Abstract
Cardiovascular disease continues to be the leading cause of death in industrialised societies. The idea that the arterial smooth muscle cell (ASMC) plays a key role in regulating many vascular pathologies has been gaining importance, as has the realisation that not enough is known about the pathological cellular mechanisms regulating ASMC function in vascular remodelling. In the past decade endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) have been recognised as a stress response underlying many physiological and pathological processes in various vascular cell types. Here we summarise what is known about how ER stress signalling regulates phenotypic switching, trans/dedifferentiation and apoptosis of ASMCs and contributes to atherosclerosis, hypertension, aneurysms and vascular calcification.
Keywords: ER stress, vascular smooth muscle cell, vascular calcification, atherosclerosis
1. INTRODUCTION
Endoplasmic reticulum (ER) stress is a cellular stress response, which is activated when the demands for protein folding exceed the capacity of the ER. It leads to activation of signalling pathways called the unfolded protein response (UPR). ER stress has been shown to be essential for many physiological processes involving professional secretory cells [1]. Differentiation of B cells into plasma cells [2], insulin secretion by pancreatic β-cells [3] and maturation of osteoblasts during bone formation [4, 5] are associated with increases in protein folding demand that induce ER stress and activate the UPR. However, pathological ER stress has also been implicated in many disease processes such as viral infections [6] and cardiovascular disease.
According to the World Health Organisation reports, more people die annually from cardiovascular disease than from any other cause, with an estimated 17.5 million deaths in 2012. Of these deaths, an estimated 7.4 million were due to coronary heart disease and 6.7 million were due to stroke [7]. These causes of death share common risk factors and underlying pathology – diseases of the vasculature. Hypertension alone is estimated to affect 25% of the adult population in industrialized societies and it is a major risk factor for stroke, myocardial infarction, congestive heart failure, and end stage renal disease [8, 9]. Vascular calcification, or mineralisation of the blood vessel wall, poses an increased risk of cardiovascular and all-cause mortality in asymptomatic adults and prevalence of coronary artery calcification (CAC) corresponds to age. Recent statistics show that among patients between 45 and 75 years of age an estimated 32% of women and 53% of men have coronary artery calcification [9]. CAC is also a measure of the burden of atherosclerosis in the coronary arteries. These statistics stress the importance of studying novel molecular mechanisms underlying vascular pathology, which will enable finding effective therapies in the future. One avenue of therapeutic potential is ER stress.
ER stress and the UPR are known to play a part in cardiovascular disease. The role of ER stress in regulating endothelial cell, macrophage and cardiomyocyte function in disease has been studied extensively and reviewed before [10-14]. However, in recent years focus has shifted towards arterial smooth muscle cells (ASMCs) and their role in regulating vascular pathology. Therefore ASMCs are the centre of attention of this review, which explores their role in vascular disease and regulation by ER stress and the UPR.
1.1. Endoplasmic Reticulum Stress and the Unfolded Protein Response
The ER is the first organelle of the secretory pathway, where most secreted and transmembrane proteins are folded and mature; processes essential for protein synthesis and cell homeostasis. ER stress occurs when the influx of unfolded proteins to the ER exceeds its capacity to fold them resulting in activation of a signalling pathway called the UPR [15, 16]. ER stress and the UPR can also be activated by disturbances in calcium homeostasis, as storing Ca2+ is an important function of the ER. Ca2+ is required for the maintenance of protein synthesis as the majority of the ER resident chaperones (for example calreticulin) are Ca2+ -binding proteins. Therefore, fluctuations in the levels of Ca2+ in the ER can severely impact folding capacity [17]. In addition, ER stress can be activated by changes in the redox status of the ER, as an oxidising environment is necessary for disulphide bonds to form [18].
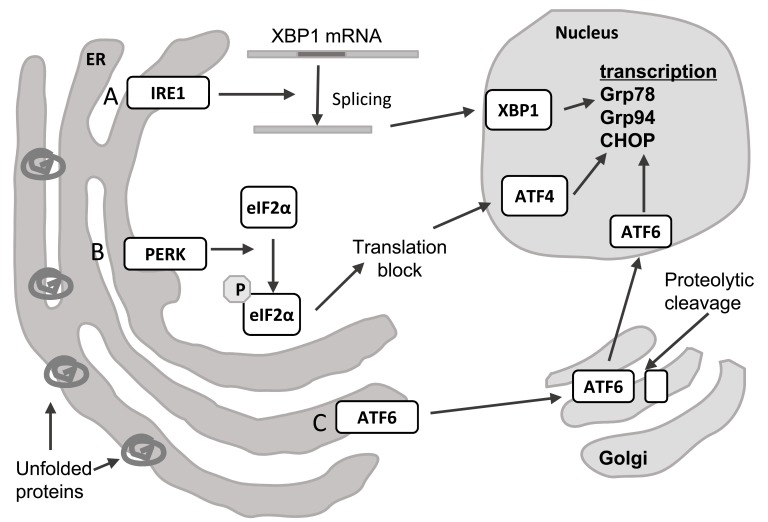
There are three proteins responsible for sensing an increased load of unfolded proteins in the ER, which define three branches of the UPR: inositol-requiring protein-1 (IRE1), activating transcription factor-6 (ATF6) and PRKR-Like Endoplasmic Reticulum Kinase (PERK). These transmembrane proteins, which have ER luminal and cytosolic domains, are called ER stress transducers as upon sensing ER stress they transmit the signal from the ER to downstream effectors (Fig. 1) [15, 16].
Fig. (1).
The unfolded protein response. ER stress caused by accumulation of unfolded proteins triggers the UPR, which consists of three branches A) IRE1 catalyses splicing of XBP1 mRNA, which results in translation of a functional transcription factor. B) PERK phosphorylates eIF2a, which leads to blocking of general translation and preferential translation of some transcripts, such as ATF4. C) ATF6 is translocated to the Golgi, where it undergoes cleavage and becomes an active transcription factor. The result of activation of each of these pathways is transcription of genes whose products help resolve ER stress. Abbreviations: ATF4 – activating transcription factor 4, ATF6 – activating transcription factor 6, CHOP - DNA Damage Inducible Transcript 3 (DDIT3), eIF2α – eukaryotic translation initiation factor 2α, Grp78 - Heat Shock Protein Family A (Hsp70) Member 5 (HSPA5), Grp94 - Heat Shock Protein 90kDa Beta Family Member 1 (HSP90B1), IRE1 - Endoplasmic Reticulum To Nucleus Signaling 1 (ERN1), PERK - Eukaryotic Translation Initiation Factor 2 Alpha Kinase 3 (EIF2AK3), XBP1 – x-box binding protein 1.
The first ER stress transducer to be described was IRE1 (also known as endoplasmic reticulum to nucleus signalling 1, ERN1). Upon unfolded protein accumulation it oligomerises and undergoes autophosphorylation [19]. This activates its effector function, which is cleavage of only one known substrate, XBP1 mRNA. This splicing of XBP1 causes a frameshift and results in a completely different protein being translated [20]. This spliced XBP1 is more stable and acts as a transcription activator for UPR regulated genes [20, 21]. The protein resulting from unspliced XBP1 mRNA is unstable and represses UPR target genes [21]. The XBP1 branch of the UPR has a role in regulating lipid biosynthetic enzymes and ER-associated degradation components, as well as ER biogenesis [16]. It has also been shown that IRE1 can mediate RIDD (regulated IRE1-dependent decay), whereby ER-localised mRNAs are degraded to limit the number of newly synthesised proteins and decrease the protein load in the ER [22].
The second branch of the UPR is represented by ATF6. When unfolded proteins accumulate in the ER, its Golgi localisation sequences are revealed and it is translocated to this organelle. In the Golgi ATF6 is processed by site 1 and site 2 proteases, which cleave off the ER luminal and transmembrane domains [23]. The resulting cytosolic fragment, which has DNA binding activity, moves to the nucleus and is a transcription factor that activates transcription of UPR-regulated genes, for example XBP1, Grp78 and Grp94 chaperones (78kDa and 94kDa glucose regulated proteins) and PDI (protein disulphide isomerase) [20, 23].
The third branch of the UPR is mediated by PERK (also known as eukaryotic initiation factor 2-alpha kinase 3, EIF2AK3). Similar to IRE1, it undergoes oligomerisation and autophosphorylation upon accumulation of unfolded proteins in the ER [24]. However, unlike IRE1, PERK also phosphorylates eukaryotic translation initiation factor 2α (eIF2α). This results in lower translation initiation levels and decreased flux of new proteins into the ER [25]. Some transcripts involved in the UPR are able to avoid this translational block and are preferentially translated when eIF2α is limiting, due to short upstream open reading frames in their 5' untranslated regions. An example of such a gene is ATF4, a transcription factor that is translationally induced by phosphorylation of eIF2α [26]. Another two are CHOP (transcription factor C/EBP homologous protein) and GADD34 (growth arrest and DNA damage–inducible 34) [16].
Activation of all three branches of the UPR results in a decrease in protein influx to the ER and increased expression of various genes encoding chaperones and other proteins that mitigate the effects of accumulation of unfolded proteins. Examples of chaperones include Grp78 and Grp94 [27, 28], protein disulphide isomerases (PDIs) and genes involved in phospholipid synthesis and ER biogenesis (phosphatidyl choline biosynthesis) [29], which leads to expansion of the ER and its protein folding capacity. Another mechanism activated in response to an overabundance of unfolded proteins is ERAD (ER-associated degradation). In this process misfolded proteins are retrotranslocated to the cytoplasm and degraded by the proteasome [30]. Large aggregates of severely unfolded proteins that are difficult to degrade via ERAD can also be disposed of via autophagy, which has been shown to be activated during ER stress [31]. However, if ER stress is too overwhelming and cannot be resolved it may lead to cell death [32].
Apoptosis is activated by ER stress via several mechanisms [33]. IRE1α activates TRAF2 (tumour necrosis factor receptor associated factor 2) which activates apoptotic-signaling kinase-1 (ASK1), causing activation downstream of stress kinases Jun-N-terminal kinase (JNK) and p38 MAPK (mitogen-activated protein kinase), that promote apoptosis [34]. CHOP has also been implicated in ER stress- induced apoptosis [35], as it increases expression of pro-apoptotic Bim, DR5 and PUMA, while decreasing expression of anti-apoptotic Bcl-2 [32, 36, 37]. In addition, ER stress can mediate cell death by disturbance in calcium metabolism; Bax and Bak have been shown to regulate Ca2+ release from the ER upon ER stress [38].
Importantly, maintenance of ER homeostasis is linked with the cellular redox potential. PDI catalyzes disulfide bond formation and becomes reduced in the process. It is then oxidised by ERO-1 (endoplasmic reticulum oxidoreductin) via transfer of electrons from reduced PDI to molecular oxygen, resulting in reactive oxygen species (ROS) generation. In addition, glutathione (GSH) and peroxiredoxin play a role in reducing PDIs, further altering the redox environment in the ER [39]. Moreover, it has been shown that disturbances in ER Ca2+ homeostasis lead to increased ROS generation by the mitochondria [40]. ROS can also feedback to sensitise the Ca2+ release channels at the ER membrane [41]. As far as downstream signalling is concerned, SKN-1/ Nrf is reported to be activated by ER stress [42], studies also report that PERK activates nuclear respiratory factor 2 (Nrf2) [43]. The relationship between ER stress and oxidative stress has been previously reviewed in detail [18, 44].
Chemical chaperones are low molecular weight proteins which interact with exposed hydrophobic fragments of the unfolded protein, thereby protecting it from aggregation [45]. In this review we describe the effect of some of these compounds (for example, TUDCA and PBA) as well as other factors shown to ameliorate ER stress in the vasculature.
1.2. ASMCs in vascular disease
In contrast to other muscle cells, ASMCs do not terminally differentiate and show phenotypic plasticity. In physiological conditions in the vessel wall they exist as contractile cells and regulate vascular tone. In response to stress and injury ASMCs lose expression of contractility-related genes such as SM22α, calponin (CNN1), and myosin light chain (MLC) [46-48] and proliferate, migrate and secrete extracellular matrix (ECM) related proteins, and thus are able to repair the injured vessel [49, 50]. Phenotypic plasticity of ASMCs underlies their functions in vascular pathology. For example, proliferation of synthetic ASMCs is the mechanism that leads to formation of a fibrous cap over an atherosclerotic plaque [51]. In addition, in response to lipid exposure, characteristic of the extracellular environment of atherosclerosis, ASMCs have been shown to differentiate into myeloid cells, showing features of macrophage-like cells, which express macrophage markers, phagocytose and present antigens [52-54]. Another example of pathological phenotype switching is trans/dedifferentiation of ASMCs into cells with characteristics of osteoblasts or chondrocytes [55-57]; this has been suggested to be one of the mechanisms underlying vascular calcification.
In addition to phenotype switching, dysregulated apoptosis of ASMCs is also a major contributor to vascular disease. ASMC apoptosis has been shown to contribute to progression of atherosclerosis and atherosclerotic plaque instability, vascular calcification and aortic aneurysm formation [58-60].
Factors regulating ASMC phenotype switching and apoptosis are important for understanding ways to manipulate vascular disease and finding therapies. Importantly, ER stress has been recently implicated in regulating ASMCs phenotype, physiology and death in vascular disease; this is discussed in the sections below.
2. ER stress in vascular disease
2.1. Atherosclerosis
The formation of the atherosclerotic plaque begins with subendothelial accumulation of lipids in the vessel wall, to form fatty streaks [61]. These lipids trigger a series of inflammatory responses, such as attraction of monocytes to activated endothelial cells overlaying fatty streaks, followed by monocyte differentiation into macrophages. Macrophages internalize the retained lipoproteins, leading to foam cell formation, and they become activated and produce inflammatory cytokines [62]. As lesions develop other inflammatory cells are recruited, the inflammatory response amplifies and does not resolve, leading to the formation of a core region in the centre of the plaque with lipid droplets, surrounded by a fibrous cap of ASMCs and a collagen-rich ECM.
ASMC proliferation also plays an important role in plaque progression [11, 63]. ASMCs migrate from the tunica media to the tunica intima, form the fibrous cap and regulate collagen biosynthesis [52, 54]. Once a plaque is established ASMC apoptosis is an important process regulating plaque stability, as ASMC death leads to plaque rupture and exposure of pro-thrombotic debris leading to myocardial infarction [59, 64].
Increased Expression of ER Stress Markers in ASMCs is Implicated in Atherosclerosis
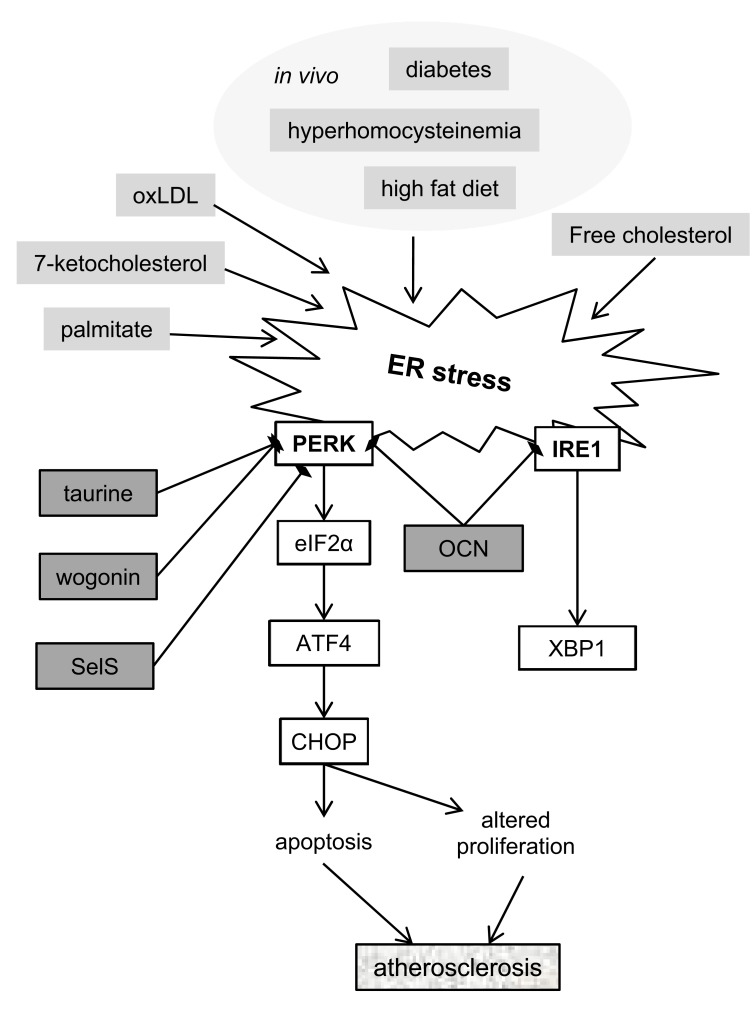
ER stress has been shown to be activated in ASMCs by common atherosclerosis risk factors (Fig. 2). For example, expression of Grp78 was observed in a mouse model of hyperhomocysteinemia in atherosclerotic lesions, predominantly in the ASMC-containing fibrous cap [65]. The involvement of ER stress in ASMCs in the context of diabetes has been shown in mouse models of this condition where increased expression of ER chaperones was observed in ASMCs in atherosclerotic plaques [66]. In addition, glucosamine, which is a by-product of excess glucose in the blood stream in diabetes, has been shown to increase ER stress in cultured aortic ASMCs [67].
Fig. (2).
Schematic representation of the contribution of ER stress to the development of atherosclerosis. ER stress is activated by known atherosclerosis risk factors. The PERK pathway has been implicated in regulation of ASMC apoptosis and proliferation. Osteocalcin (OCN), SelS, taurine and wogonin have been shown to have protective effects against ER stress in the context of atherosclerosis. Exact mechanisms linking all these factors are unknown. Abbreviations: ATF4 – activating transcription factor 4, CHOP - DNA Damage Inducible Transcript 3 (DDIT3), eIF2α – eukaryotic translation initiation factor 2α, IRE1 - Endoplasmic Reticulum To Nucleus Signaling 1 (ERN1), OCN – osteocalcin (BGLAP), oxLDL – oxidised low-density lipoprotein, PERK -Eukaryotic Translation Initiation Factor 2 Alpha Kinase 3 (EIF2AK3), SelS – selenoprotein S, XBP1 – x-box binding protein 1.
ER Stress Potentially Leads to Apoptosis of ASMCs in the Atherosclerotic Plaque
Despite the established presence of ER stress in atherosclerotic vessels, its role in plaque progression has not been fully characterized. One possible mechanism is ER stress–mediated apoptosis of ASMCs leading to decreased collagen production and thinning of the protective collagen cap in advanced lesions.
In animal models of atherosclerosis, apoptosis of ASMCs via ER stress was shown to be induced by lipids. 7-ketocholesterol was shown to activate ER stress in a study by Myoishi et al, who also observed ER stress in ASMCs in ruptured and thin cap (unstable) atherosclerotic plaques, but not in fibrous cap (stable) plaques in human coronary artery samples [68]. ER stress was also shown to be activated by 7-ketocholesterol in cultured human ASMCs, where it was mediated by ROS production and led to apoptosis [69]. Furthermore, ApoE-/- and Ldlr-/- mice lacking the ER stress-regulated apoptosis inducer CHOP showed decreased necrosis and apoptosis in atherosclerotic plaques confirming the link between ER stress and lipid metabolism in vascular disease [70]. The authors attribute these changes to macrophages, but do not rule out the participation of ASMCs. This seems more than likely in the light of findings that ASMCs can transdifferentiate into various other cell populations present in the plaque [52]. In another study free cholesterol loading of ASMCs in vitro correlated with ER stress and apoptosis [71]. Oxidised low-density lipoproteins have also been shown to activate ER stress-induced apoptosis in ASMCs [72]. Additionally, apoptosis of ASMCs was observed in a study where rabbit aortic atherosclerotic rings were treated with an ER stress inducer puromycin, however fibrous cap morphology and plaque stability were not examined [73].
ER Stress Regulates ASMC Proliferation and Vascular Remodelling
Various components of the UPR have also been implicated in regulating ASMC proliferation. Malabanan et al. showed that ATF4 expression is activated by FGF2 in response to vascular injury, and that ATF4 regulates the expression of VEGF-A, leading to increased proliferation of ASMCs and medial thickening in a rat model of balloon-induced vascular injury [74]. In a mouse model of atherosclerosis (ApoE-/- mice fed a high fat diet) ASMC-specific CHOP knockout was found to increase the levels of ASMC phenotype regulator KLF4 [75]. KLF4 is a known suppressor of ASMC contractile markers and inhibitor of proliferation of ASMCs [52, 76]. These results show that ER stress signalling can regulate signalling pathways crucial for vascular remodelling.
Conversely, there is evidence suggesting that ER stress might be protective in pathologies associated with increased ASMC proliferation. For example it has been shown that inducing ER stress in ASMCs with tunicamycin (an inhibitor of protein glycosylation) or thapsigargin (an inhibitor of SERCA) inhibited their proliferation [77, 78]. This idea is supported by a recent study of a novel protein regulated by ER stress, Gipie, which is highly expressed in synthetic ASMCs in regions of vascular injury. Gipie forms a complex with GRP78 and IRE1α, and stabilizes their interaction, reducing JNK activation and cell death induced by ER stress [79]. In a rat model of balloon injury depletion of Gipie attenuated neointima formation and stimulated ASMC apoptosis [80].
Inhibiting ER Stress has Therapeutic Benefits for Atherosclerosis
The consensus about ER stress activation in the vessel wall seems to be that it is a pathological process and that its inhibition would ameliorate vascular disease. Several compounds that decrease ER stress induced by atherosclerosis-promoting factors have been identified so far.
Osteocalcin, a protein secreted by osteoblasts, was found to decrease ER stress induced in the vasculature by high-fat diet in ApoE-/- mice [81]. Mice treated with osteocalcin were less obese and had better glucose sensitivity than untreated counterparts, suggesting that inhibiting ER stress may have real benefits with regards to atherosclerosis in the context of diabetes. Even though the protective effects of osteocalcin in vivo could have been mediated by macrophages and endothelial cells, which are also known to activate ER stress in vascular pathology, the authors show that in vitro osteocalcin has protective effects on ASMCs. However, it is worth noting that we discuss osteocalcin as a protective agent, even though it is a known vascular calcification promoting factor and overexpression of osteocalcin in ASMCs leads to increased mineralisation [82]. The authors of this study did not examine whether osteocalcin induced any calcification and therefore more research is needed to assess all the potential effects of this treatment.
FGF-21 (fibroblast growth factor 21, implicated in the pathogenesis of diabetes) has been shown to decrease hallmarks of atherosclerosis and expression of ER stress markers in the aorta in another study in ApoE-/- mice. However, the authors did not carry out any experiments to determine which cell types were involved [83].
A different naturally occurring factor implicated in inhibiting ER stress and atherosclerosis is selenoprotein S (SelS). SelS is abundantly expressed in ASMCs and silencing its expression was shown to sensitize the cells to ER stress and apoptosis [84]. Since apoptosis is a crucial mechanism of atherosclerosis progression, this provides an important treatment option.
Another study focused on taurine, a metabolite of methionine and cysteine, which has antihypertensive and antiatherogenic effects, has been shown to decrease ER stress induced by hyperhomocysteinemia in cultured rat ASMCs [85]. Finally, wogonin, a plant-derived flavonoid, has been shown to decrease ER stress and apoptosis induced by palmitate in cultured ASMCs [86].
2.2. Hypertension
As the main component of the arterial wall and cells regulating vascular tone ASMCs play an important role in the pathophysiology of hypertension. Increased proliferation of ASMCs in hypertensive blood vessels is responsible for remodelling of the vessel wall [87].
ER Stress Induces Hypertension
Mice perfused with tunicamycin showed increased systolic and diastolic blood pressure; increased expression of ER stress markers suggesting activation of the IRE1 and PERK branches of the UPR [88]. A different study showed that perfusing rats with tunicamycin also led to apoptosis, fibrosis with increased collagen production and aortic stiffening [89]. The effects of tunicamycin were reversed by administering an ER stress inhibitor, the chemical chaperone PBA. Additionally, microarray analysis showed increased expression of ATF6 and its transcriptional targets in spontaneously hypertensive rats comparing to a normotensive strain [90].
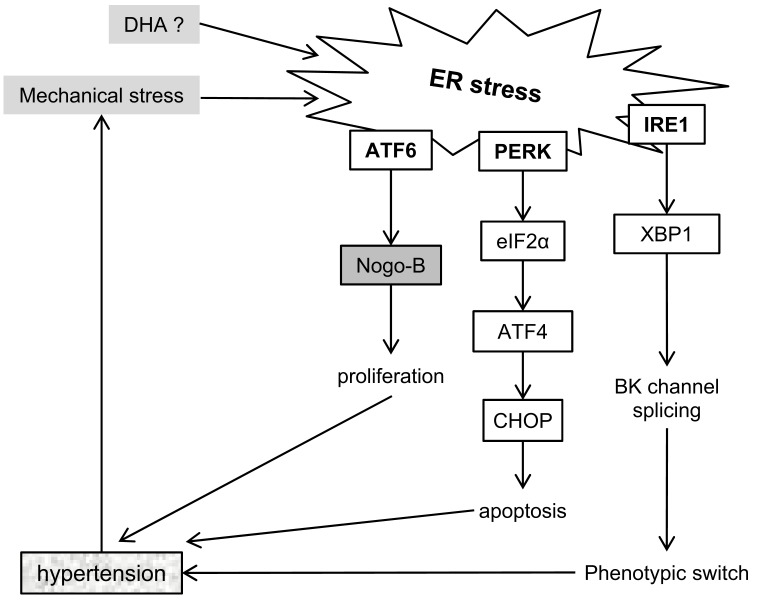
In vitro hypertension has been modelled by submitting cells to cyclic stretch of various intensities to mimic the hemodynamic conditions of a blood vessel. Both CHOP and XBP1 have been found to be upregulated by cyclic stretch in rat ASMCs [91]. XBP1 expression in ASMCs was accompanied by changes in splicing of large conductance calcium and voltage-activated potassium (BK) channels, known to be involved in regulating vascular tone and implicated in ASMC differentiation [92]. These results suggest that there might be a positive feedback loop mechanism regarding ASMCs in hypertension – mechanical stress induces ER stress, which in turn further induces hypertension.
ER Stress in Pulmonary Artery Hypertension
ER stress in ASMCs has also been implicated in pulmonary arterial hypertension (PAH), a condition that manifests as thickening and fibrosis of the pulmonary arteries, which leads to stiffness and right ventricular hypertrophy [87]. The ATF6 branch of the UPR has been shown to regulate expression of Nogo-B, an ER protein implicated in vascular remodelling and fibrosis in PAH, as it increases ASMC proliferation. Interestingly, ATF6 and Nogo-B were upregulated in pulmonary resistance arteries but not in carotid arteries, suggesting that ASMCs or their responses to stress are vessel specific [93, 94].
These in vivo studies are supported by in vitro data. Crnkovic and colleagues observed upregulation of ER stress markers in human pulmonary artery ASMCs treated with DHA (docosahexaenoic acid; [95]). DHA has been shown to have protective effects on the cardiovascular system, and the authors postulated that activation of ER stress leads to decreased proliferation and increased apoptosis of ASMCs, via an increase in ROS which in the context of PAH leads to an amelioration of disease. Fig. 3 summarizes the involvement of ER stress signalling pathways in hypertension.
Fig. (3).
Summary of current research of ER stress in hypertension. All three branches of the unfolded protein response have been shown to mediate changes in the vasculature that promote hypertension. In addition, there apears to be a positive feedback loop, where ER stress can lead to hypertension, and is increased by mechanical stress. Abbreviations: ATF4 – activating transcription factor 4, ATF6 – activating transcription factor 6, BK channel - voltage-activated potassium channel, CHOP - DNA Damage Inducible Transcript 3 (DDIT3), DHA - docosahexaenoic acid, eIF2α – eukaryotic translation initiation factor 2α, IRE1 - Endoplasmic Reticulum To Nucleus Signaling 1 (ERN1), Nogo-B – reticulon 4 (RTN4), PERK - Eukaryotic Translation Initiation Factor 2 Alpha Kinase 3 (EIF2AK3), XBP1 – x-box binding protein 1.
2.3. Aortic Aneurysms
The role of ASMCs in the development of aortic aneurysms has long been established, as increased apoptosis and replicative senescence lead to thinning of the aortic media making the aneurysm prone to rupture [96].
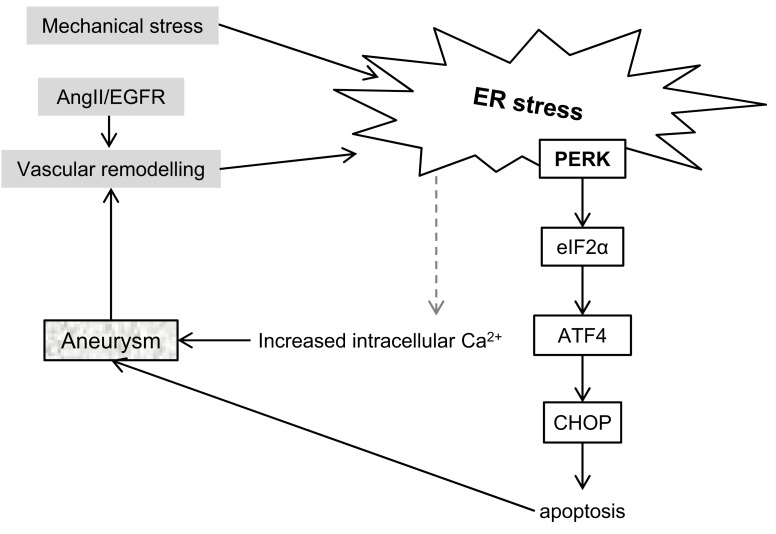
Recently it has been observed that increased expression of ER stress markers in ASMCs accompanies formation of abdominal aortic aneurysms (AAAs) both in humans and in a mouse model of this disease (Fig. 4) [97]. In the mouse model the disease phenotype could be rescued by administering erlotinib, an inhibitor of EGFR. EGFR is activated by AngII and mediates various downstream responses in ASMCs, including oxidative stress and in this model also ER stress. In a follow-up study the same group has demonstrated that AngII induces vascular remodelling by EGFR-mediated ER stress activation [98]. This was independent of hypertension, which is classically associated with AngII.
Fig. (4).
ER stress in development of aneurysms. ER stress has been observed during vascular remodelling. Conversely, ER stress signalling contributes to mechanisms which promote aneurysm formation. To date, IRE1 and ATF6 pathways have not been reported to be involved in aneurysm formation in ASMCs. Abbreviations: AngII – angiotensyn 2, ATF4 – activating transcription factor 4, CHOP - DNA Damage Inducible Transcript 3 (DDIT3), EGFR – epidermal growth factor receptor, eIF2α – eukaryotic translation initiation factor 2α, PERK - Eukaryotic Translation Initiation Factor 2 Alpha Kinase 3 (EIF2AK3).
Increased ER stress was also observed in pharmacologically induced thoracic aortic aneurysm/dissection (TAAD) as well as in human specimens of the disease [99]. The same study established that CHOP deletion prevented ASMC apoptosis and aneurysm formation, and confirmed that ER stress is activated in ASMCs by mechanical stretch.
Another study which links ER stress with AAAs examined the effect of a conditional ASMC-specific knockout of myocardin in mice [100]. Myocardin is a muscle-specific transcriptional coactivator implicated in development of the heart and vasculature. The myocardin knockout mice died within 6 months of the gene deletion due to rupture of aortic aneurysms. ASMCs of these mice undergo phenotypic change with downregulation of contractile markers and increased expression of ECM proteins, which is accompanied by upregulation of ER stress (activation of the PERK branch of the UPR and XBP1 splicing) and autophagy markers. The
authors suggest that ER stress is a result of increased protein turnover (cells degrade contractile markers and secrete ECM) rather than a trigger for the remodelling. However, ER stress and autophagy could also be the mechanisms leading to increased ASMC apoptosis observed in the media of the aortas with aneurysms.
Activation of ER stress and autophagy was also observed in a study which explored the effect of transgenic overexpression of smooth muscle myosin heavy chain (MYH11) in mouse ASMCs [101]. In humans heterozygous mutations in MYH11 cause a dominantly inherited predisposition to thoracic aneurysms and dissections. MYH overexpression in mouse ASMCs induced ER stress (the PERK branch of the UPR) and autophagy, which lead to degradation of the surplus contractile protein. The authors postulate that due to ER stress the ASMCs had increased intracellular Ca2+ levels, which led to increased contractility. Although the authors admit that overexpression of MYH11 induces ER stress, the control for their experiments were cells derived from a non-transgenic mouse, rather than a mouse overexpressing an unrelated protein. Therefore it cannot be ruled out that the effects they observed are in part an artefact of the overexpression itself, rather than specific to MYH11.
2.4. Vascular Calcification
Vascular calcification is the most extreme form of vascular pathology and remodelling. It is the process of deposition of calcium phosphate crystals in the ECM of the blood vessel wall [102, 103]. The presence of vascular calcification poses an increased risk of cardiovascular and all-cause mortality. It is ubiquitous, and among 60-year-olds, approximately 60% have vascular calcification [104, 105].
Vascular calcification can be categorised into two main types: medial and intimal, depending on its localisation in the vessel wall [106, 107]. Each type of calcification is associated with different conditions and risk factors [105]. Medial calcification, also called Mönckenberg's sclerosis, causes blood vessel wall stiffening, leading to complications such as hypertension and aortic stenosis, which in turn give rise to cardiac hypertrophy, myocardial and lower-limb ischemia, congestive heart failure and can eventually result in death [105, 106, 108]. Medial calcification is associated primarily with ageing, diabetes and chronic kidney disease (CKD). Intimal calcifications co-localise with advanced atherosclerotic plaques [105] and are associated with the same factors as atherosclerosis, such as lipids, oxidative stress and inflammation.
ASMCs play a crucial role in regulating both types of calcification [109-111]. Several mechanisms regulating vascular calcification involving ASMCs are already known, including: apoptosis [58], release of exosomes [112], loss of calcification inhibitors [113, 114], ageing-related DNA damage [115] and osteogenic differentiation [55, 57]. All stages of bone formation can be found in calcified lesions, including fully formed trabecular bone, and markers characteristic for both osteoblasts and chondrocytes are expressed by calcifying ASMCs [57, 105, 116, 117]. In addition, it has recently emerged that ER stress activation is another mechanism associated with vascular calcification. ER stress and the unfolded protein response play an important role during bone formation and vascular calcification recapitulates many of the processe involved in bone formation.
ASMC Osteogenic Transdifferentiation and Endochondral Bone Formation
During embryonic development bone formation occurs via two mechanisms: endochondral and membranous. During membranous ossification, which gives rise to flat bones of the skull, osteoblasts differentiate directly from mesenchymal stem cells (MSCs). During endochondral ossification, by which most bones in the body are made, there is an intermediate step in which MSCs differentiate into chondrocytes that proliferate and secrete ECM to form a scaffold for osteoblasts [118]. Hypertrophic chondrocytes direct perichondral cells to differentiate into osteoblasts [118, 119], and finally undergo apoptosis. Chondrocytes also secrete matrix vesicles, small membrane-bound particles, which initiate calcification of the ECM [120].
Chondrocyte and osteoblast proliferation and maturation during endochondral bone formation is regulated by a tightly orchestrated transcription programme activated in MSCs [118]. There is ample evidence that cytokines, transcription factors and bone ECM proteins are also present in the vessel wall and are involved in regulating vascular calcification. These include transcription factors Runx2 [121], Msx2, Osterix [122], Sox9 [116] and key components of BMP-2 [123] and TGFβ1 as well as Wnt [124] signalling pathways.
ER Stress in Bone Formation
When bone formation occurs during embryonic development, osteoblasts and chondrocytes need to secrete large amounts of ECM and regulatory proteins. It has been well documented, that in order to cope with the high demand of protein synthesis and secretion osteoblasts and chondrocytes employ UPR signalling to increase their protein folding capacity [4, 5]. All three branches of the UPR have been shown to activate transcription of bone specific genes [125-127]. In addition, bone-specific ER stress transducers have been described, highlighting the crucial function of the UPR in bone formation [128, 129].
ER Stress in Vascular Calcification
Given the known involvement of ER stress in osteogenic differentiation, it is no surprise that studies published to date focus on expression of osteogenic markers and apoptosis as the mechanisms linking ER stress with vascular calcification (Fig. 5).
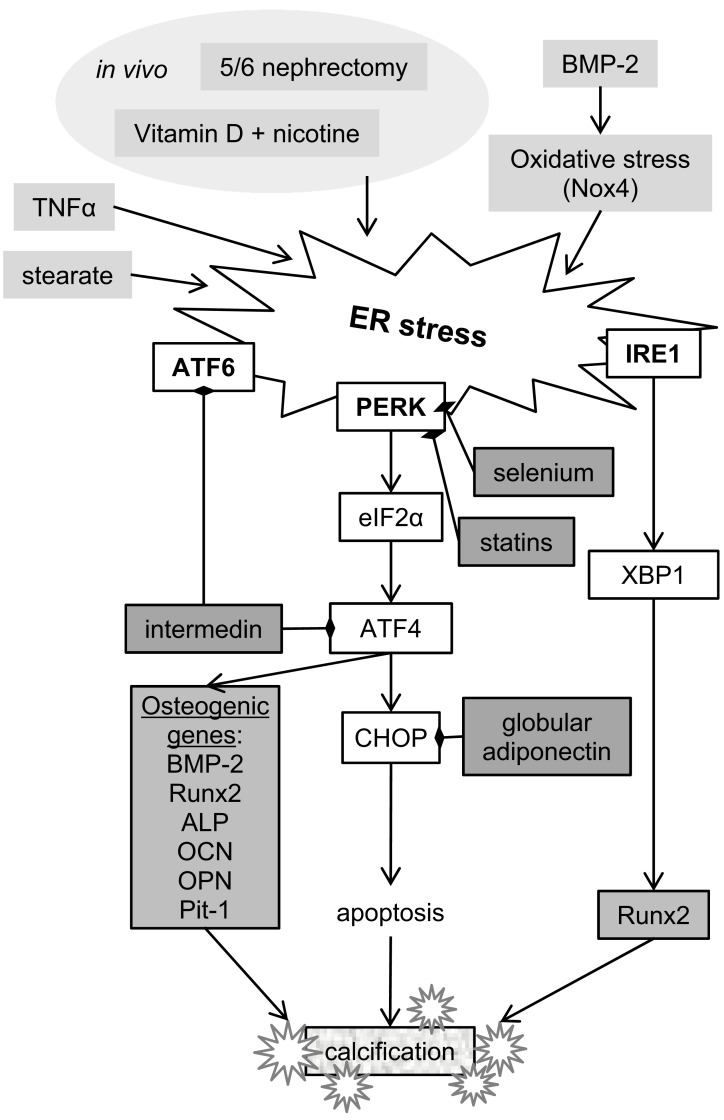
Fig. (5).
Summary of ER stress signalling facotrs involved in vascular calcification. All three unfolded protein response branches have been implicated in regulation of ASMC calcification by ER stress. Together they are suggested to regulate osteogenic differentiation and apoptosis, key events contributing to vascular calcification. Intermedin, selenium and globular adiponectin have been shown to inhibit ATF6 and PERK branches of the UPR. Abbreviations: ALP – alkaline phosphatase (ALPL), ATF4 – activating transcription factor 4, ATF6 – activating transcription factor 6, BMP-2 – bone morphogenetic protein 2, CHOP - DNA Damage Inducible Transcript 3 (DDIT3), eIF2α – eukaryotic translation initiation factor 2α, IRE1 - Endoplasmic Reticulum To Nucleus Signaling 1 (ERN1), Nox4 – NADPH oxidase, OCN – osteocalcin (BGLAP), OPN – osteopontin (SPP1), PERK - Eukaryotic Translation Initiation Factor 2 Alpha Kinase 3 (EIF2AK3), Pit-1 - type III sodium-dependent phosphate cotransporter, Runx2 - Runt related transcription factor 2, TNFα – tumor necrosis factor α, XBP1 – x-box binding protein 1.
Vascular calcification induced in rats with nicotine and vitamin D was accompanied by upregulation of Grp78 and Grp94 chaperone expression in the aorta as well as markers of ER stress-induced apoptosis, CHOP and caspase 12 [130]. ATF4 was found to be the mediator of ER stress-induced calcification, both in the rat model in vivo and in rat ASMCs in vitro [131]. The authors of this study also demonstrated that changes in calcification and ER stress markers correlated with osteogenic gene expression in ASMCs.
Further to that, the PERK-ATF4-CHOP branch of the UPR was found to be activated in aortas of ApoE-/- mice with CKD induced by 5/6 nephrectomy [132, 133]. These mice showed extensive medial calcification in the aorta, accompanied by expression of osteogenic markers. Treatment with statins attenuated both medial and intimal calcification and ER stress, suggesting that dyslipidemia is an inducer of ER stress. Both CHOP and ATF4 were found to be required for mineralisation of mouse ASMCs [133]. In addition, the latest study by Masuda et al connects ER stress-induced apoptosis and vascular calcification in mice to altered balance of saturated and unsaturated fatty acids [134]. Interestingly, this is the first study where the involvement of all three UPR pathways in mineralisation of ASMCs was shown.
The association of ER stress with ASMC calcification in vivo has been further confirmed in studies that investigated mineralisation of ASMCs in vitro. In a mouse ASMC line MOVAS-1, stearate (a saturated fatty acid) induced calcification via the PERK pathway and XBP1 splicing [135]. ATF4 knock-down blocked osteogenic differentiation and mineralization induced by stearate. Similarly TNFα induced calcification of MOVAS-1 cells by activating the PERK-ATF4-CHOP branch of the UPR [132]. In human coronary artery vascular smooth muscle cells (HCASMCs) BMP-2-mediated oxidative stress induced ER stress, and increased expression of GRP78, phospho-IRE1 and XBP1 [136]. An increase in NADPH oxidase activity and oxidative stress occurred via activation of the BMP-2 receptor 2 and Smad1 signalling. This study also showed that XBP1 binds to the Runx2 promoter in BMP-2-treated HCASMCs, linking ER stress and osteogenic differentiation. Inhibition of oxidative or ER stress decreased Runx2 expression, intracellular calcium deposition, and mineralization of BMP-2-treated HCASMCs, suggesting that ROS production could be another mechanism linking ER stress with vascular calcification.
In contrast to all other reports, there is also evidence that CHOP may protect ASMCs from calcification in some disease contexts. In mouse ASMCs lacking the ENPP1 enzyme, which synthesizes pyrophosphate, a natural calcification inhibitor, the absence of CHOP increased mineralisation [137].
Attenuating ER Stress Decreases ASMC Calcification
Potential treatments for vascular calcification, which act by attenuating ER stress, have been described in recent studies. Intermedin, a paracrine/autocrine factor, member of the calcitonin/calcitonin gene-related peptide (CGRP) family is known to lower arterial pressure and resistance by activating cAMP/PKA signalling. In a study of rat ASMCs intermedin was shown to decrease ER stress, which also led to a decrease in calcification [138]. Similarly selenium, a trace element whose deficiency has been linked to cardiovascular disease, has been found to decrease ER stress, apoptosis and calcification [139]. Finally, globular adiponectin, a peptide secreted by adipocytes, has been shown to decrease vascular calcification induced by vitamin D and nicotine in rats, which was accompanied by a decrease in ER stress and osteogenic gene expression [140].
3. CONCLUSION
In this review we presented what is known about ER stress in ASMCs in various vascular disease contexts. Specifically, we summarised which ER stress pathways and which ER stress triggers were studied in these contexts. It is apparent that ER stress signalling varies depending on the type of ASMC and the disease context with large gaps in our understanding, making it an exciting field with many potential areas for future research (Table 1).
The role of ER stress in ASMCs in vascular disease is far from understood. Current data suggest that ER stress in ASMCs is likely involved in vascular disease, however, with only a few exceptions, the studies described in this review provide few mechanistic insights and therefore many questions remain to be answered.
The issue of highest importance is establishing whether ER stress in ASMCs is a cause or a secondary effect of pathological processes in the vessel wall. The majority of the evidence suggests that ER stress causes pathological changes in ASMC function and phenotype, but it is clear that the role of ER stress signalling is not straightforward and disease-specific pathways are likely. Moreover, some key ASMC functions were studied in certain disease contexts, but not others. For example the relationships between ER stress and proliferation or senescence have been studied in the context of atherosclerosis, hypertension and aortic aneurysms, but not calcification. In addition, data is lacking on many of the ER stress activation pathways. To date most studies have focussed on the PERK-eIF2α-ATF4-CHOP pathway, with no or little mention of IRE1 or ATF6 activation. It is unclear whether they were not looked at or authors are reluctant to publish negative results. Experiments exploring the effects of blocking specific UPR pathways in the vascular system are now needed. In addition, other ER stress pathways exist, that have not been studied in ASMCs, such as the role of PTP1B [141] and many known inducers of vascular disease, such as inorganic phosphate, have not been examined for their potential in activating ER stress.
It is interesting to note that ER stress is activated in many models of diabetes [66, 67, 81, 83], which is known to cause vascular disease [105]. In addition, many factors discussed here, that were shown to activate ER stress in the vasculature, are also relevant to diabetes, for example oxidative stress or dyslipidemia. However, again, the mechanisms linking this disease with ER stress signalling in ASMCs are still mostly unknown.
More research is needed to clarify the relationship between ER stress and oxidative stress in ASMCs. Here we cite 9 studies which found causal relationships between these two processes [69, 71, 72, 85, 95, 97, 122, 136, 139]. However, few studies focus on specific signalling, most used general ER stress or oxidative stress inhibitors to confirm their findings. Several papers showed evidence of the involvement of JNK, PKCδ, Nox4, ERK and AKT [69, 72, 136, 139] either downstream or upstream of either stress pathway, with little emphasis on the complexity of either and other possible mechanisms through which these pathways may link or cross-talk.
Moreover, to date there is no evidence of a role for ER stress in normal physiological processes of ASMCs, even though the ER stress sensing machinery is present in these cells. Shedding light onto the role of ER stress in ASMC development or phenotype switching would help to understand the role of this signalling pathway in pathology.
Finally, we would also like to point out that there is not enough research on the differences in disease mechanisms between men and women and not a single research paper cited here reports anything on this topic, suggesting that they were not examined. As cardiovascular disease anecdotally manifests with different symptoms in men and women, it is very well possible it is regulated in part by different mechanisms. Therefore it would be interesting to examine whether there are any differences in ER stress.
Table 1.
Summary of ER stress in vascular pathology.
| Condition | ER stress signalling known to be involved | Known inducers | Known inhibitors | References |
|---|---|---|---|---|
| Atherosclerosis | PERK-eIF2α-ATF4-CHOP IRE1-XBP1 |
Diabetes, hyperhomocysteinemia, high fat diet, free cholesterol, oxLDL, 7-ketocholesterol, palmitate | Taurine, wogonin, SelS, osteocalcin | [65-75, 77-85] |
| Hypertension | PERK-eIF2α-ATF4-CHOP IRE1-XBP1 ATF6 |
Mechanical stress, DHA | - | [87-94] |
| Aortic aneurysms | PERK-eIF2α-ATF4-CHOP | Mechanical stress | - | [96-100] |
| Vascular calcification | PERK-eIF2α-ATF4-CHOP IRE1-XBP1 ATF6 |
TNFα, stearate, BMP-2, 5/6 nephrectomy, vitamin D+ nicotine | Selenium, statins, intermedin, globular adiponectin | [129-139] |
ACKNOWLEDGEMENTS
This work was funded by the British Heart Foundation, PhD Studentship FS/11/9/28695.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Moore K.A., Hollien J. The unfolded protein response in secretory cell function. In: Bassler B.L., editor. Annual Review of Genetics. Vol. 46. Palo Alto: Annual Reviews; 2012. pp. 165–183. [DOI] [PubMed] [Google Scholar]
- 2.Reimold A.M., Iwakoshi N.N., Manis J., et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412(6844):300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 3.Harding H.P., Zeng H.Q., Zhang Y.H., et al. Diabetes mellitus and exocrine pancreatic dysfunction in Perk-/- mice reveals a role for translational control in secretory cell survival. Mol. Cell. 2001;7(6):1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 4.Tohmonda T., Miyauchi Y., Ghosh R., et al. The IRE1a–XBP1 pathway is essential for osteoblast differentiation through promoting transcription of Osterix. EMBO Rep. 2011;12:451–457. doi: 10.1038/embor.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsang K.Y., Chan D., Bateman J.F., Cheah K.S. In vivo cellular adaptation to ER stress: survival strategies with double-edged consequences. J. Cell Sci. 2010;123(13):2145–2154. doi: 10.1242/jcs.068833. [DOI] [PubMed] [Google Scholar]
- 6.Dimcheff D.E., Askovic S., Baker A.H., Johnson-Fowler C., Portis J.L. Endoplasmic reticulum stress is a determinant of retrovirus-induced spongiform neurodegeneration. J. Virol. 2003;77(23):12617–12629. doi: 10.1128/JVI.77.23.12617-12629.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boerma T., Mathers C., AbouZahr C., et al. Health in 2015: from MDGs, millennium development goals to SDGs, Sustainable development goals. Geneva, Switzerland: WHO Press; 2015. pp. 1–216. [Google Scholar]
- 8.Lifton R.P., Gharavi A.G., Geller D.S. Molecular mechanisms of human hypertension. Cell. 2001;104(4):545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian D., Benjamin E.J., Go A.S., et al. Heart disease and stroke statistics—2016 update: A report from the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 10.Groenendyk J., Agellon L.B., Michalak M. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu. Rev. Physiol. 2013;75:49–67. doi: 10.1146/annurev-physiol-030212-183707. [DOI] [PubMed] [Google Scholar]
- 11.Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV. Role of endoplasmic reticulum stress in atherosclerosis and diabetic macrovascular complications. . BioMed Res Int. 2014. [DOI] [PMC free article] [PubMed]
- 12.Yang L.F., Zhao D.J., Ren J., Yang J. Endoplasmic reticulum stress and protein quality control in diabetic cardiomyopathy. Biochim Biophys Acta-Mol Basis Dis. 2015;1852(2):209–218. doi: 10.1016/j.bbadis.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ. Res. 2010;107(7):839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanova E.A., Orekhov A.N. The role of endoplasmic reticulum stress and unfolded protein response in atherosclerosis. Int. J. Mol. Sci. 2016;17(2):E193. doi: 10.3390/ijms17020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–530. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 16.Walter P., Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 17.Gorlach A., Klappa P., Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid. Redox Signal. 2006;8(9-10):1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra J.D., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007;9(12):2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 19.Shamu C.E., Walter P. Oligomerization and phosphorylation of the Irelp kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 1996;15(12):3028–3039. [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida H., Matsui T., Yamamoto A., Okada T., Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 21.Calfon M., Zeng H., Urano F., et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415(6867):92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 22.Hollien J., Weissman J.S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313(5783):104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 23.Haze K., Yoshida H., Yanagi H., Yura T., Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harding H.P., Zhang Y., Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397(6716):271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 25.Harding H.P., Zhang Y., Bertolotti A., Zeng H., Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 26.Vattem K.M., Wek R.C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA. 2004;101(31):11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo S., Baumeister P., Yang S., Abcouwer S.F., Lee A.S. Induction of Grp78/BiP by translational block. J. Biol. Chem. 2003;278(39):37375–37385. doi: 10.1074/jbc.M303619200. [DOI] [PubMed] [Google Scholar]
- 28.Kozutsumi Y., Segal M., Normington K., Gething M.J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 29.Sriburi R., Jackowski S., Mori K., Brewer J.W. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol. 2004;167(1):35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Travers K.J., Patil C.K., Wodicka L., Lockhart D.J., Weissman J.S., Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101(3):249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 31.Deegan S., Saveljeva S., Gorman A.M., Samali A. Stress-induced self-cannibalism: on the regulation of autophagy by endoplasmic reticulum stress. Cell. Mol. Life Sci. 2013;70(14):2425–2441. doi: 10.1007/s00018-012-1173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sano R., Reed J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta. 2013;1833(12):3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szegezdi E., Logue S.E., Gorman A.M., Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urano F., Wang X., Bertolotti A., et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 35.Zinszner H., Kuroda M., Wang X.Z., Batchvarova N., Lightfoot R.T., Remotti H., Stevens J.L., Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh A.P., Klocke B.J., Ballestas M.E., Roth K.A. CHOP potentially co-operates with FOXO3a in neuronal cells to regulate PUMA and BIM expression in response to ER stress. PLoS One. 2012;7(6):11. doi: 10.1371/journal.pone.0039586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi H., Wang H.G. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 2004;279(44):45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 38.Scorrano L., Oakes S.A., Opferman J.T., et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300(5616):135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 39.Bulleid N.J., Ellgaard L. Multiple ways to make disulfides. Trends Biochem. Sci. 2011;36(9):485–492. doi: 10.1016/j.tibs.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 40.St-Pierre J., Buckingham J.A., Roebuck S.J., Brand M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002;277(47):44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 41.Favero T.G., Zable A.C., Abramson J.J. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1995;270(43):25557–25563. doi: 10.1074/jbc.270.43.25557. [DOI] [PubMed] [Google Scholar]
- 42.Glover-Cutter K.M., Lin S., Blackwell T.K. Integration of the unfolded protein and oxidative stress responses through SKN-1/Nrf. PLoS Genet. 2013;9(9):e1003701. doi: 10.1371/journal.pgen.1003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cullinan S.B., Diehl J.A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004;279(19):20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 44.Mahdi A.A., Rizvi S.H., Parveen A. Role of endoplasmic reticulum stress and unfolded protein responses in health and diseases. Indian J. Clin. Biochem. 2016;31(2):127–137. doi: 10.1007/s12291-015-0502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cortez L., Sim V. The therapeutic potential of chemical chaperones in protein folding diseases. Prion. 2014;8(2):197–202. doi: 10.4161/pri.28938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frid M.G., Shekhonin B.V., Koteliansky V.E., Glukhova M.A. Phenotypic changes of human smooth muscle cells during development: late expression of heavy caldesmon and calponin. Dev. Biol. 1992;153(2):185–193. doi: 10.1016/0012-1606(92)90104-o. [DOI] [PubMed] [Google Scholar]
- 47.Huang Q.Q., Fisher S.A., Brozovich F.V. Forced expression of essential myosin light chain isoforms demonstrates their role in smooth muscle force production. J. Biol. Chem. 1999;274(49):35095–35098. doi: 10.1074/jbc.274.49.35095. [DOI] [PubMed] [Google Scholar]
- 48.Feil S., Hofmann F., Feil R. SM22α modulates vascular smooth muscle cell phenotype during atherogenesis. Circ. Res. 2004;94(7):863–865. doi: 10.1161/01.RES.0000126417.38728.F6. [DOI] [PubMed] [Google Scholar]
- 49.Shanahan C.M., Weissberg P.L. Smooth muscle cell heterogeneity: Patterns of gene expression in vascular smooth muscle cells in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 1998;18(3):333–338. doi: 10.1161/01.atv.18.3.333. [DOI] [PubMed] [Google Scholar]
- 50.Shanahan C.M., Weissberg P.L., Metcalfe J.C. Isolation of gene markers of differentiated and proliferating vascular smooth muscle cells. Circ. Res. 1993;73(1):193–204. doi: 10.1161/01.res.73.1.193. [DOI] [PubMed] [Google Scholar]
- 51.Dzau V.J., Braun-Dullaeus R.C., Sedding D.G. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat. Med. 2002;8(11):1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 52.Shankman L.S., Gomez D., Cherepanova O.A., et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 2015;21(6):628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andreeva E.R., Pugach I.M., Orekhov A.N. Subendothelial smooth muscle cells of human aorta express macrophage antigen in situ and in vitro. Atherosclerosis. 1997;135(1):19–27. doi: 10.1016/s0021-9150(97)00136-6. [DOI] [PubMed] [Google Scholar]
- 54.Rong J.X., Shapiro M., Trogan E., Fisher E.A. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc. Natl. Acad. Sci. USA. 2003;100(23):13531–13536. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shanahan C.M., Cary N.R., Salisbury J.R., Proudfoot D., Weissberg P.L., Edmonds M.E. Medial localization of mineralization-regulating proteins in association with Monckeberg’s sclerosis. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- 56.Iyemere V.P., Proudfoot D., Weissberg P.L., Shanahan C.M. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J. Intern. Med. 2006;260(3):192–210. doi: 10.1111/j.1365-2796.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- 57.Speer M.Y., Yang H-Y., Brabb T., et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ. Res. 2009;104(6):733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Proudfoot D., Skepper J.N., Hegyi L., Bennett M.R., Shanahan C.M., Weissberg P.L. Apoptosis regulates human vascular calcification: in vitro evidence for initiation of vascular calcification by apoptotic bodies. Circ. Res. 2000;87:1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 59.Clarke M.C., Figg N., Maguire J.J., et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat. Med. 2006;12(9):1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 60.Henderson E.L., Geng Y.J., Sukhova G.K., Whittemore A.D., Knox J., Libby P. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99(1):96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- 61.Tabas I., Williams K.J., Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116(16):1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 62.Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 63.Bennett M.R., Sinha S., Owens G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016;118(4):692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bauriedel G., Hutter R., Welsch U., Bach R., Sievert H., Lüderitz B. Role of smooth muscle cell death in advanced coronary primary lesions: implications for plaque instability. Cardiovasc. Res. 1999;41(2):480–488. doi: 10.1016/s0008-6363(98)00318-6. [DOI] [PubMed] [Google Scholar]
- 65.Zhou J., Werstuck G.H., Lhotak S., et al. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemic apolipoprotein E-deficient mice. Circulation. 2004;110(2):207–213. doi: 10.1161/01.CIR.0000134487.51510.97. [DOI] [PubMed] [Google Scholar]
- 66.Khan M.I., Pichna B.A., Shi Y., Bowes A.J., Werstuck G.H. Evidence supporting a role for endoplasmic reticulum stress in the development of atherosclerosis in a hyperglycaemic mouse model. Antioxid. Redox Signal. 2009;11(9):2289–2298. doi: 10.1089/ars.2009.2569. [DOI] [PubMed] [Google Scholar]
- 67.Werstuck G.H., Khan M.I., Femia G., et al. Glucosamine-induced endoplasmic reticulum dysfunction is associated with accelerated atherosclerosis in a hyperglycemic mouse model. Diabetes. 2006;55(1):93–101. [PubMed] [Google Scholar]
- 68.Myoishi M., Hao H., Minamino T., et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116(11):1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 69.Pedruzzi E., Guichard C., Ollivier V., et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell. Biol. 2004;24(24):10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thorp E., Li G., Seimon T.A., Kuriakose G., Ron D., Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe-/- and Ldlr-/- mice lacking CHOP. Cell Metab. 2009;9(5):474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kedi X., Ming Y., Yongping W., Yi Y., Xiaoxiang Z. Free cholesterol overloading induced smooth muscle cells death and activated both ER- and mitochondrial-dependent death pathway. Atherosclerosis. 2009;207(1):123–130. doi: 10.1016/j.atherosclerosis.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 72.Larroque-Cardoso P., Swiader A., Ingueneau C., et al. Role of protein kinase C δ in ER stress and apoptosis induced by oxidized LDL in human vascular smooth muscle cells. Cell Death Dis. 2013;4(2):e520. doi: 10.1038/cddis.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Croons V., Martinet W., Herman A.G., de Meyer G.R. Differential effect of the protein synthesis inhibitors puromycin and cycloheximide on vascular smooth muscle cell viability. J. Pharmacol. Exp. Ther. 2008;325(3):824–832. doi: 10.1124/jpet.107.132944. [DOI] [PubMed] [Google Scholar]
- 74.Malabanan K.P., Kanellakis P., Bobik A., Khachigian L.M. Activation transcription factor-4 induced by fibroblast growth factor-2 regulates vascular endothelial growth factor-A transcription in vascular smooth muscle cells and mediates intimal thickening in rat arteries following balloon injury. Circ. Res. 2008;103(4):378–387. doi: 10.1161/CIRCRESAHA.107.168682. [DOI] [PubMed] [Google Scholar]
- 75.Zhou A.X., Wang X., Lin C.S., et al. C/EBP-homologous protein (CHOP) in vascular smooth muscle cells regulates their proliferation in aortic explants and atherosclerotic lesions. Circ. Res. 2015;116(11):1736–1743. doi: 10.1161/CIRCRESAHA.116.305602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshida T., Kaestner K.H., Owens G.K. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ. Res. 2008;102(12):1548–1557. doi: 10.1161/CIRCRESAHA.108.176974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shukla N., Wan S., Angelini G.D., Jeremy J.Y. Low nanomolar thapsigargin inhibits the replication of vascular smooth muscle cells through reversible endoplasmic reticular stress. Eur. J. Pharmacol. 2013;714(1-3):210–217. doi: 10.1016/j.ejphar.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 78.Yi N., Chen S.Y., Ma A., et al. Tunicamycin inhibits PDGF-BB-induced proliferation and migration of vascular smooth muscle cells through induction of HO-1. Anat. Rec. (Hoboken) 2012;295(9):1462–1472. doi: 10.1002/ar.22539. [DOI] [PubMed] [Google Scholar]
- 79.Matsushita E., Asai N., Enomoto A., et al. Protective role of Gipie, a Girdin family protein, in endoplasmic reticulum stress responses in endothelial cells. Mol. Biol. Cell. 2011;22(6):736–747. doi: 10.1091/mbc.E10-08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Noda T., Maeda K., Hayano S., et al. New endoplasmic reticulum stress regulator, Gipie, regulates the survival of vascular smooth muscle cells and the neointima formation after vascular injury. Arterioscler. Thromb. Vasc. Biol. 2015;35(5):1246–1253. doi: 10.1161/ATVBAHA.114.304923. [DOI] [PubMed] [Google Scholar]
- 81.Zhou B., Li H., Liu J., et al. Intermittent injections of osteocalcin reverse autophagic dysfunction and endoplasmic reticulum stress resulting from diet-induced obesity in the vascular tissue via the NFkappaB-p65-dependent mechanism. Cell Cycle. 2013;12(12):1901–1913. doi: 10.4161/cc.24929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Idelevich A., Rais Y., Monsonego-Ornan E. Bone Gla protein increases HIF-1α–dependent glucose metabolism and induces cartilage and vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2011;31(9):e55–e71. doi: 10.1161/ATVBAHA.111.230904. [DOI] [PubMed] [Google Scholar]
- 83.Wu X., Qi Y.F., Chang J.R., et al. Possible role of fibroblast growth factor 21 on atherosclerosis via amelioration of endoplasmic reticulum stress-mediated apoptosis in apoE(-/-) mice. Heart Vessels. 2015;30(5):657–668. doi: 10.1007/s00380-014-0557-9. [DOI] [PubMed] [Google Scholar]
- 84.Ye Y.L., Fu F., Li X.M., Yang J., Liu H.M. Selenoprotein S is highly expressed in the blood vessels and prevents vascular smooth muscle cells from apoptosis. J. Cell. Biochem. 2016;117(1):106–117. doi: 10.1002/jcb.25254. [DOI] [PubMed] [Google Scholar]
- 85.Nonaka H., Tsujino T., Watari Y., Emoto N., Yokoyama M. Taurine prevents the decrease in expression and secretion of extracellular superoxide dismutase induced by homocysteine: amelioration of homocysteine-induced endoplasmic reticulum stress by taurine. Circulation. 2001;104(10):1165–1170. doi: 10.1161/hc3601.093976. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y.M., Wang X., Nawaz A., et al. Wogonin ameliorates lipotoxicity-induced apoptosis of cultured vascular smooth muscle cells via interfering with DAG-PKC pathway. Acta Pharmacol. Sin. 2011;32(12):1475–1482. doi: 10.1038/aps.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morrell N.W., Adnot S., Archer S.L., et al. Cellular and molecular basis of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009;54(1):S20–S31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang B., Wang S., Wang Q., et al. Aberrant endoplasmic reticulum stress in vascular smooth muscle increases vascular contractility and blood pressure in mice deficient of AMP-activated protein kinase-alpha2 in vivo. Arterioscler. Thromb. Vasc. Biol. 2013;33(3):595–604. doi: 10.1161/ATVBAHA.112.300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spitler K.M., Webb R.C. Endoplasmic reticulum stress contributes to aortic stiffening via proapoptotic and fibrotic signaling mechanisms. Hypertension. 2014;63(3):e40–e45. doi: 10.1161/HYPERTENSIONAHA.113.02558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palao T., Sward K., Jongejan A., et al. Gene expression and microRNA expression analysis in small arteries of spontaneously hypertensive rats. Evidence for ER stress. PLoS One. 2015;10(9):16. doi: 10.1371/journal.pone.0137027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng W.P., Wang B.W., Chen S.C., Chang H., Shyu K.G. Mechanical stretch induces the apoptosis regulator PUMA in vascular smooth muscle cells. Cardiovasc. Res. 2012;93(1):181–189. doi: 10.1093/cvr/cvr280. [DOI] [PubMed] [Google Scholar]
- 92.Wan X.J., Zhao H.C., Zhang P., et al. Involvement of BK channel in differentiation of vascular smooth muscle cells induced by mechanical stretch. Int. J. Biochem. Cell Biol. 2015;59:21–29. doi: 10.1016/j.biocel.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 93.Sutendra G., Dromparis P., Wright P., et al. The role of Nogo and the mitochondria–endoplasmic reticulum unit in pulmonary hypertension. Sci. Transl. Med. 2011;3(88):88ra55. doi: 10.1126/scitranslmed.3002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Munoz J.P., Zorzano A. Endoplasmic reticulum stress enters a Nogo zone. Sci. Transl. Med. 2011;3(88):4. doi: 10.1126/scitranslmed.3002708. [DOI] [PubMed] [Google Scholar]
- 95.Crnkovic S., Riederer M., Lechleitner M., et al. Docosahexaenoic acid-induced unfolded protein response, cell cycle arrest, and apoptosis in vascular smooth muscle cells are triggered by Ca(2)(+)-dependent induction of oxidative stress. Free Radic. Biol. Med. 2012;52(9):1786–1795. doi: 10.1016/j.freeradbiomed.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Michel J.B., Martin-Ventura J.L., Egido J., et al. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc. Res. 2011;90(1):18–27. doi: 10.1093/cvr/cvq337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Obama T., Tsuji T., Kobayashi T., et al. Epidermal growth factor receptor inhibitor protects against abdominal aortic aneurysm in a mouse model. Clin. Sci. (Lond.) 2015;128(9):559–565. doi: 10.1042/CS20140696. [DOI] [PubMed] [Google Scholar]
- 98.Takayanagi T., Kawai T., Forrester S.J., et al. Role of epidermal growth factor receptor and endoplasmic reticulum stress in vascular remodeling induced by angiotensin II. Hypertension. 2015;65(6):1349–1355. doi: 10.1161/HYPERTENSIONAHA.115.05344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jia L.X., Zhang W.M., Zhang H.J., et al. Mechanical stretch-induced endoplasmic reticulum stress, apoptosis and inflammation contribute to thoracic aortic aneurysm and dissection. J. Pathol. 2015;236(3):373–383. doi: 10.1002/path.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang J., Wang T., Wright A.C., et al. Myocardin is required for maintenance of vascular and visceral smooth muscle homeostasis during postnatal development. Proc. Natl. Acad. Sci. USA. 2015;112(14):4447–4452. doi: 10.1073/pnas.1420363112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kwartler C.S., Chen J.Y., Thakur D., et al. Overexpression of smooth muscle myosin heavy chain leads to activation of the unfolded protein response and autophagic turnover of thick filament-associated proteins in vascular smooth muscle cells. J. Biol. Chem. 2014;289(20):14075–14088. doi: 10.1074/jbc.M113.499277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Demer L.L., Tintut Y. Vascular calcification: Pathobiology of a multifaceted disease. Circulation. 2008;117(22):2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kapustin A.N., Shanahan C.M. Calcium regulation of vascular smooth muscle cell–derived matrix vesicles. Trends Cardiovasc. Med. 2012;22(5):133–137. doi: 10.1016/j.tcm.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 104.Rennenberg R.J., Kessels A.G., Schurgers L.J., Van Engelshoven J.M., De Leeuw P.W., Kroon A.A. Vascular calcifications as a marker of increased cardiovascular risk: A meta-analysis. Vasc. Health Risk Manag. 2009;5:185–197. doi: 10.2147/vhrm.s4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sage A.P., Tintut Y., Demer L.L. Regulatory mechanisms in vascular calcification. Nat. Rev. Cardiol. 2010;7(9):528–536. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Karwowski W., Naumnik B., Szczepański M., Myśliwiec M. The mechanism of vascular calcification - a systematic review. Med. Sci. Monit. 2012;18(1):RA1–RA11. doi: 10.12659/MSM.882181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Proudfoot D., Shanahan C.M. Biology of calcification in vascular cells: intima versus media. Herz. 2001;26(4):245–251. doi: 10.1007/pl00002027. [DOI] [PubMed] [Google Scholar]
- 108.London G.M. Arterial calcification: cardiovascular function and clinical outcome. Nefrologia. 2011;31(6):644–647. doi: 10.3265/Nefrologia.pre2011.Oct.11175. [DOI] [PubMed] [Google Scholar]
- 109.Shanahan C.M. Vascular calcification. Curr. Opin. Nephrol. Hypertens. 2005;14:361–367. doi: 10.1097/01.mnh.0000172723.52499.38. [DOI] [PubMed] [Google Scholar]
- 110.Shroff R.C., Shanahan C.M. Vascular biology of calcification. Semin. Dial. 2007;20(2):103–109. doi: 10.1111/j.1525-139X.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- 111.Shanahan C.M., Crouthamel M.H., Kapustin A., Giachelli C.M. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ. Res. 2011;109(6):697–711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kapustin A.N., Chatrou M.L., Drozdov I., et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ. Res. 2015;116(8):1312–1323. doi: 10.1161/CIRCRESAHA.116.305012. [DOI] [PubMed] [Google Scholar]
- 113.Luo G., Ducy P., McKee M.D., et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386(6620):78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 114.Ketteler M., Bongartz P., Westenfeld R., et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361(9360):827–833. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 115.Liu Y., Drozdov I., Shroff R., Beltran L.E., Shanahan C.M. Prelamin A accelerates Vascular Calcification Via Activation of the DNA Damage Response and Senescence-associated secretory phenotype in vascular smooth muscle cells. Circ. Res. 2013;112(10):e99–e109. doi: 10.1161/CIRCRESAHA.111.300543. [DOI] [PubMed] [Google Scholar]
- 116.Neven E., Dauwe S., De Broe M.E., D'Haese P.C., Persy V. Endochondral bone formation is involved in media calcification in rats and in men. Kidney Int. 2007;72(5):574–581. doi: 10.1038/sj.ki.5002353. [DOI] [PubMed] [Google Scholar]
- 117.Qiao J.H., Mertens R.B., Fishbein M.C., Geller S.A. Cartilaginous metaplasia in calcified diabetic peripheral vascular disease: morphologic evidence of enchondral ossification. Hum. Pathol. 2003;34(4):402–407. doi: 10.1053/hupa.2003.72. [DOI] [PubMed] [Google Scholar]
- 118.Karsenty G., Kronenberg H.M., Settembre C. Genetic control of bone formation. Annu. Rev. Cell Dev. Biol. 2009;25:629–648. doi: 10.1146/annurev.cellbio.042308.113308. [DOI] [PubMed] [Google Scholar]
- 119.Michigami T. Regulatory mechanisms for the development of growth plate cartilage. Cell. Mol. Life Sci. 2013;70(22):4213–4221. doi: 10.1007/s00018-013-1346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Anderson H.C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 2003;5(3):222–226. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- 121.Sun Y., Byon C.H., Yuan K., et al. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ. Res. 2012;111(5):543–552. doi: 10.1161/CIRCRESAHA.112.267237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Taylor J., Butcher M., Zeadin M., Politano A., Shaughnessy S.G. Oxidized low-density lipoprotein promotes osteoblast differentiation in primary cultures of vascular smooth muscle cells by up-regulating osterix expression in an Msx2-dependent manner. J. Cell. Biochem. 2011;112:581–588. doi: 10.1002/jcb.22948. [DOI] [PubMed] [Google Scholar]
- 123.Nakagawa Y., Ikeda K., Akakabe Y., et al. Paracrine osteogenic signals via bone morphogenetic protein-2 accelerate the atherosclerotic intimal calcification in vivo. Arterioscler. Thromb. Vasc. Biol. 2010;30(10):1908–1915. doi: 10.1161/ATVBAHA.110.206185. [DOI] [PubMed] [Google Scholar]
- 124.Shao J.S., Cheng S.L., Pingsterhaus J.M., Charlton-Kachigian N., Loewy A.P., Towler D.A. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J. Clin. Invest. 2005;115(5):1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tohmonda T., Yoda M., Mizuochi H., et al. The IRE1alpha-XBP1 pathway positively regulates parathyroid hormone (PTH)/PTH-related peptide receptor expression and is involved in pth-induced osteoclastogenesis. J. Biol. Chem. 2013;288(3):1691–1695. doi: 10.1074/jbc.C112.424606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo F.J., Han X.F., Wu Z.M., et al. ATF6a, a Runx2-activable transcription factor, is a new regulator of chondrocyte hypertrophy. J. Cell Sci. 2016;129(4):717–728. doi: 10.1242/jcs.169623. [DOI] [PubMed] [Google Scholar]
- 127.Wei J., Sheng X., Feng D., McGrath B., Cavener D.R. PERK is essential for neonatal skeletal development to regulate osteoblast proliferation and differentiation. J. Cell. Physiol. 2008;217:693–707. doi: 10.1002/jcp.21543. [DOI] [PubMed] [Google Scholar]
- 128.Saito A., Hino S.I., Murakami T., et al. Regulation of endoplasmic reticulum stress response by a BBF2H7-mediated Sec23a pathway is essential for chondrogenesis. Nat. Cell Biol. 2009;11(10):1197–1204. doi: 10.1038/ncb1962. [DOI] [PubMed] [Google Scholar]
- 129.Murakami T., Saito A., Hino S., et al. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat. Cell Biol. 2009;11(10):1205–1211. doi: 10.1038/ncb1963. [DOI] [PubMed] [Google Scholar]
- 130.Duan X., Zhou Y., Teng X., Tang C., Qi Y. Endoplasmic reticulum stress-mediated apoptosis is activated in vascular calcification. Biochem. Biophys. Res. Commun. 2009;387(4):694–699. doi: 10.1016/j.bbrc.2009.07.085. [DOI] [PubMed] [Google Scholar]
- 131.Duan X.H., Chang J.R., Zhang J., et al. Activating transcription factor 4 is involved in endoplasmic reticulum stress-mediated apoptosis contributing to vascular calcification. Apoptosis. 2013;18(9):1132–1144. doi: 10.1007/s10495-013-0861-3. [DOI] [PubMed] [Google Scholar]
- 132.Masuda M., Miyazaki-Anzai S., Levi M., Ting T.C., Miyazaki M. PERK-eIF2alpha-ATF4-CHOP signaling contributes to TNFalpha-induced vascular calcification. J. Am. Heart Assoc. 2013;2(5):e000238. doi: 10.1161/JAHA.113.000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miyazaki-Anzai S., Masuda M., Demos-Davies K.M., et al. Endoplasmic reticulum stress effector CCAAT/enhancer-binding protein homologous protein (CHOP) regulates chronic kidney disease-induced vascular calcification. J. Am. Heart Assoc. 2014;3(3):e000949. doi: 10.1161/JAHA.114.000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Masuda M., Miyazaki-Anzai S., Keenan A.L., et al. Saturated phosphatidic acids mediate saturated fatty acid-induced vascular calcification and lipotoxicity. J. Clin. Invest. 2015;125(12):4544–4558. doi: 10.1172/JCI82871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Masuda M., Ting T.C., Levi M., Saunders S.J., Miyazaki-Anzai S., Miyazaki M. Activating transcription factor 4 regulates stearate-induced vascular calcification. J. Lipid Res. 2012;53(8):1543–1552. doi: 10.1194/jlr.M025981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liberman M., Johnson R.C., Handy D.E., Loscalzo J., Leopold J.A. Bone morphogenetic protein-2 activates NADPH oxidase to increase endoplasmic reticulum stress and human coronary artery smooth muscle cell calcification. Biochem. Biophys. Res. Commun. 2011;413:436–441. doi: 10.1016/j.bbrc.2011.08.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Serrano R.L., Yu W., Terkeltaub R. Mono-allelic and bi-allelic ENPP1 deficiency promote post-injury neointimal hyperplasia associated with increased C/EBP homologous protein expression. Atherosclerosis. 2014;233(2):493–502. doi: 10.1016/j.atherosclerosis.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chang J.R., Duan X.H., Zhang B.H., et al. Intermedin1-53 attenuates vascular smooth muscle cell calcification by inhibiting endoplasmic reticulum stress via cyclic adenosine monophosphate/protein kinase A pathway. Exp. Biol. Med. (Maywood) 2013;238(10):1136–1146. doi: 10.1177/1535370213502619. [DOI] [PubMed] [Google Scholar]
- 139.Liu H., Li X., Qin F., Huang K. Selenium suppresses oxidative-stress-enhanced vascular smooth muscle cell calcification by inhibiting the activation of the PI3K/AKT and ERK signaling pathways and endoplasmic reticulum stress. J. Biol. Inorg. Chem. 2014;19(3):375–388. doi: 10.1007/s00775-013-1078-1. [DOI] [PubMed] [Google Scholar]
- 140.Lu Y., Bian Y.F., Wang Y.R., Bai R., Wang J.P., Xiao C.S. Globular adiponectin reduces vascular calcification via inhibition of ER-stress-mediated smooth muscle cell apoptosis. Int. J. Clin. Exp. Pathol. 2015;8(3):2545–2554. [PMC free article] [PubMed] [Google Scholar]
- 141.Krishnan N., Fu C., Pappin D.J., Tonks N.K. H2S-induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 2011;4(203):ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]