Abstract
Quantitative magnetic resonance imaging (MRI) has revealed abnormalities in brain volumes, cortical thickness and white matter microstructure in fetal alcohol spectrum disorders (FASD); however, no study has reported all three measures within the same cohort to assess the relative magnitude of deficits, and few studies have examined sex differences. Participants with FASD (n = 70; 30 females; 5–32 years) and healthy controls (n = 74; 35 females; 5–32 years) underwent cognitive testing and MRI to assess cortical thickness, regional brain volumes and fractional anisotropy (FA)/mean diffusivity (MD) of white matter tracts. A significant effect of group, age-by-group, or sex-by-group was found for 9/9 volumes, 7/39 cortical thickness regions, 3/9 white matter tracts, and 9/10 cognitive tests, indicating group differences that in some cases differ by age or sex. Volume reductions for several structures were larger in males than females, despite similar deficits of cognition in both sexes. Correlations between brain structure and cognitive scores were found in females of both groups, but were notably absent in males. Correlations within a given MRI modality (e.g. total brain volume and caudate volume) were prevalent in both the control and FASD groups, and were more numerous than correlations between measurement types (e.g. volumes and diffusion tensor imaging) in either cohort. This multi-modal MRI study finds widespread differences of brain structure in participants with prenatal alcohol exposure, and to a greater extent in males than females which may suggest attenuation of the expected process of sexual dimorphism of brain structure during typical development.
Keywords: Gender, Prenatal alcohol exposure, Diffusion tensor imaging, Cortical thickness, Brain volume, Cognition
Highlights
-
•
Brain volume more affected than tractography-based DTI or cortical thickness in FASD
-
•
Greater brain volume reduction in males than females, but similar cognitive deficits
-
•
Few correlations between MRI metrics suggest injuries via diverse mechanisms.
-
•
Brain structure cognitive test correlations in females notably absent in males
1. Introduction
The teratogenic effects of alcohol on the brain were first identified through a series of seminal case studies in the 1970s (Clarren et al., 1978, Jones and Smith, 1973), and have since been confirmed by a large body of research in both humans and animals. This work has demonstrated numerous direct and indirect mechanisms by which alcohol acts on the developing nervous system (Goodlett et al., 2005), producing a range of physical, behavioural and cognitive deficits collectively termed fetal alcohol spectrum disorders (FASD) (Riley and McGee, 2005). However, interactions between exposure, genetic and environmental factors result in enormous variability in both clinical presentation and structural brain damage, challenging both the study and treatment of this common developmental disorder.
Quantitative neuroimaging has revealed numerous structural abnormalities associated with FASD, including reductions in total brain, white matter, cortical and deep grey matter volumes (Lebel et al., 2011) as well as abnormalities in cortical thickness (e.g. Sowell et al., 2008, Zhou et al., 2011) and white matter microstructure (Wozniak and Muetzel, 2011). With the exception of brain volume, which seems to be consistently reduced across all FASD cohorts and studies, a lot of variability exists in the literature with respect to diffusion tensor imaging parameters and cortical thickness—including magnitude, parameter, region, and direction of difference. No FASD study has examined all three of these MRI measures together in the same cohort, precluding assessment of the relative severity of each abnormality, or the coherence between them, i.e. if individuals with greater deficits in one parameter (e.g. volume) also have greater deficits in other parameters (e.g. diffusion metrics). Consistent patterns of deficit across subjects may suggest similar vulnerability to prenatal alcohol exposure across various brain structures, whereas inconsistent patterns of deficit may suggest that each MRI metric reflects uniquely vulnerable properties of brain structure, potentially influenced by factors such as timing of exposure.
In addition, there is a paucity of data on sex differences of brain structure in FASD, despite well-established sex differences in brain structure, function and neurochemistry in healthy populations that are proposed to partially underlie sexual dimorphism of neurodevelopmental and degenerative disorders (Cahill, 2006, Cosgrove et al., 2007). The lack of prior work looking at sex differences of brain structure in FASD likely stems from small sample sizes and a focus on sub-grouping samples according to other important variables of interest such as diagnostic category, presence/absence of dysmorphology, and other clinical measures. However, animal models of prenatal alcohol exposure have revealed several sex-dependent neurophysiological and neurochemical sequelae, many of which appear to be more severe in males than females. Examples of PAE effects observed to be greater in males than females include reduced preoptic area of the hypothalamus (Barron et al., 1988), 40% reductions of long term potentiation (Sickmann et al., 2014), increases in dopamine D1R binding (Converse et al., 2014), reduced sensitivity to testosterone (Lan et al., 2009), as well as greater degrees of structural abnormality in the corpus callosum (Zimmerberg and Reuter, 1989). Prenatal alcohol exposure also appears to produce complex sexually dimorphic effects such as up-regulation of the hypothalamic-pituitary axis that depends on different concomitant environmental factors in males than females (Weinberg et al., 2008). Examination of differences in the effects of prenatal alcohol exposure on human brain structure remains relatively unexplored, though a handful of studies have identified greater relative volume reductions of cortical and deep grey matter regions in males than females with FASD (Chen et al., 2012, Dudek et al., 2014, Nardelli et al., 2011), suggesting a need for further investigation. In addition, longitudinal work suggests that individuals with FASD have altered trajectories of brain development with age during childhood and adolescence (Lebel et al., 2012, Treit et al., 2013, Treit et al., 2014) suggesting a need to investigate change with age in this population, that is often overlooked due to small sample sizes and/or narrow age ranges.
The purpose of this study is to evaluate diffusion tensor imaging of white matter, cortical thickness and regional brain volumes in a sizeable (n = 70) cohort of individuals with FASD from 5 to 32 years of age, with the aims of examining: i) age and sex-differences in abnormalities of brain structure associated with FASD, ii) the relative magnitude of abnormalities observed with each imaging method, and iii) relationships between abnormalities identified with each MRI method as well as links to cognition.
2. Materials and methods
2.1. Subjects
Participants were 70 individuals with FASD (5–32 years, mean 14 ± 6 years, 40 males) and 74 controls (5–32 years, 13 ± 5 years, 38 males). Alcohol-exposed participants were primarily recruited (n = 40) through two FASD diagnostic clinics at the Glenrose Rehabilitation Hospital in Edmonton, Alberta, and were previously diagnosed according to the Canadian Guidelines (Chudley et al., 2005) and the 4 digit code (Astley, 2004). The remaining FASD participants (n = 30) were recruited through school and social work services, and were diagnosed clinically by primary care physicians and practitioners in other developmental disability clinics. In total, 10 had a diagnosis of fetal alcohol syndrome (FAS), 6 partial fetal alcohol syndrome (pFAS), 54 alcohol related neurobehavioural disorder (ARND) or “FASD” without further specification. Twenty of the 70 FASD participants were included in previous publications on volume and cortical thickness (Nardelli et al., 2011, Zhou et al., 2011), and 50 were newly recruited for this study. The second scans of the 17 FASD participants in our previous longitudinal study (Treit et al., 2013) are included here; note that these subjects (but not scans) overlap with those in Lebel et al. (2008a). Controls were recruited through advertising on public bulletin boards in locations such as community centres, hospitals, recreation centres etc., by letters sent home from several public schools, and by word of mouth. All 74 controls were newly recruited for this study. Forty-nine of these control participants were included in a previous publication on DTI and inhibition in healthy development (Treit et al., 2014), but none have been included as controls in any previous FASD papers. Controls were screened for neurological, psychiatric and developmental disorders, head injuries and contraindications to MRI as well as prenatal exposure to alcohol, nicotine, and illicit drugs. Control subjects with exposure to > 2 drinks per occasion or > 6 drinks in total during gestation were excluded. Seven controls included here were prenatally exposed to alcohol within the threshold for inclusion (average exposure: 3 drinks total), 12 were prenatally exposed to nicotine (all via cigarettes in varying frequencies and amounts) and none were prenatally exposed to illicit drugs. Written informed consent was obtained from all participants or parents/legal guardians (in addition to written assent from participants under 18 years of age) prior to study procedures. This study was approved by the Health Research Ethics Board at the University of Alberta.
2.2. Demographic and cognitive data
Ethnicity, annual household income, living situation and other demographic information was collected on the same day as the study visit through questionnaires given to participants or their parents/legal guardians in 72/74 control participants and 44/70 FASD participants (Table 1). Collection of demographic data was initiated part way through recruitment of the FASD sample, thus 26 FASD subjects are missing demographic (but not cognitive) data here.
Table 1.
Participant demographic and clinical information.
| Control (n = 74) | FASD (n = 70) | Group difference | |
|---|---|---|---|
| Age (years) | 13.2 ± 6.0 (5–32) | 14.2 ± 6.3 (5–32) | t = 1.04; p = 0.300 |
| Males (%) | 40 (57%) | 38 (51%) | X2 = 0.49; p = 0.486 |
| Demographic information | n = 72 | n = 44 | |
| Ethnicity | |||
| Caucasian | 58 (81%) | 18 (41%) | X2 = 19.00; p < 0.001 |
| Aboriginal | 4 (6%) | 21 (48%) | X2 = 28.73; p < 0.001 |
| Other | 10 (14%) | 5 (11%) | X2 = 0.15; p = 0.694 |
| Living situation | |||
| Biological parent(s) | 62 (86%) | 5 (11%) | X2 = 62.54; p < 0.001 |
| Adopted/biological relative | 1 (1%) | 21 (48%) | X2 = 38.16; p < 0.001 |
| Foster care | 0 (0%) | 12 (27%) | X2 = 21.90; p < 0.001 |
| Group home | 0 (0%) | 2 (5%) | X2 = 3.33; p = 0.068 |
| Independent | 9 (13%) | 4 (9%) | X2 = 0.319; p = 0.572 |
| Foster care placements per person (mean, range) | 0 (0–0) | 1.5 (0–5) | t = 8.36; p < 0.001 |
| Annual household income (median) | $76,000–100,000 | $51,000–75,000 | X2 = 17.28; p = 0.016 |
| Basic needs not met at any timea | 1 (1%) | 24 (55%) | X2 = 62.25; p < 0.001 |
| Psychiatric co-morbiditiesa | |||
| ADHD | 0 (0%) | 38 (87%) | X2 = 81.40; p < 0.001 |
| Reactive attachment disorder | 0 (0%) | 10 (23%) | X2 = 16.36; p < 0.001 |
| Anxiety | 3 (4%)b | 13 (30%) | X2 = 16.43; p = 0.001 |
| Other | 0 (0%) | 19 (43%) | X2 = 33.86; p < 0.001 |
| Psychiatric medicationsa | |||
| Atypical antipsychotics | 0 (0%) | 24 (55%) | X2 = 50.79; p < 0.001 |
| Stimulants | 0 (0%) | 20 (45%) | X2 = 40.54; p < 0.001 |
| Antidepressants | 0 (0%) | 11 (25%) | X2 = 20.37; p < 0.001 |
| Other | 0 (0%) | 9 (20%) | X2 = 16.35; p < 0.001 |
| Number of psychiatric medications per person (mean, range) | 0 (0–0) | 1.7 (0–4) | t = 11.27; p < 0.001 |
Self/parent reported.
Self or parent reported anxiety for which medical attention/counselling was sought. None were taking anxiolytic medication or had a formal diagnosis.
All participants in both groups underwent approximately 1.5 h of cognitive testing on the same day as their MRI, administered by a trained research assistant. Participants of all ages were administered the Woodcock Reading Mastery Test Revised (WRMT-R) Word ID; Woodcock Johnson (WJ) Quantitative Concepts; Rey Complex Figure Test (RCFT) and Recognition Trial; Comprehensive Receptive and Expressive Vocabulary Test (CREVT); and the Wide Range Intelligence Test (WRIT). In addition, participants under 15 years of age were administered the Working Memory Test Battery for Children (WMTB-C) Digit and Block recall, and those under 16 years of age were administered either the NEPSY Tower, Auditory Attention/Response Set and Visual Attention, or the NEPSY II Animal Sorting, Auditory Attention and Response Set and Inhibition (NEPSY was updated to NEPSY II mid-recruitment). Participants over 16 years of age were administered the Delis-Kaplan Executive Functioning System (D-KEFS) Trail Making, Verbal Fluency and Card Sorting. Given that different combinations of executive function tasks were administered to different subjects, a composite executive function score was calculated from the mean of i) NEPSY Executive Composites (Tower, Auditory Attention and Visual Attention), ii) NEPSY II Response Set, Animal Sorting, Inhibition-Inhibition, and Inhibition-Switching or iii) D-KEFS Verbal Fluency condition 3 (Category Switching), Trail Making condition 4 (Number-Letter Switching), and Card Sorting (Free Sorting and Sort Recognition combined) scaled scores for each subject. This average scaled score was used to provide a single measure of executive function for each subject, under the assumption that performance across these subtests would be highly correlated. Correlations among the subtests within each of the executive function batteries were in the moderate-to-strong range, r's = 0.31–0.76.
2.3. Image acquisition
All participants were scanned on the same 1.5 T Siemens Sonata in the Peter S. Allen MR Research Centre at the University of Alberta. Scans included DTI, T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), and FLAIR-DTI (not used here) for a total scan time of ~ 26 min. DTI was acquired using a dual spin-echo, single shot echo-planar imaging sequence with: 40 3-mm axial-oblique slices with no inter-slice gap; TR = 6400 ms; TE = 88 ms; 6 non-collinear diffusion sensitizing gradient directions with b = 1000 s/mm2; 8 averages; FOV = 220 × 220 mm2; matrix of 128 × 128 with 75% phase partial Fourier zero-filled to 256 × 256 for a total acquisition time of 6:06 min. T1-weighted images for volume analysis were acquired using a high resolution (1 × 1 × 1 mm3) MPRAGE sequence with: TR = 1870 ms; TE = 4.38 ms; TI = 1100 ms; total acquisition of 4:29 min. Raw diffusion weighted images were inspected with single-slice movie loops prior to pre-processing and tensor calculation to check for signal dropout, artifacts and gross subject motion. Likewise MPRAGE images were visually inspected for image quality and subject motion prior to processing. The 144 scans included here passed this initial visual inspection.
2.4. DTI tractography
A semi-automated tractography method (adapted from Lebel et al., 2008b) was used to delineate 9 major white matter tracts for each participant. Subject images were motion/distortion corrected in ExploreDTI v8.3 (Leemans et al., 2009), and then normalized to the ICBM-DTI81 template using a deformable tensor-based registration algorithm in DTI-TK (Zhang et al., 2006; http://dti-tk.sourceforge.net), saving warping parameters for each subject. Seed, target and exclusion regions were drawn on the template according to a priori information on tract location (Wakana et al., 2004), and then applied to each subject's native FA colourmap using an inverse of their normalization parameters, such that the same seed, target and exclusion regions were applied to all subjects. Adequate template registration was ensured via visual inspection of movie loops, and tractography seed ROIs were drawn slightly larger than typically required in order to accommodate for slight variations between subjects, according to previously described methods (Lebel et al., 2008b). White matter tracts included the genu (gCC), body (bCC), and splenium (sCC) of the corpus callosum, corticospinal tracts (CST), superior and inferior longitudinal fasciculus (SLF; ILF), inferior fronto-occipital fasciculus (SFO; IFO), cingulum, and uncinate fasciculus (UF). Tractography was carried out in native space for each subject, using a deterministic streamline method in ExploreDTI. Left and right hemispheres were tracked separately for all tracts except the corpus callosum. A minimum FA threshold of 0.25 was used to initiate and continue tracking, and an angle threshold of 60° was set for the UF and SLF and 30° for all other tracts. All tracts were manually inspected for consistency with known anatomy and additional exclusions of spurious fibres were made as necessary. Exclusions due to insufficient reconstruction (e.g. a very small number of streamlines) were made in < 1–6% of subjects, varying by tract. Fractional anisotropy (FA) and mean diffusivity (MD) were measured for each tract counting each voxel only once.
2.5. Cortical thickness
T1-weighted MPRAGE images were processed in CBrain using the CIVET 1.1.11 pipeline, with normalization to the ICBM-152 template according to previously described methods (Zhou et al., 2015). Cortical thickness was measured across 40,962 vertices per hemisphere and smoothed using a kernel of 20 mm full width at half maximum to preserve cortical topology. Cortical surface data were then segmented into 39 areas per hemisphere with an automated anatomical labelling (AAL) template (Tzourio-Mazoyer et al., 2002), averaging all vertices within each region. AAL regions were used for all statistical analysis in order to reduce multiple comparisons and increase signal-to-noise compared to individual analysis of over 80,000 vertices.
2.6. Brain volumes
T1-weighted MPRAGE images were processed using Freesurfer v5.1 (Anthinoula A Martinos Centre for Biomedical Imaging, Charlestown M.A.), according to a previously described segmentation algorithm (Fischl et al., 2002). Total brain (excluding cerebrospinal fluid and cerebellum), white matter, cortical grey matter, thalamus, caudate, putamen, globus pallidus, hippocampus and amygdala volumes were segmented separately in each hemisphere. Freesurfer was used for this analysis given that CIVET does not perform volume segmentation for deep grey matter structures.
2.7. Statistical analysis
Statistical analysis was carried out in IBM SPSS 22.0. Group differences (FASD and control) in demographic data were determined with independent sample t-tests for continuous variables, or Chi Squared tests for categorical variables (Table 1).
Left and right structures were first averaged (FA/MD of 9 white matter tracts and cortical thickness of 39 AAL regions) or summed (9 volumes) prior to statistical analysis to reduce the number of multiple comparisons. Step-wise linear regression was then used to test for the main effect of Age, Sex, Group, as well as Age-by-Group and Sex-by-Group interactions for each outcome variable (FA/MD, volumes, cortical thickness and cognitive scores). This approach is user-independent: every model starts with the same parameters which are iteratively removed from the model if they do not significantly predict the dependent variable, leading to a potentially unique final model for each tract/structure. p-values of variables in each final model were FDR-corrected, and significance was determined at a corrected p-value threshold of 0.05. Following this, any variables with significant effects of Group, Age-by-Group, or Sex-by-Group were converted to Z scores using the mean and standard deviation of the control group separately in males and females (Z = , where x is the individual FASD participant raw score; μ and σ are the control group mean and standard deviation, respectively), in order to permit comparisons across variables (e.g. MRI and cognitive testing) on the same scale. For the FASD group, Z scores greater than or equal to ± 0.5 standard deviation (28 variables total; 3 DTI, 9 volumes, 7 cortical thickness regions and 9 cognitive tests) were then ranked according to their magnitude separately in males and females for visualization. This arbitrary threshold was chosen to exclude statistically significant differences that may be of little clinical significance due to their small-magnitude (i.e. < 0.5 SD from control mean). Z scores were not corrected for age when reported as group means, given roughly equal matching of age and sex distributions between the control and FASD groups. Lastly, correlations within and between these 28 Z scores were tested using partial correlations, controlling for age (assessed separately in males and females), and FDR-corrected for a p-value threshold of 0.05. Correlations were corrected for age given that analysis was conducted on individual Z scores within groups which may be influenced by subject age.
3. Results
3.1. Demographic data
Age and sex did not differ between the FASD and control groups. Other demographic data revealed significant differences in ethnicity, living situation, annual household income, comorbidities and psychiatric medication use between the FASD and control groups (Table 1).
3.2. Cognitive testing
Significant main effects of group indicated better performance in the control than FASD group on all cognitive tests except the RCFT Immediate, Delay and Recognition (Table 2). Significant main effects of age were found for Word ID (p < 0.001; decreasing with age), RCFT Immediate recall t score (p < 0.001; increasing with age) and RCFT Delay t score (p < 0.001; increasing with age). Age-by-group interactions were significant for RCFT Immediate and Delay t scores (both p < 0.001), as well as Executive Functioning Composite score (p = 0.001), all indicating greater increase with age in the control but not FASD group. No cognitive tests had significant effects of sex, or sex-by-group interactions.
Table 2.
Age-standardized cognitive test scores in controls and FASD.
| Test | Mean ± SD |
Regression analysisd |
||||||
|---|---|---|---|---|---|---|---|---|
| Control (n = 74) | FASD (n = 70) | Group | Age | Sex | Age-by-group | Sex-by-group | ||
| WRITa (age 4 +) | General IQ | 112 ± 13 (n = 74) | 87 ± 18 (n = 45) | β = 0.633 p < 0.001 |
--- | --- | --- | --- |
| WRMT-Ra (age 5 +) | Word ID | 107 ± 13 (n = 74) | 88 ± 16 (n = 69) | β = 0.513 p < 0.001 |
β = − 0.319 p < 0.001 | --- | --- | --- |
| WJa (age 5 +) | Quantitative concepts | 108 ± 13 (n = 74) | 83 ± 14 (n = 65) | β = 0.691 p < 0.001 |
--- | --- | --- | --- |
| CREVTa (age 5 +) | Receptive & expressive composite | 102 ± 10 (n = 74) | 84 ± 12 (n = 50) | β = 0.620 p < 0.001 |
--- | --- | --- | --- |
| NEPSY/DKEFSb (age 5 +) | Executive function composite | 10.2 ± 2.2 (n = 74) | 7.3 ± 3.0 (n = 66) | β = 0.335 p < 0.001 |
--- | --- | β = 0.280 p = 0.001 |
--- |
| RCFTc (age 6 +) | Immediate recall | 47 ± 12 (n = 71) | 36 ± 14 (n = 33) | --- | β = − 0.355 p = 0.011 |
--- | β = 0.681 p < 0.001 |
--- |
| Delayed recall | 47 ± 11 (n = 71) | 36 ± 15 (n = 33) | --- | β = − 0.364 p = 0.011 |
--- | β = 0.645 p < 0.001 |
--- | |
| Recognition | 51 ± 13 (n = 71) | 48 ± 15 (n = 33) | --- | --- | --- | --- | --- | |
| WMTB-Ca (5-15 years) | Digit | 98 ± 16 (n = 59) | 86 ± 13 (n = 43) | β = 0.352 p < 0.001 |
--- | --- | --- | --- |
| Block | 98 ± 16 (n = 59) | 83 ± 15 (n = 41) | β = 0.438 p < 0.001 |
--- | --- | --- | --- | |
WRIT = Wide Range Intelligence Test; WRMT-R = Woodcock Reading Mastery Test- Revised; WJ = Woodcock Johnson; CREVT = Comprehensive Receptive and Expressive Vocabulary Test; NEPSY = A NEuroPSYchological Assessment; DKEFS = Delis Kaplan Executive Function System; RCFT = Rey Complex Figure Task; WMTB-C = Working Memory Test Battery for Children.
Standard score, population mean = 100 ± 15 (higher score = better performance).
Scaled score, population mean = 10 ± 3 (higher score = better performance).
T score, population mean = 50 ± 10 (higher score = better performance).
Only variables included in the final model are shown here with associated β statistics and corrected p-values. Variables excluded from final model are indicated with ‘---’. Note that final models are unique to each test, thus β values for a given variable cannot be quantitatively compared across tests.
3.3. Diffusion tensor Tractography of white matter
A significant main effect of age on FA (increasing) from 5 to 32 was found for the bCC (p < 0.001), sCC (p = 0.006), CST (p < 0.001), IFO (p < 0.001), cingulum (p < 0.001) and uncinate (p < 0.001) (Table 3). In these tracts age-by-group interactions were non-significant, indicating similar age-related increases in both groups. Age-by-group interactions were significant for FA of the genu (p = 0.001), ILF (p = 0.006) and SLF (p < 0.001), indicating steeper increases of FA with age for the SLF and ILF in the control group, and for the genu in the FASD group (Table 3; Fig. 1, Fig. 2). MD decreased significantly with age (p < 0.001) in all tracts, with no significant age-by-group interactions, indicating similar slopes in both groups (Table 4).
Table 3.
Fractional anisotropy of white matter tracts (left and right combined) in controls and FASD.
| Tract | FA (mean ± SD) |
Regression analysisa |
|||||
|---|---|---|---|---|---|---|---|
| Control (n = 74) | FASD (n = 70) | Group | Age | Sex | Age-by-group | Sex-by-group | |
| Genu corpus callosum | 0.52 ± 0.02 | 0.51 ± 0.03 | --- | --- | β = − 0.227 p = 0.006 | β = 0.266 p = 0.001 | --- |
| Body corpus callosum | 0.49 ± 0.02 | 0.49 ± 0.02 | --- | β = 0.423 p < 0.001 | β = − 0.236 p = 0.003 | --- | --- |
| Splenium corpus callosum | 0.52 ± 0.02 | 0.52 ± 0.02 | --- | β = 0.194 p = 0.022 | --- | --- | --- |
| Cingulum | 0.44 ± 0.02 | 0.44 ± 0.02 | --- | β = 0.405 p < 0.001 | β = − 0.284 p < 0.001 | --- | --- |
| Corticospinal tract | 0.50 ± 0.02 | 0.50 ± 0.03 | --- | β = 0.351 p < 0.001 | β = − 0.183 p = 0.022 | --- | --- |
| Inferior longitudinal fasciculus | 0.43 ± 0.02 | 0.42 ± 0.02 | --- | --- | --- | β = 0.236 p = 0.006 | --- |
| Inferior fronto-occipital fasciculus | 0.46 ± 0.03 | 0.46 ± 0.02 | --- | β = 0.524 p < 0.001 | --- | --- | --- |
| Superior longitudinal fasciculus | 0.42 ± 0.02 | 0.42 ± 0.02 | --- | --- | --- | β = 0.539 p < 0.001 | β = − 0.283 p < 0.001 |
| Uncinate fasciculus | 0.38 ± 0.02 | 0.38 ± 0.02 | --- | β = 0.447 p < 0.001 | --- | --- | --- |
Only variables included in the final model are shown here with associated β statistics and corrected p-values. Variables excluded from final model are indicated with ‘---’. Note that final models are unique to each structure, thus β values for a given variable cannot be quantitatively compared across tracts.
Fig. 1.
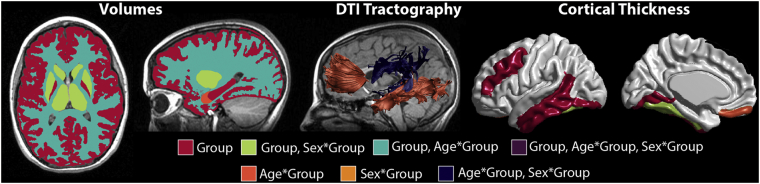
Map of stepwise regression results indicating effects of group, age-by-group, and sex-by-group (or combination thereof) on combined bilateral brain measures. Significant main effects of group were found for FA or MD of 0/9 tracts, volume of 8/9 structures and thickness of 6/39 cortical regions. Age-by-group interactions were significant for FA of 3/9 tracts, volume of 2/9 structures, and thickness of 1/39 cortical regions. Lastly, sex-by-group interactions were significant for FA of 1/9 tracts, volume of 3/9 structures, and thickness of 1/39 cortical regions.
Fig. 2.
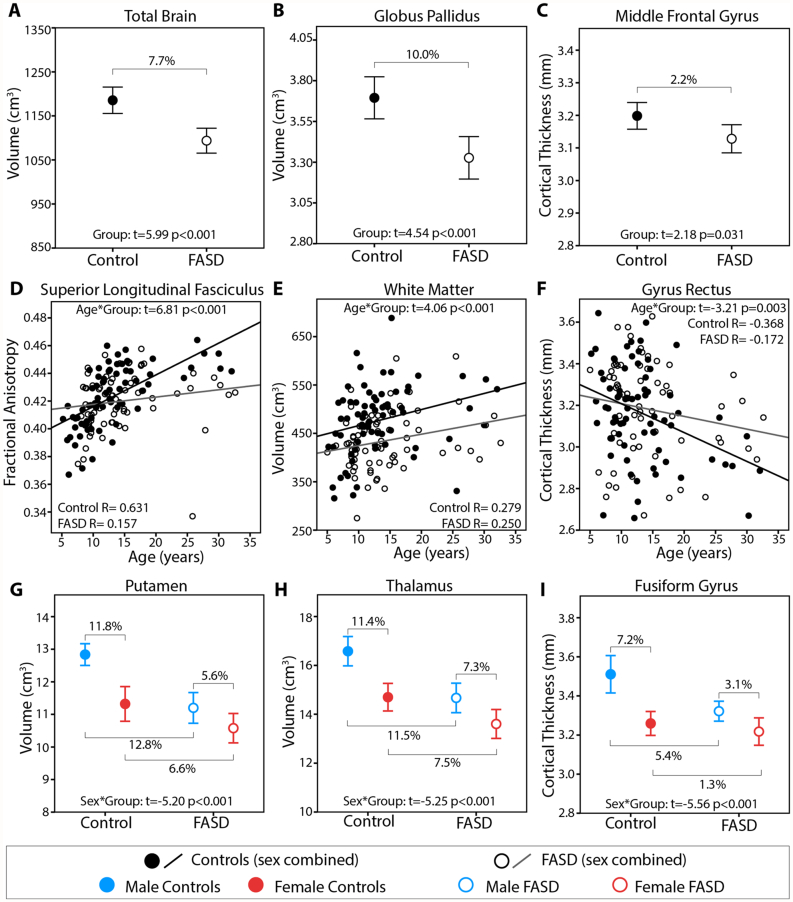
Examples of raw data for group differences (A–C), age-by-group interactions (D–F) and sex-by-group interactions (G–I). Reduced total brain volume (A), globus pallidus volumes (B), and middle frontal gyrus cortical thickness (C) were found in the FASD group relative to controls. Less age related change of FA from the SLF (D), white matter volume (E), and gyrus rectus thickness (F) was found in the FASD than control group. Greater magnitude sex differences were seen in the control than FASD group for several structures including putamen volume (G), thalamus volume (H), and fusiform gyrus cortical thickness (I) indicating reduced sexual dimorphism in FASD.
Table 4.
Mean diffusivity of white matter tracts (left and right combined) in controls and FASD.
| Tract | MD (mean ± SD, mm2/s × 10−3) |
Regression analysisa |
|||||
|---|---|---|---|---|---|---|---|
| Control (n = 74) | FASD (n = 70) | Group | Age | Sex | Age-by-group | Sex-by-group | |
| Genu corpus callosum | 0.81 ± 0.04 | 0.80 ± 0.04 | --- | β = − 0.439 p < 0.001 |
--- | --- | --- |
| Body corpus callosum | 0.86 ± 0.05 | 0.86 ± 0.05 | --- | β = − 0.537 p < 0.001 |
--- | --- | --- |
| Splenium corpus callosum | 0.89 ± 0.05 | 0.87 ± 0.04 | --- | β = − 0.490 p < 0.001 |
--- | --- | --- |
| Cingulum | 0.78 ± 0.04 | 0.77 ± 0.03 | --- | β = − 0.714 p < 0.001 |
--- | --- | --- |
| Corticospinal tract | 0.81 ± 0.04 | 0.80 ± 0.03 | --- | β = − 0.528 p < 0.001 |
--- | --- | --- |
| Inferior longitudinal fasciculus | 0.85 ± 0.04 | 0.85 ± 0.03 | --- | β = − 0.497 p < 0.001 |
--- | --- | --- |
| Inferior fronto-occipital fasciculus | 0.81 ± 0.04 | 0.81 ± 0.03 | --- | β = − 0.511 p < 0.001 |
--- | --- | --- |
| Superior longitudinal fasciculus | 0.79 ± 0.04 | 0.78 ± 0.03 | --- | β = − 0.684 p < 0.001 |
--- | --- | --- |
| Uncinate fasciculus | 0.84 ± 0.04 | 0.84 ± 0.04 | --- | β = − 0.479 p < 0.001 |
--- | --- | --- |
Only variables included in the final model are shown here with associated β statistics and corrected p-values. Variables excluded from final model are indicated with ‘---’. Note that final models are unique to each structure, thus β values for a given variable cannot be quantitatively compared across tracts.
No main effects of group (FASD and controls) were found for FA or MD of any of the 9 tracts. A significant effect of sex was found for FA of the bCC (p = 0.003), gCC (p = 0.006), CST (p = 0.022) and Cingulum (p < 0.001); in all these cases males had higher FA than females in both groups. A significant sex-by-group interaction was found for FA of the SLF (p < 0.001), indicating group differences were greater among males (2.5% higher in control than FASD) than in females (1.5% higher in control than FASD) (Table 3; Fig. 1, Fig. 2). There were no significant effects of sex or sex-by-group interactions for MD of any tract (Table 4).
3.4. Brain volumes
Main effect of group indicated significantly reduced volume of nearly all structures (p < 0.001–0.033) in the FASD group, except the amygdala (Table 5, Fig. 1, Fig. 2). Main effects of age across 5 to 32 years were significant for cortical grey matter (p < 0.001), putamen (p = 0.024) and globus pallidus volumes (p < 0.001); all indicating a decrease in volume with age in both groups. Age-by-group interactions were significant for white matter (p < 0.001; Fig. 1, Fig. 2E) and amygdala volumes (p = 0.006; Fig. 1), both cases indicating steeper age-related increases of volume in the control than FASD group. Sex differences were significant for total brain (p < 0.001), cortical grey matter (p < 0.001), white matter (p < 0.001), globus pallidus (p < 0.001) hippocampus (p < 0.001) and amygdala volumes (p < 0.001); in both groups volumes were larger on average in males than females, as expected. Sex-by-group interactions were significant for the thalamus (p < 0.001 Fig. 2H), caudate (p < 0.001) and putamen (p < 0.001; Fig. 2G); in all these deep grey matter structures the magnitude of group differences (FASD and controls) was greater among males than females. Specifically, male controls had 11.5% larger thalamus, 17.0% larger caudate, and 12.8% larger putamen volumes than males in the FASD group, whereas female controls only had 7.5% larger thalamus, 11.9% larger caudate, and 6.6% larger putamen volumes than female FASD participants.
Table 5.
Regional cortical thickness (left and right combined) in controls and FASD.
| Region (AAL) | Cortical thickness (mean ± SD, mm) |
Regression analysisa |
|||||
|---|---|---|---|---|---|---|---|
| Control (n = 74) | FASD (n = 70) | Group | Age | Sex | Age-by-group | Sex-by-group | |
| Gyrus rectus | 3.16 ± 0.23 | 3.18 ± 0.23 | --- | --- | --- | β = − 0.268 p = 0.003 |
--- |
| Olfactory cortex | 3.05 ± 0.21 | 3.03 ± 0.21 | --- | β = − 0.230 p = 0.008 |
--- | --- | --- |
| SFG orbital | 3.21 ± 0.20 | 3.20 ± 0.19 | --- | β = − 0.423 p < 0.001 |
--- | --- | --- |
| SFG medial orbital | 3.41 ± 0.23 | 3.37 ± 0.23 | --- | β = − 0.397 p < 0.001 |
--- | --- | --- |
| MFG orbital | 3.27 ± 0.21 | 3.22 ± 0.21 | --- | β = − 0.449 p < 0.001 |
--- | --- | --- |
| IFG orbital | 3.48 ± 0.20 | 3.45 ± 0.19 | --- | β = − 0.406 p < 0.001 |
--- | --- | --- |
| SFG dorsolateral | 3.21 ± 0.18 | 3.14 ± 0.17 | --- | β = − 0.454 p < 0.001 |
--- | --- | --- |
| MFG | 3.20 ± 0.17 | 3.13 ± 0.18 | β = 0.167 p = 0.031 |
β = 0.425 p < 0.001 |
--- | --- | --- |
| IFG opercular | 3.40 ± 0.19 | 3.38 ± 0.20 | --- | β = − 0.380 p < 0.001 |
--- | --- | --- |
| IFG triangular | 3.29 ± 0.19 | 3.24 ± 0.19 | --- | β = − 0.471 p < 0.001 |
--- | --- | --- |
| SFG medial | 3.55 ± 0.21 | 3.47 ± 0.23 | --- | β = − 0.451 p < 0.001 |
--- | --- | --- |
| SMA | 3.47 ± 0.21 | 3.43 ± 0.22 | --- | β = − 0.427 p < 0.001 |
--- | --- | --- |
| Paracentral lobule | 2.98 ± 0.22 | 2.95 ± 0.21 | --- | β = − 0.436 p < 0.001 |
--- | --- | --- |
| Precentral gyrus | 3.00 ± 0.16 | 2.94 ± 0.17 | --- | β = − 0.296 p < 0.001 |
--- | --- | --- |
| Rolandic operculum | 3.33 ± 0.19 | 3.34 ± 0.19 | --- | β = − 0.368 p < 0.001 |
--- | --- | --- |
| Postcentral gyrus | 2.69 ± 0.18 | 2.64 ± 0.21 | --- | β = − 0.408 p < 0.001 |
--- | --- | --- |
| Superior parietal gyrus | 2.76 ± 0.21 | 2.70 ± 0.20 | --- | β = − 0.558 p < 0.001 |
--- | --- | --- |
| Inferior parietal supramarginal angular | 2.88 ± 0.17 | 2.83 ± 0.19 | --- | β = − 0.550 p < 0.001 |
--- | --- | --- |
| Supramarginal | 3.13 ± 0.18 | 3.10 ± 0.19 | --- | β = − 0.483 p < 0.001 |
--- | --- | --- |
| Angular gyrus | 3.11 ± 0.19 | 3.04 ± 0.22 | --- | β = − 0.521 p < 0.001 |
--- | --- | --- |
| Precuneus | 3.06 ± 0.18 | 3.02 ± 0.16 | --- | β = − 0.618 p < 0.001 |
--- | --- | --- |
| Superior occipital | 2.75 ± 0.15 | 2.76 ± 0.16 | --- | β = − 0.212 p = 0.012 |
β = − 0.242 p = 0.005 |
--- | --- |
| Middle occipital gyrus | 3.07 ± 0.17 | 3.01 ± 0.18 | --- | β = − 0.421 p < 0.001 |
β = − 0.200 p = 0.011 |
--- | --- |
| Inferior occipital gyrus | 3.19 ± 0.25 | 3.09 ± 0.20 | β = 0.223 p = 0.007 |
--- | β = − 0.334 p < 0.001 |
--- | --- |
| Calcarine fissure and surrounding cortex | 2.76 ± 0.20 | 2.72 ± 0.18 | --- | --- | β = − 0.259 p = 0.003 |
--- | --- |
| Cuneus | 2.76 ± 0.15 | 2.78 ± 0.14 | --- | β = − 0.292 p = 0.001 |
--- | --- | --- |
| Lingual gyrus | 2.98 ± 0.21 | 2.90 ± 0.16 | β = 0.223 p = 0.008 |
--- | β = − 0.296 p < 0.001 |
--- | --- |
| Fusiform gyrus | 3.38 ± 0.26 | 3.28 ± 0.17 | β = 0.629 p < 0.001 |
β = − 0.182 p = 0.018 |
--- | --- | β = − 0.580 p < 0.001 |
| Heschl gyrus | 3.16 ± 0.16 | 3.15 ± 0.18 | --- | β = − 0.240 p = 0.006 |
--- | --- | --- |
| Superior temporal gyrus | 3.32 ± 0.17 | 3.27 ± 0.21 | --- | β = − 0.292 p = 0.001 |
--- | --- | --- |
| Middle temporal gyrus | 3.36 ± 0.17 | 3.29 ± 0.20 | β = 0.172 p = 0.026 |
β = − 0.400 p < 0.001 |
β = − 0.201 p = 0.010 |
--- | --- |
| Inferior temporal gyrus | 3.59 ± 0.22 | 3.50 ± 0.25 | β = 0.178 p = 0.022 |
β = − 0.262 p = 0.001 |
β = − 0.357 p < 0.001 |
--- | --- |
| Temp pole STG | 3.79 ± 0.28 | 3.74 ± 0.36 | --- | --- | β = − 0.236 p = 0.007 |
--- | --- |
| Temp pole MTG | 3.93 ± 0.28 | 3.86 ± 0.38 | --- | --- | β = − 0.242 p = 0.006 |
--- | --- |
| Parahippocampal | 3.24 ± 0.17 | 3.20 ± 0.17 | --- | β = − 0.263 p = 0.003 |
β = − 0.199 p = 0.017 |
--- | --- |
| Anterior cingulate and paracingulate | 3.57 ± 0.22 | 3.55 ± 0.19 | --- | β = − 0.455 p < 0.001 |
--- | --- | --- |
| Median cingulate and paracingulate | 3.26 ± 0.16 | 3.27 ± 0.15 | --- | β = − 0.538 p < 0.001 |
--- | --- | --- |
| Posterior cingulate gyrus | 2.83 ± 0.15 | 2.82 ± 0.14 | --- | β = − 0.270 p = 0.001 |
--- | --- | --- |
| Insula | 3.84 ± 0.26 | 3.80 ± 0.24 | --- | --- | β = − 0.191 p = 0.026 |
--- | --- |
Only variables included in the final model are shown here with associated β statistics and corrected p-values. Variables excluded from final model are indicated with ‘---’. Note that final models are unique to each structure, thus β values for a given variable cannot be quantitatively compared across regions.
3.5. Cortical thickness
Significant main effects of group were found for cortical thickness (combined left and right) in the middle frontal gyrus (p = 0.031), inferior occipital gyrus (p = 0.007), lingual gyrus (p = 0.008), fusiform gyrus (p < 0.001), middle temporal gyrus (p = 0.026) and inferior temporal gyrus (p = 0.022); in all cases thicker in the control than FASD group (Table 6; Fig. 1, Fig. 2). Effects of age were significant in 32/39 AAL regions (p < 0.001–0.008), indicating expected patterns of thinner cortex at older ages in both groups. An age-by-group interaction was significant for the gyrus rectus (p = 0.003; Fig. 2F), indicating steeper slopes in the control than FASD group. Effects of sex were significant for the superior occipital gyrus (p = 0.005), middle occipital gyrus (p = 0.011), inferior occipital gyrus (p < 0.001), calcarine fissure and surrounding cortex (p = 0.003), lingual gyrus (p < 0.001), middle temporal gyrus (p = 0.010), inferior temporal gyrus (p < 0.001), temporal pole superior temporal gyrus (p = 0.007), temporal pole middle temporal gyrus (p = 0.006), parahippocampal gyrus (p = 0.017) and insula (p = 0.026); in all cases thicker in males of both groups. Sex-by-group interactions were significant for the fusiform gyrus (p < 0.001; Fig. 2I), again indicating that group differences in cortical thickness were larger among males (5.4% thicker in the control than FASD group) than females (1.5% thicker in the control than FASD group).
Table 6.
Brain volumes (left and right combined) in controls and FASD.
| Structure | Volume (mean ± SD, cm3) |
Regression analysisa |
|||||
|---|---|---|---|---|---|---|---|
| Control (n = 74) | FASD (n = 70) | Group | Age | Sex | Age-by-group | Sex-by-group | |
| Total brain | 1185 ± 129 | 1094 ± 118 | β = 0.390 p < 0.001 |
--- | β = − 0.542 p < 0.001 |
--- | --- |
| White matter | 477 ± 71 | 435 ± 60 | β = 0.169 p = 0.033 |
--- | β = − 0.485 p < 0.001 |
β = 0.319 p < 0.001 |
--- |
| Cortical grey matter | 540 ± 63 | 496 ± 68 | β = 0.317 p < 0.001 |
β = − 0.369 p < 0.001 |
β = − 0.472 p < 0.001 |
--- | --- |
| Thalamus | 15.7 ± 2.0 | 14.2 ± 1.8 | β = 0.729 p < 0.001 |
--- | --- | --- | β = − 0.534 p < 0.001 |
| Caudate | 7.9 ± 1.2 | 6.8 ± 1.2 | β = 0.752 p < 0.001 |
--- | --- | --- | β = − 0.462 p < 0.001 |
| Putamen | 12.1 ± 1.5 | 10.9 ± 1.4 | β = 0.719 p < 0.001 |
β = − 0.164 p = 0.024 |
--- | --- | β = − 0.518 p < 0.001 |
| Globus pallidus | 3.7 ± 0.6 | 3.3 ± 0.5 | β = 0.321 p < 0.001 |
β = − 0.276 p < 0.001 |
β = − 0.367 p < 0.001 |
--- | --- |
| Hippocampus | 8.6 ± 1.0 | 7.9 ± 0.9 | β = 0.326 p < 0.001 |
--- | β = − 0.382 p < 0.001 |
--- | --- |
| Amygdala | 3.1 ± 0.4 | 3.0 ± 0.4 | --- | --- | β = − 0.439 p < 0.001 |
β = 0.213 p = 0.006 |
--- |
Only variables included in the final model are shown here with associated β statistics and corrected p-values. Variables excluded from final model are indicated with ‘---’. Note that final models are unique to each structure, thus β values for a given variable cannot be quantitatively compared across structures.
3.6. FASD group Z score ranking
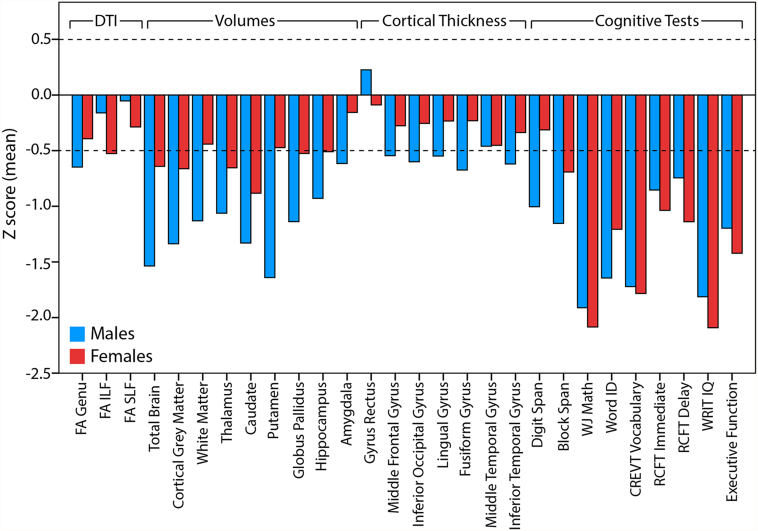
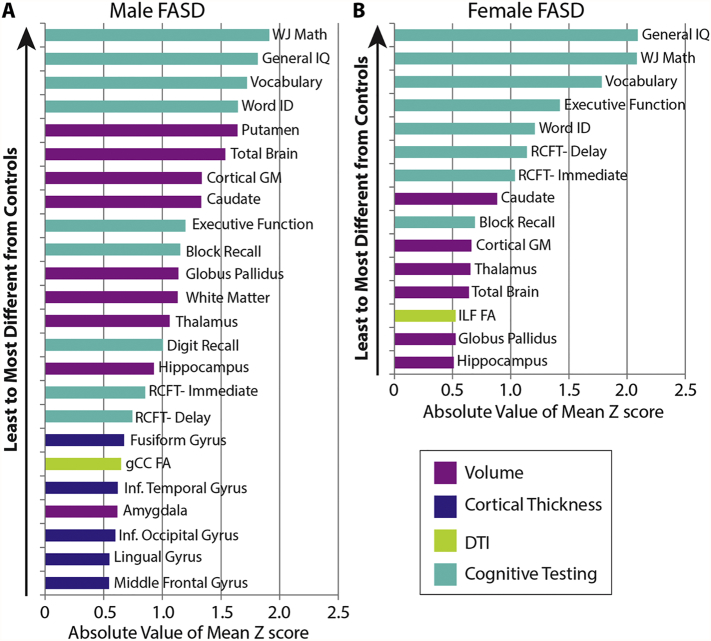
In order to permit comparison of the magnitude of group differences (FASD and control) among variables with different scales, Z scores were calculated for variables with significant main or interaction effect of group, for a total of 28 variables: 3 DTI, 9 volumes, 7 cortical thickness and 9 cognitive tests. Z scores were calculated in the FASD group from the control mean and standard deviation, separately in males and females, and plotted on the same scale (Fig. 3). Note that Z scores are not shown for controls as they average to zero by definition. The largest Z scores for FASD (i.e. biggest differences from controls) were observed for cognitive tests and volumes (~ 0.5–2 SD smaller/lower than the control mean), followed by cortical thickness and DTI parameters (~ 0.25–0.5 SD). The magnitude of Z scores was similar between male and female FASD participants for all cognitive tests, but was larger in males for brain volumes and select regions of cortical thickness, again suggesting greater magnitude group differences in male than female FASD participants. Including only FASD Z scores greater than ± 0.5 yielded a total of 24 variables in males and 15 variables in females (Fig. 4). In addition to having more Z scores greater than ± 0.5 SD from the control mean, males had larger magnitudes of Z scores than females for several structures, e.g. putamen volume with ~ 2 SD in males and < 0.5 SD in females. Z scores were not larger in females for any variable.
Fig. 3.
FASD group Z scores are shown for 28 variables with significant effects of group, age-by-group, or sex-by-group from stepwise regression. The largest Z scores are observed for cognitive tests and brain volumes, followed by cortical thickness and DTI parameters. Of note, larger Z scores are seen in males than females for several structural brain parameters (e.g. putamen volume) but not cognitive testing where performance was similarly poor for males and females with FASD.
Fig. 4.
Rank ordered FASD group Z scores in males (A) and females (B) of variables with Z scores greater than an arbitrary cut-off of ± 0.5. More variables meet this criterion in males (24) than females (15). In addition, larger magnitude Z scores are seen in males relative to females for several structures. Cognitive scores are shown to have the largest absolute volume Z scores in both males and females, though it should be noted that all participants have an FASD diagnosis that requires significant cognitive impairment a priori.
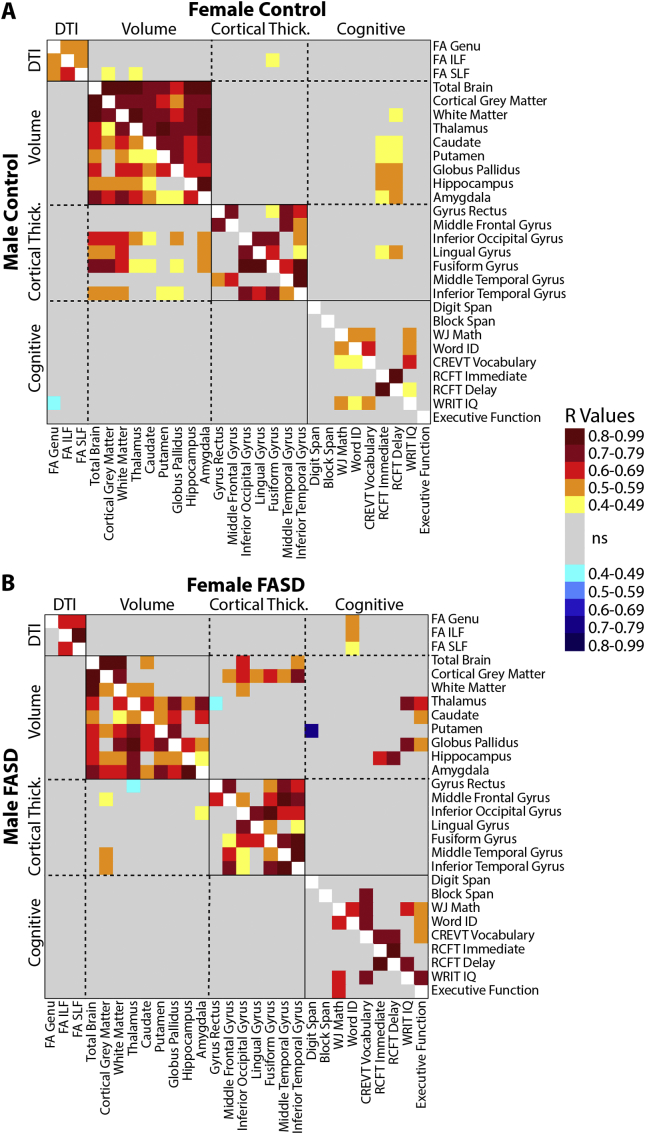
3.7. Z-score correlation analysis
Partial correlations (controlling for age) were performed between all Z scores (28 variables total), and p-values were FDR corrected for multiple comparisons. This analysis revealed several significant correlations between the various cognitive scores (36 total possible correlations), e.g. IQ and math in both males (7 correlations in controls, 5 in FASD) and females (8 in controls, 13 in FASD) (Fig. 5). Likewise, significant correlations among the various volumes (e.g. total brain and cortical grey matter) were found for males and females in both groups, though notably the greatest number and strongest correlations were found for female controls (36/36) and the least were found for female FASD participants (18/36 possible correlations). Interestingly, total brain volume correlated with the volume of all other structures (as may be expected) in control males and females, as well as in FASD males, but was only significantly correlated with cortical grey matter, white matter, and caudate volume in females with FASD. This may suggest a more regionally-dependent pattern of deep grey matter volume deficit in females with FASD. Correlations among cortical thickness regions (e.g. middle temporal gyrus and middle frontal gyrus) and DTI parameters of white matter tracts (e.g. FA of the SLF and ILF) were similar in all sub-groups. Correlations between volumes and cortical thickness are observed in male controls, but are notably absent in males with FASD. Correlations between the other modalities (e.g. DTI and volumes) were largely absent in all groups.
Fig. 5.
Partial correlation matrices (controlling for age) of Z scores (28 variables total that showed significant effects of group, age-by-group or sex-by-group from stepwise regression) in controls (A) and FASD participants (B); shown separately per plot in males (lower left) and females (upper right). R values for correlations that survived FDR correction are shown here by colour. In both groups, more correlations are seen within than between modalities (e.g. within various volumes than between volumes and cognitive scores) albeit in volumes these correlations appear to be stronger in the control than FASD group, possibly reflecting disproportionate and variable reductions of regional brain volumes in the FASD group. Interestingly, significant correlations between cognitive scores and brain volumes are more frequent in females of both groups (FASD and controls) than in males of either group.
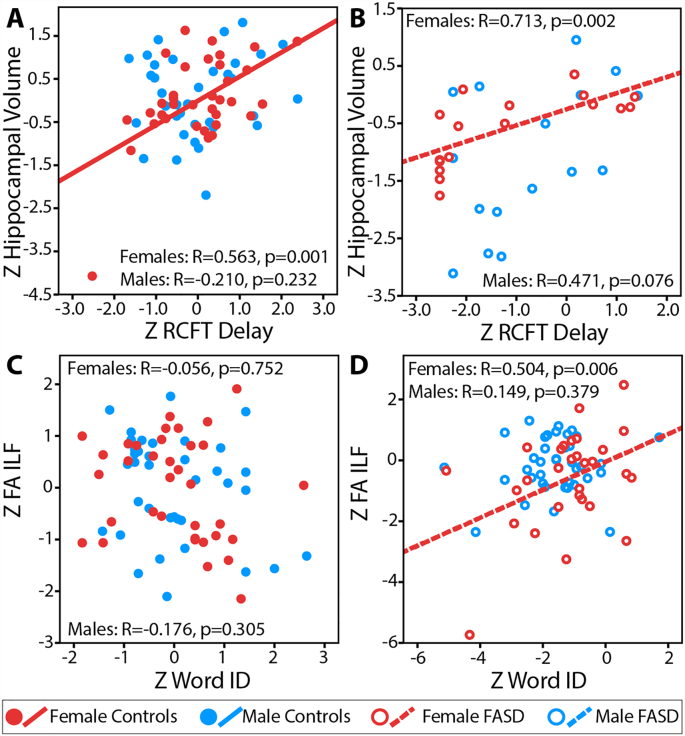
Thirteen significant correlations were found between cognitive scores and MRI variables in female controls and 11 in female FASD participants, whereas only one correlation was significant in male controls and none in male FASD participants (Fig. 5). In female controls, RCFT Immediate and Delay scores are shown to positively correlate with several deep grey matter volumes, some of which also hold in female FASD participants. For example, hippocampal volume Z scores correlated with RCFT Delay Z scores in female controls (Fig. 6A) and female FASD participants (Fig. 6B), indicating that female participants with larger hippocampal volumes also had better visuospatial memory. This correlation did not hold in males of either group. In females with FASD, FA of the genu, ILF, and SLF are all found to positively correlate with Word ID scores (Fig. 6C, D), indicating a link between white matter and reading that is not observed in any other group. Likewise, female FASD participants are found to have positive correlations between executive functioning and IQ scores with caudate, thalamus, and globus pallidus volumes, which are among the most affected MRI metrics in females (Fig. 4B).
Fig. 6.
Examples of partial correlations of Z scores controlling for age for controls (A,C) and FASD (B,D) showing significant positive correlations between MRI metrics and cognitive tests in females but not males. (A, B) Correlations between hippocampal volume and RCFT delay scores indicate larger hippocampus volumes in subjects with better memory performance among females but not males in both the FASD and control groups. (C, D) A positive correlation between FA of the inferior longitudinal fasciculus (ILF) and Word ID indicates that better reading ability is associated with higher FA only among females with FASD, but not among female controls or males in either group (ns = non-significant).
4. Discussion
This cross-sectional MRI study of 70 children, adolescents and young adults with FASD provides novel comparisons between diffusion tensor imaging, cortical thickness, brain volume and cognition within a single sample, with a focus on sex differences and coherence between measures. Findings demonstrate: i) robust reductions of total brain, cortical grey matter, white matter and deep grey matter volumes (8–14% smaller in FASD), relatively smaller magnitude (2–3%), less extensive reductions of cortical thickness, and no main effect of group on DTI of 9 white matter tracts; (ii) more extensive structural anomalies in males than females with FASD, despite similar levels of cognitive deficits; and (iii) significant correlations between brain structure (mainly subcortical volumes) and select cognitive scores in both FASD and control females, but not males, and few correlations between different structural measures (e.g. cortical thickness and diffusion abnormalities) within any group.
Many previous studies have reported ~ 8–15% smaller brain volumes in FASD (e.g. Archibald et al., 2001, Astley et al., 2009, Roussotte et al., 2011), suggesting that volume reduction is a robust finding which could reflect alcohol-induced alterations in neuronal proliferation, migration and cell death in utero (Goodlett et al., 2005). Similar slopes of volume change with age in the FASD and control group here confirm longitudinal findings (Gautam et al., 2015a, Treit et al., 2013), except for white matter and amygdala which are shown to have larger group differences at older ages here. Previous cortical thickness studies of FASD have yielded inconsistent group differences, with some studies finding reductions (Gautam et al., 2015b, Robertson et al., 2016, Zhou et al., 2011), increases (Fernandez-Jaen et al., 2011, Sowell et al., 2008, Yang et al., 2012) or no difference in cortical thickness in the FASD group relative to controls (Rajaprakash et al., 2014, Wozniak et al., 2013). Inconsistencies in previous literature may potentially stem from differences in methodology, patient populations and developmental effects of age. For example, thicker cortex in the FASD group of samples spanning younger ages (e.g. 8–16 years in Yang et al., 2012) may stem from accelerated thinning in controls during adolescence that is not observed in subjects with FASD (as demonstrated in Treit et al., 2014); likewise, thinner cortex in the FASD group of older samples (e.g. 7–30 in Zhou et al., 2011) may be revealed after delayed thinning occurs. Here we see several regions with thinner cortex in the FASD group, in agreement with both our earlier cross-sectional work (Zhou et al., 2011) and others (Gautam et al., 2015b, Robertson et al., 2016), as well as less age-related change of cortical thickness in the FASD group in keeping with our previous longitudinal study (Treit et al., 2014), albeit in a cross-sectional cohort here.
In contrast to our previous cross-sectional DTI tractography study with similar methods (n = 24, 5–13 years; Lebel et al., 2008a), and several other DTI studies in the literature (for review, see Wozniak and Muetzel, 2011), we did not find omnibus group differences in DTI parameters of several white matter tracts. We see expected changes in DTI parameters with age (i.e. increasing FA, decreasing MD) in the majority of white matter tracts in both groups; however, age-by-group interactions suggest group differences that emerge only in older subjects for 3 tracts (genu corpus callosum, ILF and SLF– Fig. 2D; left and right combined here) potentially stemming from slower rates of white matter maturation in the FASD group. Of note, this does not agree with our previous longitudinal study, which found greater reduction of MD with age in FASD participants (Treit et al., 2013), though in a younger cohort of 5–15 years.
Previous studies have revealed numerous sex differences in brain structure and neurophysiology in healthy populations, though not all findings have been widely consistent across the literature. Nonetheless, examples include (but are not limited to): ~ 10% reductions of brain volume in females relative to males (Giedd et al., 2012), higher FA of white matter in males (Lenroot et al., 2007), select regions of thicker cortex in females (Sowell et al., 2007), differences of sex steroid distribution in deep grey matter, and earlier peaks of volume in females relative to males (Giedd et al., 2012). While sex is typically factored into image analysis as a covariate for these reasons, only a handful of MRI studies of FASD have examined sex-differences in brain structure, likely due to sample size limitations and interest in sub-grouping samples based on other important variables (e.g. diagnostic category). Sex-by-group interactions here indicate smaller sex differences within the FASD group (i.e. attenuated sexual dimorphism) relative to controls for thalamus, caudate and putamen volumes (e.g. Fig. 2G,H). In healthy populations, the putamen and globus pallidus are shown to be disproportionately larger in males even after controlling for sex differences in total brain volume (Rijpkema et al., 2012), potentially leading to larger group differences (FASD and controls) in males if this expected sexual differentiation of brain structure is disrupted by prenatal alcohol exposure. The basal ganglia has been implicated in numerous psychiatric and developmental disorders that have higher prevalence in males (e.g. Parkinson's disease, autism, ADHD), as well as with disorders that differ phenotypically between males and females (e.g. obsessive compulsive disorder) (Rijpkema et al., 2012), potentially stemming from sex differences in the neurochemical properties of these structures. Reduced sensitivity to the neurophysiological effects of testosterone has been shown in male rats following prenatal alcohol exposure (Lan et al., 2009), and may confer a mechanism by which males with prenatal alcohol exposure show greater volume reductions in structures highly innervated with sex steroids, given testosterone's role in increasing neuronal density, size, migration and synaptogenesis during development (Goldstein et al., 2001, McCarthy, 2008). Decreased systemic tissue responsiveness to testosterone (despite increased levels) has recently been observed in adolescents with prenatal alcohol exposure (Carter et al., 2014); however, further work is needed to investigate the influence of prenatal alcohol exposure on testosterone bioavailability and responsiveness in the brain.
Animal models suggest that prenatal alcohol exposure may have a greater impact on several other aspects of brain structure/physiology in males, including long-term potentiation in the hippocampus (Sickmann et al., 2014), hypothalamic-pituitary axis regulation (Weinberg et al., 2008), corpus callosum size (Zimmerberg and Mickus, 1990, Zimmerberg and Reuter, 1989, Zimmerberg and Scalzi, 1989), and dopamine receptor binding in the prefrontal cortex (Converse et al., 2014). Human studies have shown greater relative reductions in regional cortical (Chen et al., 2012) and deep grey matter volumes (Dudek et al., 2014, Nardelli et al., 2011) in males than females with FASD. Moreover, greater differences in longitudinal cortical volume trajectories in males (prenatal alcohol exposure relative to controls) than females (Lebel et al., 2012) further suggest a trend towards greater teratogenic effects of alcohol on brain structure in males.
Despite sex-specific effects on brain structure, we did not observe sex differences of cognitive deficits in this FASD sample, where IQ, mathematics and vocabulary were among the most impaired domains in both males and females in keeping with previous reports (Kodituwakku, 2007). Among healthy populations, sex differences are not typically observed in most cognitive domains despite mean differences in brain volumes, suggesting a complex relationship between sexual dimorphism of brain structure and function. The majority of cognitive tests here focus on academic or basic cognitive skills, so it remains possible that sex differences would be revealed by querying other aspects of cognition (e.g. emotion-related/affective executive functions or emotional regulation skills). We did, however, find several significant brain-behaviour correlations in females with FASD that were absent in males, e.g. hippocampus volume and visuospatial memory (Figs. 5B & 6B). Interestingly, correlations between hippocampal volume and visual memory have been demonstrated in adults with prenatal alcohol exposure (Coles et al., 2011), albeit with males and females combined. Other studies have demonstrated sex differences in behavioural impairment associated with prenatal alcohol exposure, including affective (depression and anxiety) behaviours in rats (Hellemans et al., 2010, Osborn et al., 1998), as well as auditory processing (Tesche et al., 2015) and eye tracking performance in humans (Paolozza et al., 2015).
Overall very few correlations were observed between structural measurement types (Fig. 5), suggesting that abnormalities in one domain (e.g. diffusion metrics) do not necessarily co-occur with abnormalities in other aspects of brain structure (e.g. volume reduction). This lack of cohesion may indicate ‘independent’ injuries that depend on timing of exposure and the developmental trajectories of each variable among other factors, which may partially explain challenges thus far in establishing a common set of neurobehavioural and structural features that would serve as a biomarker for FASD.
Several limitations of this study must be acknowledged. Tractography-based diffusion analysis has the advantage of querying an entire white matter tract, but this may in turn reduce sensitivity to local abnormalities that may otherwise be detected by voxel-based or along-the tract methods, and may account for fewer group differences here than in previous work using other methods. Moreover, lack of group differences in diffusion parameters may also partially stem from averaging left and right tracts, which could overshadow hemisphere-specific diffusion abnormalities found in other studies of FASD (e.g. Lebel et al., 2008a) as well as in healthy samples (e.g. Catani et al., 2007). The use of the ICBM-DTI81 template for tractography analysis and ICBM-152 template for cortical thickness and volume analysis may be less ideal than creating study-specific templates given the age-range of participants in this study. However, manual inspection of the images suggested adequate registration. Further, the semi-automated tractography analysis purposefully uses larger seed ROIs to capture all of a tract regardless of imperfect registration between subjects. Age and age-by-group effects were modelled linearly, despite known non-linear trajectories of maturation in development over the 5 to 32 year age span (e.g. Lebel and Beaulieu, 2011), and puberty stage was not measured despite evidence that it may better model development than age in some structures (Goddings et al., 2014) and could therefore influence group and sex differences. Large longitudinal studies of FASD will be needed to tease apart the effects of age and puberty on reduced sexual dimorphism of the brain in FASD.
In addition, the age distribution is not evenly sampled here, resulting in a greater number of children/adolescents than adults in both groups, which could influence results or overshadow group differences in the presence of age-by-group interactions. As a post-hoc analysis we repeated regression analysis on brain volumes after excluding the 16 subjects > 21 years of age, and found no difference in group differences for 6/9 structures. However, the 3/9 (white matter, thalamus, hippocampus) that lost an effect of group showed a gain or strengthening of age-by-group interaction, suggesting that age mediates group differences even within only younger subjects. No changes in main effects of group were seen for FA or MD of any tract. Including only subjects < 21 years in cortical thickness analysis revealed two additional cortical areas with significant effects of group (superior frontal gyrus medial and dorsolateral), and one area that lost a main effect of group (middle temporal gyrus) for a total of 7 rather than 6 cortical areas with an effect of group. Future studies should strive to evenly sample across large age spans to further tease apart these effects.
Demographic data (household income, ethnicity, etc.) was missing in 26/70 FASD participants, limiting the ability to properly characterize this sample. In addition to prenatal alcohol exposure, many individuals with FASD have had adverse childhood experiences and other exposures that cannot be accurately controlled or even assessed in human studies, but nonetheless require adequate reporting to contextualize results. Indeed, we find that ~ 80% of the FASD sample for which we have demographic data report living in an adoptive, foster or group home, compared to ~ 1% of our control sample. In addition, ethnicity is imbalanced in this sample, with 81% of our control sample and 41% of our FASD sample self-reporting as Caucasian. However, as a post-hoc analysis we added ethnicity to the stepwise regression model for brain volumes (our most robust findings), and found that it was not a significant predictor for any of the 9 structures (data not shown). In addition, ADHD is a common co-morbidity in this population, with estimates that > 70% of individuals with FASD meet criteria for comorbid ADHD (Burd et al., 2003, Fryer et al., 2007). Thus, in addition to recruiting ethnically diverse samples, future studies may also want to consider the value of recruiting both ADHD and healthy control groups for comparisons of brain structure. Finally, the executive function scores reported here are the average of several sub-tests that differed slightly between subjects. Although it is not ideal to compare scores derived from different measures (of the same construct), we elected to include these standardized measures rather than exclude all executive function scores.
5. Conclusions
Comprehensive brain imaging of 70 FASD subjects that examined three primary brain metrics in one study confirmed previous findings of reduced brain volumes and thinner cortex in FASD, but did not show group DTI differences in white matter tracts. Few correlations are seen between the various brain measures, suggesting that each modality may be uniquely susceptible to prenatal alcohol exposure. Novel sex differences in the magnitude of structural brain damage and correlations with specific cognitive scores suggest that prenatal alcohol may impair sexual dimorphism of brain structure.
Acknowledgements
This study was funded by the Canadian Institutes of Health Research. Salary support was provided by Alberta Innovates-Health Solutions (co-authors ST & CB), Canada Research Chairs program (CB), and the Women's and Children's Health Research Institute (ST). We gratefully acknowledge the time and efforts of the families who participated in this study
References
- Archibald S.L., Fennema-Notestine C., Gamst A., Riley E.P., Mattson S.N., Jernigan T.L. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev. Med. Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Astley S. Fetal Alcohol Syndrome Diagnostic and Prevention Network, University of Washington; Seattle USA: 2004. Diagnostic Guide for Fetal Alcohol Spectrum Disorders: The 4-Digit Diagnostic Code. [Google Scholar]
- Astley S.J., Aylward E.H., Olson H.C., Kerns K., Brooks A., Coggins T.E., Davies J., Dorn S., Gendler B., Jirikowic T., Kraegel P., Maravilla K., Richards T. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2009;33:1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron S., Tieman S.B., Riley E.P. Effects of prenatal alcohol exposure on the sexually dimorphic nucleus of the preoptic area of the hypothalamus in male and female rats. Alcohol. Clin. Exp. Res. 1988;12:59–64. doi: 10.1111/j.1530-0277.1988.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Burd L., Klug M.G., Martsolf J.T., Kerbeshian J. Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicol. Teratol. 2003;25:697–705. doi: 10.1016/j.ntt.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat. Rev. Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Carter R.C., Jacobson J.L., Dodge N.C., Granger D.A., Jacobson S.W. Effects of prenatal alcohol exposure on testosterone and pubertal development. Alcohol. Clin. Exp. Res. 2014;38:1671–1679. doi: 10.1111/acer.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Allin M.P.G., Husain M., Pugliese L., Mesulam M.M., Murray R.M., Jones D.K. Symmetries in human brain language pathways correlate with verbal recall. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.C., Coles C.D., Lynch M.E., Hu X.P. Understanding specific effects of prenatal alcohol exposure on brain structure in young adults. Hum. Brain Mapp. 2012;33:1663–1676. doi: 10.1002/hbm.21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudley A.E., Conry J., Cook L.L., Loock C., Rosales T., LeBlanc N. Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. Can. Med. Assoc. J. 2005;172:S1–S21. doi: 10.1503/cmaj.1040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarren S.K., Alvord E.C., Sumi S.M., Streissguth A.P., Smith D.W. Brain malformations related to prenatal exposure to ethanol. J. Pediatr. 1978;92:64–67. doi: 10.1016/s0022-3476(78)80072-9. [DOI] [PubMed] [Google Scholar]
- Coles C.D., Goldstein F.C., Lynch M.E., Chen X., Kable J.A., Johnson K.C., Hu X. Memory and brain volume in adults prenatally exposed to alcohol. Brain Cogn. 2011;75:67–77. doi: 10.1016/j.bandc.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Converse A.K., Moore C.F., Holden J.E., Ahlers E.O., Moirano J.M., Larson J.A., Resch L.M., DeJesus O.T., Barnhart T.E., Nickles R.J., Murali D., Christian B.T., Schneider M.L. Moderate-level prenatal alcohol exposure induces sex differences in dopamine D-1 receptor binding in adult rhesus monkeys. Alcohol. Clin. Exp. Res. 2014;38:2934–2943. doi: 10.1111/acer.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove K.P., Mazure C.M., Staley J.K. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek J., Skocic J., Sheard E., Rovet J. Hippocampal abnormalities in youth with alcohol-related neurodevelopmental disorder. J. Int. Neuropsychol. Soc. 2014;20:181–191. doi: 10.1017/S1355617713001343. [DOI] [PubMed] [Google Scholar]
- Fernandez-Jaen A., Fernandez-Mayoralas D.M., Quinones Tapia D., Calleja-Perez B., Garcia-Segura J.M., Arribas S.L., Munoz Jareno N. Cortical thickness in fetal alcohol syndrome and attention deficit disorder. Pediatr. Neurol. 2011;45:387–391. doi: 10.1016/j.pediatrneurol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fryer S.L., McGee C.L., Matt G.E., Mattson S.N. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics. 2007;119:E733–E741. doi: 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- Gautam P., Lebel C., Narr K.L., Mattson S.N., May P.A., Adnams C.M., Riley E.P., Jones K.L., Kan E.C., Sowell E.R. Volume changes and brain-behavior relationships in white matter and subcortical gray matter in children with prenatal alcohol exposure. Hum. Brain Mapp. 2015;36:2318–2329. doi: 10.1002/hbm.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P., Warner T.D., Kan E.C., Sowell E.R. Executive function and cortical thickness in youths prenatally exposed to cocaine, alcohol and tobacco. Dev. Cogn. Neurosci. 2015;16:155–165. doi: 10.1016/j.dcn.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Raznahan A., Mills K.L., Lenroot R.K. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol. Sex Differ. 2012;3 doi: 10.1186/2042-6410-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A.-L., Mills K.L., Clasen L.S., Giedd J.N., Viner R.M., Blakemore S.-J. The influence of puberty on subcortical brain development. NeuroImage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.M., Seidman L.J., Horton N.J., Makris N., Kennedy D.N., Caviness V.S., Faraone S.V., Tsuang M.T. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb. Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Goodlett C.R., Horn K.H., Zhou F.C. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp. Biol. Med. 2005;230:394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- Hellemans K.G.C., Verma P., Yoon E., Yu W.K., Young A.H., Weinberg J. Prenatal alcohol exposure and chronic mild stress differentially alter depressive- and anxiety-like behaviors in male and female offspring. Alcohol. Clin. Exp. Res. 2010;34:633–645. doi: 10.1111/j.1530-0277.2009.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.L., Smith D.W. Recognition of fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kodituwakku P.W. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neurosci. Biobehav. Rev. 2007;31:192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Lan N., Hellemans K.G.C., Ellis L., Viau V., Weinberg J. Role of testosterone in mediating prenatal ethanol effects on hypothalamic-pituitary-adrenal activity in male rats. Psychoneuroendocrinology. 2009;34:1314–1328. doi: 10.1016/j.psyneuen.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Rasmussen C., Wyper K., Walker L., Andrew G., Yager J., Beaulieu C. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol. Clin. Exp. Res. 2008;32:1732–1740. doi: 10.1111/j.1530-0277.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lebel C., Roussotte F., Sowell E.R. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol. Rev. 2011;21:102–118. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Mattson S.N., Riley E.P., Jones K.L., Adnams C.M., May P.A., Bookheimer S.Y., O'Connor M.J., Narr K.L., Kan E., Abaryan Z., Sowell E.R. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. J. Neurosci. 2012;32:15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans A., Jeurissen B., Sijbers J., Jones D. 17th Annual Meeting of International Society of Magnetic Resonance Medicine, Hawaii, USA. 2009. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data; p. 3537. [Google Scholar]
- Lenroot R.K., Gogtay N., Greenstein D.K., Wells E.M., Wallace G.L., Clasen L.S., Blumenthal J.D., Lerch J., Zijdenbos A.P., Evans A.C., Thompson P.M., Giedd J.N. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M.M. Estradiol and the developing brain. Physiol. Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli A., Lebel C., Rasmussen C., Andrew G., Beaulieu C. Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol Spectrum disorders. Alcohol. Clin. Exp. Res. 2011;35:1404–1417. doi: 10.1111/j.1530-0277.2011.01476.x. [DOI] [PubMed] [Google Scholar]
- Osborn J.A., Kim C.K., Steiger J., Weinberg J. Prenatal ethanol exposure differentially alters behavior in males and females on the elevated plus maze. Alcohol. Clin. Exp. Res. 1998;22:685–696. [PubMed] [Google Scholar]
- Paolozza A., Munn R., Munoz D.P., Reynolds J.N. Eye movements reveal sexually dimorphic deficits in children with fetal alcohol spectrum disorder. Front. Neurosci. 2015;9:76. doi: 10.3389/fnins.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaprakash M., Chakravarty M.M., Lerch J.P., Rovet J. Cortical morphology in children with alcohol-related neurodevelopmental disorder. Brain Behav. 2014;4:41–50. doi: 10.1002/brb3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijpkema M., Everaerd D., van der Pol C., Franke B., Tendolkar I., Fernandez G. Normal sexual dimorphism in the human basal ganglia. Hum. Brain Mapp. 2012;33:1246–1252. doi: 10.1002/hbm.21283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley E.P., McGee C.L. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp. Biol. Med. 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Robertson F.C., Narr K.L., Molteno C.D., Jacobson J.L., Jacobson S.W., Meintjes E.M. Prenatal alcohol exposure is associated with regionally thinner cortex during the preadolescent period. Cereb. Cortex. 2016;26:3083–3095. doi: 10.1093/cercor/bhv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte F.F., Sulik K.K., Mattson S.N., Riley E.P., Jones K.L., Adnams C.M., May P.A., O'Connor M.J., Narr K.L., Sowell E.R. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum. Brain Mapp. 2011;33:920–937. doi: 10.1002/hbm.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickmann H.M., Patten A.R., Morch K., Sawchuk S., Zhang C., Parton R., Szlavik L., Christie B.R. Prenatal ethanol exposure has sex-specific effects on hippocampal long-term potentiation. Hippocampus. 2014;24:54–64. doi: 10.1002/hipo.22203. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Kan E., Woods R.P., Yoshii J., Bansal R., Xu D., Zhu H., Thompson P.M., Toga A.W. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb. Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Mattson S.N., Kan E., Thompson P.M., Riley E.P., Toga A.W. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb. Cortex. 2008;18:136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesche C.D., Kodituwakku P.W., Garcia C.M., Houck J.M. Sex-related differences in auditory processing in adolescents with fetal alcohol spectrum disorder: a magnetoencephalographic study. NeuroImage Clin. 2015;7:571–587. doi: 10.1016/j.nicl.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit S., Lebel C., Baugh L., Rasmussen C., Andrew G., Beaulieu C. Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol Spectrum disorders. J. Neurosci. 2013;33:10098–10109. doi: 10.1523/JNEUROSCI.5004-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit S., Zhou D.M., Lebel C., Rasmussen C., Andrew G., Beaulieu C. Longitudinal MRI reveals impaired cortical thinning in children and adolescents prenatally exposed to alcohol. Hum. Brain Mapp. 2014;35:4892–4903. doi: 10.1002/hbm.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wakana S., Jiang H.Y., Nagae-Poetscher L.M., van Zijl P.C.M., Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Weinberg J., Sliwowska J.H., Lan N., Hellemans K.G.C. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J. Neuroendocrinol. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J.R., Muetzel R.L. What does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders? Neuropsychol. Rev. 2011;21:133–147. doi: 10.1007/s11065-011-9162-1. [DOI] [PubMed] [Google Scholar]
- Wozniak J.R., Mueller B.A., Bell C.J., Muetzel R.L., Hoecker H.L., Boys C.J., Lim K.O. Global functional connectivity abnormalities in children with fetal alcohol Spectrum disorders. Alcohol. Clin. Exp. Res. 2013;37:748–756. doi: 10.1111/acer.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.L., Roussotte F., Kan E., Sulik K.K., Mattson S.N., Riley E.P., Jones K.L., Adnams C.M., May P.A., O'Connor M.J., Narr K.L., Sowell E.R. Abnormal cortical thickness alterations in fetal alcohol spectrum disorders and their relationships with facial dysmorphology. Cereb. Cortex. 2012;22:1170–1179. doi: 10.1093/cercor/bhr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Yushkevich P.A., Alexander D.C., Gee J.C. Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med. Image Anal. 2006;10:764–785. doi: 10.1016/j.media.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Zhou D., Lebel C., Lepage C., Rasmussen C., Evans A., Wyper K., Pei J., Andrew G., Massey A., Massey D., Beaulieu C. Developmental cortical thinning in fetal alcohol spectrum disorders. NeuroImage. 2011;58:16–25. doi: 10.1016/j.neuroimage.2011.06.026. [DOI] [PubMed] [Google Scholar]
- Zhou D., Lebel C., Treit S., Evans A., Beaulieu C. Accelerated longitudinal cortical thinning in adolescence. NeuroImage. 2015;104:138–145. doi: 10.1016/j.neuroimage.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B., Mickus L.A. Sex-differences in corpus-callosum - influence of prenatal alcohol exposure and maternal undernutrition. Brain Res. 1990;537:115–122. doi: 10.1016/0006-8993(90)90347-e. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B., Reuter J.M. Sexually dimorphic behavioral and brain asymmetries in neonatal rats - effects of prenatal alcohol exposure. Dev. Brain Res. 1989;46:281–290. doi: 10.1016/0165-3806(89)90291-5. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B., Scalzi L.V. Commissural size in neonatal rats - effects of sex and prenatal alcohol exposure. Int. J. Dev. Neurosci. 1989;7:81–86. doi: 10.1016/0736-5748(89)90046-4. [DOI] [PubMed] [Google Scholar]