenetic analysis, swine erysipelas
INTRODUCTION
The Gram-positive facultative intracellular pathogen Erysipelothrix rhusiopathiae is ubiquitous in nature and can cause a variety of diseases in humans and many species of wild and domestic animals (1). In swine, E. rhusiopathiae can cause acute septicemia, subacute urticaria, or chronic endocarditis and polyarthritis, all of which result in great economic losses to the swine industry worldwide (1).
For epidemiological studies of the disease, clinical isolates of E. rhusiopathiae are traditionally serotyped with a double agar-gel precipitation test using type-specific rabbit antisera and peptidoglycan antigens extracted with hot water by autoclave treatment (2). Serotyping plays an important role not only in discriminating E. rhusiopathiae from Erysipelothrix tonsillarum, which is a closely related nonpathogenic species that is frequently isolated from the tonsils of healthy pigs (3), but also in detecting clinically important serovars, i.e., 1a, 1b, and 2, which are the dominant serovars among the E. rhusiopathiae strains isolated from diseased pigs (4–9). Serovar 1a is most commonly isolated from acute swine erysipelas (1); however, the molecular basis for the apparently enhanced pathogenicity of the serovar 1a strains remains unknown.
In Japan, the number of cases of acute swine erysipelas has been increasing since 2008. Between July 2009 and April 2011, cases of acute septicemia and/or urticaria were reported in at least 11 prefectures (out of a total of 47 prefectures), and we have confirmed that all of the cases were caused by E. rhusiopathiae serovar 1a strains (our unpublished data). The reason for the increase in cases is unclear. In Japan, an attenuated live vaccine (E. rhusiopathiae Koganei 65-0.15 strain, serovar 1a) has long been used to control the disease, and vaccine-derived strains have caused chronic forms of the disease (5, 6). Thus, there is also a concern that the live vaccine may have regained its virulence and caused acute forms of the disease.
In the United States, a marked increase in erysipelas due to serovar 1a strains has also been observed from 2001 to 2004 (7). The failure to use vaccines and improper vaccine management, including the timing of vaccination, have been suggested as possible reasons, although the outbreaks remain unexplained. In Germany, erysipelas is also emerging in poultry industries (10). Recently, E. rhusiopathiae has been reported to be the cause of the large-scale die-offs of muskoxen in the Canadian Arctic (11). Thus, the marked increase of erysipelas in animals appears to be a concern worldwide; therefore, a better understanding of the epidemiology and pathogenesis of the recent outbreaks is required.
For the epidemiological studies of E. rhusiopathiae, single-locus sequence-based genotyping of E. rhusiopathiae using the amino acid sequence of a hypervariable region in surface-protective antigen A (SpaA) has been widely used (12). Based on the sequences in this region, serovar 1a clinical isolates possessing SpaA with a methionine at position 203 (M203-SpaA), in which the third position in the codon is guanine (G) at nucleotide position 609 of the gene, have been reported to be widespread in pigs in Japan (13, 14).
To gain insights into the epidemiology of the recent increase in incidences of acute swine erysipelas in Japan and investigate the genetic relatedness of the strains from the cases, we genetically characterized the E. rhusiopathiae serovar 1a clinical strains isolated between 1990 and 2011 from pigs with acute cases of infection. We further investigated the possible association of the live Koganei 65-0.15 vaccine strain with the increased incidence. To this end, we performed pulsed-field gel electrophoresis (PFGE)-based genotyping and spaA genotyping of Koganei 65-0.15 and the clinical isolates. Furthermore, we obtained their genome sequences and performed a high-resolution phylogenetic analysis based on the genome-wide single-nucleotide polymorphisms (SNPs) of these strains.
RESULTS
PFGE analysis.
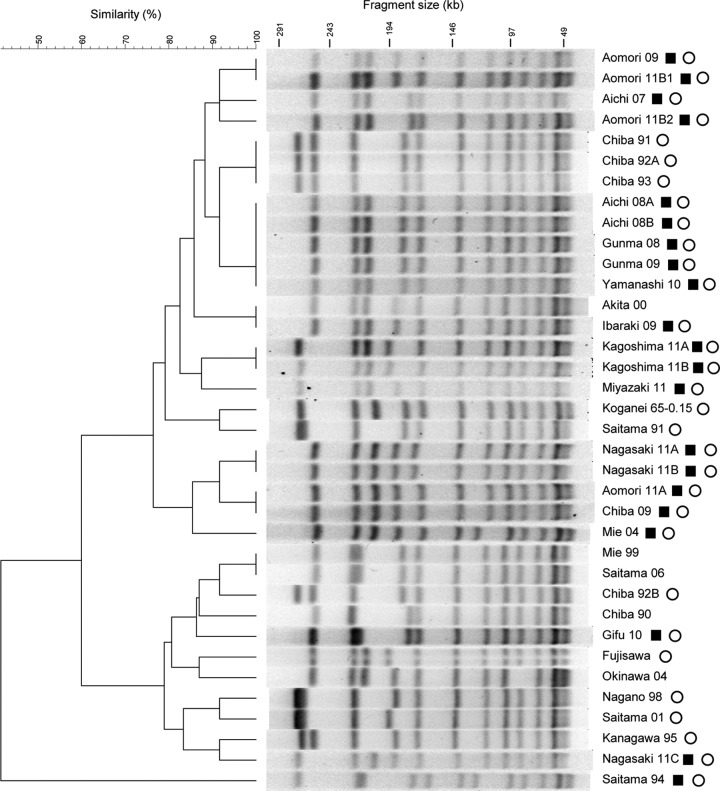
SmaI is widely used for single-digestion PFGE analyses of E. rhusiopathiae (7, 24). The genomic DNA of the Fujisawa and Koganei 65-0.15 strains and 13 E. rhusiopathiae strains that were randomly selected from the clinical strains isolated after 2007 (designated “recent strains” in this article) was digested with SmaI and analyzed by PFGE. The PFGE patterns of the clinical isolates were similar, and only small differences were observed compared with the PFGE pattern of the Koganei 65-0.15 strain (data not shown). To identify other restriction enzymes that might be suitable for PFGE typing of the organisms, we performed in silico restriction digestion of the completely sequenced genome of strain Fujisawa to identify more suitable enzymes for PFGE analysis and additionally tested three candidate enzymes: CpoI, ApaI, and NgoMIV. The results revealed that CpoI digestion yielded the most discriminatory result in the PFGE analysis of the 15 strains (data not shown). We therefore analyzed the CpoI digestion patterns of the 34 field strains and the Koganei 65-0.15 and Fujisawa strains. Although we observed some level of variation in the banding patterns between the strains, with the lowest similarity of 40%, we identified several strain pairs/sets that showed identical patterns (Fig. 1). However, recent clinical strains (M203-SpaA-type strains) did not cluster together (see below).
FIG 1.
Dendrogram obtained via pulsed-field gel electrophoresis of CpoI-digested DNA from E. rhusiopathiae serovar 1a Japanese field strains isolated from pigs with acute erysipelas. The M203-SpaA-type strains are indicated with black squares. The strains possessing the prophage PP_Erh_Fujisawa (20) are indicated with an open circle.
spaA genotyping.
Sequence analysis of the hypervariable region of spaA revealed that all of the recent clinical strains were the M203-SpaA type, while the two older strains, E. rhusiopathiae Saitama 94 and Mie 04, which were isolated in 1994 and 2004, respectively, were also the same SpaA type (Table 1). Two strains (E. rhusiopathiae Aomori 11A and Aomori 11B2), which were isolated from different farms in the same prefecture, contained a unique nonsynonymous SNP at codon 242 of spaA. Interestingly, this SNP was not identified in the other strain (E. rhusiopathiae Aomori 11B1) isolated from the same farm from which Aomori 11B2 was isolated. We also identified three additional SNPs in the spaA coding region among the 34 strains examined: one synonymous SNP at codon 185 and two nonsynonymous SNPs at codons 195 and 257 (which introduced alanine-to-aspartic acid and leucine-to-isoleucine substitutions, respectively). The I257 allele was predominant among the 34 strains and was identified in all of the M203-type strains and in six out of the 13 non-M203 strains.
TABLE 1.
Sequencing analysis of the hypervariable region of the spaA gene of 36 Erysipelothrix rhusiopathiae strains
| Straina | Yr of isolation/construction | SNP profile at positionb: |
||||
|---|---|---|---|---|---|---|
| 185 | 195 | 203 | 242 | 257 | ||
| Fujisawa | Before 1972 | Pro (CCC) | Asp (GAT) | Ile (ATT) | Glu (GAG) | Leu (CTT) |
| Koganei 65-0.15 | 1974 | Pro (CCC) | Asp (GAT) | Ile (ATT) | Glu (GAG) | Ile (ATT) |
| Chiba 90 | 1990 | Pro (CCC) | Ala (GCT) | Ile (ATT) | Glu (GAG) | Ile (ATT) |
| Chiba 91 | 1991 | Pro (CCC) | Asp (GAT) | Ile (ATT) | Glu (GAG) | Leu (CTT) |
| Saitama 91 | 1991 | Pro (CCC) | Asp (GAT) | Ile (ATT) | Glu (GAG) | Leu (CTT) |
| Chiba 92A | 1992 | Pro (CCC) | Asp (GAT) | Ile (ATT) | Glu (GAG) | Leu (CTT) |
| Chiba 92B | 1992 | Pro (CCT) | Ala (GCT) | Ile (ATT) | Glu (GAG) | Ile (ATT) |
| Chiba 93 | 1993 | Pro (CCC) | Asp (GAT) | Ile (ATT) | Glu (GAG) | Leu (CTT) |
| Saitama 94 | 1994 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Kanagawa 95 | 1995 | Pro (CCC) | Asp (GAT) | Ile (ATT) | Glu (GAG) | Leu (CTT) |
| Nagano 98 | 1998 | Pro (CCC) | Asp (GAT) | Ile (ATT) | Glu (GAG) | Leu (CTT) |
| Mie 99 | 1999 | Pro (CCC) | Ala (GCT) | Ile (ATT) | Glu (GAG) | Ile (ATT) |
| Akita 00 | 2000 | Pro (CCC) | Ala (GCT) | Ile (ATT) | Glu (GAG) | Ile (ATT) |
| Saitama 01 | 2001 | Pro (CCC) | Asp (GAT) | Ile (ATT) | Glu (GAG) | Leu (CTT) |
| Okinawa 04 | 2004 | Pro (CCC) | Ala (GCT) | Ile (ATT) | Glu (GAG) | Ile (ATT) |
| Mie 04 | 2004 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Saitama 06 | 2006 | Pro (CCC) | Ala (GCT) | Ile (ATT) | Glu (GAG) | Ile (ATT) |
| Aichi 07 | 2007 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Aichi 08A | 2008 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Aichi 08B | 2008 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Gunma 08 | 2008 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Gunma 09 | 2009 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Ibaraki 09 | 2009 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Chiba 09 | 2009 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Aomori 09 | 2009 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Yamanashi 10 | 2010 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Gifu 10 | 2010 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Nagasaki 11A | 2011 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Nagasaki 11B | 2011 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Nagasaki 11C | 2011 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Kagoshima 11A | 2011 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Kagoshima 11B | 2011 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Miyazaki 11 | 2011 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Aomori 11A | 2011 | Pro (CCC) | Asp (GAT) | Met (ATG) | Asp (GAT) | Ile (ATT) |
| Aomori 11B1 | 2011 | Pro (CCC) | Asp (GAT) | Met (ATG) | Glu (GAG) | Ile (ATT) |
| Aomori 11B2 | 2011 | Pro (CCC) | Asp (GAT) | Met (ATG) | Asp (GAT) | Ile (ATT) |
The Arabic numerals following the names of the prefectures indicate the last two digits of the year of isolation.
The different nucleotides and amino acids relative to the Fujisawa strain are indicated in boldface. Ala, alanine; Asp, aspartic acid; Glu, glutamic acid; Ile, isoleucine; Leu, leucine; Met, methionine; Pro, proline.
Phylogeny based on genome-wide SNPs.
To determine the genetic relatedness of the recent clinical strains, we sequenced the genomes of the 34 clinical isolates and the Koganei 65-0.15 strain using the Illumina HiSeq 2000 or MiSeq platform and analyzed the genome-wide SNPs of these strains. By mapping the Illumina reads from the 34 clinical strains and the Koganei 65-0.15 strain to the reference genome sequence of the Fujisawa strain (1,787,941 bp in length), we identified a total of 904 SNPs. Of these, 793 and 111 SNPs were in protein-coding sequences (CDSs) and intergenic regions, respectively (see Table S2 in the supplemental material). In each analyzed strain, >95.6% of the Illumina reads (mostly >99%) were mapped to the reference genome, and the mapped regions covered >98.0% (mostly >99%) of the reference genome (Table S1).
A recent study clarified the existence of three distinct clades (clades 1, 2, and 3) and an “intermediate” clade between clade 2 and the dominant clade 3 within the species E. rhusiopathiae (16). To examine the phylogenetic position of the Japanese strains, we constructed a phylogenetic tree by including the strains belonging to clades 2 and 3 and the two recently sequenced Chinese strains, SY1027 and GXBY-1, both of which were isolated from septicemic pigs (17, 18). In that study, the Fujisawa and SY1027 strains were found to belong to the intermediate clade (16).
A phylogenetic tree generated based on the genome-wide SNP sites revealed that all of the Japanese clinical isolates fell within clonal lineages in the intermediate clade (Fig. 2). They were clustered into three major lineages (lineages I, II, and IV; Fig. 2). The Koganei 65-0.15 strain was phylogenetically separated from these clinical isolates to form a single-membered cluster (lineage III). The M203-SpaA-type strains, including all of the recent clinical isolates and two older strains, Mie 04 and Saitama 94, constituted one of the major lineages (lineage IV). In lineage IV, the two older strains were clearly separated from the recent clinical isolates (lineages IVa and IVb, respectively). More importantly, the recent clinical isolates were further divided into two sublineages (lineages IVb-1 and IVb-2) that corresponded to their geographic origins, i.e., a Kyushu island group (Miyazaki, Kagoshima, and Nagasaki strains) and a Honshu island group (other strains). It is also noteworthy that all of the L257-SpaA strains were found in lineage I, and this SpaA type was not identified in the other lineages. Thus, the three major lineages found in this analysis were characterized by I203/L257 (lineage I), I203/I257 (lineage II), and M203/I257 (lineage IV) spaA genotypes. The Chinese SY1027 and GXBY-1 strains belonged to lineages II and IVb-2, respectively.
FIG 2.
(A) Genome-wide SNP-based phylogenetic analysis of 38 E. rhusiopathiae strains analyzed. The phylogenetic tree was constructed by using the maximum-likelihood method with 1,000 bootstrap replicates in the RAxML program. Strains belonging to clade 3, clade 2, or the intermediate group (16) were used as an outgroup for rooting the tree. (B) Enlargement of all of the lineage branches without an outgroup from the phylogenetic tree. Chinese strains that were isolated from recent acute cases are boxed. The spaA genotypes are indicated in parentheses below the lineage names. The values in the nodes represent the bootstrap values expressed as percentages. The scale is provided as the number of substitutions per variable site.
SNP analysis.
Among the total 71 SNPs identified in the genome of the Koganei 65-0.15 strain, 14 SNPs were synonymous, 51 SNPs were nonsynonymous, and 24 SNPs were strain specific (Table S2). These strain-specific SNPs appeared to be evenly scattered across the genome, except for those in an ABC transporter gene (ERH_1125), in which 4 nonsynonymous SNPs were identified.
In the Japanese clinical isolates, the total numbers of SNPs per strain varied between 100 and 186, with an average of 135 SNPs (Table S2). Out of the SNPs, the strains isolated after 2007 commonly possessed 20 nonsynonymous SNPs, two of which were identified within the genes encoding RspB (19) and collagen-binding protein (ERH_1436), both of which probably play important roles in bacterial adhesion to host cells and subsequent biofilm formation in vivo (19).
Analysis of prophage PP_Erh_Fujisawa.
Because a 36.5-kb prophage named PP_Erh_Fujisawa was previously found in strain Fujisawa (20), and because mobile genetic elements, such as phages and plasmids, often play critical roles in the genomic diversification of bacteria, we investigated the distribution of PP_Erh_Fujisawa in the clinical strains analyzed in this study. Out of the 34 strains analyzed, 29 (85.3%) strains, including all the M203-SpaA-type strains, possessed PP_Erh_Fujisawa (Fig. 1). Only a few SNPs and small insertion/deletions (INDels) were observed in the PP_Erh_Fujisawa prophages of these 29 strains. Among the remaining five strains, three strains (Saitama 06, Chiba 90, and Mie 99) each possessed a PP_Erh_Fujisawa-like prophage. The prophages of these three strains, all of which were integrated in the same chromosomal locus as that of PP_Erh_Fujisawa, were almost identical (more than 99% nucleotide sequence identity throughout the whole prophage genome) and have significantly diverged in nucleotide sequences compared to PP_Erh_Fujisawa (see Fig. S1 in the supplemental material). One strain (Akita 00) also contained a prophage at the same chromosomal locus, but extensive recombinations appeared to have occurred internal parts of the prophage genome (Fig. S1). Strain Okinawa 04 completely lacked PP_Erh_Fujisawa; no phage-related sequences were found at its integration site, and an intact attachment site (attB) was detected (Fig. S1). Intriguingly, these five strains all belong to the same lineage (lineage II). These results indicated that PP_Erh_Fujisawa is highly conserved in the E. rhusiopathiae serovar 1a strains isolated in Japan and that the deletion of PP_Erh_Fujisawa or its genomic diversification by recombination has taken place only in lineage II. The Chinese SY1027 strain in the same lineage also completely lacked phage-related sequences, but the GXBY-1 strain in lineage IVb-2 contained PP_Erh_Fujisawa prophage.
It is also noteworthy that PP_Erh_Fujisawa and its relatives found in this study are all similar in size and integrated at the same chromosomal locus and that no CpoI restriction sites were found in any of these prophage genomes. Therefore, their genomic differences do not affect the PFGE patterns of each strain. The exception was in the case of strain Okinawa 04, from which PP_Erh_Fujisawa has been completely deleted; the size of one band which corresponds to the PP_Erh_Fujisawa-containing fragment (an 85-kb band in Fig. 1) was changed due to the prophage deletion.
DISCUSSION
To investigate the genetic relatedness of the clinical strains from the recent increased cases of acute swine erysipelas in Japan and further investigate the possible association of the live Koganei 65-0.15 vaccine strain with these cases, we genetically characterized the E. rhusiopathiae serovar 1a strains isolated from pigs with acute cases of infection, which were collected from various regions in Japan over 2 decades.
We first evaluated the ability of the conventional PFGE genotyping method to investigate the genetic relatedness of the 34 clinical strains. In E. rhusiopathiae strains, no correlation has been found between multilocus sequence typing and PFGE (10). The results of this study also showed no clear correlations between the geographic origins or years of isolation of the strains and the PFGE patterns. This may be because of chromosomal rearrangements resulting from a high level of core genome recombination among E. rhusiopathiae strains (16). It is also possible that the serovar 1a strains analyzed in this study are not phylogenetically related because of homoplasy in serotype (16). Thus, the PFGE-based genotyping provided little information regarding the genetic relatedness between the recent Japanese isolates and older isolates or the vaccine strain; therefore, we next performed spaA genotyping. spaA genotyping analysis revealed that the clinical strains isolated after 2007 showed a specific spaA genotype (M203/I257-SpaA) and could be differentiated from older strains or the Koganei 65-0.15 vaccine strain; however, two older strains, Saitama 94 and Mie 04, also showed the same genotype. To further investigate the genetic relatedness among the strains, we employed a high-resolution genotyping method, i.e., genome-wide SNP analysis of these clinical isolates. Recently, using whole-genome sequence data from 83 E. rhusiopathiae isolates from a wide range of hosts and geographic origins, Forde et al. revealed that E. rhusiopathiae consists of three distinct clades that are not clearly segregated by host species or geographic origin (16). The authors showed that isolates in clade 1 exclusively contained a single copy of the surface-protective antigen type B gene (spaB), while all other isolates among the two other clades (clades 2 and 3) had a single copy of the spaA gene. In their phylogenetic analysis, they further showed that some strains were grouped as intermediate isolates between clade 2 and the dominant clade 3. In this study, we found that the E. rhusiopathiae Japanese strains examined exhibited remarkably low levels of genetic diversity and confirmed that all of the Japanese and Chinese clinical isolates examined in this study belong to clonal lineages within the intermediate clade.
The phylogenetic analysis revealed that the Japanese clinical isolates can be classified into three major lineages (lineages I, II, and IV) and further indicated that the Koganei 65-0.15 strain formed a single-membered cluster (lineage III). These findings exclude the possibility that the vaccine strain regained its virulence and became widespread in the country. Most interestingly, the results from the spaA genotyping of the Japanese isolates were consistent with the classification results obtained with the genome-wide SNP analysis, i.e., the I203/L257-SpaA-type and I203/I257-SpaA-type strains constituted lineages I and II, respectively. Furthermore, the M203/I257-SpaA-type strains constituted the major lineage (lineage IV), with two older strains (lineage IVa) that were clearly separated from recent isolates (lineage IVb), and the recent isolates that were further subdivided into two sublineages according to their geographic origins, Kyushu island and Honshu island (lineages IVb-1 and IVb-2). Interestingly, the Chinese isolate (GXBY-1) was also found to be the M203/I257-SpaA type and belong to lineage IVb-2. Thus, it appears that the recent Japanese isolates comprise at least two lineages that may have evolved and spread separately in two different geographic regions, Kyushu and Honshu, from an ancestor common to the Chinese strains.
Sequence analysis of the spaA sequence data deposited in the NCBI database revealed that many of the recent Chinese isolates are M203/I257-SpaA-type strains (Table S3 in the supplemental material). However, Janßen et al. (10) reported that among 165 E. rhusiopathiae field isolates primarily from Germany, only 2.4% (4/165) were of the M203/I257-SpaA type (grouped as group V in their study). Furthermore, we did not find this SpaA type in the 135 E. rhusiopathiae isolates collected from livestock and wildlife from multiple continents (16), including the isolates from arctic and boreal ungulates (21) (Table S4). The results of this study indicate that spaA genotyping for strains, such as I203/L257 (lineage I), I203/I257 (lineage II), and M203/I257 (lineage IV), is a practical alternative to whole-genome sequencing analysis of E. rhusiopathiae strains, but its application may be limited to E. rhusiopathiae isolates from eastern Asian countries.
It is unclear whether particular selective pressures have led to the recent expansion of the E. rhusiopathiae M203/I257-SpaA strains in Japan. Interestingly, among the 20 nonsynonymous SNPs specifically identified in the Japanese lineage IVb strains, two were found within the genes encoding virulence-associated surface proteins (20), i.e., RspB (19) and collagen-binding protein (ERH_1436). Because surface proteins in Gram-positive pathogens play important roles in virulence (22), it might be possible that the lineage IVb strains have higher virulence than other Japanese strains and that the observed mutations in these surface proteins, including that in the SpaA protein, contribute to the increase in the virulence potential. Recently, Forde et al. excluded the possibility that the widespread mortalities associated with E. rhusiopathiae in wild animals in Arctic Canada and Alaska were not linked by a common emerging E. rhusiopathiae strain (21). They hypothesized that other factors, including climate change, that make hosts permissive to opportunistic pathogens may be the reasons for the deaths of the wild animals. This hypothesis may be supported by our finding that the Chinese strains isolated from recent acute cases belonged to lineages II and IVb-2. However, in this study, we observed that the recent increase in acute swine erysipelas in Japan was caused by clonal lineage IVb strains of E. rhusiopathiae. Taken together, it is possible that other environmental and/or host factors, including underlying immunosuppressive viral infections among pig populations, may be involved in the disease outbreaks.
MATERIALS AND METHODS
Bacterial strains and DNA preparation.
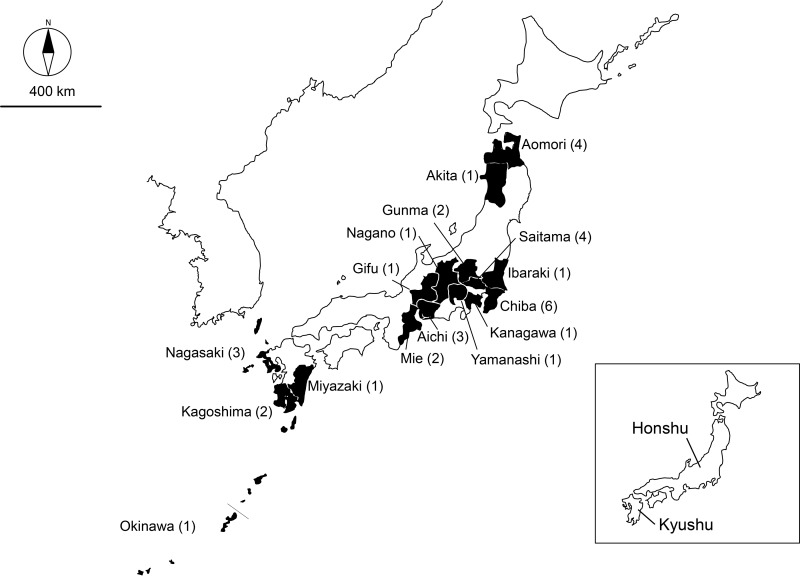
The E. rhusiopathiae strains included in this study were all typed as serovar 1a. These included the Fujisawa strain, which was isolated from a septicemic pig before 1972, the Koganei 65-0.15 strain, which is the Japanese live vaccine strain that was developed in 1974, and a total of 34 field strains. The complete genome sequence of the Fujisawa strain was previously determined by our group (20). All of the 34 field strains were isolated from pigs with acute septicemia and/or urticaria from different geographic regions in Japan (Fig. 3) during the period from 1990 to 2011. The field strains, which were named after the prefecture and the year of their isolation, were isolated by the veterinarians at prefectural government diagnostic laboratories and sent to the National Institute of Animal Health for serotyping.
FIG 3.
Map of Japan indicating the prefectures from which the field strains were isolated. The numbers in parentheses indicate the numbers of strains analyzed.
The E. rhusiopathiae strains were grown at 37°C for 16 h in brain heart infusion broth (Becton, Dickinson and Company, Baltimore, MD) supplemented with 0.1% Tween 80 and 0.3% Tris-HCl (pH 8.0). The genomic DNA of the E. rhusiopathiae strains was prepared as previously described (23), except for the following modifications: after cell lysis with 10% sodium dodecyl sulfate, the samples were mixed with an equal volume of phenol-chloroform solution and centrifuged, and the DNA was recovered by ethanol precipitation.
Pulsed-field gel electrophoresis.
To identify restriction enzymes suitable for PFGE typing of the organisms, we performed in silico restriction digestion of the genome of strain Fujisawa (GenBank accession no. AP012027) using the in silico Molecular Cloning Genomics Edition (IMCGE) software (15); based on the result, we selected four enzymes, SmaI, CpoI, ApaI, and NgoMIV, as candidates.
Bacterial cultures were boiled for 1 min before plug preparation, and DNA plugs were prepared according to the method described by Okatani et al. (24). The DNA plugs were sliced, and each plug was placed in 0.1× Tris-EDTA buffer and incubated for 1 h at room temperature. After removal of the buffer, the DNA was digested with 15 U of SmaI (TaKaRa Bio, Inc., Shiga, Japan), CpoI (TaKaRa Bio), ApaI (TaKaRa Bio), or NgoMIV (New England BioLabs, Ipswich, MA) for 4 h at the temperatures recommended by the manufacturers. The DNA fragments were separated on a CHEF-DR III system (Bio-Rad Laboratories, Hercules, CA) in a 1% agarose gel, which was prepared with pulsed-field certified agarose (Bio-Rad) and 0.5× Tris-borate-EDTA buffer (TaKaRa Bio). Electrophoresis was performed for 22 h at 14°C and 6 V/cm, with initial and final pulse times of 1 and 15 s, respectively, for SmaI and ApaI, and with initial and final pulse times of 10 and 20 s, respectively, for CpoI and NgoMIV. Thereafter, the gel was stained with ethidium bromide for 15 min, destained in distilled water, and photographed under UV light. The DNA banding patterns were analyzed using the BioNumerics software version 5.1 (Applied Maths, Sint-Martens-Latem, Belgium), as previously described (25).
spaA genotyping.
A 1,085-bp DNA fragment encompassing the 432-bp hypervariable region at nucleotide positions 502 to 933 in the spaA gene (12) was PCR amplified with primers 0094F (5′-TCGGCTACAGAAGTTTTATGCAGG-3′) and 0094R (5′-TGCTACCTTCTTCCAACCCGTAAC-3′). The sequences of these primers correspond to nucleotide positions 395 to 418 and 1456 to 1479 of the spaA gene of strain Fujisawa, respectively. The PCR was performed in a 50-μl reaction mixture containing 50 ng of template DNA, 0.3 μM concentrations of each primer, 0.4 mM concentrations of each deoxynucleoside triphosphate (dNTP), PCR buffer, and 1.0 U of KOD FX DNA polymerase (Toyobo, Osaka, Japan). The following conditions were employed: initial denaturation at 94°C for, and three steps of amplification (30 cycles) at 98°C for 10 s, 55°C for 30 s, and 68°C for 1 min. The products were directly sequenced with an ABI Prism 3130xl genetic analyzer (Applied Biosystems, Foster City, CA) using a BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems), according to the manufacturer's instructions.
Genome sequencing, SNP discovery, and phylogenetic analysis.
To obtain the draft genome sequences of Koganei 65-0.15 and Kagoshima 11A, sequencing libraries were generated according to the standard protocols suggested by Illumina (San Diego, CA) and subjected to paired-end sequencing (100 cycles × 2) on an Illumina HiSeq 2000 platform. The draft sequences of other isolates were generated using the Illumina MiSeq platform. The sequencing libraries were prepared using a Nextera XT DNA sample preparation kit (Illumina) and pooled for multiplexed paired-end sequencing (251 cycles × 2), according to the manufacturer's instructions. Platanus_trim (http://platanus.bio.titech.ac.jp/?page_id=30) was used for trimming adaptor sequences and filtering low-quality reads (quality cutoff value, 25).
The Illumina reads were mapped to the genome sequence of strain Fujisawa (the reference sequence) with the Burrows-Wheeler Aligner (version 0.6.2) (26), using default parameters. SAMtools (version 0.1.19) (27) was used to call SNPs. The SNPs were filtered according to the following criteria: (i) all insertions and deletions were excluded, (ii) the minimum distance between each of the SNPs was set to 100 bp, and (iii) the consensus-base ratio (i.e., the ratio of the number of reads supporting the SNP base to the total number of reads mapped to the reference sequence for each position) was >0.9. The second criterion was included in this step to exclude SNPs that may have been introduced by recombination.
For the Koganei 65-0.15 and Kagoshima 11A strains, we obtained 73.7 and 96.0 million reads that each resulted in 4,033- and 5,073-fold sequence depths, respectively. For the other strains, we obtained an average of 1.60 (range, 0.66 to 3.78) million reads per genome, with a minimum coverage of 63.9-fold (average, 138.0-fold) (Table S1).
For the phylogenetic tree construction, the completed genome sequences of two Chinese strains, SY1027 (accession no. CP005079) (17) and GXBY-1 (accession no. CP014861) (18), both of which were recently isolated from acute cases of swine septicemia, were obtained from GenBank. Sequence alignment to the Fujisawa genome sequence and SNP detection were performed using the programs NUCmer and Show-SNPs, respectively, in the MUMmer package (version 3.23) (28). As an outgroup, raw read sequences of U.S. strain HC-585 (accession no. SAMN03837082), isolated from a swine with septicemia in 1975 (7, 16), were retrieved from GenBank. Trimming of adaptor sequences, filtering of low-quality reads, and mapping to the Fujisawa genome sequence were performed as described above.
A total of 2,239 SNP sites were identified among the 36 genomes analyzed in this study. All SNPs were concatenated into a single sequence for each strain to be used for the construction of a phylogenetic tree using the maximum-likelihood method, with 1,000 bootstrap replicates in the RAxML program (29).
Accession number(s).
The short-read archives have been deposited in the DNA Data Bank of Japan (DDBJ) under accession numbers DRA003556 and DRA003561 for the Koganei 65-0.15 strain and the 34 field strains, respectively.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NARO.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00130-17.
REFERENCES
- 1.Wood RL, Henderson LM. 2006. Erysipelas, p 629–638. In Straw BE, Zimmerman JJ, D'Allaire S, Taylor DJ (ed), Diseases of swine, 9th ed Iowa State University Press, Ames, IA. [Google Scholar]
- 2.Kucsera G. 1973. Proposal for standardisation of the designations used for serotypes of Erysipelothrix rhusiopathiae (Migula) Buchanan. Int J Syst Bacteriol 23:184–188. doi: 10.1099/00207713-23-2-184. [DOI] [Google Scholar]
- 3.Takahashi T, Fujisawa T, Tamura Y, Suzuki S, Muramatsu M, Sawada T, Benno Y, Mitsuoka T. 1992. DNA relatedness among Erysipelothrix rhusiopathiae strains representing all twenty-three serovars and Erysipelothrix tonsillarum. Int J Syst Bacteriol 42:469–473. doi: 10.1099/00207713-42-3-469. [DOI] [PubMed] [Google Scholar]
- 4.Bender JS, Shen HG, Irwin CK, Schwartz KJ, Opriessnig T. 2010. Characterization of Erysipelothrix species isolates from clinically affected pigs, environmental samples, and vaccine strains from six recent swine erysipelas outbreaks in the United States. Clin Vaccine Immunol 17:1605–1611. doi: 10.1128/CVI.00206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imada Y, Takase A, Kikuma R, Iwamaru Y, Akachi S, Hayakawa Y. 2004. Serotyping of 800 strains of Erysipelothrix isolated from pigs affected with erysipelas and discrimination of attenuated live vaccine strain by genotyping. J Clin Microbiol 42:2121–2126. doi: 10.1128/JCM.42.5.2121-2126.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiraiwa K, Ogawa Y, Eguchi M, Hikono H, Kusumoto M, Shimoji Y. 2015. Development of an SNP-based PCR assay for rapid differentiation of a Japanese live vaccine strain from field isolates of Erysipelothrix rhusiopathiae. J Microbiol Methods 117:11–13. doi: 10.1016/j.mimet.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Opriessnig T, Hoffman LJ, Harris DL, Gaul SB, Halbur PG. 2004. Erysipelothrix rhusiopathiae: genetic characterization of midwest U.S. isolates and live commercial vaccines using pulsed-field gel electrophoresis. J Vet Diagn Invest 16:101–107. doi: 10.1177/104063870401600202. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi T, Nagamine N, Kijima M, Suzuki S, Takagi M, Tamura Y, Nakamura M, Muramatsu M, Sawada T. 1996. Serovars of Erysipelothrix strains isolated from pigs affected with erysipelas in Japan. J Vet Med Sci 58:587–589. doi: 10.1292/jvms.58.587. [DOI] [PubMed] [Google Scholar]
- 9.Wood RL, Harrington R Jr. 1978. Serotypes of Erysipelothrix rhusiopathiae isolated from swine and from soil and manure of swine pens in the United States. Am J Vet Res 39:1833–1840. [PubMed] [Google Scholar]
- 10.Janßen T, Voss M, Kühl M, Semmler T, Philipp HC, Ewers C. 2015. A combinational approach of multilocus sequence typing and other molecular typing methods in unravelling the epidemiology of Erysipelothrix rhusiopathiae strains from poultry and mammals. Vet Res 46:84. doi: 10.1186/s13567-015-0216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutz S, Bollinger T, Branigan M, Checkley S, Davison T, Dumond M, Elkin B, Forde T, Hutchins W, Niptanatiak A, Orsel K. 2015. Erysipelothrix rhusiopathiae associated with recent widespread muskox mortalities in the Canadian Arctic. Can Vet J 56:560–563. [PMC free article] [PubMed] [Google Scholar]
- 12.Nagai S, To H, Kanda A. 2008. Differentiation of Erysipelothrix rhusiopathiae strains by nucleotide sequence analysis of a hypervariable region in the spaA gene: discrimination of a live vaccine strain from field isolates. J Vet Diagn Invest 20:336–342. doi: 10.1177/104063870802000313. [DOI] [PubMed] [Google Scholar]
- 13.To H, Sato H, Tazumi A, Tsutsumi N, Nagai S, Iwata A, Nagano T. 2012. Characterization of Erysipelothrix rhusiopathiae strains isolated from recent swine erysipelas outbreaks in Japan. J Vet Med Sci 74:949–953. doi: 10.1292/jvms.11-0533. [DOI] [PubMed] [Google Scholar]
- 14.Uchiyama M, Yamamoto K, Ochiai M, Yamamoto T, Hirano F, Imamura S, Nagai H, Ohishi K, Horiuchi N, Kijima M. 2014. Prevalence of Met-203 type spaA variant in Erysipelothrix rhusiopathiae isolates and the efficacy of swine erysipelas vaccines in Japan. Biologicals 42:109–113. doi: 10.1016/j.biologicals.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Ohyama A, Kurokawa K, Enai K, Saitoh H, Kanaya S, Amin MAU, Ogasawara N. 2006. Bioinformatics tool for genomic era: a step towards the in silico experiments–focused on molecular cloning. J Comp Aid Chem 7:102–115. doi: 10.2751/jcac.7.102. [DOI] [Google Scholar]
- 16.Forde T, Biek R, Zadoks R, Workentine ML, De Buck J, Kutz S, Opriessnig T, Trewby H, van der Meer F, Orsel K. 2016. Genomic analysis of the multi-host pathogen Erysipelothrix rhusiopathiae reveals extensive recombination as well as the existence of three generalist clades with wide geographic distribution. BMC Genomics 17:461. doi: 10.1186/s12864-016-2643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwok AH, Li Y, Jiang J, Jiang P, Leung FC. 2014. Complete genome assembly and characterization of an outbreak strain of the causative agent of swine erysipelas–Erysipelothrix rhusiopathiae SY1027. BMC Microbiol 14:179. doi: 10.1186/1471-2180-14-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang HB, Xie J, Wang L, Liu F, Wu J. 2016. Complete genome sequence of Erysipelothrix rhusiopathiae strain GXBY-1 isolated from acute swine erysipelas outbreaks in south China. Genom Data 8:70–71. doi: 10.1016/j.gdata.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimoji Y, Ogawa Y, Osaki M, Kabeya H, Maruyama S, Mikami T, Sekizaki T. 2003. Adhesive surface proteins of Erysipelothrix rhusiopathiae bind to polystyrene, fibronectin, and type I and IV collagens. J Bacteriol 185:2739–2748. doi: 10.1128/JB.185.9.2739-2748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa Y, Ooka T, Shi F, Ogura Y, Nakayama K, Hayashi T, Shimoji Y. 2011. The genome of Erysipelothrix rhusiopathiae, the causative agent of swine erysipelas, reveals new insights into the evolution of Firmicutes and the organism's intracellular adaptations. J Bacteriol 193:2959–2971. doi: 10.1128/JB.01500-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forde TL, Orsel K, Zadoks RN, Biek R, Adams LG, Checkley SL, Davison T, De Buck J, Dumond M, Elkin BT, Finnegan L, Macbeth BJ, Nelson C, Niptanatiak A, Sather S, Schwantje HM, van der Meer F, Kutz SJ. 2016. Bacterial genomics reveal the complex epidemiology of an emerging pathogen in Arctic and boreal ungulates. Front Microbiol 7:1759. doi: 10.3389/fmicb.2016.01759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischetti VA. 2006. Surface proteins on Gram-positive bacteria, p 12–25. In Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood JI (ed), Gram-positive pathogens, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 23.Galán JE, Timoney JF. 1990. Cloning and expression in Escherichia coli of a protective antigen of Erysipelothrix rhusiopathiae. Infect Immun 58:3116–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okatani AT, Uto T, Taniguchi T, Horisaka T, Horikita T, Kaneko K, Hayashidani H. 2001. Pulsed-field gel electrophoresis in differentiation of Erysipelothrix species strains. J Clin Microbiol 39:4032–4036. doi: 10.1128/JCM.39.11.4032-4036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusumoto M, Fukamizu D, Ogura Y, Yoshida E, Yamamoto F, Iwata T, Ooka T, Akiba M, Hayashi T. 2014. Lineage-specific distribution of insertion sequence excision enhancer in enterotoxigenic Escherichia coli isolated from swine. Appl Environ Microbiol 80:1394–1402. doi: 10.1128/AEM.03696-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. 2004. Versatile and open software for comparing large genomes. Genome Biol 5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.