ABSTRACT
Extended-spectrum β-lactamases (ESBLs) and plasmid-mediated AmpC β-lactamases (pAmpC) are enzymes able to hydrolyze a large variety of β-lactam antibiotics, including third-generation cephalosporins and monobactams. Broilers and broiler meat products can be highly contaminated with ESBL- and pAmpC-producing Escherichia coli strains, also known as extended-spectrum cephalosporin (ESC)-resistant E. coli strains, and can be a source for human infections. As few data on interventions to reduce the presence of ESC-resistant E. coli in broilers are available, we used transmission experiments to examine the role of competitive exclusion (CE) on reducing transmission and excretion in broilers. A broiler model to study the transmission of ESC-resistant E. coli was set up. Day-old chickens were challenged with an ESBL-producing E. coli strain isolated from healthy broilers in the Netherlands. Challenged and not challenged chicks were housed together in pairs or in groups, and ESBL-producing E. coli transmission was monitored via selective culturing of cloacal swab specimens. We observed a statistically significant reduction in both the transmission and excretion of ESBL-producing E. coli in chicks treated with the probiotic flora before E. coli challenge compared to the transmission and excretion in untreated controls. In conclusion, our results support the use of competitive exclusion as an intervention strategy to control ESC-resistant E. coli in the field.
IMPORTANCE Extended-spectrum β-lactamases (ESBLs) and plasmid-mediated AmpC β-lactamases are a primary cause of resistance to β-lactam antibiotics among members of the family Enterobacteriaceae in humans, animals, and the environment. Food-producing animals are not exempt from this, with a high prevalence being seen in broilers, and there is evidence pointing to a possible foodborne source for human contamination. We investigated the effect of administration of a commercial probiotic product as an intervention to reduce the amount of ESBL-producing Escherichia coli in broilers. Our results showed a substantial reduction in the level of colonization of broiler intestines by ESBL-producing E. coli after administration of commercial probiotic product. The protective effect provided by these probiotics could be implemented on a larger scale in poultry production. Reductions in the levels of ESBL-producing Enterobacteriaceae in the food chain would considerably benefit public health.
KEYWORDS: ESBL, Escherichia coli, poultry, transmission, excretion, competitive exclusion, intervention, β-lactamases, broiler chicken
INTRODUCTION
Extended-spectrum β-lactamases (ESBLs) and plasmid-mediated AmpC β-lactamases (pAmpC) are enzymes able to hydrolyze a large variety of β-lactam antibiotics, including third-generation cephalosporins and monobactams (1). Numerous enzymes have been described to date, including the most clinically significant variants involved in β-lactam resistance in Enterobacteriaceae: blaCTX-M (2), blaSHV (3), blaTEM (4), and blaCMY (5). The successful spread of ESBLs and pAmpCs has been explained by the effective association of β-lactamase genes with conjugative plasmids (6) that drove the dissemination of these enzymes to virtually all ecological niches in the last few decades (7).
Among the members of the Enterobacteriaceae, extended-spectrum cephalosporin (ESC)-resistant Escherichia coli isolates are the most frequently found ESBL or pAmpC producers in livestock (8, 9). Several studies reported the dissemination of multidrug-resistant and ESC-resistant E. coli strains in poultry all over Europe (10–13). Although these bacteria rarely pose a direct risk to animal health, indirect evidence pointed to a possible foodborne source for human colonization (14) as early as the 1970s (15, 16). Due to intestinal carriage and the high levels of contamination of retail meat (17–19), poultry has been indicated to be an E. coli reservoir for humans (20).
Broiler chicken meat is the final product of a complex poultry production pyramid. Recent studies carried out in the Netherlands, Sweden, and Switzerland have reported ESC-resistant Enterobacteriaceae, including E. coli, to be present at the top of the pyramid and to be vertically transmitted, despite the absence of a clear selective pressure (21–23). While the top of the pyramid still has a relatively low prevalence of ESC-resistant E. coli, these bacteria can enter the production chain from the environment at all levels, resulting in a variably exposed production system (24–26). Control or complete removal of ESC-resistant E. coli has become a major goal in poultry production.
In addition to the use of good manufacturing practices, intervention strategies applied to control Campylobacter and Salmonella (27–30) have seen their first applications in controlling ESC-resistant E. coli in the poultry production system in recent years. The reduction of ESC-resistant E. coli has been associated with the use of acidified drinking water as the sole drinking water source in a risk factor study in Belgian broiler farms (31). Competitive exclusion (CE) is defined as the protective effect of a natural intestinal bacterial flora in limiting colonization with certain bacterial pathogens (32). In broiler studies, CE is achieved by the administration of probiotics, i.e., live microorganisms which, when administered in adequate amounts, confer a health benefit on the host (33). Several commercial probiotic products have been developed to reestablish the normal intestinal bacteria of poultry, and a few have been applied to control ESC-resistant or pathogenic E. coli. Nuotio and colleagues have shown a substantial reduction in the levels of colonization of broiler ceca with ESBL-producing E. coli as a result of the use of the Broilact product in young broiler chickens (34). A reduction in the level of colonization with pathogenic E. coli bacteria was also demonstrated to be an effect of commercial CE culture administration in day-old broilers (35).
As of today, little knowledge about the transmission dynamics of ESC-resistant E. coli in broilers is available. Huijbers and colleagues reported the results of the first longitudinal study conducted on an organic broiler farm and explored the effect of direct broiler contact relative to the environment on ESC-resistant E. coli transmission (13). The aim of our study was to set up an in vivo broiler model to study ESC-resistant E. coli transmission and to analyze the role of competitive exclusion on preventing this transmission in broilers. Competitive exclusion by the Aviguard product was chosen as an intervention strategy on the basis of its ability to reduce the number of Salmonella and pathogenic E. coli challenge organisms in the cecal content of broiler chicks (35–37). Aviguard is a commercial freeze-dried fermentation product derived from healthy, pathogen-free birds (http://www.msd-animal-health.co.za/products/aviguard/020_product_details.aspx). It contains a mixture of live, commensal, partially characterized bacteria for use as a spray treatment or drinking water application for poultry for establishment or reestablishment of the normal intestinal bacteria of poultry.
The aim of this work was to set up an animal trial to study the rates of transmission of ESC-resistant E. coli among broilers and test the effect of Aviguard treatment on the transmission in chick pairs and groups.
RESULTS
Transmission of ESBL-producing E. coli in chick pairs.
Transmission experiments with challenged and susceptible (S) chick pairs were performed to study the rate of direct transmission of ESBL-producing E. coli and to set up a baseline for future trials. The experimental setup also enabled the study of indirect contact transmission (via droplets and/or aerosols) between cages thanks to the presence of sentinel chicks housed in the same pen (Fig. 1A). ESBL-producing E. coli transmission was investigated in both infectious (I) and S chicks over a period of 13 days from the time of challenge of I chicks on day 1 (D1) (Table 1). All I chicks were successfully colonized, and all started shedding ESBL-producing E. coli within 24 h after challenge (D2; Table 1). The presence of ESBL-producing E. coli was already detected in S chicks after 24 h from the time that they were housed together with I chicks (D5, 1st sampling time point; Table 1). Estimation of the transmission rate parameter β yielded a value of 1.33 per day (95% confidence interval [CI], 0.600 to 2.51). These trials confirmed that ESBL-producing E. coli quickly spread within chicks, and all of them were positive for ESBL-producing E. coli at the end of the trial (D13; Table 1).
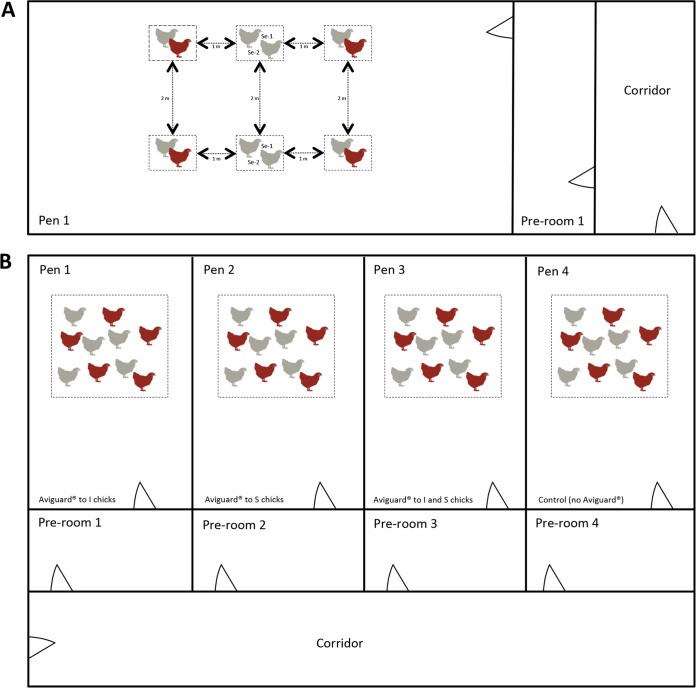
FIG 1.
Schematic representation of the animal trials used to monitor the ESBL-producing E. coli transmission rate. (A) Representation of one round of trials with animal pairs. Each pen consisted of 4 pairs of I chicks and S chicks and 2 pairs of sentinel (Se) chicks, for a total of 6 cages per pen. (B) Representation of one round of group trials. Five challenged (I) chicks were housed together with 5 susceptible (S) chicks inside a cage in each pen; each group (treated and control groups) was housed in separate pens. Red, challenged (I) chicks; gray, susceptible or sentinel chicks. The schematic representation is not to scale. For details on cage size, distances, and ventilation, refer to “Chickens and housing conditions” in Materials and Methods.
TABLE 1.
Detection and quantification of ESBL-producing E. coli in pairsa
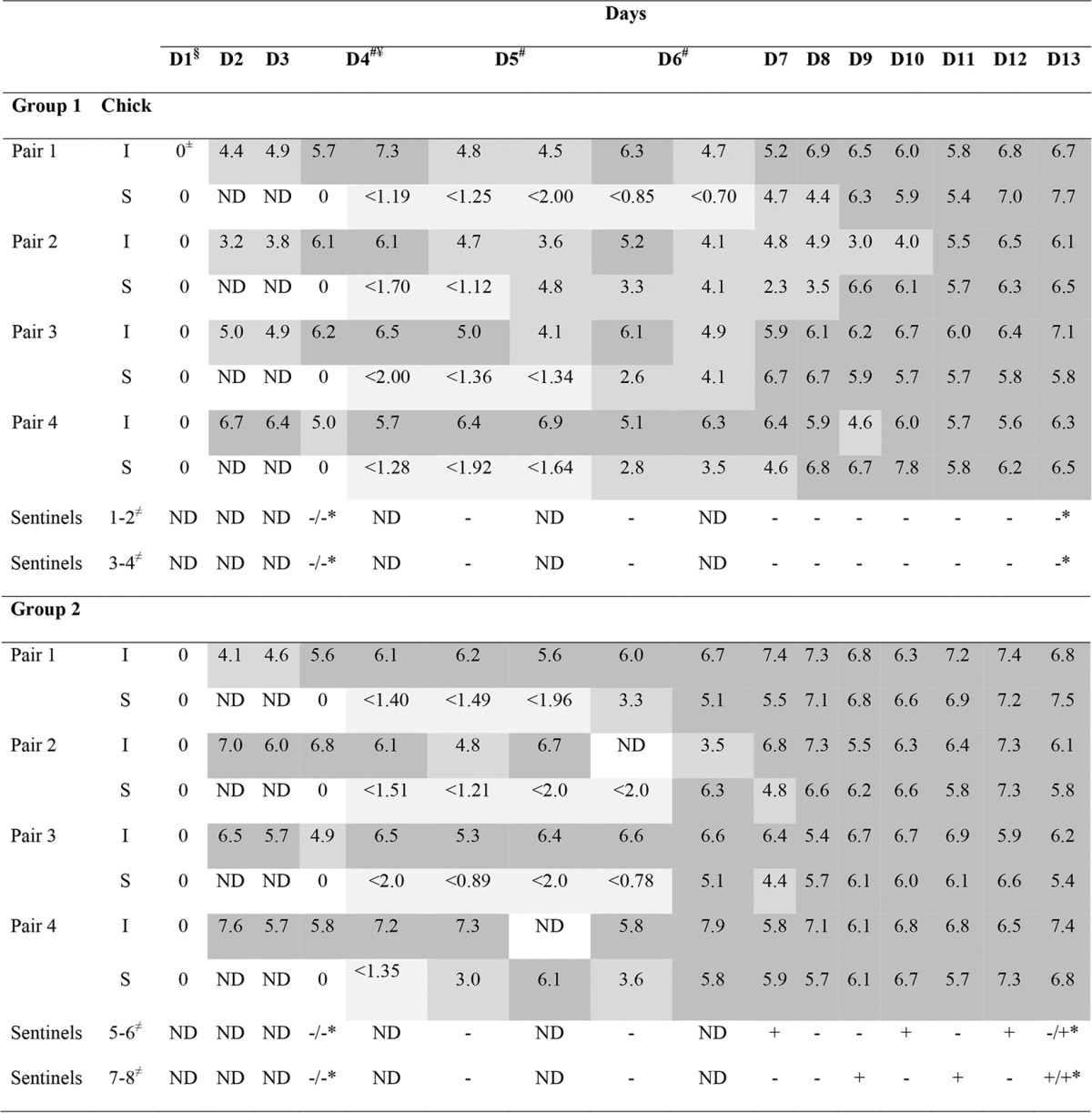
Different shades of gray indicate the concentration of ESBL-producing E. coli, expressed as the log10 number of CFU per gram of feces; darker colors correspond to higher ESBL-producing E. coli concentrations. I, infected and infectious, ESBL-producing E. coli-challenged chick; S, susceptible chick; ND, not determined; + or − for sentinel chicks, the presence or absence of ESBL-producing E. coli in mixed droppings of sentinel chicks, respectively (+/− represent the results for each sentinel chick in the pair); §, D1 is the day of ESBL-producing E. coli challenge of 5-day-old chicks; cloacal swab specimens were taken right before challenge; ±, 0 values for I or S chicks are not expressed as the log10 number of CFU per gram of feces but indicate no ESBL-producing E. coli detection; ¥, from D4, S, I, and sentinel chicks were housed together; #, days 4 to 6 each had two sampling points (9 a.m. and 4 p.m.); ≠, sentinel chicks were housed in pairs in one cage; results are derived from two mixed droppings from two sentinel chicks, unless otherwise stated; *, single cloacal swab specimens were taken from each sentinel chick per pair.
ESBL-producing E. coli indirect transmission to sentinel chicks placed in separate cages in the same pen as I and S chicks on day 4 was investigated. Initial cloacal swab specimens were negative for all sentinel chicks. For the duration of the study, fresh mixed droppings from each cage with sentinel chicks were analyzed, and a fluctuation in the presence of ESBL-producing E. coli was observed (D6 to D13; Table 1). At the end of the trial, three out of four sentinel chicks in pen 2 were colonized with ESBL-producing E. coli, whereas all four sentinel chicks in pen 1 remained negative.
Competitive exclusion has an effect in reducing ESBL-producing E. coli transmission and excretion.
Competitive exclusion was investigated to understand its potential role in reducing excretion and the rate of transmission of ESBL-producing E. coli. Probiotic flora was administered to the chicks 24 h before challenge (D0) with ESBL-producing E. coli (D1) since in previous experimental trials probiotic administration after challenge resulted in no effect on transmission (unpublished data).
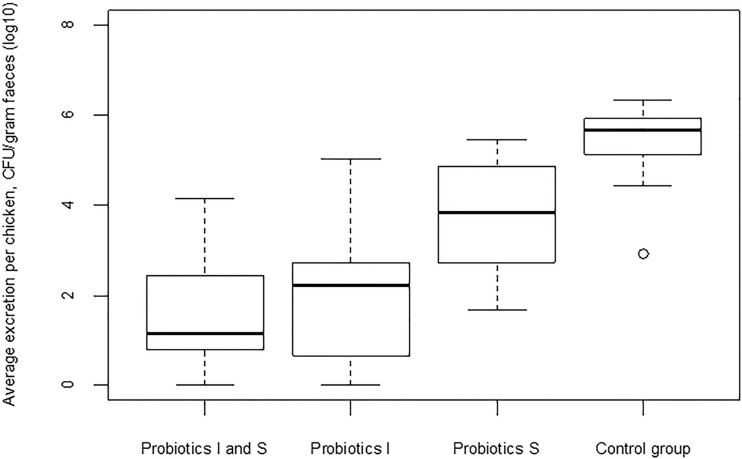
To have a comprehensive idea of the different effects on excretion and susceptibility to colonization, competitive flora was administered to I and S chicks, only I chicks, and only S chicks in three separate groups (Fig. 1B). The results of detection of ESBL-producing E. coli in the three groups during transmission experiments in the presence or absence of competitive exclusion are presented in Table 2. Aviguard reduced the rates of transmission of ESBL-producing E. coli. Competitive exclusion was effective when I chicks were treated alone or together with S chicks, as the transmission rate in these groups significantly differed from that in the control group (P < 0.001 for both) (Table 3). Although the transmission rate was reduced by probiotic treatment only in S chicks (β = 4.68; 95% CI, 2.09 to 9.95), there was no significant difference in the transmission rate from that for the control group (β = ∞; 95% CI, 4.53 to ∞). Similar effects were observed for the excretion of ESBL-producing E. coli, as shown in Fig. 2. The median of the average excretion for the control group was 5.68 CFU/g feces, whereas those for the treated groups were 1.17 CFU/g feces (I and S chicks), 2.22 CFU/g feces (I chicks), and 3.86 CFU/g feces (S chicks); the medians for the groups were significantly different {H(3) = 51.8 [H(3) is the nonparametric Kruskal-Wallis test parameter; see Materials and Methods], P < 0.001}. Pairwise comparison of results for the treated groups with those for the control group showed a significant difference between the medians for all treated groups and the control group (W = 4 for I and S chicks, W = 7 for I chicks, and W = 38 for S chicks [W is the Wilcoxon parameter; see Materials and Methods]; P < 0.001 for all groups).
TABLE 2.
Detection and quantification of ESBL-producing E. coli in groups treated by use of competitive exclusiona
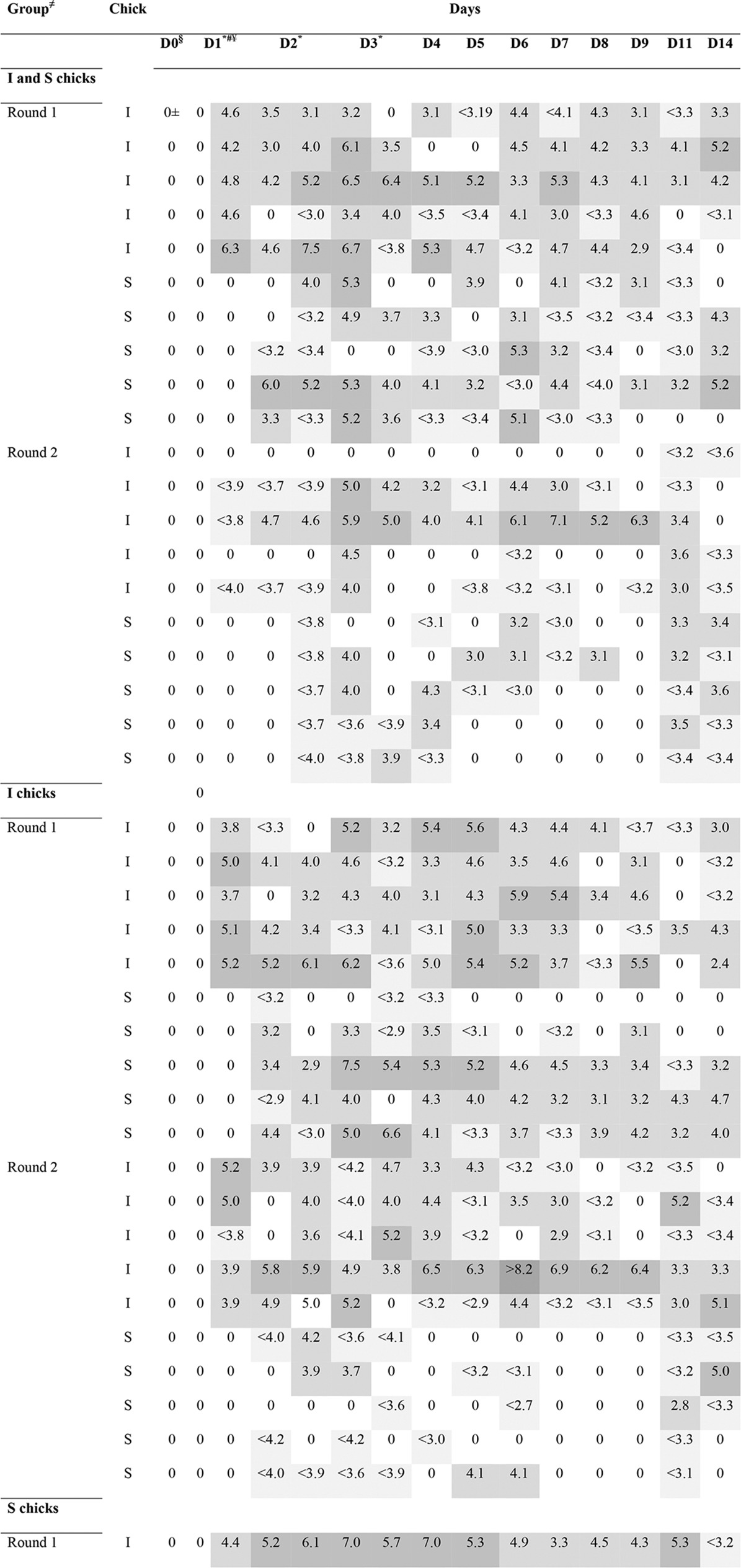
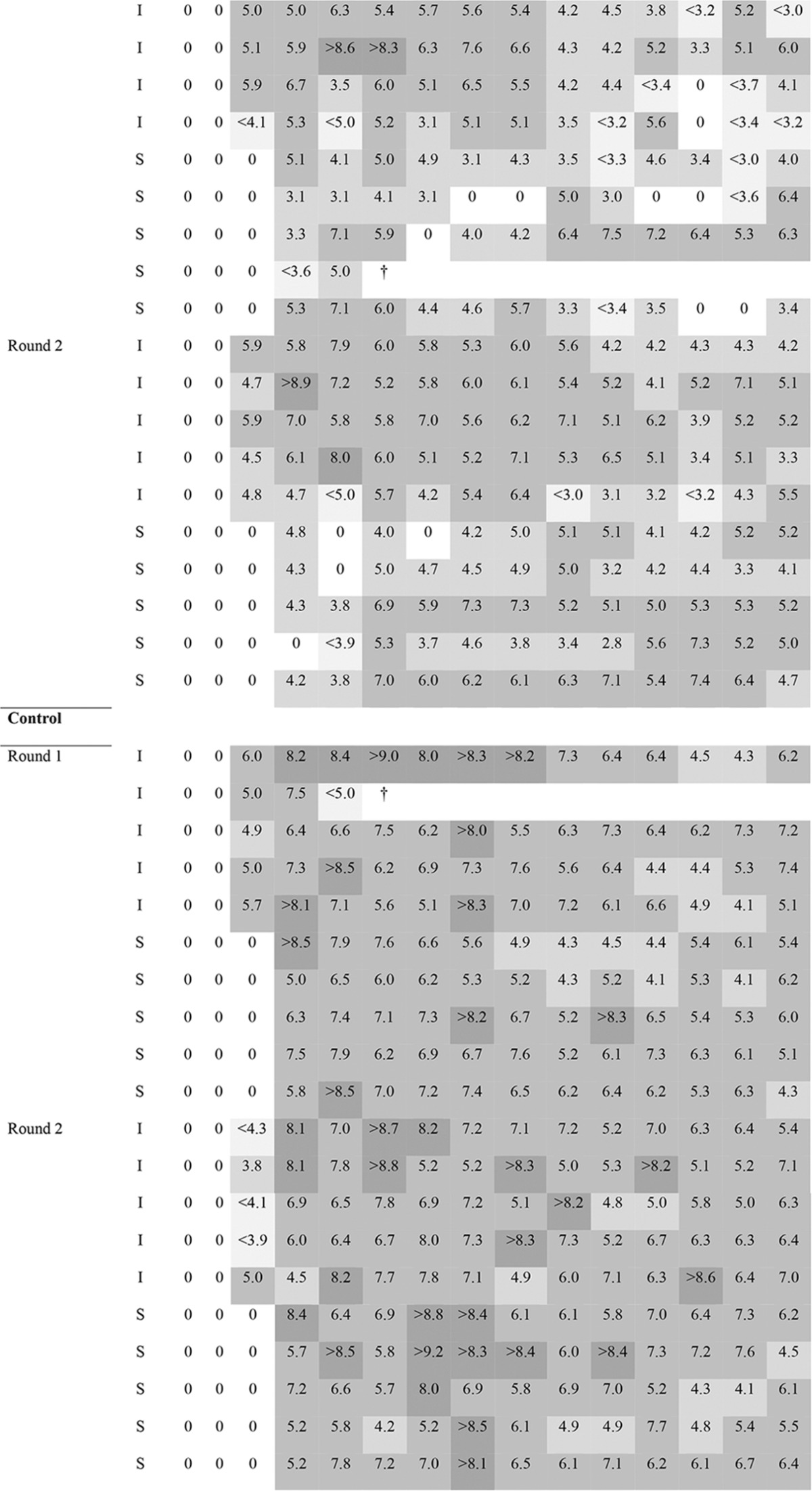
Different shades of gray indicate the concentration of ESBL-producing E. coli, expressed as the log10 number of CFU per gram of feces; darker colors correspond to higher concentrations of ESBL-producing E. coli bacteria. I, infected or infectious, ESBL-producing E. coli-challenged chick; S, susceptible chick; ND, not determined. ≠, group indicates if Aviguard was administered to I and/or S chicks; control groups were administered the ESBL-producing E. coli challenge strain only on D1; ±, 0 values for I or S chicks are not expressed as the log10 number of CFU per gram feces but indicate no ESBL-producing E. coli detection; §, D0 is the day of Aviguard administration; cloacal swab specimens were taken right before administration; #, D1 is the day of ESBL-producing E. coli challenge; cloacal swab specimens were taken right before challenge; ¥, on D1, at 1 h after challenge, I chicks were housed together with S chicks; *, days 1 to 3 had each two sampling points (9 a.m. and 4 p.m.); †, the bird died.
TABLE 3.
Effect of competitive exclusion on rate of ESBL-producing E. coli transmission in broilers
| Groupa | βb (day−1) | 95% CIc |
|---|---|---|
| Control | ∞ | 4.53–∞ |
| I and S | 0.669* | 0.334–1.18 |
| I | 0.331* | 0.151–0.617 |
| S | 4.68 | 2.09–9.95 |
Group indicates whether Aviguard was administered to I and/or S chicks. Control groups were not treated with Aviguard.
β, transmission rate parameter; *, β was significantly different between the treatment and control groups (P < 0.001).
CI, confidence interval.
FIG 2.
Effect of competitive exclusion on the excretion of ESBL-producing E. coli in broilers. The plot shows the average level of excretion per chicken for the different groups, each with a control group and three treated groups (I chicks, S chicks, and I and S chicks). The heavy line indicates the median; the box extends from the lower to the upper quartile; the whiskers extend from the box to show the range of the data, with the maximum being 1.5 times the box length; the small circle indicates an outlier in the control group.
DISCUSSION
By early administration of nonpathogenic strains belonging to the normal intestinal bacteria, competitive exclusion prevents colonization of the gut of broilers with transient harmful bacteria. This approach has proved to be effective in terms of limiting colonization of broilers with pathogenic E. coli strains (35, 38, 39) as well as Salmonella (40). Similar results were obtained in studies to control colonization with and shedding of different nonpathogenic ESC-resistant E. coli strains in the ceca of broilers (34).
Based on these preliminary results, we set up an in vivo animal model and trials longer than 1 week, as suggested by Nuotio and colleagues (34), in order to observe the extension of the protective effect of competitive exclusion and its effect on transmission to control ESBL-producing E. coli in broilers. The use of an E. coli strain carrying blaCTX-M-1 on an IncI1 plasmid (41), which is known to be the most prevalent plasmid-ESBL gene association in broilers, was the option closest to the real situation in the field. Our study reports the effect of competitive exclusion on the transmission of ESBL-producing E. coli between broilers.
ESBL-producing E. coli isolates spread between chick pairs throughout the trial, with the final rate of colonization of the birds being 100%. ESBL-producing E. coli isolates easily spread between the cages in one pen, indicating that indirect transmission between subgroups of physically separated chickens occurred. Due to this cross-contamination, only one group was housed per pen for the follow-up group trials.
ESBL-producing E. coli excretion was significantly reduced in groups treated with Aviguard (I and S birds or I birds) compared to that in the control groups, with medians decreasing from 5.68 to 1.17 CFU/g feces. This may lead to a significant reduction in the rate of shedding of ESBL-producing E. coli in the environment and represents a promising result for reduced exposure of humans and other animals to ESBL-producing E coli.
Competitive exclusion strongly reduced the transmission rate parameter β, which corresponds to the number of birds infected by one infectious bird per unit of time (Table 3). However, despite the reduction of β, during the trial ESBL-producing E. coli could still spread to the majority of chickens in the flock due to the long period of excretion from individual birds. The effect on both excretion and transmission was larger when challenged birds (I birds or I and S birds) were treated beforehand than when only susceptible (S) birds were treated, indicating that the effect of competitive exclusion depends more on the reduced shedding of I birds than on the reduced susceptibility of S birds. Translating these results to a practical level, since birds carrying ESBL-producing E. coli cannot be recognized in a flock, treatment of all birds should be pursued as soon as possible before the first uptake of ESBL-producing E. coli occurs, i.e., at very early stages of the production chain, ideally, at the hatchery.
Although different animal trials (with Broilact versus Aviguard, 5 versus 14 days of follow-up, and various challenge strains) were set up, our results are in line with the overall reduction in the amounts of E. coli (35, 39) or ESBL-producing E. coli (34) observed in broilers treated by use of the competitive exclusion strategy. In our study, both transmission and excretion were calculated and shown to be reduced by competitive exclusion, whereas previous studies analyzed only E. coli colonization rates, which did not allow a direct comparison of different parameters. As previously suggested (42), the effectiveness of competitive exclusion might rely on different adhesive and/or metabolic properties of the bacterial flora, but further studies in this direction, specifically, studies of E. coli-versus-E. coli competition, should be pursued.
In conclusion, these transmission experiments demonstrated that competitive exclusion has a clear effect in reducing the transmission of ESBL-producing E. coli in broilers under controlled conditions. The information gathered in the present work provides a starting point for controlling ESBL-producing E. coli dissemination in broilers through probiotic administration. Our results point out that the use of probiotics alone will not be enough to completely control ESBL-producing E. coli dissemination and that a multimeasure approach will be needed. As we are aware of the differences between controlled trial conditions and field conditions and the necessity to evaluate the effect on different or multiple E. coli strains, which would represent the real scenario in a broiler gut, the next step will be to investigate the effectiveness of this strategy in the field, ideally, along different steps of the poultry production pyramid. Interventions aimed at reducing or completely removing ESBL-producing E. coli should be focused at the top of the pyramid but also at measures to prevent reintroduction from the environment. As we know that competitive exclusion is effective only before ESBL-producing E. coli colonization, it remains a challenge whether the same results will be achieved since contact with ESC-resistant E. coli can occur as early as in the hatchery.
MATERIALS AND METHODS
E. coli challenge.
E. coli strain E75.01/pE38.27 (multilocus sequence type 539), which carries the ESBL gene blaCTX-M-1 on an IncI1 plasmid and which is resistant to ciprofloxacin and cefotaxime (41), was used to challenge the chicks in all transmission experiments. MacConkey agar (product no. 212123; Becton Dickinson) was used to culture the E. coli challenge strain. The ciprofloxacin (1 mg/liter) and cefotaxime (1 mg/liter) used for selective plating throughout the study were obtained from Sigma-Aldrich (Poole, Dorset, UK). Challenged birds were inoculated with 0.5 ml of a 106- or 108-CFU/ml dilution of the inoculum strain prepared in saline solution, which was administered in the crop through the beak with a blunt needle (see “Experimental design to estimate transmission rates in pairs” and “Experimental design to evaluate competitive exclusion treatment in groups” below for details). E. coli E75.01/pE38.27 was cultured on heart infusion agar (HIS) with 5% sheep blood (Becton Dickinson GmbH, Heidelberg, Germany) supplemented with cefotaxime and ciprofloxacin (1 mg/liter each).
Cloacal swab and manure sample analysis.
Cloacal swab samples, obtained using sterile dry cotton swabs, were analyzed individually and were weighed before and after sampling to determine the amount of feces collected. Each swab was suspended in 1 ml saline solution (0.85% NaCl), and to quantify the number of CFU of ESBL-producing E. coli, a 10-fold dilution series (10−1 to 10−5) was prepared in saline solution by inoculating 10 μl of each dilution on MacConkey plates with cefotaxime and ciprofloxacin (1 mg/liter each), which were incubated overnight (O/N) at 37°C. A semiquantitative method was used, and on the basis of the highest dilution from which E. coli growth was observed and the amount of feces on the swabs, the number of CFU per gram of feces was calculated. The average weight of the fecal samples on the cloacal swab specimens taken from chicks ranged from 0.004 to 0.268 g.
To confirm the results for samples negative by quantification analysis, we also performed O/N enrichment by adding 100 μl of the original swab specimen suspension to 3 ml Luria-Bertani broth (LB) with cefotaxime and ciprofloxacin (1 mg/liter each) for O/N selective enrichment at 37°C. If the quantification result was negative and the enrichment culture result was positive, the value for the sample was reported with a < symbol and corrected for the amount of feces on the swab. If the quantification and enrichment results were negative, the value for the sample was reported to be 0.
Droppings collected from sentinel chicks in experiments with pairs of chicks were screened by resuspending 1 g of manure (fecal material only, no litter) in 9 ml peptone-glycerol, followed by inoculation of 10 μl on MacConkey plates with cefotaxime and ciprofloxacin (1 mg/liter each) and O/N incubation at 37°C. Results were defined as positive or negative for the presence or absence of the ESBL-producing E. coli challenge strain.
Chickens and housing conditions.
Eighteen-day-old embryonated eggs from a specific-pathogen-free (SPF) broiler parent flock were hatched at the experimental facility. The absence of ESBL-producing Enterobacteriaceae in the flock was confirmed. Directly after the chicks hatched, egg shells and hatching papers were tested for the absence of ESBL. Chicks were tested for the absence of ESBL-producing Enterobacteriaceae by the collection of cloacal swab specimens on different days after hatching, depending on the animal trial setup.
Animal trials were performed in controlled pens (4.70 m by 7.40 m) inside experimental facilities. Every pen had its own separate preroom where the researchers changed their clothes and shoes before entering the pen. Cleaning and disinfection of the room and preroom with formaldehyde, including the chicken cages, feeders, and drinkers, were performed before each trial. After disinfection, the floors, walls, cages, feed, and drinking equipment were swabbed with sterile swabs soaked in sterile phosphate-buffered saline (PBS) and tested for the absence of Enterobacteriaceae as indicators of external contamination. Controlled ventilation in the experimental facility was ensured by the use of air intakes equipped with HEPA filters. Cages were placed on the pen floor, and the floor of the cages was covered with wood shavings. The researchers changed their gloves for sterile gloves between evaluations of chick pairs and changed their clothes between evaluations of pens, and once a day the floor between the cages was disinfected with a Sumatab D4 (JohnsonDiversey, Utrecht, the Netherlands) solution (2 tablets/10 liters water).
In the trials with chick pairs, six cages (0.75 m by 0.75 m by 0.50 m) with two chicks each were housed in one pen of the experimental facility at a distance of 1 to 2 m from each other (Fig. 1A). In the group trials, only one cage (density, 17 chickens/m2) with 10 chicks (5 S chicks and 5 I chicks) was housed per pen to prevent transmission from one group to another (Fig. 1B).
Day-old chickens received gamma-irradiated commercial feed without coccidiostats; feed and water were available ad libitum during all animal trials. Water was supplied using height-adjustable drinking cups connected to a separate reservoir for each cage, and feed was supplied in a closed feed pan with openings for eating. Chicks were euthanized by means of injection of an overdose of pentobarbital (75 to 100 mg/kg of body weight, or approximately 0.4 to 0.5 ml/kg) in the wing vein.
Experimental design to estimate transmission rates in pairs.
Twenty-four 18-day-old embryonated eggs were hatched at the experimental facility. Chicks were tested for the absence of ESBL-producing Enterobacteriaceae on days 1 and 3 after hatching. On day 1 of the transmission experiment (day 5 after hatching), eight chicks were challenged with 0.5 ml containing 108 CFU/ml the ESBL-producing E. coli inoculum strain, and cloacal swab specimens were taken on days 2 and 3 to confirm excretion of the challenge strain. From here on, all chicks challenged with the ESBL-producing E. coli strain are referred to as infectious (I) for statistical analysis of the transmission model (see “Statistical analysis” below for a further explanation). On day 4 after challenge, eight I chicks were paired with eight susceptible (S) chicks and housed in separate groups in two separate pens, each consisting of 4 pairs (1 I chick and 1 S chick; i.e., the number of chicks [n] equals 2). Also on day 4 after challenge, two pairs of sentinel chicks were placed in two cages in the same pen with the 4 I chick and S chick pairs to check for transmission between cages. In total, 6 cages were present in each experimental pen (see Fig. 1A for a schematic representation). I and S chicks were monitored by taking cloacal swab specimens daily, whereas fresh droppings were taken daily from the litter of sentinel chicks. Cloacal swab specimens were taken from the sentinel chicks on days 4 and 13 of the study. Sentinel chicks were always sampled first, followed by S chicks and then I chicks. The experiment was terminated 13 days after challenge.
Experimental design to evaluate competitive exclusion treatment in groups.
Aviguard is a commercial competitive exclusion product (MSD Animal Health Nederland, Boxmeer, the Netherlands), consists of a natural, lyophilized intestinal microflora derived from SPF chicks, and is manufactured by fermentation. Aviguard was suspended in water according to the manufacturer's instructions, and 0.5 ml was administered to the chickens with a crop needle. The absence of ESBL-producing Enterobacteriaceae in Aviguard was tested by resuspending 1 g lyophilized powder in 9 ml peptone-glycerol, plating the suspension on MacConkey plates with cefotaxime and ciprofloxacin (1 mg/liter each), and incubating O/N at 37°C.
Each trial consisted of a control group (5 I chicks and 5 S chicks challenged with E. coli but not treated with Aviguard) and three treatment groups (5 I chicks and 5 S chicks challenged with E. coli and treated with Aviguard). Birds in the control and treatment groups were placed in different pens (see Fig. 1B for a schematic representation). Aviguard was administered on day 0 of the animal trial, before E. coli challenge, either to I chicks only (group I), to S chicks only (group S), or to both I and S chicks (group I and S). D0 of the animal trial corresponded to the day after hatching (chicks were a maximum of 24 h old and had not yet received any feed), when the birds were moved from the hatching unit to the experimental facility, mimicking what happens in real life when they are moved from the hatchery to the farm. On day 1 of the animal trial, I chicks were challenged with the ESBL-producing E. coli strain (0.5 ml containing 106 CFU/ml), and at 1 h after challenge, they were placed in the experimental pen with the S chicks. All chicks were monitored for ESBL carriage by the collection of cloacal swab specimens twice a day on days 1 to 3 (9 a.m. and 4 p.m.), once a day from days 4 to 9, and on days 11 and 14. The experiment was terminated on day 14. The trial was performed in duplicate (rounds 1 and 2; Table 2) at different times.
Statistical analysis.
The aim of the transmission experiments was to quantify the rate of transmission (parameter β) of ESBL-producing E. coli between birds and the effects of intervention on the transmission rate. The transmission rate parameter β is defined as the mean number of new infections produced by a typical infectious individual per unit of time. The results of transmission experiments have previously been analyzed using generalized linear models (43, 44); however, we choose to use a maximum likelihood estimation with a more realistic transmission model. For the benefit of the transmission model, all chicks challenged with the ESBL-producing E. coli strain are referred to as infectious (I), but this should be interpreted as indicating that the birds were colonized by ESBL-producing E. coli and did not have a microbiological infection. Briefly, the analysis of the transmission experiment was based on a stochastic SEIR transmission model, in which individuals are either susceptible (S), latently infected (i.e., infected but not yet infectious) (E), or infected and infectious (I) and recovered (R). Within this model, a chick remains infectious after the first positive samples until the last positive sample. After the last positive sample, the chick is assumed to be noninfectious and not susceptible. The rate of infection acquisition per bird per day (also called the force of infection) was calculated by adding the transmission rate parameter β for all infectious birds. Differently from previous methods (43, 44), we did not assume that birds were infectious from the moment of the first positive sample but assumed that the transition to the infectious class was uniformly distributed within an interval. The force of infection toward susceptible birds was calculated by use of the start of the infectious period, taken from a uniform distribution between the time of the last negative sample (tnegative) and the time of the first positive sample (tpositive). To allow for a latency period between day 0 and 0.5, we recalculated this interval by subtracting 0.5 day (the maximum latency period [lmax]) from the moment of the last negative sample and 0 day (the minimum latency period [lmin]) from the moment of the first positive sample. This more complicated model was fitted to the data with a maximum likelihood method (see the Appendix for mathematical details). All calculations were coded in Mathematica (version 7) (45).
To compare the excretion of ESBL-producing E. coli by the chicks in the different groups, the average level of excretion per chick over all samples during the experiment was calculated and is given as the log10 number of CFU per gram of feces. To test for differences between the different groups, the nonparametric Kruskal-Wallis test was performed [defined by value H(3), where 3 represents the degrees of freedom], and pairwise comparisons between the groups were made by a Wilcoxon test (defined by value W), because of the nonnormality of the data. The P values were adjusted using the Bonferroni method.
Ethics of experimentation.
The study plan describing the animal trials (number 2013094.b) was approved by the Ethical Review Committee (ERC) of Wageningen Bioveterinary Research in accordance with the Act on Experimental Animals.
ACKNOWLEDGMENTS
We thank Joop Testerink, Marga Japing, and Arie Kant for technical assistance in the microbial analysis and the animal caretakers of Wageningen Bioveterinary Research for their valuable technical assistance with the animal trials. We are also grateful to Jantien Backer (National Institute for Public Health and the Environment, RIVM) for programming the original analysis software CATE, Antonio Zagaris (Wageningen Bioveterinary Research) for insightful statistical analysis discussion, and Cindy Dierikx (National Institute for Public Health and the Environment, RIVM) for insightful advice in experimental planning.
J.A.V.D.G. and D.J.M. conceived the study. A.V.E.-Z., B.S., and K.T.V. helped process the samples and collect the data. D.C., G.J.B., E.A.J.F., and J.A.V.D.G. analyzed the data. D.C. and J.A.V.D.G. wrote the manuscript. E.A.J.F. and G.J.B. wrote the appendix. D.J.M. reviewed the manuscript. All authors discussed, read, contributed to, and approved the final manuscript.
This work was funded by the Dutch Ministry of Economic Affairs (grant number BO-20-016-001 to J.A.V.D.G.).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We have no conflict of interest to declare.
APPENDIX
Estimation of transmission rate parameter β.
The analysis of the transmission experiments is based upon the stochastic SEIR model. Each susceptible bird can transit from state S to state E with a certain probability. The rate of this transition [λ(t)] is the sum of the force of infection produced by n individual infectious birds.
| (1) |
The force of infection [λ(t)] incorporates the contagiousness of infectious birds, i.e., how likely it is that an infectious bird would contaminate another bird with the ESBL-producing E. coli strain, and the susceptibility of susceptible birds, i.e., how likely it is that a bird would become colonized after contamination with an ESBL-producing E. coli strain. In this appendix, we use the slightly inadequate terminology of “infection” to indicate the process of contamination and colonization.
In our model, we assumed that a bird becomes infectious somewhere during an interval, indicated by (tnegative, tpositive), and assuming that this occurs with a uniform distribution, this is equivalent to assuming a linear increase in the force of infection produced by this single bird. The force of infection produced by an infected bird i in a group of n birds at a certain time t is
| (2) |
This is a linear interpolation between the time of the last negative sample and the time of the first positive sample of chick i. The boundaries of the interval, (tE1, tE2), are determined by these sample times and a preset range of the latency period (lmin, lmax), with tE1 = tnegative − lmax and tE2 = tpositive − lmin. The probability of transition from the susceptible state (S) to the exposed state (E) in the aforementioned interval of (tE1, tE2) for a specific bird is determined by multiplication of the probability (Pr) of not being infected since the time of first contact with infectious birds (tFC) and being infected during the interval (tE1,tE2):
| (3) |
If we observe in total C transitions from negative to positive samples, the log-likelihood function of these observations, ln[L(β)], is
| (4) |
The log-likelihood function is maximized for the point estimate of β, and the 95% confidence intervals are calculated on the basis of a χ12 distribution of the difference with the log likelihood at the maximum likelihood estimator and any other value of the log-likelihood function. The confidence interval is found for those values, where the log-likelihood function equals 0.05 for the χ12 distribution.
Hypothesis testing was done using the likelihood ratio (Λ) test, for which the test statistic is then given by
| (5) |
This test statistic is approximately χ12 distributed.
REFERENCES
- 1.Fisher JF, Meroueh SO, Mobashery S. 2005. Bacterial resistance to β-lactam antibiotics: compelling opportunism, compelling opportunity. Chem Rev 105:395–424. doi: 10.1021/cr030102i. [DOI] [PubMed] [Google Scholar]
- 2.Canton R, Gonzalez-Alba JM, Galan JC. 2012. CTX-M enzymes: origin and diffusion. Front Microbiol 3:110. doi: 10.3389/fmicb.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liakopoulos A, Mevius D, Ceccarelli D. 2016. A review of SHV extended-spectrum β-lactamases: neglected yet ubiquitous. Front Microbiol 7:1374. doi: 10.3389/fmicb.2016.01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K, Fisher JF. 2011. Epidemiological expansion, structural studies, and clinical challenges of new β-lactamases from Gram-negative bacteria. Annu Rev Microbiol 65:455–478. doi: 10.1146/annurev-micro-090110-102911. [DOI] [PubMed] [Google Scholar]
- 5.Jacoby GA. 2009. AmpC beta-lactamases. Clin Microbiol Rev 22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carattoli A. 2013. Plasmids and the spread of resistance. Int J Med Microbiol 303:298–304. doi: 10.1016/j.ijmm.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother 53:2227–2238. doi: 10.1128/AAC.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazarus B, Paterson DL, Mollinger JL, Rogers BA. 2015. Do human extraintestinal Escherichia coli infections resistant to expanded-spectrum cephalosporins originate from food-producing animals? A systematic review. Clin Infect Dis 60:439–452. doi: 10.1093/cid/ciu785. [DOI] [PubMed] [Google Scholar]
- 9.Olsen RH, Bisgaard M, Löhren U, Robineau B, Christensen H. 2014. Extended-spectrum β-lactamase-producing Escherichia coli isolated from poultry: a review of current problems, illustrated with some laboratory findings. Avian Pathol 43:199–208. doi: 10.1080/03079457.2014.907866. [DOI] [PubMed] [Google Scholar]
- 10.Dahms C, Hubner NO, Kossow A, Mellmann A, Dittmann K, Kramer A. 2015. Occurrence of ESBL-producing Escherichia coli in livestock and farm workers in Mecklenburg-Western Pomerania, Germany. PLoS One 10:e0143326. doi: 10.1371/journal.pone.0143326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghodousi A, Bonura C, Di Noto AM, Mammina C. 2015. Extended-spectrum β-lactamase, AmpC-producing, and fluoroquinolone-resistant Escherichia coli in retail broiler chicken meat, Italy. Foodborne Pathog Dis 12:619–625. doi: 10.1089/fpd.2015.1936. [DOI] [PubMed] [Google Scholar]
- 12.Maciuca IE, Williams NJ, Tuchilus C, Dorneanu O, Guguianu E, Carp-Carare C, Rimbu C, Timofte D. 2015. High prevalence of Escherichia coli-producing CTX-M-15 extended-spectrum beta-lactamases in poultry and human clinical isolates in Romania. Microb Drug Resist 21:651–662. doi: 10.1089/mdr.2014.0248. [DOI] [PubMed] [Google Scholar]
- 13.Huijbers PMC, Graat EAM, van Hoek AHAM, Veenman C, de Jong MCM, van Duijkeren E. 2016. Transmission dynamics of extended-spectrum β-lactamase and AmpC β-lactamase-producing Escherichia coli in a broiler flock without antibiotic use. Prev Vet Med 131:12–19. doi: 10.1016/j.prevetmed.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Hasman H, Mevius D, Veldman K, Olesen I, Aarestrup FM. 2005. Beta-lactamases among extended-spectrum beta-lactamase (ESBL)-resistant Salmonella from poultry, poultry products and human patients in the Netherlands. J Antimicrob Chemother 56:115–121. doi: 10.1093/jac/dki190. [DOI] [PubMed] [Google Scholar]
- 15.Smith HW. 1969. Transfer of antibiotic resistance from animal and human strains of Escherichia coli to resident E. coli in the alimentary tract of man. Lancet i:1174–1176. [DOI] [PubMed] [Google Scholar]
- 16.Shooter RA, Cooke EM, Rousseau SA, Breaden AL. 1970. Animal sources of common serotypes of Escherichia coli in the food of hospital patients. Possible significance in urinary-tract infections. Lancet ii:226–228. [DOI] [PubMed] [Google Scholar]
- 17.Overdevest I, Willemsen I, Rijnsburger M, Eustace A, Xu L, Hawkey P, Heck M, Savelkoul P, Vandenbroucke-Grauls C, van der Zwaluw K, Huijsdens X, Kluytmans J. 2011. Extended-spectrum beta-lactamase genes of Escherichia coli in chicken meat and humans, The Netherlands. Emerg Infect Dis 17:1216–1222. doi: 10.3201/eid1707.110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kluytmans JA, Overdevest IT, Willemsen I, Kluytmans-van den Bergh MF, van der Zwaluw K, Heck M, Rijnsburger M, Vandenbroucke-Grauls CM, Savelkoul PH, Johnston BD, Gordon D, Johnson JR. 2013. Extended-spectrum beta-lactamase-producing Escherichia coli from retail chicken meat and humans: comparison of strains, plasmids, resistance genes, and virulence factors. Clin Infect Dis 56:478–487. doi: 10.1093/cid/cis929. [DOI] [PubMed] [Google Scholar]
- 19.Smet A, Martel A, Persoons D, Dewulf J, Heyndrickx M, Catry B, Herman L, Haesebrouck F, Butaye P. 2008. Diversity of extended-spectrum beta-lactamases and class C beta-lactamases among cloacal Escherichia coli isolates in Belgian broiler farms. Antimicrob Agents Chemother 52:1238–1243. doi: 10.1128/AAC.01285-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leverstein-van Hall MA, Dierikx CM, Stuart JC, Voets GM, van den Munckhof MP, van Essen-Zandbergen A, Platteel T, Fluit AC, van de Sande-Bruinsma N, Scharinga J, Bonten MJM, Mevius DJ. 2011. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect 17:873–880. doi: 10.1111/j.1469-0691.2011.03497.x. [DOI] [PubMed] [Google Scholar]
- 21.Dierikx CM, van der Goot JA, Smith HE, Kant A, Mevius DJ. 2013. Presence of ESBL/AmpC-producing Escherichia coli in the broiler production pyramid: a descriptive study. PLoS One 8:e79005. doi: 10.1371/journal.pone.0079005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson O, Borjesson S, Landen A, Bengtsson B. 2014. Vertical transmission of Escherichia coli carrying plasmid-mediated AmpC (pAmpC) through the broiler production pyramid. J Antimicrob Chemother 69:1497–1500. doi: 10.1093/jac/dku030. [DOI] [PubMed] [Google Scholar]
- 23.Zurfluh K, Wang J, Klumpp J, Nuesch-Inderbinen M, Fanning S, Stephan R. 2014. Vertical transmission of highly similar blaCTX-M-1-harboring IncI1 plasmids in Escherichia coli with different MLST types in the poultry production pyramid. Front Microbiol 5:519. doi: 10.3389/fmicb.2014.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dierikx C, van der Goot J, Fabri T, van Essen-Zandbergen A, Smith H, Mevius D. 2013. Extended-spectrum-β-lactamase- and AmpC-β-lactamase-producing Escherichia coli in Dutch broilers and broiler farmers. J Antimicrob Chemother 68:60–67. doi: 10.1093/jac/dks349. [DOI] [PubMed] [Google Scholar]
- 25.Mo SS, Norstrom M, Slettemeas JS, Lovland A, Urdahl AM, Sunde M. 2014. Emergence of AmpC-producing Escherichia coli in the broiler production chain in a country with a low antimicrobial usage profile. Vet Microbiol 171:315–320. doi: 10.1016/j.vetmic.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Laube H, Friese A, von Salviati C, Guerra B, Kasbohrer A, Kreienbrock L, Roesler U. 2013. Longitudinal monitoring of extended-spectrum-beta-lactamase/AmpC-producing Escherichia coli at German broiler chicken fattening farms. Appl Environ Microbiol 79:4815–4820. doi: 10.1128/AEM.00856-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santini C, Baffoni L, Gaggia F, Granata M, Gasbarri R, Di Gioia D, Biavati B. 2010. Characterization of probiotic strains: an application as feed additives in poultry against Campylobacter jejuni. Int J Food Microbiol 141(Suppl):S98–S108. doi: 10.1016/j.ijfoodmicro.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 28.Van Bunnik BA, Katsma WE, Wagenaar JA, Jacobs-Reitsma WF, de Jong MC. 2012. Acidification of drinking water inhibits indirect transmission, but not direct transmission of Campylobacter between broilers. Prev Vet Med 105:315–319. doi: 10.1016/j.prevetmed.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Milillo SR, Ricke SC. 2010. Synergistic reduction of Salmonella in a model raw chicken media using a combined thermal and acidified organic acid salt intervention treatment. J Food Sci 75:M121–M125. doi: 10.1111/j.1750-3841.2009.01510.x. [DOI] [PubMed] [Google Scholar]
- 30.Chaveerach P, Keuzenkamp DA, Lipman LJ, Van Knapen F. 2004. Effect of organic acids in drinking water for young broilers on Campylobacter infection, volatile fatty acid production, gut microflora and histological cell changes. Poult Sci 83:330–334. doi: 10.1093/ps/83.3.330. [DOI] [PubMed] [Google Scholar]
- 31.Persoons D, Haesebrouck F, Smet A, Herman L, Heyndrickx M, Martel A, Catry B, Berge AC, Butaye P, Dewulf J. 2011. Risk factors for ceftiofur resistance in Escherichia coli from Belgian broilers. Epidemiol Infect 139:765–771. doi: 10.1017/S0950268810001524. [DOI] [PubMed] [Google Scholar]
- 32.Nurmi E, Nuotio L, Schneitz C. 1992. The competitive exclusion concept: development and future. Int J Food Microbiol 15:237–240. doi: 10.1016/0168-1605(92)90054-7. [DOI] [PubMed] [Google Scholar]
- 33.Sanders ME. 2008. Probiotics: definition, sources, selection, and uses. Clin Infect Dis 1(Suppl 2):S58–S61. doi: 10.1086/523341. [DOI] [PubMed] [Google Scholar]
- 34.Nuotio L, Schneitz C, Nilsson O. 2013. Effect of competitive exclusion in reducing the occurrence of Escherichia coli producing extended-spectrum beta-lactamases in the ceca of broiler chicks. Poult Sci 92:250–254. doi: 10.3382/ps.2012-02575. [DOI] [PubMed] [Google Scholar]
- 35.Hofacre CL, Johnson AC, Kelly BJ, Froyman R. 2002. Effect of a commercial competitive exclusion culture on reduction of colonization of an antibiotic-resistant pathogenic Escherichia coli in day-old broiler chickens. Avian Dis 46:198–202. doi: 10.1637/0005-2086(2002)046[0198:EOACCE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira AJ, Ferreira CS, Knobl T, Moreno AM, Bacarro MR, Chen M, Robach M, Mead GC. 2003. Comparison of three commercial competitive-exclusion products for controlling Salmonella colonization of broilers in Brazil. J Food Prot 66:490–492. doi: 10.4315/0362-028X-66.3.490. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura A, Ota Y, Mizukami A, Ito T, Ngwai YB, Adachi Y. 2002. Evaluation of Aviguard, a commercial competitive exclusion product for efficacy and after-effect on the antibody response of chicks to Salmonella. Poult Sci 81:1653–1660. doi: 10.1093/ps/81.11.1653. [DOI] [PubMed] [Google Scholar]
- 38.Nisbet D. 2002. Defined competitive exclusion cultures in the prevention of enteropathogen colonisation in poultry and swine. Antonie Van Leeuwenhoek 81:481–486. doi: 10.1023/A:1020541603877. [DOI] [PubMed] [Google Scholar]
- 39.Hakkinen M, Schneitz C. 1996. Efficacy of a commercial competitive exclusion product against a chicken pathogenic Escherichia coli and E. coli O157:H7. Vet Rec 139:139–141. doi: 10.1136/vr.139.6.139. [DOI] [PubMed] [Google Scholar]
- 40.Stern NJ, Cox NA, Bailey JS, Berrang ME, Musgrove MT. 2001. Comparison of mucosal competitive exclusion and competitive exclusion treatment to reduce Salmonella and Campylobacter spp. colonization in broiler chickens. Poult Sci 80:156–160. doi: 10.1093/ps/80.2.156. [DOI] [PubMed] [Google Scholar]
- 41.Dierikx C, van Essen-Zandbergen A, Veldman K, Smith H, Mevius D. 2010. Increased detection of extended spectrum beta-lactamase producing Salmonella enterica and Escherichia coli isolates from poultry. Vet Microbiol 145:273–278. doi: 10.1016/j.vetmic.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Mead GC. 2000. Prospects for ‘competitive exclusion’ treatment to control salmonellas and other foodborne pathogens in poultry. Vet J 159:111–123. doi: 10.1053/tvjl.1999.0423. [DOI] [PubMed] [Google Scholar]
- 43.De Jong MC, Kimman TG. 1994. Experimental quantification of vaccine-induced reduction in virus transmission. Vaccine 12:761–766. doi: 10.1016/0264-410X(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 44.Van der Goot JA, Koch G, de Jong MC, van Boven M. 2005. Quantification of the effect of vaccination on transmission of avian influenza (H7N7) in chickens. Proc Natl Acad Sci U S A 102:18141–18146. doi: 10.1073/pnas.0505098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer EAJ, Backer JA, Hagenaars TJ, Engel B. 2009. Maximum likelihood estimation of transmission parameters with classical analysis of transmission experiments, abstr ISVEE 12. Abstr Int Symp Vet Epidemiol Econ Proc, Durban, South Africa. [Google Scholar]