Abstract
Background
Mobile apps can increase access to care, facilitate self-management, and improve adherence to treatment. Stress urinary incontinence (SUI) affects 10-35% of women and, currently, an app with instructions for pelvic floor muscle training (PFMT) is available as first-line treatment. A previous randomized controlled study demonstrated that the app benefitted symptom severity and quality of life (QoL); in this study we investigate the cost-effectiveness of the app.
Objective
The objective of this study was to evaluate the health economy of the app for treating SUI.
Methods
This deterministic cost-utility analysis, with a 1-year societal perspective, compared the app treatment with no treatment. Health economic data were collected alongside a randomized controlled trial performed in Sweden from March 2013 to October 2014. This study included 123 community-dwelling women participants of 18 years and above, with stress urinary incontinence ≥1 time per week. Participants were self-assessed with validated questionnaires and 2-day leakage diaries, and then randomized to 3 months of treatment (app group, n=62) or no treatment (controls, n=61). The app focused on pelvic floor muscle training, prescribed 3 times daily. We continuously registered treatment delivery costs. Data were collected on each participant’s training time, incontinence aids, and laundry at baseline and at a 3-month follow-up. We measured quality of life with the International Consultation on Incontinence Modular Questionnaire on Lower Urinary Tract Symptoms and Quality of Life, and calculated the quality-adjusted life years (QALYs) gained. Data from the 3-month follow-up were extrapolated to 1 year for the calculations. Our main outcome was the incremental cost-effectiveness ratios compared between app and control groups. One-way and multiway sensitivity analyses were performed.
Results
The mean age of participants was 44.7 years (SD 9.4). Annual costs were €547.0 for the app group and €482.4 for the control group. Annual gains in quality-adjusted life years for app and control groups were 0.0101 and 0.0016, respectively. Compared with controls, the extra cost per quality-adjusted life year for the app group ranged from −€2425.7 to €14,870.6, which indicated greater gains in quality-adjusted life years at similar or slightly higher cost.
Conclusions
The app for treating stress urinary incontinence is a new, cost-effective, first-line treatment with potential for increasing access to care in a sustainable way for this patient group.
Keywords: mobile application; pelvic floor; urinary incontinence, stress; self care; cost-benefit analysis
Introduction
One possible way to meet the future demands in the health care sector could be to empower patients by increasing self-management with mHealth [1]. Worldwide, there are approximately 5 billion mobile phone subscribers, and the smartphones are constant companions for many individuals [2]. The App Store and Google Play websites offer around 100,000 health apps, but few have been scientifically evaluated [3]. Mobile health apps could facilitate self-management and adherence to treatment; in addition, they could increase access to care for individuals with limited access or for those unwilling to seek ordinary health care [4].
Stress urinary incontinence (SUI), that is, urine leakage upon sneezing, coughing, or exertion [5], affects 10-35% of women [6,7]. This condition is suited to self-management. The diagnosis is based on patient-reported measures and does not require a physical examination [8]. The first-line treatment is pelvic floor muscle training (PFMT), which is safe, effective [8-10], and can be completed without health care personnel supervision [8]. Although SUI can decrease quality of life (QoL) [11], only around 20% of individuals seek care [12]. In some cases, the leakage is not considered as a major problem, but in other cases, the patient is too embarrassed to seek care [13]. Our research group has developed the mobile app, Tät, which serves as a first-line treatment for SUI, based on self-management. This app provides information and instructions for PFMT [14].
One concern in deciding the treatments to be delivered in the health care systems is the cost. One common way to evaluate cost is the cost-utility analysis, which compares the costs and effects of at least two treatment alternatives. This analysis allows comparison of diverse interventions [15]. Costs can be considered either from a health care perspective, which only includes costs borne by the health care system, or from a societal perspective, which includes other costs. Currently, the former perspective is recommended in the United Kingdom by the National Institute for Health and Clinical Exellence (NICE) [16], and the latter is recommended in the United States [17] and Sweden [18]. The utility of the treatment is defined as the added time gained with an improved QoL, calculated as quality-adjusted life years (QALYs).
In this study, we performed a cost-utility analysis of SUI treatment with the app, Tät, compared with no treatment.
Methods
Design
This deterministic cost-utility analysis had a 1-year societal perspective. It was performed according to the principles outlined by Drummond et al [15].
Population
We collected data for this analysis alongside a randomized controlled trial on SUI treatment with the Tät app. The trial was registered at clinicaltrials.gov (ID: NCT01848938), and the trial results were described in detail elsewhere [14].
Briefly, we recruited community-dwelling women, aged 18 years and above, with SUI of once or more than once a week, via our website. Interested women completed an online screening questionnaire. When they met the study criteria, we sent them a letter of information, a form to provide informed consent, and a 2-day leakage diary. After returning these, they completed a Web-based questionnaire that provided their background characteristics, medical history, symptom severity, and QoL. Exclusion criteria were ongoing pregnancy, maximum voiding volume <0.3 L, macroscopic hematuria, irregular menstrual bleeding, difficulty passing urine, previous incontinence surgery, previous or present malignancy in the lower abdomen, severe psychiatric disorder, or impaired mobility or sensibility in the legs or lower abdomen.
We consecutively randomized eligible women to either three months of treatment with the app (app group, n=62), or no treatment (control group, n=61). The app Tät contained information on SUI, provided a PFMT program, with 6 basic and 6 advanced levels, and it prescribed PFMT 3 times daily during treatment. At the end of treatment, the instructions were to continue PFMT 2 or 3 times per week for maintenance training [19]. The control group received no intervention. After the 3-month follow-up, we offered the participants in the control group the app, on an optional basis. We collected 3-month follow-up data with a Web-based questionnaire. There was no face-to-face contact with the participants at any time.
Symptom Severity
We measured symptom severity at baseline and at 3 months, with the validated [20] and recommended [8,10,21] questionnaire, the International Consultation on Incontinence Modular Questionnaire on Urinary Incontinence, Short Form (ICIQ-UI SF). It contained 3 items such as frequency, amount of leakage, and overall QoL impact. The total score ranged from 0 to 21, with higher scores indicating greater severity. The total scores were used to categorize the severity of the condition (1-5: slight; 6-12: moderate; 13-18: severe; and 19-21: very severe) [22]. After 3 months, the app group reported clinically relevant and significantly greater improvements in symptoms compared with the control group [14].
Costs
We evaluated costs from a 1-year societal perspective. Costs included the cost of mailing the 2-day leakage diary, and the estimated time spent by our study administrator in emailing each participant the link to the Web-based questionnaire. The cost for our study administrator’s time was calculated based on her gross hourly wage. We did not include costs for the app development because these are one-time costs and are comparable with, for example, the costs for basic education of health care personnel; these are costs which are normally not included in health economic analyses. No other costs for the delivery of treatment were identified.
We collected baseline and follow-up data on the use of incontinence aids and any extra laundry due to leakage. In a previous study, we collected data on the different types of incontinence aids (large, medium, or small) used by women with SUI [23]. We then calculated a mean price per unit, based on the prices for incontinence aids listed on the website of a large pharmacy brand (Apoteket). The price for laundry was derived from the literature [24].
When a societal perspective is applied, an estimate of the cost for the individual’s time should be included in the health economic analysis [14]. At the 3-month follow-up, participants estimated how much time they had spent on PFMT during the last 4 weeks. We used this estimate to calculate the PFMT performed during the treatment period. To estimate PFMT for the entire year for the app group, we assumed that the participants would follow the prescription for maintaining PFMT over the remaining part of the year and we adjusted the time spent on PFMT accordingly. For the control group, we assumed the time spent on PFMT would remain constant throughout the year. To estimate the cost for each participant’s time, we calculated the gross hourly wages for women with the same educational level in Sweden [25], a method which is commonly used [14].
For all other costs, we assumed that costs measured at the 3-month follow-up would remain constant throughout the year. We added up all the costs, and the sum represented the total societal cost. All costs are given in euro, and they were based on the 2013 year-end prices. At that time, the exchange rate for 1 EUR was 8.94 SEK (Swedish krona).
Quality of life, Utility Weights, and QALYs
To evaluate QoL, we used the validated [26-28] and recommended [8,10,24] condition-specific questionnaire, the International Consultation on Incontinence Modular Questionnaire on Lower Urinary Tract Symptoms and Quality of Life (ICIQ-LUTSqol). This questionnaire contained 19 items on aspects of everyday life that might be influenced by urinary leakage, such as travel, work, meetings with family and friends, exercise, sexual performance, mood, energy, and sleep. Items are scored 1-4 (1: not at all or never; 2: slightly or sometimes; 3: moderately or often; and 4: a lot or all the time). The overall score ranged from 19 to 76, with higher scores indicating more impact. The questionnaire, was derived from the Kings Health Questionnaire [26], which is widely used in health economic analyses; both questionnaires used the same method for calculating QALY [29].
We based our QALY calculations on data from the ICIQ-LUTSqol, and we applied a preference-based index derived by Brazier et al [29], which incorporates 9 of the 19 items into a “health state” classification. We used the algorithm of this index to translate the health state classification into a utility weight, which ranged from 0 (worst imaginable health state) to 1 (best imaginable health state). We assumed that the utility weight calculated from 3-month follow-up data would remain stable for the remainder of the year in both groups.
Main Outcome
Our main outcome was the incremental cost-effectiveness ratio (ICER), defined as the difference in cost-effectiveness between the app group and the control group. We calculated the ICER as presented in Figure 1.
Figure 1.

Equation for calculation of the incremental cost-effectiveness ratio (ICER) using costs and quality-adjusted life-years (QALYs) for the app and control group respectively.
Statistics
For group comparisons at baseline, we used the Student t test for continuous variables, the chi-square test for categorical variables, and the Mann-Whitney U test for ordinal variables. For analyses of treatment effects within each group, we used paired t test. For comparisons of treatment effects between groups, we used a linear mixed-model analysis, which incorporated all available data for the outcomes of symptom severity and QoL. The utility weights are expressed as the mean value with a 95% CI. Costs were assumed to change linearly, and QALYs were calculated based on an “area-under-the-curve.”
P values <.05 were considered statistically significant. We collected and analyzed data in SPSS for Mac, version 23.0 (IBM) and in Excel for Mac, version 14.6.3 (Microsoft Corporation).
Sensitivity Analysis
We considered the fact that PFMT could be performed while doing other things and that laundry caused by leakage could be washed with other garments. Therefore, we performed a one-way sensitivity analysis by varying input data on the time spent on PFMT, the time spent on laundry, and the cost of laundry, one at a time, to test the potential impact of these uncertainties. In addition, we performed a multiway analysis that incorporated all three variables.
Ethics
The Regional Ethical Review Board, Umeå University, approved of the study (number 2012-325-31M). All participants gave informed consent.
Results
Study Population
We performed this study in Sweden, from March 2013 to October 2014. We randomized 123 participants to receive the app (app group, n=62) or with no treatment (control group, n=61). Baseline characteristics, including age, education, symptom severity, and baseline QoL scores did not differ significantly between groups (Table 1).
Table 1.
Baseline characteristics of study participants.
| Variable | App group (n=62) | Control group (n=61) | |
| Age, mean years (SDa) | 44.8 (9.7) | 44.7 (9.1) | |
| University education ≥3 years, n (%) | 52 (84) | 46 (75) | |
| BMIb, mean kg/m2 (SD) | 24.0 (4.1) | 24.5 (4.4) | |
| Daily smoker, n (%) | 2 (3) | 3 (5) | |
| Symptom severity, n (%)c | |||
| Slight | 3 (5) | 0 (0) | |
| Moderate | 36 (58) | 42 (69) | |
| Severe | 23 (37) | 19 (31) | |
| Overall score ICIQ-UI SFc, mean (SD) | 11.1 (3.0) | 11.0 (2.6) | |
| Overall score ICIQ-LUTSqold, mean(SD) | 34.1 (6.1) | 34.8 (6.1) | |
aSD: standard deviation.
bBMI: body mass index.
cBased on overall score from the International Consultation on Incontinence Modular Questionnaire on Urinary Incontinence, Short Form (ICIQ-UI SF).
dICIQ-LUTSqol: International Consultation on Incontinence Modular Questionnaire on Lower Urinary Tract Symptoms, Quality of Life.
At follow-up, we had lost one participant from each group. In addition, in the app group, we were missing outcome data on the ICIQ-LUTSqol for 3 participants.
Costs
The total assessment cost per participant was €6.4. The app group had higher total costs than the control group, mainly due to the extra time spent on PFMT. The total annual cost per participant in each group is presented in Table 2.
Table 2.
Costs per participant included in a cost-effectiveness analysis with a 1-year societal perspective, for App group versus Control group in women with stress urinary incontinence.
| Variable | Price per unita | Amount used | Cost | |||
| App group | Control group | App group | Control group | |||
| Assessment | 6.4 | 1 | 1 | 6.4 | 6.4 | |
| Treatment delivery | 0 | - | - | 0 | 0 | |
| Participant costs | ||||||
| Participant's time for PFMTb,c, mean (h) | 29.61 | 15.66 | 9.91 | 463.7 | 293.4 | |
| Participant's time for laundryc, mean (h) | 29.61 | 1.30 | 3.38 | 38.5 | 100.1 | |
| Incontinence aidsd, mean (n) | 0.134 | 114.40 | 169.60 | 15.4 | 22.7 | |
| Extra laundry loadse, mean (n) | 2.21 | 10.40 | 27.04 | 23.0 | 59.8 | |
| Total cost | 547.0 | 482.4 | ||||
aPrices are in euro (€), based on the 2013 year-end prices. Exchange rate was 1 EUR=8.94 SEK.
bPFMT: pelvic floor muscle training.
cBased on 2013 mean income for Swedish women with a similar educational level.
dBased on mean consumption at baseline (prices acquired from Apoteket).
eData from the literature [24].
Quality of Life, Utility Weights, and QALYs
In the app group, there was significant improvement in QoL at follow-up (mean ICIQ-LUTSqol reduction: 4.8, 95% CI 3.4-6.2). In contrast, the control group did not display a significant reduction in scores (mean ICIQ-LUTSqol reduction: 0.7, 95% CI −0.5 to 1.8). The difference between groups was highly significant (P<.001).
The utility weights and QALY changes for each group are presented in Figure 2. The QALYs gained corresponded to an extra 3.9 days in the best imaginable health state for the app group, and only 0.6 days for the control group.
Figure 2.
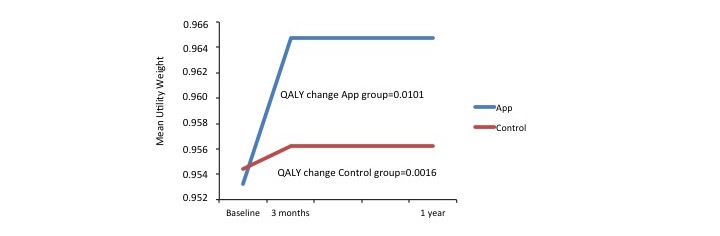
Changes in utility weights reflect gains in quality-adjusted life years (QALYs). Assessments were recorded at baseline, and at 3-month follow-ups, and estimated at one-year, for individuals that received either the app treatment (top, blue line) or no treatment (bottom, red line) for stress urinary incontinence.
Main Outcome and Sensitivity Analysis
In Table 3, we illustrate the ICERs for the base case and the sensitivity analysis. In all the analyses, except one (participant’s time for PFMT halved), the costs in the app group were slightly higher than costs in the control group. However, in all cases, the app treatment was more effective compared with no treatment or control group.
Table 3.
Incremental cost-effectiveness ratios (ICERs) for the app group versus the control group, including the base case and a sensitivity analysis.
| Group | Total cost (€a) | QALYb-gain | ∆ Cost (€) | ∆ QALY-gain | ICERc | ||
| Base case | |||||||
| Control group | 482.4 | 0.00158 | |||||
| App group | 547.0 | 0.01006 | |||||
| App group vs control group | 64.6 | 0.00849 | 7615.5 | ||||
| Sensitivity analysis | |||||||
| One-way: participant’s time for PFMTdhalved | |||||||
| Control group | 335.7 | 0.00158 | |||||
| App group | 315.1 | 0.01006 | |||||
| App group vs control group | −20.6 | 0.00849 | −2425.7 | ||||
| One-way: cost for laundry halved | |||||||
| Control group | 452.4 | 0.00158 | |||||
| App group | 535.4 | 0.01006 | |||||
| App group vs control group | 83.1 | 0.00849 | 9785.5 | ||||
| One-way: participant’s time for laundry not included | |||||||
| Control group | 382.3 | 0.00158 | |||||
| App group | 508.5 | 0.01006 | |||||
| App group vs control group | 126.2 | 0.00849 | 14870.6 | ||||
| Multiway: participant’s time for PFMT and cost for laundry halved, participant's time for laundry not included | |||||||
| Control group | 205.7 | 0.00158 | |||||
| App group | 265.1 | 0.01006 | |||||
| App group vs control group | 59.4 | 0.00849 | 6999.5 | ||||
a€ refers to euro at 2013 year-end price.
bQALY: quality-adjusted life years.
cICER: ∆ Cost/∆ QALY-gain.
dPFMT: pelvic floor muscle training.
Discussion
Principal Findings
In this health economic evaluation, we demonstrated that SUI self-management with a mobile app that provided information and instructions for PFMT was a cost-effective first-line treatment alternative, compared with a control group that received no treatment. The results were consistent and stable in different scenarios with varying costs.
Strengths and Limitations With the Study
This study had several strengths. The calculations were based on known costs and on data collected directly from the participants. Our research group had previous experience with non–face-to-face SUI treatment; there were no disruptions or major technical problems during the study and the loss to follow-up was low. We applied existing guidelines and used validated and recommended outcomes. The diagnosis of SUI was well substantiated and the population was clinically relevant because the vast majority (120/123, 97.6%) of participants had moderate to severe symptoms and actively sought treatment.
This study also had some limitations. One was the relatively low number of participants (n=123), which increases the uncertainty of the data and might have affected the results. Another was that we did not have 1-year follow-up data. Instead, we assumed that costs and utility weights measured at the 3-month follow-up would remain constant over the year. This assumption was based on our previous study of long-term effects of Internet-based PFMT for SUI [30], where improvements achieved after 3 months of treatment were maintained after 1 and 2 years. We had no reason to believe that the outcome of the current app treatment would be different from that of the previous study. Moreover, although the no-treatment alternative was plausible, given the fact that only around 20% of affected women seek care [12], it would have been interesting to compare outcomes between the app and a care-as-usual alternative. However, care-as-usual varies substantially, because there is no gold standard for SUI treatment. Another limitation was that our population had a higher educational level (98/123, 79.7% had ≥3 years of university education) than the general population of Swedish women (≈30% of women aged 25-44 years have ≥3 years of university education) [31]. However, there are no indications that the educational level might affect the ability to perform PFMT [32].
Strengths and Weaknesses Compared With the Literature
The total cost per participant was higher in the app group (€547.0) than that in the control group (€482.4). Although savings on laundry and incontinence aids were larger in the app group, participants in this group spent more time on PFMT. However, our estimation of the cost for participant time might have been somewhat biased, due to the relatively high educational level of our participants, compared with that of the general population. Nevertheless, the results were consistent in all tested scenarios, and the costs were comparable with those reported in other studies on conservative SUI treatments. For example, in a previous study, we compared Internet-based programs and postal-treatment programs for PFMT, where we found that the total costs were €596.5 and €596.2, respectively [20]. Moreover, in a Dutch study on a care-as-usual SUI treatment, the total cost was €453 (including productivity losses, travel costs, patient out-of-pocket costs, and health care costs, but not time for PFMT) [33].
In this study, we most likely overestimated the QALY gains in the control group due to the fact that we considered it significant in the incremental analysis despite of controls not showing a significant improvement in QoL. The app-group gain in QALY (0.0101) might seem small, but it was comparable to QALY gains observed in other studies on SUI treatment. In a primary care setting, Albers-Heitner et al [33] reported incremental QALY gains of 0.01-0.02, when intense PFMT was performed under guidance of a specialist nurse and compared with a general practitioner (GP) care-as-usual alternative. Arlandis-Guzman et al [34] reported QALY gains of 0.01014, 0.00846, and 0.00957, with the antimuscarinic drugs fesoterodine, tolterodine, and solifenacin, respectively. However, second-line treatments with sling surgery could produce larger QALY gains (0.0504) [35]. Nevertheless, although the QALY gains with conservative treatments are low, in sheer numbers, the patient group that can potentially benefit from treatment is large; thus, the attainable total QALY gain is substantial.
We estimated that the extra cost per QALY for the app treatment was €7615.5, and the sensitivity analyses indicated a potential range of −€2425.7 to €14,870.6. In one of the scenarios tested (time for PFMT halved), the negative ICER value implied that, compared with doing nothing for this group of patients, the app treatment could increase the QoL for the individual at a reduced cost for the society. In the other scenarios, QALY gains were larger in the app group, but at greater cost, compared with the control group. The affordability of an additional cost per QALY depends on the willingness to pay for a more effective treatment, which might differ in different countries. In the United Kingdom, interventions with ICERs ≤€16,500-25,000 (£20,000–30,000) are typically recommended by NICE [16], and in US, those with ICERs of ≤€36,500 ($50,000) are usually recommended [36,37]. In Sweden, incremental costs of ≤€11,200 (100,000 SEK) are considered low, and incremental costs of ≥€60,000 (500,000 SEK) are considered high [38]. Data are scarce on the cost effectiveness of other health apps, due to the limited number of studies conducted.
We did not calculate an ICER from a health care perspective, because the assessment cost was the same (€6.3) in both the groups, and no costs could be identified for the delivery of treatment, that is, ∆ Cost=0. When the app is implemented outside a study setting, the parameters will change. For example, additional costs for updates, bug fixes, and technical support must be taken into account. We estimate that an IT technician would require approximately 4 h per month for this maintenance, but on the other hand, as the number of users increases, the cost per person will diminish. Furthermore, the total cost for the health care system to deliver an app treatment is likely to be low compared with face-to-face treatment. For example, in Sweden, the estimated cost for a GP consultation was €173 [39]; in United Kingdom, the estimated cost for 3 months of PFMT under supervision of a trained nurse was €158 to €293 [40].
Future Research and Clinical Implications
The Tät app has been released free of charge in both Swedish and English. Although the cost per participant will decrease as the number of users increases, the effects might decline outside the study setting. We are currently continuing to follow the effects reported by users. In addition, the long-term effects of the app need to be established, and the app should also be evaluated as a possible complement to other treatments. Future perspectives include developing the app for treating other types of urinary incontinence.
SUI treatment with the Tät app will not suit all women, but it offers a cost-effective first-line treatment to many women. To our knowledge, this study was the first to evaluate the cost effectiveness of an app treatment for a common health condition. Modern health care systems face many challenges, and it is important for clinicians to deliver care in sustainable ways. The development of self-management apps could be a feasible way to deliver high-quality care in a cost effective, affordable manner to large patient groups. It could also be a way to provide treatment to women with limited access to care, for example, women in low or middle-income countries. While to adding value to the individual patient, these apps could reduce the need for support from primary care, and thus, those resources could be conserved for individuals with explicit needs.
Conclusion
Self-management of SUI with an app for PFMT is a cost-effective first-line treatment alternative.
Acknowledgments
We would like to thank the participating women for making this study possible. Many thanks also to the Department of ICT Services and System Development (ITS) at Umeå University for helping in developing the app. We would also like to express our gratitude to our colleagues, Ina Asklund and Emma Nyström, for assistance in the study and to our study administrator, Susanne Johansson. This study was funded by grants from the Swedish Council for Working Life and Social Research, the Region Jämtland Härjedalen, and Visare Norr, Northern County Councils, Sweden. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
The application Tät was developed by Eva Samuelsson, Malin Sjöström, and Göran Umefjord in cooperation with the Department of ICT Services and System Development (ITS), Umeå University, Sweden. The application is a registered trademark by the Swedish Patent and Registration office for E Samuelsson at Umeå University. It is also CE-marked as a medical device Class 1, according to Swedish regulation LVFS 2003:11. It is available for free in English and Swedish at Appstore and Google Play.
Abbreviations
- GP
general practitioner
- ICER
incremental cost-effectiveness ratio
- ICIQ-LUTSqol
International Consultation on Incontinence Modular Questionnaire on Lower Urinary Tract Symptoms and Quality of Life
- ICIQ-UI SF
International Consultation on Incontinence Modular Questionnaire on Urinary Incontinence, Short Form
- NICE
National Institute for Health and Clinical Exellence
- PFMT
pelvic floor muscle training
- QALY
quality-adjusted life year
- QoL
quality of life
- SUI
stress urinary incontinence
Footnotes
Conflicts of Interest: None declared.
References
- 1.Steinhubl SR, Muse ED, Topol EJ. Can mobile health technologies transform health care? JAMA. 2013 Dec 11;310(22):2395–6. doi: 10.1001/jama.2013.281078. [DOI] [PubMed] [Google Scholar]
- 2.WHO. From innovation to implementation http://www.euro.who.int/__data/assets/pdf_file/0012/302331/From-Innovation-to-Implementation-eHealth-Report-EU.pdf?ua=1 .
- 3.Whitehead L, Seaton P. The Effectiveness of Self-Management Mobile Phone and Tablet Apps in Long-term Condition Management: A Systematic Review. J Med Internet Res. 2016;18(5):e97. doi: 10.2196/jmir.4883. http://www.jmir.org/2016/5/e97/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg AS. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res. 2015;17(2):e52. doi: 10.2196/jmir.3951. http://www.jmir.org/2015/2/e52/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haylen BT, de RD, Freeman RM, Swift SE, Berghmans B, Lee J, Monga A, Petri E, Rizk DE, Sand PK, Schaer GN, International UA, International CS. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4–20. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- 6.Hannestad YS, Rortveit G, Sandvik H, Hunskaar S, Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. J Clin Epidemiol. 2000 Nov;53(11):1150–7. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 7.Abrams P, Cardozo L, Khoury S, Wein Aj. Incontinence 4th edition. Paris, France: Health Publications Ltd; 2009. Epidemiology of Urinary (UI) and Faecal (FI) Incontinence and Pelvic Organ Prolapse (POP) [Google Scholar]
- 8.Shamliyan T, Wyman J, Kane R. AHRQ. Nonsurgical treatments for urinary incontinence in adult women: diagnosis and comparative effectiveness http://www.effectivehealthcare.ahrq.gov/ehc/products/169/834/urinary-incontinence-treatment-report-130909.pdf . [PubMed]
- 9.Dumoulin C, Hunter KF, Moore K, Bradley CS, Burgio KL, Hagen S, Imamura M, Thakar R, Williams K, Chambers T. Conservative management for female urinary incontinence and pelvic organ prolapse review 2013: summary of the 5th International consultation on incontinence. Neurourol Urodyn. 2016 Jan;35(1):15–20. doi: 10.1002/nau.22677. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for HealthClinical Excellence. [2017-01-16]. Urinary incontinence: the management of urinary incontinence in women https://www.nice.org.uk/guidance/cg171/resources/urinary-incontinence-in-women-management-35109747194821 .
- 11.Coyne KS, Kvasz M, Ireland AM, Milsom I, Kopp ZS, Chapple CR. Urinary incontinence and its relationship to mental health and health-related quality of life in men and women in Sweden, the United Kingdom, and the United States. Eur Urol. 2012 Jan;61(1):88–95. doi: 10.1016/j.eururo.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 12.Hannestad YS, Rortveit G, Hunskaar S. Help-seeking and associated factors in female urinary incontinence. The Norwegian EPINCONT Study. Epidemiology of Incontinence in the County of Nord-Trøndelag. Scand J Prim Health Care. 2002 Jun;20(2):102–7. [PubMed] [Google Scholar]
- 13.Kinchen KS, Burgio K, Diokno AC, Fultz NH, Bump R, Obenchain R. Factors associated with women's decisions to seek treatment for urinary incontinence. J Womens Health (Larchmt) 2003 Sep;12(7):687–98. doi: 10.1089/154099903322404339. [DOI] [PubMed] [Google Scholar]
- 14.Asklund I, Nyström E, Sjöström M, Umefjord G, Stenlund H, Samuelsson E. Mobile app for treatment of stress urinary incontinence: a randomized controlled trial. Neurourol Urodyn. 2016 Sep 09;:a. doi: 10.1002/nau.23116. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Drummond MS, Torrance G, O'Brien B, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes, 3rd edition. Oxford: Oxford University Press; 2005. [Google Scholar]
- 16.National Institute for Health and Clinical Exellence. 2013. [2017-01-16]. Guide to the methods of technology appraisal https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 .
- 17.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996 Oct 09;276(14):1172–7. [PubMed] [Google Scholar]
- 18.The Dental and Pharmaceutical Benefits Agency TLV. [2017-01-11]. Health economics http://www.tlv.se/In-English/medicines-new/health-economics/
- 19.Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, Fleck SJ, Franklin B, Fry AC, Hoffman JR, Newton RU, Potteiger J, Stone MH, Ratamess NA, Triplett-McBride T, American College of Sports Medicine American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002 Feb;34(2):364–80. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 20.Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23(4):322–30. doi: 10.1002/nau.20041. [DOI] [PubMed] [Google Scholar]
- 21.Abrams P, Andersson KE, Birder L, Brubaker L, Cardozo L, Chapple C, Cottenden A, Davila W, de RD, Dmochowski R, Drake M, Dubeau C, Fry C, Hanno P, Smith JH, Herschorn S, Hosker G, Kelleher C, Koelbl H, Khoury S, Madoff R, Milsom I, Moore K, Newman D, Nitti V, Norton C, Nygaard I, Payne C, Smith A, Staskin D, Tekgul S, Thuroff J, Tubaro A, Vodusek D, Wein A, Wyndaele JJ, Members OC, Fourth International Consultation on Incontinence Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn. 2010;29(1):213–40. doi: 10.1002/nau.20870. [DOI] [PubMed] [Google Scholar]
- 22.Klovning A, Avery K, Sandvik H, Hunskaar S. Comparison of two questionnaires for assessing the severity of urinary incontinence: the ICIQ-UI SF versus the incontinence severity index. Neurourol Urodyn. 2009;28(5):411–5. doi: 10.1002/nau.20674. [DOI] [PubMed] [Google Scholar]
- 23.Sjöström M, Umefjord G, Lindholm L, Samuelsson E. Cost-effectiveness of an Internet-based treatment program for stress urinary incontinence. Neurourol Urodyn. 2015 Mar;34(3):244–50. doi: 10.1002/nau.22540. doi: 10.1002/nau.22540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subak L, Van Den Eeden S, Thom D, Creasman JM, Brown JS, Reproductive Risks for Incontinence Study at Kaiser Research Group Urinary incontinence in women: direct costs of routine care. Am J Obstet Gynecol. 2007 Dec;197(6):596.e1–9. doi: 10.1016/j.ajog.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Statistics Sweden Statistical Database SCB. Labour market 2013 http://www.statistikdatabasen.scb.se/pxweb/sv/ssd/START__AM__AM0112/TidsserieUtbniva/table/tableViewLayout1/?rxid=0408f212-2586-4d09-bc21-82819c677eb3 .
- 26.Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol. 1997 Dec;104(12):1374–9. doi: 10.1111/j.1471-0528.1997.tb11006.x. [DOI] [PubMed] [Google Scholar]
- 27.Coyne K, Kelleher C. Patient reported outcomes: the ICIQ and the state of the art. Neurourol Urodyn. 2010 Apr;29(4):645–51. doi: 10.1002/nau.20911. [DOI] [PubMed] [Google Scholar]
- 28.Sjöström M, Stenlund H, Johansson S, Umefjord G, Samuelsson E. Stress urinary incontinence and quality of life: a reliability study of a condition-specific instrument in paper and web-based versions. Neurourol Urodyn. 2012 Nov;31(8):1242–6. doi: 10.1002/nau.22240. [DOI] [PubMed] [Google Scholar]
- 29.Brazier J, Czoski-Murray C, Roberts J, Brown M, Symonds T, Kelleher C. Estimation of a preference-based index from a condition-specific measure: the King's health questionnaire. Med Decis Making. 2008;28(1):113–26. doi: 10.1177/0272989X07301820. [DOI] [PubMed] [Google Scholar]
- 30.Sjöström M, Umefjord G, Stenlund H, Carlbring P, Andersson G, Samuelsson E. Internet-based treatment of stress urinary incontinence: 1- and 2-year results of a randomized controlled trial with a focus on pelvic floor muscle training. BJU Int. 2015 Dec;116(6):955–64. doi: 10.1111/bju.13091. doi: 10.1111/bju.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SCB. Educational attainment of the population 2014 http://www.scb.se/Statistik/UF/UF0506/2014A01S/UF0506_2014A01_SM_UF84SM1601.pdf .
- 32.Henderson JW, Wang S, Egger MJ, Masters M, Nygaard I. Can women correctly contract their pelvic floor muscles without formal instruction? Female Pelvic Med Reconstr Surg. 2013;19(1):8–12. doi: 10.1097/SPV.0b013e31827ab9d0. http://europepmc.org/abstract/MED/23321652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albers-Heitner CP, Joore MA, Winkens RA, Lagro-Janssen AL, Severens JL, Berghmans LC. Cost-effectiveness of involving nurse specialists for adult patients with urinary incontinence in primary care compared to care-as-usual: an economic evaluation alongside a pragmatic randomized controlled trial. Neurourol Urodyn. 2012 Apr;31(4):526–34. doi: 10.1002/nau.21204. [DOI] [PubMed] [Google Scholar]
- 34.Arlandis-Guzman S, Errando-Smet C, Trocio J, Arumi D, Rejas J. Cost-effectiveness analysis of antimuscarinics in the treatment of patients with overactive bladder in Spain: a decision-tree model. BMC Urol. 2011 May 20;11:9. doi: 10.1186/1471-2490-11-9. https://bmcurol.biomedcentral.com/articles/10.1186/1471-2490-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montesino-Semper MF, Jimenez-Calvo JM, Cabases JM, Sanchez-Iriso E, Hualde-Alfaro A, García-García D. Cost-effectiveness analysis of the surgical treatment of female urinary incontinence using slings and meshes. Eur J Obstet Gynecol Reprod Biol. 2013 Nov;171(1):180–6. doi: 10.1016/j.ejogrb.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 36.Eichler H, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7(5):518–28. doi: 10.1111/j.1524-4733.2004.75003.x. https://linkinghub.elsevier.com/retrieve/pii/S1098-3015(10)60216-1. [DOI] [PubMed] [Google Scholar]
- 37.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008 Apr;8(2):165–78. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 38.The National Board of Health and Welfare Socialstyrelsen. Nationella riktlinjer för sjukdomsförebyggande metoder https://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/18484/2011-11-11.pdf .
- 39.Nilsson FO, Linnér L, Samuelsson E, Milsom I. Cost-effectiveness analysis of newer anticholinergic drugs for urinary incontinence vs oxybutynin and no treatment using data on persistence from the Swedish prescribed drug registry. BJU Int. 2012 Jul;110(2):240–6. doi: 10.1111/j.1464-410X.2011.10729.x. doi: 10.1111/j.1464-410X.2011.10729.x. [DOI] [PubMed] [Google Scholar]
- 40.Imamura M, Abrams P, Bain C, Buckley B, Cardozo L, Cody J, Cook J, Eustice S, Glazener C, Grant A, Hay-Smith J, Hislop J, Jenkinson D, Kilonzo M, Nabi G, N'Dow J, Pickard R, Ternent L, Wallace S, Wardle J, Zhu S, Vale L. Systematic review and economic modelling of the effectiveness and cost-effectiveness of non-surgical treatments for women with stress urinary incontinence. Health Technol Assess. 2010 Aug;14(40):1–188, iii. doi: 10.3310/hta14400. doi: 10.3310/hta14400. [DOI] [PubMed] [Google Scholar]


