Abstract
The dose-response effects of exercise in reduced gravity on musculoskeletal health have not been well documented. It is not known whether or not individualized exercise prescriptions can be effective in preventing the substantial loss in bone mineral density and muscle function that have been observed in space flight and in bed rest. In this study, typical daily loads to the lower extremities were quantified in free-living subjects who were then randomly assigned to control or exercise groups. Subjects were confined to 6-degree head-down bed rest for 84 days. The exercise group performed individually prescribed 1 g loaded locomotor exercise to replace their free-living daily load. Eleven subjects (5 exercise, 6 control) completed the protocol. Volumetric bone mineral density results from quantitative computed tomography demonstrated that control subjects lost significant amounts of bone in the intertrochanteric and total hip regions (p < 0.0125), whereas the exercise group showed no significant change from baseline in any region (p > 0.0125). Pre-and post-bed rest muscle volumes were calculated from analysis of magnetic resonance imaging data. The exercise group retained a larger percentage of their total quadriceps and gastrocnemius muscle volume (− 7.2% ± 5.9, − 13.8% ± 6.1, respectively) than their control counterparts (− 23.3% ± 5.9, − 33.0 ± 8.2, respectively; p < 0.01). Both groups significantly lost strength in several measured activities (p < 0.05). The declines in peak torque during repeated exertions of knee flexion and knee extension were significantly less in the exercise group than in the control group (p < 0.05) but work done was not significantly different between groups (p > 0.05). The decline in VO2max was 17% ± 18 in exercising subjects (p < 0.05) and 31% ± 13 in control subjects (p = 0.003; difference between groups was not significant p = 0.26). Changes in blood and urine measures showed trends but no significant differences between groups (p > 0.05). In summary, the decline in a number of important measures of musculoskeletal and cardiovascular health was attenuated but not eliminated by a subject-specific program of locomotor exercise designed to replace daily load accumulated during free living. We conclude that single daily bouts of exposure to locomotor exercise can play a role in a countermeasures program during bed rest, and perhaps space flight, but are not sufficient in their own right to ensure musculoskeletal or cardiovascular health.
Keywords: Space flight, Bed rest, Exercise, Biomechanics, Simulation, Gravity
Highlights
-
•
Daily loads were quantified in subjects who were then randomly assigned to control or exercise groups.
-
•
Eleven subjects (5 exercise, 6 control) completed the protocol of 84-days head-down bedrest.
-
•
In 2 hip regions, bone loss was significant in controls but not in exercising subjects.
-
•
The exercise group retained a larger percentage ofquadriceps and gastrocnemius muscle volumes and VO2max than controls.
-
•
1x day locomotor exercise attenuates changes but does not maintain musculoskeletal or cardiovascular health during bedrest.
1. Introduction
The detrimental effects of spaceflight on the musculoskeletal system have been known for almost half a century (LeBlanc et al., 2007). Pharmacological countermeasures have been recently explored (Leblanc et al., 2013), but exercise has been the mainstay of both the U.S. and Russian countermeasures programs (Macias et al., 2005). Although questions about bone strength and integrity remain, recent findings suggest that heavy resistance exercise and good nutrition help maintain bone mineral density (Smith et al., 2012, Smith et al., 2014). Other evidence shows that exercise alone has not been completely successful in preventing bone and muscle loss during flight (Orwoll et al., 2013). An excellent analog for the study of on-orbit musculoskeletal changes that occur during long-duration spaceflight is head-down bed rest (Pavy-Le Traon et al., 2007). The purpose of the present study was to determine whether individualized mechanical load replacement through locomotor exercise can serve as a countermeasure to losses in bone mineral density, bone quality (including cortical and trabecular components), and muscle atrophy during 84 days of bed rest.
2. Materials and methods
2.1. Subjects
Twelve subjects were enrolled in an 84-day bed rest study that was approved by the Institutional Review Boards at NASA Johnson Space Center, the Cleveland Clinic, and the University of Washington. Six men and six women (76.5 ± 13.6 kg, 174.5 ± 7.2 cm, and 30.2 ± 6.8 years; and 66.9 ± 6.9 kg, 165.5 ± 7.3 cm, and 31.3 ± 9.9 years, respectively) provided written informed consent before they participated. There was no significant difference in weight, height, or age between treatment groups. One female subject was lost after 5 weeks of bed rest because of a pre-existing medical condition that had not been detected during screening.
Subjects were required to pass a rigorous screening protocol before they were considered for inclusion in the study. They were required to pass psychological and physical examinations. Subjects took both the clinical and validity sections of the Minnesota Multiphasic Personality Inventory-2 (MMPI-2) and completed an interview with the study psychiatrist. Subjects were medically cleared via a modified Air Force Class III physical examination, including screening for cardiovascular issues with a Bruce treadmill stress test. Subjects were required to have a whole-body bone mineral density (BMD) Z-score within 1 standard deviation of age-adjusted normal. Additional exclusion criteria included metabolic disease, a medical or orthopaedic condition, certain medications, pregnancy, gastroesophageal reflux disease, and a history of renal stones or thrombosis. Subjects were also tested for tuberculosis, hepatitis B, and HIV. Female subjects who were taking hormonal birth control or hormone replacement therapy or were perimenopausal or menopausal were excluded from participation. Subjects were nonsmokers or had not smoked or used tobacco products in 6 months, as confirmed by urine cotinine testing. Subjects were interviewed about their activity levels, and only mild to moderate nonhabitual exercisers were accepted into the study.
2.2. Procedures
2.2.1. Before bed rest
One to 2 months before the start of the bed rest phase of the study, subjects began participating in study activities. Subjects were asked about any changes in their physical activity and weight in the previous 12 months. They filled out activity logs for about 1 month, recording their daily activity in 30-minute increments on the activity log sheets. Four representative days were selected from each subject's activity logs for collection of ambulatory data recording (ADR) while the subject was still free-living in their normal environment. On an ADR day, the subject arrived at the laboratory immediately after waking and performing their morning routine. The subjects were outfitted with a Novel Load Monitor system (Novel GmbH, Munich, Germany), a non-encumbering long-duration data logger that measured the net vertical force during each foot contact under the right and left feet individually at 100 Hz. The unit was attached to a pair of rubber-sealed insoles, which were sized to the subject's shoes. The subject wore the system for all waking hours and performed shut-down and doffing procedures just before preparing for bed, resulting in 12.70 ± 1.31 h of data. This provided a comprehensive record of the subject's daily force profile. The subject returned to the clinic the next day for data download and system re-initialization. Four to five days of ADR data were collected for each subject. The collected foot forces were analyzed via a series of custom MATLAB (MathWorks, Natick, MA, USA) scripts using the enhanced daily load stimulus (EDLS) algorithm (Genc et al., 2009) to calculate the subject's total stimulus value for each day. These values were used in the calculation of the subject's exercise prescription (Genc et al., 2015).
Subjects underwent magnetic resonance imaging (MRI) and quantitative computerized tomography (QCT) scans so that their muscle volumes and bone mineral density could be assessed. Before the subject was scanned in the MRI, a pair of water-filled cylinders was scanned for calibration purposes. All scans were performed using a 1.5-tesla magnet, with a 3-mm slice thickness, no inter-slice gap, and a 512 × 512 acquisition matrix. T2-weighted and short TI inversion recovery (STIR) scans of the lumbar spine were taken from the endplate of T12 to the endplate of L3 using a surface acquisition coil. The remaining regions—thigh, shank and upper extremity—were scanned using T1-weighted spine echo sequence and STIR, and a body coil was used. The thigh was scanned from the superior aspect of the femoral head to the inferior border of the patella, and the shank was scanned from the superior border of the patella to the distal end aspect of the lateral malleolus. For the calculation of BMD, a series of scans using a Siemens Somatom Sensation 16 CT scanner (Siemens AG, Munich, Germany) was performed and analyzed using specialized Mindways bone densitometry computer software (Mindways Software, Inc., Austin, TX, USA). Before a subject was scanned, a rectangular calibration phantom was scanned longitudinally, with a quality assurance phantom placed perpendicularly at its longitudinal midpoint. The subject was then scanned while lying atop the rectangular calibration phantom. Volumetric scans of the lumbar spine and both hips were performed. The left hip was used for analysis. Both scans used a spiral acquisition technique with a peak kilovoltage of 120 kVp at 180 mA, and a 3-mm slice thickness.
Maximal isokinetic torque measurements were recorded for the hip, knee, ankle, and elbow using an isokinetic dynamometer (Biodex Medical Systems, Inc., Shirley, NY, USA). Subjects underwent a familiarization session to ascertain their correct dynamometer positions and ranges of motion, which were used for both pre- and post-bed rest testing sessions. During testing, each movement was performed five times with a 2-second pause at the end of each motion. Bilateral testing was performed. For the knee and elbow, peak concentric and eccentric torques were determined at a velocity of 60 °/s during extension and flexion. Peak concentric and eccentric torques at the hip during flexion and extension and abduction and adduction were also determined at 60 °/s. Peak ankle concentric and eccentric torques were determined during plantarflexion and dorsiflexion performed at 30 °/s. Isometric testing was performed for each joint at set angles of 0°, 45°, 45°, and 90° for the ankle, knee, hip, and elbow, respectively. Additionally, endurance was assessed while subjects performed 21 reciprocating, maximal knee extension and flexion contractions at 180 °/s. Changes in torque and work were evaluated.
Measures of postural stability were assessed while subjects stood quietly for 30 s on a force plate (AMTI, Watertown, MA, USA) mounted flush with the floor. Subjects stood barefoot, with their arms at their sides, heels placed 15 cm apart, feet each rotated externally 10°, and wore a fall safety harness attached to an overhead rail. Measures of postural stability were quantified by measuring total center-of-pressure excursion during the 30-second period. Subjects were tested under three experimental conditions: (1) eyes open, head upright; (2) eyes open, head back; and (3) eyes closed, head upright. For the eyes-open conditions, subjects fixed their gaze on a stationary target in the center of their field of vision. For the head-back condition, the head-neck angle was standardized to 45 degrees of extension. The conditions were ordered using a randomized balanced design. Each subject performed three 30-second trials per condition, which were averaged.
Aerobic capacity was assessed via cycle ergometry. Subjects performed a graded exercise test while cycling upright. First, peak heart rate and VO2 peak were determined using the standard NASA protocol in which the work rate was increased by 25 W every minute until peak was attained. The remaining cycle ergometer exercise tests, performed immediately before and immediately after bed rest, used a submaximal exercise protocol, as is used by NASA post-flight, to determine aerobic capacity. The same cycle ergometer, ECG, blood pressure and metabolic gas analysis devices/techniques were used for all VO2 tests.
Before entering bed rest, subjects had a baseline indirect calorimetry performed to determine resting energy expenditure (REE). While this test was being performed, subjects were in a relaxed, supine position without arms or legs being crossed. The subjects breathed into a mask covering their face to measure VO2. The REE was calculated from the measurement of oxygen uptake and used to determine the subjects' caloric intake needs for maintenance of their current body weight during bed rest.
All subjects were suspended in the Cleveland Clinic Zero-gravity Locomotion Simulator (ZLS, Fig. 1) to ensure that they were comfortable and no anxiety was caused by the system. Briefly, the ZLS simulates zero gravity by suspending the subject horizontally with the use of pulleys and a suspension system, and tethering the subject to the surface of the treadmill via a pneumatic Subject Load Device (pSLD) (Genc et al., 2006, McCrory et al., 2004).
Fig. 1.
The zero-gravity locomotion simulator (ZLS) in which subjects assigned to the exercise group walked and ran during the study. Subjects assigned to the control group were suspended in the ZLS for a similar period of time but did not exercise.
After all ADR collection was complete, subjects were randomly assigned by a biostatistician to the control or the exercise group, balanced for gender, with the exception that the first two male subjects were placed in the control group as the pSLD was still in development. Subjects assigned to the exercise group were fitted with a custom harness, which was worn in the ZLS to apply the load from the pSLD to the subject's waist and shoulders to provide the gravity replacement load and traction between the subject's feet and the treadmill. Before bed rest started, the exercise subjects participated in three ZLS exercise familiarization sessions. During these sessions, the subjects were exposed to conditions similar to those they would experience during their bed rest exercise sessions by practicing walking and running while their harness was loaded to 50%, 75%, and 100% of their body weight (BW). Subjects also wore the Novel Load Monitor system while in the ZLS and insoles to collect foot force data.
One week before bed rest started, subjects had their vitamin D levels measured, and vitamin D supplementation was prescribed if necessary. Female subjects also underwent another pregnancy test.
2.2.2. Bed rest
Subjects were confined to 6° head-down-tilt bed rest for a total of 84 days in the Cleveland Clinic Clinical Research Unit (CRU). Subjects were free to move in the plane of their bed surface but remained at head-down tilt for almost all activities. However, they were allowed to prop themselves up on one elbow while eating and they bathed horizontally in an inflatable bath up to five days per week. Subjects maintained a strict 16 h awake, 8 h sleeping schedule, with no napping allowed. Subjects were continuously monitored via infrared cameras 24 h per day. The cameras were positioned to record if the subject's head came > 12 in. off the surface of the bed, even while the privacy curtains were drawn.
Starting with lunch on bed rest day 1 (BR 1), subjects were fed a strict weight-maintaining metabolic diet similar to diets used in previous bed rest studies (Inniss et al., 2009). There was no dietary run-in period before the start of bed rest. These diets were based on the World Health Organization's recommendations and consisted of seven to ten menus of three meals of regular food and additional snacks during the day and evening. Subjects were expected to eat the entirety of the food provided. They were not allowed any alcoholic beverages, herbal drinks, caffeinated beverages, or other beverages not offered in the metabolic diet. Subjects were required to consume a minimum of 2000–3000 cm3 (67–100 oz) of water per day based on individual body weights. Diets were designed to maintain body weight (which was measured daily) within 3% of the subject's body weight on day 3 of bed rest.
All subjects were transported by head-down gurney to a laboratory located in the same medical complex and suspended in the ZLS five days per week. Control subjects were suspended for 20 min each day, allowing them to experience a horizontal exposure and time away from the bed rest facility similar to those of their exercising counterparts. Exercise subjects were assisted in the donning of their harness while maintaining a horizontal position on the gurney before being suspended in the ZLS. Subjects followed an individualized exercise prescription based on the EDLS value computed from their free-living foot force data. The goal was to replace their individual total daily load from free living in a single bout of treadmill exercise. The prescribed EDLS achieved 7 days of free-living loading in five sessions. Each exercise session included a warm-up of walking and running, a series of intervals that alternated up to 5 min of walking or running with an equal or greater duration of rest, and a cool-down of running followed by walking. For the first 2 weeks of the bed rest, the exercise prescriptions were ramped up from 50% BW loading (during the first exercise session which occurred on bed rest day 2) and increasing to 100% BW loading (by bed rest day 12) and achieving 100% of the prescribed EDLS. For the remaining 10 weeks of bed rest, the subjects continued to exercise at 100% BW loading following individualized protocols with increasing speeds based on their ability and preference, blending walking and running to achieve 100% of their individual daily prescribed EDLS. Subjects were instrumented with the Novel Load Monitor system and insoles for all exercise sessions. The resulting foot force data were used to confirm that daily EDLS was achieved and to inform the custom exercise prescription program when new speeds were attempted by the subject. Details of the individual prescriptions and further discussion of the approach are presented in Genc et al. (2015).
All subjects performed non-weightbearing stretch exercises in their rooms designed to minimize the risk of vascular pathology while maintaining the head-down position. These exercises included 4 to 10 repetitions of hip, gluteal, abdominal, leg, arm, low back, wrist, shoulder, neck, and ankle isometric and stretch movements, performed two times per day, and once-daily arm ergometry. Massage therapy (excluding the bottoms of the feet) and psychological occupational therapy were provided twice per week.
Blood and 24-hour pooled urine samples were collected at least once during the pre-bed rest period, weekly during bed rest, and before subject returning to vertical ambulation after 84 days of bed rest. No exercise was performed on the 2 days before sample collection. The weekly blood samples were analyzed for serum bone-specific alkaline phosphatase (BSAP), osteoprotegerin (OPG), RANKL, and sclerostin. Weekly urine samples were analyzed for creatinine, calcium (Ca, absolute and normalized to creatinine), N-telopeptide (NTx - absolute and normalized to creatinine), and urine stone profile for the early detection of increased risk of renal stone formation. Weekly samples were analyzed immediately after collection and also stored for batch analysis after the completion of data collection for all subjects. Daily urine samples were also frozen and archived for future use.
2.2.3. After bed rest
On bed rest day 83, subjects underwent the post-test MRI to assess end of bed rest muscle volumes. On the morning of bed rest day 84, the subjects were gradually returned to vertical while being monitored by a nurse and an exercise physiologist. Once subjects were ambulatory, they were transported via wheelchair to the laboratory for post-bed rest posture testing followed by the isokinetic testing of the ankle. On the second day after bed rest, the post-bed rest QCT, DXA, and isokinetic testing of the elbow and the knee, including fatigue testing, were performed. On the third day after bed rest, the follow-up submaximal cycle ergometry aerobic capacity test and isokinetic testing of the hip were performed. The subjects also began their rehabilitation program. The following day, subjects were discharged from the CRU. They continued to undergo mandatory daily rehabilitation sessions under the supervision of an exercise physiologist for 2 weeks and were offered the option of 6 additional weeks of access to the rehabilitation center and its staff to continue their recovery.
2.3. Analysis
The MRI data were analyzed following the same protocol set forth in Gopalakrishnan et al. (2010). In brief, the scans of the circular calibration phantoms were analyzed to determine the distortion factor present in each series of scans. The pre- and post-bed rest scans were aligned using the same anatomical reference frame, which in following Gopalakrishnan et al. (2010) was based on the endosteal canal of the long bones. The medial gastrocnemius and quadriceps were traced individually on a computer screen using custom MATLAB software (MathWorks Inc., Natick, MA, USA). The distortion factor previously determined was applied to the traced muscle contours. Three-dimensional muscle volumes were reconstructed and calculated using Rhinoceros (Robert McNeel & Associates, Seattle, WA, USA), and pre- and post-bed rest volumes were compared.
The QCT scans acquired from the subjects were analyzed using QCT PRO (Mindways Inc., Austin, TX, USA). Datasets from the first 4 subjects were analyzed by 4 observers, 9 times each on 3 different days to determine inter- and intra-observer reliability. The outcome of this analysis indicated that multiple readings from one observer are reliable and hence the data from the rest of the subjects were analyzed by one observer.
The average peak torque for strength measures was calculated by removing the lowest and highest values for each side and averaging the remaining measurements.
The weekly blood samples were batch analyzed at the end of the study by the NASA JSC Nutritional Biochemistry Lab (Smith et al., 2012, Smith et al., 2014) for BSAP (via enzymatic rate method using a Quidel BAP EIA Kit), OPG (using an Alpco Osteoprotegerin EIA Kit), RANKL (using a BioVendor Human sRANKL (total) Kit, Roche Modular D Chemistry Analyzer [Basel, Switzerland]), and for sclerostin (using an Alpco Sclerostin EIA Kit). The weekly urine samples were analyzed for Ca and NTx (by flame atomic absorption analysis and the Osteomark ELISA kit [Ostex Int., Seattle, WA, USA], respectively). An ACE Alera Clinical Chemistry Analyzer Reagent was used to analyze creatinine.
2.4. Statistical analysis
Statistical analyses were performed using R, an open-source software package for statistical computing and graphics (Free Software Foundation, Boston, MA, USA), and/or IBM SPSS Statistics (IBM Corp., Armonk, NY, USA).
The following hypotheses were tested for the pre-/post-bed rest measures (QCT, MRI, strength, posture, and aerobic capacity):
-
1.
For each subject the (post-pre) difference was calculated for each endpoint. A two-sample t-test on the differences was used to test for a statistically significant difference between the control and exercise treatment groups. Unequal variances were assumed. The same analysis was conducted using proportion change [(Post-Pre)/Pre] as an endpoint.
-
2.
A set of hypothesis tests were conducted to see if there was any evidence of decrements, on average, for each group. Tests were conducted for both paired differences and proportion change.
Blood and urine biomarkers were examined for the presence of outliers. Initial boxplots were examined for each endpoint using all the data and then by dividing the data into subsets based on treatment, gender, week, and subject. Since the analyses for treatment effect involved looking at endpoints by subject, it was decided that further outlier assessment would be based on data subsetted by subject. The Shapiro-Wilks test for normality of a given endpoint was used. If there was no evidence of non-normality, a boxplot of the data was generated to identify points outside of 1.5 × the interquartile range (IQR). If the data showed evidence of non-normality, then an adjusted boxplot to flag potential outliers was used (Vandervieren and Hubert, 2004, Hubert and Vandervieren, 2006). Because the variability of the week 1 data values was large, further outlier assessment was based on data from bed rest weeks 2–13.
Cook's distance and leverage values were examined for linear regressions by subject, using the weekly data values. A Cook's distance threshold value of D(i) = 4 / (n − p) = 4 / (12 − 2) = 0.4 was used to identify influential points.
Because there were four tests per set of BMD data (total hip, femoral neck, trochanter, inter-trochanteric), a Bonferroni-adjusted alpha level of 0.0125 was used for establishing significance.
Scatterplots of endpoint by week were created, and all points identified as outliers based on 1.5 IQR or as influential regression points were marked. Blood and urine samples identified as potential outliers were reanalyzed and the above analyses completed again before any points were labeled as outliers. Original batch analysis data were used for any data points still identified by boxplots or Cook's distance as potential outliers after reanalysis. No data points were eliminated from statistical analyses. Marker data were then analyzed using a mixed-effects linear model for repeated measures using a random intercept and random slope.
The study was initially powered to detect a difference in the integral BMD between control and exercise groups of 0.13% per month. This would have required a total sample size of 22 subjects (11 per group). The funding agency (NASA Human Research Program) requested an unplanned interim analysis at the study midpoint. Shortly afterward the study was defunded because of shifting program priorities. This paper reports the results from the eleven subjects who completed the protocol.
3. Results
A total of eleven subjects completed the bed rest protocol. The participation of one female exercise subject was discontinued at week 5 due to a pre-existing medical condition unrelated to the study that was not one of the screening exclusion criteria. The data from this subject were not included in the results reported below.
3.1. Activity
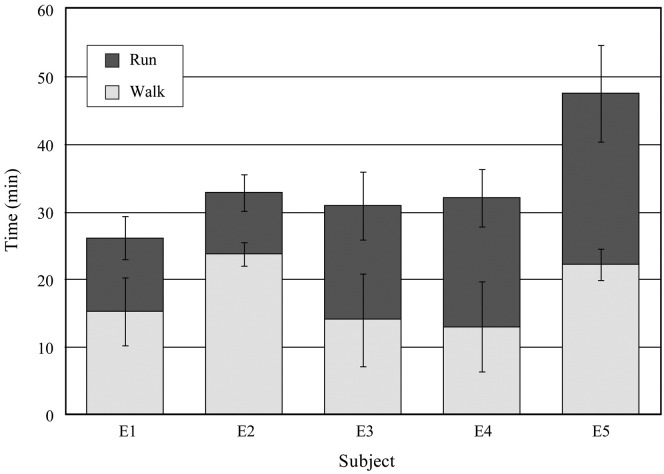
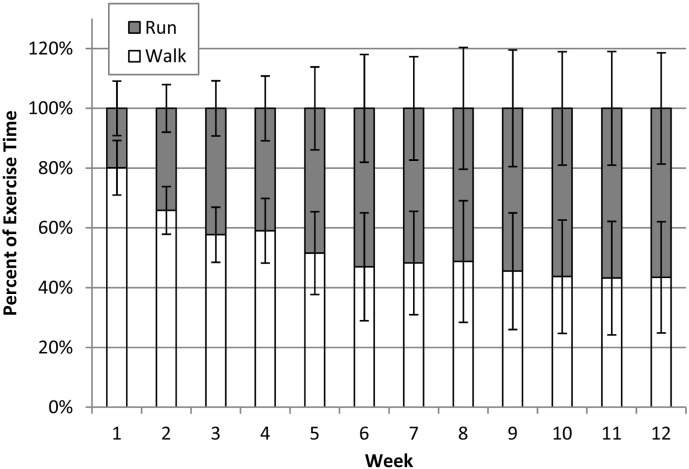
There was no significant difference in daily EDLS before bedrest between the control and exercise groups. The distribution of running and walking and the total time each was performed by the subjects in the exercise group are shown in Fig. 2. Because exercise was based on the subjects' measured habitual activity, the actual exercise required to replace the EDLS varied considerably. Average exercise durations were between 26.2 and 47.6 min per session and total distance covered over all sessions was between 93.9 and 182.7 miles. On average, subjects spent 53% of their time walking and 47% running, although, as shown in Fig. 3, this ratio changed during the course of the study.
Fig. 2.
Average and standard deviation of the time spent by each exercise group subject running and walking during the exercise sessions (5 per week).
Fig. 3.
Exercise group average and standard deviation of the time spent walking and running each week during bed rest.
3.2. Bone mineral density
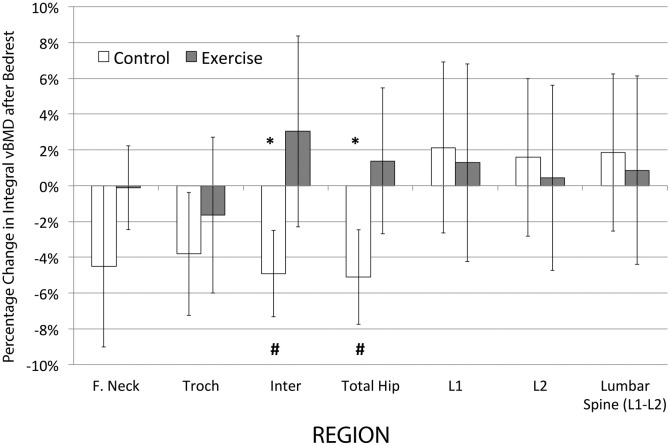
Pre- and post-bed rest changes in regional vBMD are shown in Table 1 and Fig. 4. At subject entry to the study, there were no significant differences between the control and exercise groups in any of the BMD regions. A comparison of the percentage change from before to after bed rest in regional integral bone mineral density indicated that the changes in the control group were in the intertrochanteric and total hip regions (p < 0.0125 with Bonferroni correction). However, in the exercise group, pre- to post-bed rest changes in all hip regions were not significantly different from zero (p > 0.0125). Comparison of the two groups showed that the changes in two of the four hip regions were significantly different between control and exercise groups, with more loss of BMD seen in the control group (p < 0.0125) for intertrochanteric and total hip regions. Comparisons in absolute units yielded similar results.
Table 1.
Changes in integral bone loss (vBMD [post-pre bed rest]) in regions of the hip and lumbar spine in control and exercise groups of subjects.
| Region | Control |
Exercise |
|||||
|---|---|---|---|---|---|---|---|
| Mean g/cm3 |
SD g/cm3 |
Delta/mo % |
Mean g/cm3 |
SD g/cm3 |
Delta/mo % |
||
| Hip | |||||||
| Integral | F Necka | –16.03 | 16.95 | − 1.46% | –0.20 | 8.29 | − 0.05% |
| Troch | –9.75 | 9.56 | − 1.32% | –4.02 | 10.39 | − 0.58% | |
| Inter | –20.05b, c | 11.28 | − 1.50% | 13.26b | 21.95 | 1.03% | |
| Total | –17.61b, c | 10.61 | − 1.64% | 4.88b | 13.32 | 0.48% | |
| Cortical | F Neck | –24.91 | 72.96 | − 0.63% | 22.81 | 37.11 | 1.19% |
| Troch | –10.50 | 52.33 | − 0.36% | 45.16 | 48.08 | 1.96% | |
| Inter | –15.59 | 21.44 | − 0.54% | 23.24 | 30.93 | 0.90% | |
| Total | –15.68 | 31.1 | − 0.51% | 29.02 | 32.71 | 1.21% | |
| Trabecular | F Neck | –6.59c | 4.85 | − 1.20% | –0.12 | 5.73 | − 0.17% |
| Troch | –4.90 | 5.68 | − 1.02% | –2.47 | 6.78 | − 0.63% | |
| Inter | –4.89 | 8.8 | − 0.88% | –0.32 | 13.4 | − 0.22% | |
| Total | –5.26 | 6.88 | − 1.01% | –1.20 | 9.24 | − 0.36% | |
| Spine | |||||||
| Integral | L1 | 3.8 | 8.06 | 0.65% | 2.84 | 10.14 | 0.42% |
| L2 | 2.85 | 6.82 | 0.57% | 1.19 | 9.45 | 0.10% | |
| L1 + L2 | 3.33 | 7.04 | 0.65% | 2.01 | 9.61 | 0.30% | |
F. Neck, femoral neck; Troch, trochanter; Inter, intertrochanteric region.
Significant difference between groups p < 0.0125.
Significant difference from baseline p < 0.0125.
Fig. 4.
Percentage change in integral bone loss (vBMD [post-pre bed rest]) in regions of the hip and lumbar spine in control and exercise subjects.
Significant difference between groups *p < 0.0125. Significant difference from baseline #p < 0.0125.
F. Neck, femoral neck; Troch, trochanter; Inter, intertrochanteric region.
When compared to no difference from before bed rest, there were no significant changes in the exercising subjects, whereas the control subjects lost a significant amount of trabecular vBMD in the femoral neck (p < 0.0125) and a significant amount of integral vBMD in the inter-trochanteric and total hip regions (p < 0.0125). No significant changes occurred in the lumbar spine vBMD for either control or exercise subjects in all compartments.
3.3. Muscle volumes
Before bed rest there were no significant differences in gastrocnemius and quadriceps muscle volume between the groups. Both groups lost muscle volume during bed rest, and the pre- to post-bed rest changes were significantly different from zero in both groups (p < 0.01 in control subjects for both muscle groups; in exercise subjects p < 0.01 and p < 0.05 for the gastrocnemius and total quadriceps, respectively). Exercise subjects lost 13.8% ± 6.1 and 7.2% ± 5.9 in the gastrocnemius and total quadriceps, respectively, while control subjects lost 33.0% ± 8.2 and 23.3% ± 5.9 in the gastrocnemius and total quadriceps, respectively. Muscle volume loss was significantly reduced by exercise (p < 0.01 for the difference between groups in absolute change in volume for both muscles; p < 0.05 and p < 0.01 for the difference between groups in percentage loss of volume for gastrocnemius and total quadriceps, respectively).
3.4. Muscle strength
There were no significant differences between control and exercise groups in isokinetic strength at the knee, hip, elbow, or ankle prior to bed rest (p > 0.05). Table 2 reports the absolute and percentage changes in recorded strength (in Newton meters) from before and after bed rest for both groups. Both groups lost strength (p < 0.05) in several regions tested (10 of 22 tests for exercise subjects and 12 of 22 tests for control subjects). However, the losses were significantly greater in control subjects than in exercise subjects for the following activities: concentric knee extension, isometric knee flexion, and isometric plantar flexion and eccentric dorsiflexion of the ankle (p < 0.05). Comparisons in absolute and percentage change units yielded similar results. Although not significant, trends for a favorable exercise effect were observed in all other knee movements measured.
Table 2.
Absolute and percentage changes in muscle strength with bed rest in control and exercise groups of subjectsa.
| Joint | Motion | Action | Absolute (nM) |
Percentage change (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control |
Exercise |
Control |
Exercise |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Knee | EXT | CON | − 38.08 | 12.77 | − 20.17 | 12.77 | − 33.90 | 5.36 | − 19.68 | 9.74 |
| ISOM | − 33.61 | 18.39 | − 18.38 | 11.10 | − 26.95 | 8.27 | − 19.51 | 8.73 | ||
| FLX | CON | − 15.87 | 8.87 | − 8.26 | 4.77 | − 27.71 | 10.44 | − 17.78 | 10.43 | |
| ISOM | − 18.18 | 5.26 | − 9.30 | 5.78 | − 27.92 | 4.64 | − 17.62 | 8.37 | ||
| Hip | EXT | CON | − 12.31 | 12.10 | − 5.15 | 3.27 | − 18.07 | 13.75 | − 8.06 | 5.06 |
| ISOM | − 6.56 | 10.57 | − 5.62 | 11.68 | − 6.08 | 18.06 | − 8.67 | 12.65 | ||
| FLX | CON | − 0.54 | 8.55 | − 7.64 | 4.56 | 2.88 | 26.30 | − 16.93 | 13.43 | |
| ISOM | − 4.95 | 5.12 | − 6.87 | 8.01 | − 9.60 | 10.57 | − 16.84 | 20.15 | ||
| ABD | CON | − 5.07 | 9.49 | − 4.46 | 11.95 | − 7.88 | 20.33 | − 6.71 | 26.42 | |
| ISOM | − 5.60 | 13.44 | − 9.54 | 7.14 | − 8.03 | 22.70 | − 17.94 | 14.03 | ||
| ADD | CON | − 6.18 | 10.10 | − 3.45 | 8.94 | − 11.11 | 17.07 | − 9.28 | 14.79 | |
| ISOM | − 6.80 | 6.57 | − 0.71 | 5.73 | − 9.52 | 9.96 | − 1.85 | 10.37 | ||
| Elbow | EXT | CON | 0.09 | 4.52 | − 4.84 | 4.96 | − 0.47 | 15.83 | − 4.72 | 13.73 |
| ISOM | − 1.61 | 3.00 | − 3.58 | 3.88 | − 3.55 | 10.64 | − 11.53 | 13.65 | ||
| FLX | CON | − 7.86 | 5.66 | − 5.06 | 0.43 | − 6.74 | 17.64 | − 19.31 | 28.78 | |
| ISOM | − 4.36 | 5.72 | − 7.86 | 5.66 | − 13.08 | 13.12 | − 26.29 | 18.19 | ||
| Ankle | PLANT | CON | − 33.24 | 32.52 | − 6.86 | 5.31 | − 43.87 | 16.08 | − 15.55 | 13.25 |
| ECC | − 5.47 | 6.05 | 1.43 | 6.35 | − 24.00 | 22.58 | 1.51 | 21.99 | ||
| ISOM | − 32.47 | 21.36 | − 6.31 | 11.82 | − 42.32 | 16.91 | − 9.10 | 23.22 | ||
| DORSI | CON | − 5.07 | 8.80 | − 4.83 | 9.20 | − 24.31 | 28.93 | − 16.86 | 31.47 | |
| ECC | − 83.63 | 64.68 | − 14.94 | 16.55 | − 60.41 | 16.89 | − 20.98 | 14.80 | ||
| ISOM | − 2.33 | 2.69 | − 1.39 | 3.90 | − 13.31 | 21.34 | − 12.32 | 27.34 | ||
Gray shading indicates significant difference between control and exercise groups (p < 0.05).
3.5. Knee fatigue
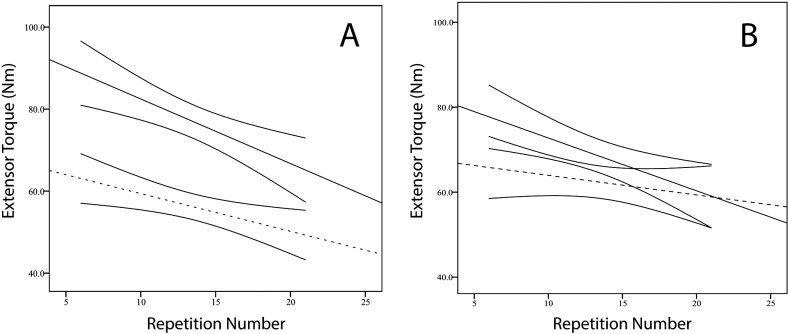
There was no difference in the rate of fatigue (slope) for flexion or extension between the control and exercise groups before bed rest. However, after bed rest the mean slopes for both flexion and extension fatigue in the control group were significantly more negative than the corresponding mean slopes for the exercise group (p < 0.05). Compared to the exercise group, the post-bed rest declines in the control group were 0.26 Nm and 0.20 Nm per contraction greater for knee extension (Fig. 5) and flexion, respectively. There were no significant differences between groups for the slopes of work as a function of repetition, but trends in both flexion and extension indicated a favorable exercise effect.
Fig. 5.
Change in maximal knee extensor torque (nM) during repeated contractions for assessing endurance. A). Average of control subjects before (solid, straight line) and after bed rest (dashed, straight line). Regression coefficients: − 1.574 and − 0.919 Nm/repetition, respectively. B). Exercise subjects before (solid, straight line) and after bed rest (dashed, straight line). Regression coefficients: − 1.243 and − 0.662 Nm/repetition, respectively. The pre-bed rest slopes were not different between groups, but the control subjects showed significantly greater decline than the exercise group after bed rest. The solid, curved pairs of lines indicate 95% confidence intervals.
3.6. Posture
There were no significant differences between groups for any of the posture measures before bed rest. A nonsignificant trend was observed for exercise subjects to exhibit smaller changes than control subjects in their pre- to post-bed rest posture testing, though all subjects experienced an increase in the total excursion of their center of pressure. The exercise subjects changed by 52% ± 61, 74% ± 53, and 80% ± 53, respectively for eyes open, eyes closed, and head back eyes open, while the similar values for the control subjects were 151% ± 181, 164% ± 274, and 208% ± 396.
3.7. Aerobic capacity
Maximal oxygen consumption (VO2max) was measured during cycle ergometry before and after bed rest. The prescribed exercise regime attenuated, but did not prevent, decrements in aerobic capacity with bed rest. Exercise subjects' VO2max decreased by − 17% ± 18 (p = 0.05) whereas control subjects decreased VO2max by − 31% ± 13 (p = 0.003). The difference between groups was not significant (p = 0.26).
3.8. Bone biochemical markers
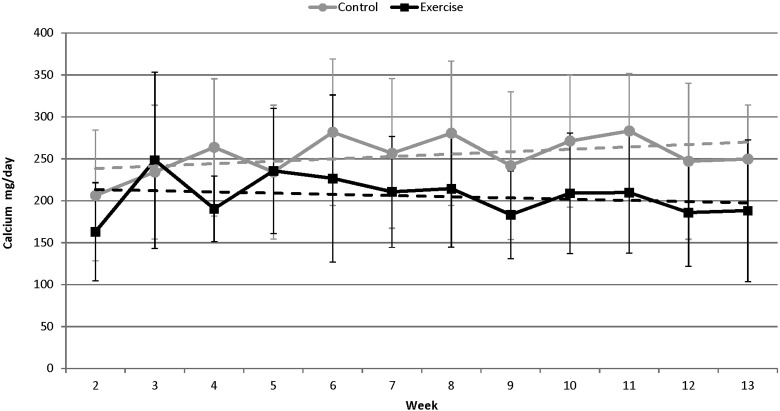
When the slopes of the control and exercise subjects' individual data sets were used as derived variables, the data indicated that exercise subjects showed a decrease in Ca excretion (mg/day), whereas control subjects increased Ca excretion (Fig. 6). However, the difference between the means of the slopes for the two groups was not statistically significant (p > 0.05; Table 3).
Fig. 6.
Changes in calcium excretion (mg/day, solid lines) over the course of an 84-day bed rest campaign. The slopes (dashed lines) of the control and exercise subjects' individual data sets were used as derived variables and showed that control subjects (gray) had an average increase in Ca excretion (slope = 2.89 ± 10.01) while exercise subjects (black) had a decrease in Ca excretion (slope = − 1.44 ± 3.59) over the course of the study. The slopes are not significantly different from each other (p > 0.05).
Table 3.
Average slopes indicating change in bone biochemical analytes during bed rest.
| Analyte | Control |
Exercise |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Creatinine (mg/day) | − 9.87 | 32.42 | − 3.35 | 23.44 |
| Calcium (mg/day) | 2.89 | 10.01 | − 1.44 | 3.59 |
| NTx (nmol/mmolCr) | 0.97 | 0.46 | 0.58 | 0.31 |
| BSAP (U/L) | 0.43 | 0.53 | 0.41 | 0.59 |
| RANKL (pmol/L) | 2.16 | 1.12 | − 0.14 | 5.40 |
| OPG (pmol/L) | 0.03 | 0.05 | 0.01 | 0.03 |
| Sclerostin (pmol/L) | 1.61 | 2.68 | 0.72 | 0.86 |
Both groups showed an increase in NTx (nmol/mmol Cr) with slopes significantly different from zero (p < 0.01 and p < 0.05 for control and exercise subjects, respectively), and control subjects exhibiting (not significantly) greater changes in NTx levels than exercise subjects.
Both groups showed an increase in the bone formation marker BSAP; however, the differences between the means of the slopes of the two groups were not significant (Table 3). Exercise subjects showed a decrease in the bone resorption marker RANKL while control subjects showed an increase in RANKL. The differences between groups were not significant, but the change in RANKL levels for control subjects was significantly different from baseline (p < 0.01). Both groups showed no change in OPG but sclerostin, an inhibitor of bone formation, increased. Control subjects had larger increases in sclerostin than exercise subjects, but the differences were not significant.
4. Discussion
The key finding of this study is that individually prescribed locomotor exercise was effective in preventing some important changes in the musculoskeletal system as a result of 84 days of bed rest but that changes to a number of other critical components of the musculoskeletal and cardiovascular systems were not adequately attenuated by locomotor exercise alone. This suggests that other forms of exercise, such as resistance training, and/or pharmacological intervention will be necessary to assure skeletal health during long-duration space missions.
The prescribed exercise program prevented significant bone loss in two out of four regions studied, judging by the findings that no significant changes occurred in the hip QCT-derived measures in exercise subjects after bed rest compared to baseline, and that the exercise and control subjects were significantly different from each other. The amount of loss in the control subjects was similar to that observed after spaceflight by Lang et al. (2004) in the integral and cortical components (integral: 1.46% and 1.32% loss per month in this study in the femoral neck and trochanter respectively versus 1.2% and 1.5% loss per month after space travel; cortical: 0.63% and 0.36% loss per month in the femoral neck and trochanter respectively in this study versus 0.3% and 0.45% loss per month after space travel). Trabecular bone loss was, however, considerably greater in space than in our bed rest subjects (1.2% and 1.0% loss per month in the current study versus 2.7% and 2.2% loss per month after space travel in the femoral neck and trochanter respectively) (Lang et al., 2004). The effect of our exercise program was, on average, to eliminate the cortical loss and to attenuate the trabecular loss. Such a pattern would not be optimal for use during space flight where the trabecular component loss is the largest (Lang et al., 2004).
Our findings demonstrate greater mitigation of loss of total hip vBMD than in previous long-duration bed rest studies with exercise interventions (Smith et al., 2008, Shackelford et al., 2004). While this may be a result of different investigators using different measures (Orwoll et al., 2013), it is also possible that our novel, individualized exercise program was responsible.
It is notable that there was no significant change in either group in vBMD in the lumbar spine over the course of the 84-day bed rest period. This is in contrast to changes observed during long duration space flight (Lang et al., 2004). This may point to a limitation of the bed rest model in which subjects are continually exerting moments about the spinal joints as they change position in bed during the day. Subjects could be said to be performing “mini sit-ups” all day long while they move their torso in bed. This effect was likely dominant over any exercise-related benefit.
In the WISE 2005 bed rest study, Smith et al. (2008) were able to significantly attenuate loss of hip and leg BMD in exercisers versus controls, but both groups lost BMD significantly compared to baseline (~ 1% loss per month compared to a gain of 0.5% per month in the present study) (Smith et al., 2008). These investigators also used two exercise modalities, treadmill running in lower body negative pressure (LBNP) and flywheel resistance exercise, and so the potentially separate effects on bone could not be identified. Total hip BMD was increased in the subjects in the current study (0.5% per month gain) in contrast to the 0.23% loss per month reported by Shackelford et al. (2004) in a 119-day bed rest study in subjects who performed heavy resistance training (Shackelford et al., 2004). Loss in the greater trochanter was, however, similar in the two studies. In both of the previous bed rest studies mentioned above, DXA was used for the assessment of BMD whereas CT was used in the present study.
In contrast to the observed preservation of some bone parameters, muscle volume, strength, and endurance were not, in general, preserved during bed rest by the exercise intervention. The response of skeletal muscle to the locomotor countermeasure was, in general, inferior to previous resistance exercise regimens.
For example, the exercise group lost about 20% of their knee extensor strength over the 84-day bed rest period (− 0.23% per day) despite their exposure to locomotor exercise, compared to a 33% loss in the control group (− 0.4% per day). In other bed rest studies in which resistance exercise was used [19-22] subjects maintained or increased their knee extensor strength. The cross-sectional area of the quadriceps muscles decreased in our study by 23.3% (− 0.28% per day) and 7.20% (− 0.09% per day) in the control and exercise groups, respectively. However, bed rest subjects exposed to resistance exercise exhibited no significant changes in quadriceps cross-sectional area [18E-20, (Ploutz-Snyder et al., 2014).
Our control subjects lost 43.9% of their concentric plantar flexion strength (− 0.52% per day) while exercise subjects lost 15.5% (− 0.19% per day). This indicates that locomotor exercise was relatively better at preventing calf muscle decrements than preventing losses in quadriceps (the relative loss in peak concentric strength [control delta / exercise delta] was 1.7 for the quadriceps but 2.8 for the calf). With the exception of the study by Lee et al. in which exercising subjects lost 4.27% (− 0.07% per day) (Lee et al., 2014), results from other bed rest studies noted increases in plantar flexion strength with resistance exercise regimens (+ 28.90%, + 0.48% per day (Trappe et al., 2007); and + 9.36%, + 0.10% per day (Alkner and Tesch, 2004)) after bed rest.
Decreases in cross-sectional area in the calf muscles in our study (33.0% [− 0.39% per day] and 13.8% [− 0.16% per day] for control and exercise subjects, respectively) were also greater than those found in the quadriceps (23.3% and 7.20% in the control and exercise subjects, respectively, as described above). Exercising subjects in previous studies using resistance exercise also exhibited reduced calf muscle volumes (− 8% [− 0.14% per day] (Trappe et al., 2007); − 6.60% [− 0.06% per day] (Shackelford et al., 2004)) after bed rest. Losses in hip and elbow actions in the current study were, in general, less than those seen in knee extension and plantar flexion.
Trappe et al. (2009) reported changes in calf muscle structure and function after 6-month missions to the International Space Station (ISS) (Trappe et al., 2009). They found decrements in isokinetic calf strength of 0.18% per day and changes in calf muscle volume of − 0.06% per day. The crewmembers in Trappe et al.'s study performed regular treadmill exercise and low-intensity resistive exercise using the Interim Resistance Exercise Device (iRED) on the ISS. The changes observed have values quite similar to our exercising subjects despite the fact that the loading during running on the ISS was almost certainly lower (Gopalakrishnan et al., 2010) than the full body weight loading experienced by our subjects. Our own data from on-orbit measurements in crewmembers with exercise profiles similar to those reported by Trappe et al. showed changes in concentric muscle contraction torques of − 0.05% and 0.11% per day in the knee extensors and ankle plantar flexors, respectively (Gopalakrishnan et al., 2010). The accompanying changes in muscle volumes were − 0.03% per day, − 0.1% per day, and − 0.06% per day in the quadriceps, soleus, and gastrocnemius muscles, respectively. Thus the exercising subjects in the present study exhibited considerably larger losses of strength and volume in both the quadriceps and calf muscle groups than did exercising crewmembers on orbit.
A comparison of the decrements during repeated isokinetic knee flexion and extension contractions between the control and exercise groups showed that the exercise regimen offered attenuation—but not protection—from decline in peak joint torques but not in work done.
Previous bed rest studies have shown that exercise increases markers of bone formation and decreases markers of bone resorption (Smith et al., 2008, Shackelford et al., 2004). The trends for differences between exercise and control groups observed in Ca excretion, NTx, RANKL, and sclerostin were all in the expected directions, with exercise tending to reduce excretion of all of these analytes. Other comparisons (such as BSAP and OPG) showed no trends. However, none of the trends were significant between exercise and control groups. The primary reason for this finding was likely the low power of the experiment to detect differences. The inclusion of male and female subjects likely also affected the statistical power. In a previous study of bed-rested identical male twins, with one twin performing horizontal treadmill exercise while in a lower body negative pressure chamber (Smith et al., 2003), significant effects of the exercise on bone resorption were found. The identical study, with female twin pairs, found similar trends, but did not achieve statistical significance (Zwart et al., 2007). Furthermore, the lack of a pre-bed rest dietary “run in” period probably also contributed to the lack of statistically significant differences between groups, since the subjects were not eating standardized diets until day 1 of bed rest. For this reason, the comparison of slopes started at week 2 of bed rest, further attenuating any trends that may have been present. Variability in testing procedure should be minimal since the samples were batch analyzed by a single lab after completion of the study.
Both groups increased their postural sway after bed rest. There was, however, a large between-subject variability in the postural response to bed rest, particularly among the control subjects (as indicated by the large variances under all conditions tested). However, a nonsignificant trend for less oscillation of the center of pressure was noted in the exercise subjects, in whom the mean changes were always < 100%, whereas the mean changes in control subjects were between 151% and 208% in the three sensory conditions. Despite this trend, it is clear that exercise did not protect against deleterious changes in postural stability.
The primary limitation of this study was a lack of statistical power to determine changes in measures that have substantial inherent variability. In resource-intensive experiments, such as bed rest studies, the ability to complete a planned series of experiments with an adequate number of subjects is sometimes outside the control of the investigator, and such was the case in the present study. The lack of a dietary run-in period (mentioned above) was also a significant limitation, particularly for the blood chemistry data. The accuracy of estimation of the daily load stimulus, which enabled us to expose subjects to a loading regimen that represented a typical personalized load, depended on two assumptions: (1) that the subjects' activity on the four ambulatory monitoring days was typical of their normal activity profile, and (2) that the exponent used in the EDLS calculation was appropriate. While the former limitation could be overcome by more days of pre-bed rest monitoring, more work is needed to validate the choice of an exponent in the EDLS formula (Genc et al., 2015). Post-bed rest data were collected over a 3-day period and thus all responses do not represent the true acute response to reambulation. It should be recalled that the subjects in the study were fairly sedentary. Future studies should explore the response in more active subjects who are typical of the astronaut population. The exercise durations required for such subjects will be much longer than used here, because loads from an entire day of daily living are replaced in a single bolus exercise dose. Although this is the typical situation on orbit, distributed exercise periods should be examined since they have been shown in animal studies to enhance bone formation (Srinivasan et al., 2015). Future investigation may also benefit from musculoskeletal models that incorporate recent findings showing heterogeneity of skeletal responses depending upon the different bone loading (Lang et al., 2014).
5. Conclusions
This experiment has demonstrated that individually prescribed, fully loaded, locomotor exercise based on the daily load stimulus prevents loss of cortical bone in two key regions of the hip during 84 days of bed rest. However, it attenuates, but does not prevent, degradation of muscle force production and loss of muscle volume in the quadriceps and calf muscles. Declines in postural stability and maximal exercise capacity were also not prevented by the exercise program. If bed rest is an adequate simulation of long-duration space flight, these results suggest that single daily bouts of exposure to locomotor exercise can play a role in a countermeasures program during space flight but they are not sufficient in their own right to ensure musculoskeletal or cardiovascular health.
6. Acknowledgments
This work was supported by funding from the National Aeronautics and Space Administration (NASA) through grants NNJ05HE72G and NNX08BA58G, the National Space Biomedical Research Institute through NASA NCC 9-58, and in part by the National Institutes of Health, National Center for Research Resources, CTSA1UL1RR024989. The authors would like to acknowledge the contributions of R. Gopalakrishnan, M. Kuklis, B.L. Davis, A.M. Hanson, D.R. Carter, D. Muzina, G. Blackburn, and the staffs of the Cleveland Clinic CRU and the NASA JSC Nutritional Biochemistry Lab. The authors thank J. Krauhs for editorial review.
Authors' roles: Study design: PRC, AJR, and AAL. Study conduct and data collection: PRC, AJR, SCN, KOG, RKE, TMO, HI, and AAL. Data analysis and interpretation: PRC, AJR, SCN, KOG, TMO, BC, TC, HI, SMS, and AAL. Drafting manuscript: PRC, AJR, and SCN. Revising manuscript content and approval of final version: all authors. PRC takes responsibility for the integrity of the data analysis.
References
- Alkner B.A., Tesch P.A. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur. J. Appl. Physiol. 2004;93(3):294–305. doi: 10.1007/s00421-004-1172-8. [DOI] [PubMed] [Google Scholar]
- Genc K.O., Mandes V.E., Cavanagh P.R. Gravity replacement during running in simulated microgravity. Aviat. Space Environ. Med. 2006;77(11):1117–1124. [PubMed] [Google Scholar]
- Genc K.O., Humphreys B.T., Cavanagh P.R. Enhanced daily load stimulus to bone in spaceflight and on earth. Aviat. Space Environ. Med. 2009;80(11):919–926. doi: 10.3357/asem.2380.2009. [DOI] [PubMed] [Google Scholar]
- Genc K., Humphreys B., Rice A., Novotny S., Englehaupt R., Gopalakrishnan R. Validating the enhanced daily load stimulus model using the bedrest analog of spaceflight. J. Biomech. 2015 [Google Scholar]
- Gopalakrishnan R., Genc K.O., Rice A.J., Lee S.M.C., Evans H.J., Maender C.C. Muscle volume, strength, endurance, and exercise loads during 6-month missions in space. Aviat. Space Environ. Med. 2010;81(2):91–102. doi: 10.3357/asem.2583.2010. [DOI] [PubMed] [Google Scholar]
- Hubert M., Vandervieren E. Technical Report TR-06-11. KU Leuven, Section of Statistics; Leuven: 2006. An adjusted boxplot for skewed distributions. [Google Scholar]
- Inniss A.M., Rice B.L., Smith S.M. Dietary support of long-duration head-down bed rest. Aviat. Space Environ. Med. 2009;80(Suppl. 5):A9–14. doi: 10.3357/asem.br04.2009. [DOI] [PubMed] [Google Scholar]
- Lang T.F., LeBlanc A.D., Evans H.J., Lu Y., Genant H., Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J. Bone Miner. Res. 2004;19(6):1006–1012. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- Lang T.F., Saeed I.H., Streeper T., Carballido-Gamio J., Harnish R.J., Frassetto L.A. Spatial heterogeneity in the response of the proximal femur to two lower-body resistance exercise regimens. J. Bone Miner. Res. 2014;29(6):1337–1345. doi: 10.1002/jbmr.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc A.D., Spector E.R., Evans H.J., Sibonga J.D. Skeletal responses to space flight and the bed rest analog: a review. J. Musculoskelet. Neuronal Interact. 2007;7(1):33–47. [PubMed] [Google Scholar]
- Leblanc A., Matsumoto T., Jones J., Shapiro J., Lang T., Shackelford L. Bisphosphonates as a supplement to exercise to protect bone during long-duration spaceflight. Osteoporos. Int. 2013;24(7):2105–2114. doi: 10.1007/s00198-012-2243-z. [DOI] [PubMed] [Google Scholar]
- Lee S.M.C., Schneider S.M., Feiveson A.H., Macias B.R., Smith S.M., Watenpaugh D.E. WISE-2005: countermeasures to prevent muscle deconditioning during bed rest in women. J. Appl. Physiol. 2014;116(6):654–667. doi: 10.1152/japplphysiol.00590.2013. [DOI] [PubMed] [Google Scholar]
- Macias B.R., Groppo E.R., Eastlack R.K., Watenpaugh D.E., Lee S.M.C., Schneider S.M. Space exercise and earth benefits. Curr. Pharm. Biotechnol. 2005;6(4):305–317. doi: 10.2174/1389201054553653. [DOI] [PubMed] [Google Scholar]
- McCrory J.L., Derr J., Cavanagh P.R. Locomotion in simulated zero gravity: ground reaction forces. Aviat. Space Environ. Med. 2004;75(3):203–210. [PubMed] [Google Scholar]
- Orwoll E.S., Adler R.A., Amin S., Binkley N., Lewiecki E.M., Petak S.M. Skeletal health in long-duration astronauts: nature, assessment, and management recommendations from the NASA bone summit. J. Bone Miner. Res. 2013;28(6):1243–1255. doi: 10.1002/jbmr.1948. [DOI] [PubMed] [Google Scholar]
- Pavy-Le Traon A., Heer M., Narici M.V., Rittweger J., Vernikos J. From space to earth: advances in human physiology from 20 years of bed rest studies (1986–2006) Eur. J. Appl. Physiol. 2007;101(2):143–194. doi: 10.1007/s00421-007-0474-z. [DOI] [PubMed] [Google Scholar]
- Ploutz-Snyder L.L., Downs M., Ryder J., Hackney K., Scott J., Buxton R. Integrated resistance and aerobic exercise protects fitness during bed rest. Med. Sci. Sports Exerc. 2014;46(2):358–368. doi: 10.1249/MSS.0b013e3182a62f85. [DOI] [PubMed] [Google Scholar]
- Shackelford L.C., LeBlanc A.D., Driscoll T.B., Evans H.J., Rianon N.J., Smith S.M. Resistance exercise as a countermeasure to disuse-induced bone loss. J. Appl. Physiol. 2004;97(1):119–129. doi: 10.1152/japplphysiol.00741.2003. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Davis-Street J.E., Fesperman J.V., Calkins D.S., Bawa M., Macias B.R. Evaluation of treadmill exercise in a lower body negative pressure chamber as a countermeasure for weightlessness-induced bone loss: a bed rest study with identical twins. J. Bone Miner. Res. 2003;18(12):2223–2230. doi: 10.1359/jbmr.2003.18.12.2223. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Zwart S.R., Heer M., Lee S.M.C., Baecker N., Meuche S. WISE-2005: supine treadmill exercise within lower body negative pressure and flywheel resistive exercise as a countermeasure to bed rest-induced bone loss in women during 60-day simulated microgravity. Bone. 2008;42(3):572–581. doi: 10.1016/j.bone.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Heer M.A., Shackelford L.C., Sibonga J.D., Ploutz-Snyder L., Zwart S.R. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J. Bone Miner. Res. 2012;27(9):1896–1906. doi: 10.1002/jbmr.1647. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Zwart S.R., Heer M., Hudson E.K., Shackelford L., Morgan J.L. Men and women in space: bone loss and kidney stone risk after long-duration spaceflight. J. Bone Miner. Res. 2014;29(7):1639–1645. doi: 10.1002/jbmr.2185. [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Ausk B.J., Bain S.D., Gardiner E.M., Kwon R.Y., Gross T.S. Rest intervals reduce the number of loading bouts required to enhance bone formation. Med. Sci. Sports Exerc. 2015;47(5):1095–1103. doi: 10.1249/MSS.0000000000000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe T.A., Burd N.A., Louis E.S., Lee G.A., Trappe S.W. Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol (Oxford) 2007;191(2):147–159. doi: 10.1111/j.1748-1716.2007.01728.x. [DOI] [PubMed] [Google Scholar]
- Trappe S., Costill D., Gallagher P., Creer A., Peters J.R., Evans H. Exercise in space: human skeletal muscle after 6 months aboard the International Space Station. J. Appl. Physiol. 2009;106(4):1159–1168. doi: 10.1152/japplphysiol.91578.2008. [DOI] [PubMed] [Google Scholar]
- Vandervieren E., Hubert M. An adjusted boxplot for skewed distributions. In: Antoch J., editor. Proceedings in Computational Statistics 2004. Springer-Verlag; Heidelberg: 2004. pp. 1933–1940. [Google Scholar]
- Zwart S.R., Hargens A.R., Lee S.M.C., Macias B.R., Watenpaugh D.E., Tse K. Lower body negative pressure treadmill exercise as a countermeasure for bed rest-induced bone loss in female identical twins. Bone. 2007;40(2):529–537. doi: 10.1016/j.bone.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]