Abstract
Virus persistence in chronic hepatitis B patients is due to the sustaining level of covalently closed circular DNA (cccDNA) within the nuclei of infected hepatocytes. In this study, we used a modified 1.3-fold hepatitis B virus (HBV) genome, with a BclI genetic marker embedded in the redundancy region, to examine the transcriptional activity of cccDNA and the effect of the HBx protein on transcriptional regulation. After harvesting total RNA from transfected cells or stable lines, we specifically identified and monitored the transcripts from cccDNA by using reverse transcription-PCR (RT-PCR) combined with the restriction enzyme digestion method. In this approach, we have found that (i) RT-PCR combined with detection of the BclI marker is a highly specific method for distinguishing cccDNA-derived transcripts from the original integrated viral genome, (ii) the transcriptional ability of cccDNA was less efficient than that from the integrated viral genome, and (iii) the transcriptional activity of cccDNA was significantly regulated by the HBx protein, a potential transcription activator. In conclusion, we provided a tool with which to elucidate the transcriptional regulation of cccDNA and clarified the transcriptional regulation mechanism of HBx on cccDNA. The results obtained may be helpful in the development of a clinical intervention for patients with chronic HBV infections.
Chronic hepatitis B (CHB) is one of the most serious viral infections of humans worldwide. More than 350 million people in the world suffer from chronic hepatitis B virus (HBV) infection, and the number is still increasing (11, 20). CHB is also a high risk factor for cirrhosis and hepatocellular carcinoma (29). CHB patients maintain a pool of covalently closed circular DNA (cccDNA) in the nuclei of infected hepatocytes which presumably serves as the template for HBV gene expression (40). Within the HBV-infected hepatocyte, the pregenomic RNA (pgRNA) is transcribed from cccDNA, which has been reverse transcribed into the relaxed-circle (RC) form of viral DNA within the viral particles (36). The mature core particles can either be secreted out of the cell through the endoplasmic reticulum or reenter the nuclei for amplification of their own cccDNA pool (12, 34, 42). In addition to its role in the initial step of transcription, studies suggest that HBV cccDNA may also be involved in virus persistence in CHB patients. (−)-2′,3′-Dideoxy-3′-thiacytidine (lamivudine), an approved oral drug, is a nucleoside analogue that has been shown to effectively inhibit HBV replication in patients (22, 27). However, cessation of drug treatment resulted in the rapid reappearance of HBV DNA in the serum (1, 24). In vitro studies suggested that cccDNA might be responsible for the recurrence of HBV (1, 28). Thus, cccDNA may play a pivotal role in the life cycle of HBV. However, the metabolism of cccDNA and its function in HBV replication are still poorly understood. Moreover, no suitable model system in which to study the transcriptional regulation of cccDNA is available.
HBV-encoded X protein is a candidate transcriptional activator involved in the regulation of gene expression. Although the detailed mechanisms by which HBx transactivates gene expression are just beginning to emerge, several lines of evidence have demonstrated that the viral HBx protein is capable of activating a wide range of viral and cellular gene expression (8, 9, 30). Reports have suggested that HBx might stimulate transcription through a direct interaction with components of the transcriptional machinery. Indeed, it has been shown recently that HBx can bind to the RPB5 subunit of RNA polymerase II and stimulate transcription (25). Moreover, HBx has been suggested to interact not only with general transcription factors, including TFIIB, TFIID, and TFIIH (25, 26, 31, 39), but also with upstream transcription factors, such as C/EBP, CREB, ATF-2, and p53 (7, 13, 14, 23, 39). Also, HBx has been reported to be capable of activating a further diverse group of transcription factors, including AP-1, AP-2, NF-κB, ATP/CREB, SRF, and SP1 (4, 5, 9, 16, 19, 21, 41). Interestingly, several transcription factor binding elements responsive to HBx-activated transcription factors have been identified within the viral genome or cccDNA (3, 13, 15, 26). It is quite possible that HBx may interact with cellular transcription factors and contribute to the transcriptional regulation of cccDNA. Nevertheless, the details of how HBx acts to modify the transcriptional activity of cccDNA are still unclear.
Our goal in this study was to understand the viral gene expression associated with cccDNA and any regulatory effect of HBx on cccDNA. We have introduced a BclI restriction site into the redundancy region of the viral genome and developed a reverse transcription-PCR (RT-PCR) method in combination with BclI digestion that allows us to distinguish the transcripts of cccDNA from those of the input plasmid and/or the integrated genome. By stably transfecting with a BclI-modified HBV genome, we have obtained several stable cccDNA-producing clones that permit us to examine the transcriptional activity of cccDNA. In addition, we have also investigated the transcriptional regulation of HBx protein on cccDNA. A better understanding of viral gene regulation in CHB patients may be useful for the development of an intervention protocol in the future.
MATERIALS AND METHODS
Plasmid construction.
The BclI-harboring plasmids (p1.3HBcl-5, p1.3HBcl-3, and p1.3HBcl-5/3) were derived from plasmid pHBV1.3, containing a 1.3-fold HBV genome (ayw subtype [17]) in a modified pUC13 vector backbone, in which the transcription of pgRNA was controlled by the virus's own core promoter and enhancer I and II regulatory elements. The BclI restriction site was constructed by a single nucleotide substitution (nucleotide [nt] 3119; A to G) within the 5′-end redundancy region and/or the corresponding 3′-end redundancy region (nt 63; A to G). Plasmid p1.3SspI was constructed by a 9-base (TTAATATTT) in-frame insertion at nt 3129 of the HBV sequence on a p1.3HBcl-5 vector backbone. p1.3HBcl-P2 was mutated from p1.3HBcl-5 (nt 2024; G to C) to generate a single-amino-acid substitution (amino acid 540; D to H) within the YMDD motif of viral polymerase to abolish its reverse transcription ability (32). p1.3HBclI-X− was constructed from p1.3HBcl-5 by a start codon mutation (nt 2656; ATG to TTG) and a point mutation (nt 2966; TCA to TGA) to create a stop codon within the open reading frame of HBx without any effect on the coding of the Pol gene. The HBx mutant plasmid pX-Δ(100-115) has an internal deletion in the region corresponding to amino acid residues 100 to 115 of pX (2, 38). The numbers indicate the nucleotide number in the sequence of the full-length HBV genome; the start codon of HBc is nt 1.
Cell culture.
Stable HBV-producing cell lines were derived from HepG2 hepatoblastoma cells by stably transfecting with the plasmid containing the 1.3-fold HBV genome, which also contains the BclI genetic marker at either the 5′ or the 3′ terminus, and were then selected with 1 mg of G418/ml. Cells were maintained in Iscove's modified Dulbecco's modified Eagle's medium (Gibco-BRL) containing 10% fetal calf serum (Gibco-BRL), 2 mM l-glutamine, 1% nonessential amino acids, 100 IU of penicillin, 100 μg of streptomycin/ml, and 2.5 μg of amphotericin B (Fungizone)/ml at 37°C in a 5% CO2 incubator.
RNA isolation and Northern blot analysis.
In the transient transfection experiments, cells were harvested 3 days posttransfection and total RNAs were extracted by using a Trizol RNA extraction kit (Invitrogen). Fifteen micrograms of total RNA was separated on 1.2% formaldehyde agarose gels by electrophoresis. After electrophoresis, the RNA samples were transferred to nylon membranes. After UV cross-linking, the membranes were prehybridized at 42°C for 4 h in prehybridization solution (5× SSPE [1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA {pH 7.7}], 0.5% sodium dodecyl sulfate [SDS], 10× Denhardt's solution, 400 μg of salmon sperm DNA/ml, and 100 μg of tRNA/ml in 50% formamide) and then hybridized in hybridization solution (3.5× SSPE, 0.5% SDS, 10× Denhardt's solution, 8% dextran sulfate, 300 μg of salmon sperm DNA/ml, and 100 μg of tRNA/ml in 50% formamide) with a 32P-radiolabeled DNA probe (2 × 108 cpm/μl; prepared using random oligonucleotide priming of the whole HBV genome or the glyceraldehyde-3-phosphate dehydrogenase [GAPDH] gene). After 16 h of hybridization, the membranes were washed three times with 0.2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% SDS at 52°C (20 min each time) and then exposed to X-ray film for 16 h at −80°C.
Hirt extraction.
To isolate cccDNA from stable HBV transfectants, we used a previously described procedure with modifications (43). Cells were washed twice with ice-cold GKNP, and the residual washing solution was then removed as completely as possible. Cells were lysed by addition of 3 ml of Hirt solution (0.6% SDS, 10 mM EDTA, 10 mM Tris-HCl [pH 7.5]) for 5 min. After complete lysis of the cells, 750 μl of 5 M NaCl was added to the cell lysate and mixed gently. After the whole mixture was incubated on ice overnight, the insoluble components were pelleted by centrifuging at 3,000 × g for 15 min at 4°C. The supernatant, which contained the cccDNA, was extracted twice with phenol and once with phenol-chloroform and then precipitated by adding 2 volumes of absolute ethanol.
Isolation of cytoplasmic replicative intermediates and Southern blot analysis.
Transiently transfected cells were harvested 3 days after plasmid transfection. Cells were washed twice with ice-cold GKNP, then resuspended in 3 ml of NET buffer (10 mM Tris-Hcl [pH 8.0], 1 mM EDTA, 100 mM NaCl, 0.5% Nonidet P-40) containing 5 mM MgCl2 and kept at room temperature for 10 min. The cell lysate was centrifuged at 3,000 × g for 4 min to remove nuclei. The remaining supernatant was treated with 10 μg of DNase I (Roche)/ml and 20 μg of RNase A (Roche)/ml at 37°C for 1 h. Following DNase I and RNase A treatment, the sample was immunoprecipitated with anti-HBc antiserum (from patient serum) and then treated with 50 mM EDTA, followed by digestion with 50 μg of proteinase K (Roche)/ml at 50°C overnight. The supernatant was extracted twice with phenol, once with phenol-chloroform, and once with chloroform. Finally, the cytoplasmic viral replicative intermediates were precipitated with ethanol and dissolved in 1× TE (0.1 mM EDTA, 1 mM Tris-HCl [pH 8.0]). DNA samples were separated on 1.2% agarose gels by electrophoresis. The loaded nucleic acid was normalized to equal amounts by adjusting according to the cell numbers. After electrophoresis, the agarose gel was soaked in denaturing buffer (0.5 M NaOH, 1.5 M NaCl) twice for 15 min each time and then neutralized with neutralizing buffer (10 M Tris-HCl [pH 8.0], 1.5 M NaCl). The DNA samples were then transferred to nylon membranes (Hybond-XL; Amersham Pharmacia Biotech) and UV cross-linked. The membranes were prehybridized at 42°C for 4 h in prehybridization solution and then hybridized in hybridization solution with a 32P-radiolabeled DNA probe (2 × 108 cpm/μl; prepared using random oligonucleotide priming of the whole HBV genome). After 16 h of hybridization, membranes were washed three times (20 min for each time) with 0.2× SSC and 0.1% SDS at 52°C and then exposed to X-ray film for 16 h at −80°C.
Chromosomal DNA preparation.
To obtain chromosomal DNA with less contamination of HBV replicative intermediates and fewer cccDNAs, cells were seeded loosely and pretreated with 20 μM lamivudine at intervals of 3 days for a period of 7 to 10 days. Cells were then rinsed with GKNP twice, scraped with lifter, transferred to a 15-ml tube, and centrifuged at 3,000 × g for 5 min. Cell pellets were resuspended in 0.5 ml of NET buffer containing 5 mM MgCl2. The nuclei were recovered by centrifugation of the mixture at 3,000 × g for 5 min, and the pellet was resuspended in NET buffer containing 5 mM MgCl2. This procedure was repeated twice to remove the cytoplasmic HBV replication intermediates. The washed nuclei were resuspended in NET buffer supplemented with 0.5% SDS and 200 μg of proteinase K/ml and were incubated at 50°C overnight. The DNA mixture was then extracted with phenol, phenol-chloroform, and chloroform (once for each extraction). Finally, the DNA was precipitated with alcohol combined with sodium acetate. After centrifugation, the pellet was washed with 70% alcohol and dried in air. The pellet was dissolved completely in 0.1× TE for Southern blot analysis.
RT-PCR and restriction enzyme digestion.
cDNA templates were obtained by reverse transcription of total mRNA with oligo(dT) 18-mers and SuperScript II reverse transcriptase (Invitrogen); then they were amplified by PCR with primer pair HBV2338/F′ (AGCGTGGTTATCCTGCGTTGATG)-T20-Taq/HBV5 (GCGGCCGCCCTGCAGTTTTTTTTTTTTTTTTTTTTAGCTC). The thermocycling parameters were 94°C for 1 min; 5 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 30 s; 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s; and 72°C for 10 min. The PCR products were eluted from an agarose gel after electrophoresis and subjected to BclI/SspI double digestion. To reveal the relative amounts of RT-PCR products from the integrated genome or cccDNA, the restricted samples were separated again by electrophoresis on a 2% agarose gel. The amounts of 603-bp fragments were monitored as a loading control for each lane.
RESULTS
Strategy for indirect detection of viral transcripts from HBV cccDNA.
The current cell model for studying HBV replication cannot distinguish between cccDNA-derived transcripts and transcripts from a transfected plasmid (or the integrated genome). In order to identify the viral transcripts from cccDNA, a BclI restriction enzyme site was introduced into the 5′ terminus of the redundancy region of a 1.3-fold HBV genome to generate plasmid p1.3HBcl-5. The BclI genetic marker was designed to trace the transcriptional origin (cccDNA or transfected plasmid) of the viral transcripts. In this case, viral pgRNA transcribed from plasmid p1.3HBcl-5 was identified by the BclI restriction site at the 5′ terminus only, not at the 3′ terminus. However, there are two BclI genetic markers, at both the 5′ and the 3′ termini, in transcripts derived from cccDNA. Based on the current HBV replication model, a large portion of the pgRNA is removed by viral RNase H activity during reverse transcription (18). The 5′-terminal redundant region, which contains the BclI marker in p1.3HBcl-5 transcripts, is retained to act as a primer for plus-strand DNA synthesis (33). After double-stranded DNA synthesis and cccDNA formation, the BclI genetic marker is retained within the region between DR1 and epsilon. Transcripts from cccDNA overlap this region to form the 5′- and 3′-terminal redundancies, and both contain BclI markers. Consequently, the two populations of viral RNAs are identifiable: that derived from cccDNA contains the BclI restriction site at both the 5′- and 3′-terminal redundant regions, while that derived from the transfected plasmid contains only one BclI genetic marker, at the 5′ terminus.
Since the two populations of viral transcripts can be distinguished by the BclI marker at their 3′ termini, we designed a pair of PCR primers (HBV2338/F and T20-Tag/HBV5) to specifically amplify the 3′ termini of HBV transcripts. After RT-PCR amplification, the size of the PCR product is 935 bp. By use of SspI/BclI double digestion, the transcriptional origin of viral transcripts (plasmid or cccDNA) can be easily determined. The PCR product is cut into two bands of 603 and 332 bp by SspI digestion. If a BclI site is present, the 332-bp product is further cut into two fragments of 200 and 132 bp after BclI digestion. Thus, the amounts of 200- and 132-bp DNA fragments present will be proportional to the amount of mRNA derived from the cccDNA carrying the BclI genetic marker at the 3′ terminus of redundancy (Fig. 1). Since both the cccDNA- and plasmid-derived transcripts generate the same 603-bp fragment after RT-PCR combined with SspI restriction, equal amounts of the 603-bp fragment in each lane can serve as a control for identical loadings. In the preliminary study, we had transiently transfected plasmid mixtures (pHBV1.3 and p1.3HBcl-3) with different ratios in the presence of 20 μM lamivudine (which prevents cccDNA formation) and analyzed the plasmid-derived viral transcripts by RT-PCR and a BclI restriction assay. Our data demonstrated that the relative signals of restricted RT-PCR DNA fragments on the agarose gel were proportional to the amounts of RNA from the different ratios of input plasmids (data not shown).
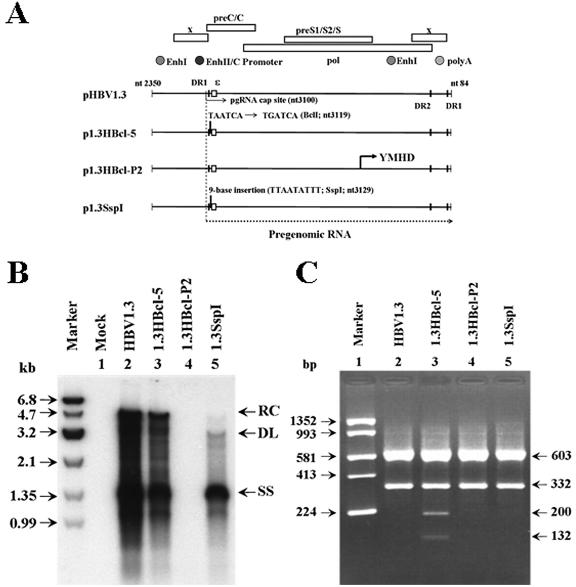
FIG. 1.
Schematic representation of the strategy for detection of origins of viral transcripts. For p1.3HBcl-5 transient transfection, the plasmid, containing a 1.3-fold HBV genome with the BclI genetic marker in the 5′ terminus redundancy, is introduced into HepG2 cells. Viral transcripts from the plasmid have only one BclI marker within the 5′ terminus redundancy region, while transcripts derived from cccDNA generate a terminally repeated sequence where the embedded BclI site is presented at both the 5′ and 3′ ends. The PCR primers (HBV2338/F and T20-Taq/HBV5) are designed to specifically amplify the 3′ terminus of the HBV transcript, and the size of the resulting PCR product is 935 bp. After restriction enzyme digestion with SspI and BclI, the PCR product is divided into three bands of 603, 200, and 132 bp. After 2% agarose gel electrophoresis, the restricted PCR products are separated with a equal amount of the 603-bp fragment as a loading control. The 332-bp product, which lacks the BclI site, represents transcripts from the integrated plasmid, whereas the two fragments of 200 and 132 bp represent transcripts from cccDNA.
The RT-PCR-BclI digestion method can distinguish between cccDNA-derived and plasmid-derived viral transcripts in transient transfection experiments.
To verify that viral transcripts originated from cccDNA, we examined the RT-PCR-BclI digestion method by using several plasmids containing the wild-type or modified 1.3-fold HBV genome in a transient transfection system (Fig. 2A). In addition to pHBV1.3 (wild-type HBV) and p1.3HBcl-5 (HBV with the BclI genetic marker in its 5′ redundancy region), two control plasmids, p1.3SspI and p1.3HBcl-P2, were also used. p1.3SspI and p1.3HBcl-P2 were constructed with p1.3HBcl-5 as the backbone vector and are unable to generate cccDNA. Plasmid p1.3SspI contains a 9-base insertion (with an SspI restriction site) at nt 3129 of the HBV genome, and p1.3HBcl-P2 is derived from p1.3HBcl-5 by a single-amino-acid substitution in the YMDD motif (YMDD to YMHD) (32). After transient transfection of plasmids into HepG2 cells, Southern blot analysis of the HBV replicative intermediates revealed that the viral replication pattern of p1.3HBcl-5 was similar to that of wild-type pHBV1.3 (Fig. 2B, lane 3), whereas p1.3SspI transfectants displayed primarily the duplex linear (DL) form of DNA and large amounts of single-stranded molecules (Fig. 2B, lane 5). Accordingly, p1.3SspI-transfected HepG2 cells could not produce cccDNA in the absence of its precursor, RC DNA. Thus, the RNA prepared from p1.3SspI-transfected HepG2 cells would be an appropriate control for the RT-PCR and BclI digestion analyses. A similar cccDNA-null control could be produced by using the p1.3HBcl-P2-transfectant. This YMHD mutant has a significant defect in the reverse transcription ability of the HBV polymerase (32) which can block viral replication and cccDNA production (Fig. 2B, lane 4). To verify the transcriptional ability of cccDNA, HepG2 cells were transfected with pHBV1.3, p1.3HBcl-5, p1.3HBcl-P2, or p1.3SspI individually, and their total RNAs were extracted at day 3 posttransfection. Northern blot analysis showed that the expression profiles of the HBV mRNAs were similar in all transfectants (data not shown). After RT-PCR amplification, whether the BclI marker was present at the 3′ end of viral transcripts was demonstrated by SspI/BclI double digestion. The appearance of 200- and 132-bp fragments suggested that partial viral mRNAs in the p1.3HBcl-5 transfectant were specifically transcribed from cccDNA (Fig. 2C, lane 3). When cDNA prepared from the p1.3HBcl-P2 or p1.3SspI transfectant was used, the 332-bp fragment could not be digested by BclI, showing that the 3′-terminal BclI marker is undetectable (Fig. 2C, lanes 4 and 5). Therefore, it is likely that those transfectants which lost their ability to generate the cccDNA molecule have also lost their ability to transfer the 5′-terminal BclI marker to the 3′-terminal redundant region. Thus, the results suggested that our method was able to specifically detect the transcripts transcribed from cccDNA by monitoring the presence of the BclI marker at the 3′ ends of HBV transcripts.
FIG. 2.
Identification of viral transcripts from cccDNA by transient transfection analysis. (A) Diagrams of 1.3-fold HBV genome constructions. p1.3HBcl-5, derived from plasmid pHBV1.3 (with a 1.3-fold HBV genome), contains a BclI genetic marker within the 5′-end redundant region. p1.3HBcl-P2 and p1.3SspI are mutants based on p1.3HBcl-5, both of which have lost the ability to form cccDNA and therefore served as negative controls. (B) Individual constructs were transiently transfected into HepG2 cells. Three days posttransfection, viral replicative intermediates were extracted and analyzed for their replication abilities by Southern blot analysis. (C) Total RNA of each transfectant was extracted for RT-PCR using the primer pair HBV2338/F-T20-Taq/HBV5. Following SspI/BclI double digestion, the restricted PCR products were separated by electrophoresis.
Establishment and characterization of stable HBV transfectants with an embedded BclI marker.
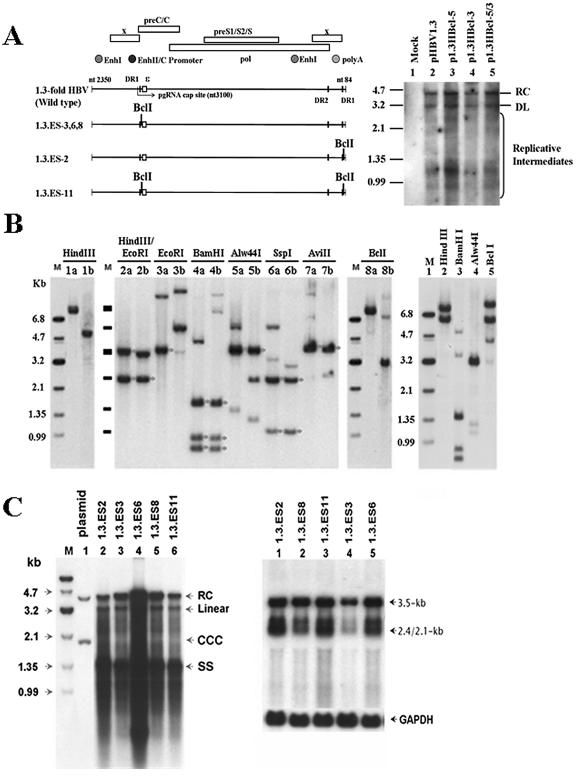
Next, we generated several BclI-modified plasmids with a 1.3-fold HBV genome and evaluated their replication capacities (Fig. 3A). Southern blotting of transient transfectants demonstrated that the viral replication abilities of those plasmids with the BclI mutation in the 3′ position only (p1.3HBcl-3) and in both the 5′ and 3′ positions (p1.3HBcl-5/3) were comparable to those of pHBV1.3 and p1.3HBcl-5 (Fig. 3A, right panel). This suggests that all BclI-bearing mutants could replicate as normally as wild-type HBV. Since the input plasmid would interfere with the observation of cccDNA in the transient transfection experiments, several HBV-producing cell lines were produced by stably transfecting HepG2 cells with plasmids carrying the BclI genetic marker (Fig. 3A, left panel). According to the position of BclI within the integrated viral genome, three types of stable transfectants may be established. The stable cell lines 1.3.ES3, 1.3.ES6, and 1.3.ES8 could be distinguished by the inclusion of the BclI marker in the 5′-terminal redundancy region of the integrated viral genome, whereas the 1.3.ES2 cell line carried the BclI site at the 3′-terminal redundancy. A control cell line, 1.3.ES11, was established in which the BclI restriction sites were introduced into both the 5′-terminal and 3′-terminal redundant regions of the viral genome. Preliminary characterization of the chromosomal integration of the HBV genome revealed that a single integrated copy of the HBV genome could be detected in the 1.3.ES2, 1.3.ES3, 1.3.ES6, and 1.3.ES11 cell lines, since only a single band was observed after genomic digestion with HindIII, which is not a restriction site within the HBV genome (data not shown) (Fig. 3B, left panel). To examine the integrity of the integrated HBV genome, several restriction enzymes (EcoRI, BamHI, Alw44I, SspI, and AviII) that are able to cut the HBV genome were used to digest the chromosomal DNA from the 1.3.ES2, 1.3.ES8, and 1.3.ES11 cell lines (Fig. 3B, center and right panels). For the 1.3.ES2 cell line, two fragments, of 3.3 and 2.1 kb, were generated after cutting with HindIII and EcoRI (Fig. 3B, lane 2a), whereas in 1.3.ES11, fragments of 3.1 and 2.1 kb were detected after HindIII/EcoRI double digestion, suggesting that the proximal sequence located in the downstream region of HBV in 1.3.ES11 cells was missing after integration (Fig. 3B, lane 2b). However, the integrated HBV genomes in both cell lines are likely to be intact after restriction enzyme digestion (BamHI, Alw44I, SspI, and AviII) (Fig. 3B, lanes 4b to 7b), since the resulting restriction fragments were detected with their predicted sizes after hybridization with a full-length HBV probe (Fig. 3B, center panel). To examine whether both the 5′ and 3′ ends of the integrated HBV genome contained the BclI sites in 1.3.ES11 cells, chromosomal DNA was digested with BclI and then probed with the HBV sequence (Fig. 3B, lanes 8a and 8b). A 3.2-kb fragment was detected from 1.3.ES11 chromosomal DNA (Fig. 3B, lane 8b), suggesting that BclI sites had been acquired at both the 5′ and 3′ ends of the HBV genome during the establishment of the clone. The integrated HBV genome in 1.3.ES8 cells is also likely to be intact and contains two integration sites on the chromosome (Fig. 3B, right panel). As in the primary transient transfection experiment, Southern blot analysis of total DNA extracted from our stable cell lines revealed that those HBV-producing cell lines with an embedded BclI marker could support replicative-intermediate formation (Fig. 3C, left panel). Analysis of the viral RNA expression pattern by Northern blotting also showed the existence of both the viral 3.5-kb pgRNA and 2.1- to 2.4-kb HBs transcripts (Fig. 3C, right panel). Our data suggest that the replication capacities of these BclI-carrying stable HBV transfectants are as good as that of the wild-type HBV genome.
FIG. 3.
Characterization of stable HBV transfectants bearing the BclI marker(s) within a terminal repeated region(s). (A) (Left) Schematic representation of modified 1.3-fold HBV plasmids and the position(s) of their BclI genetic marker(s). (Right) The replication capacity of each BclI-modified plasmid (p1.3HBcl-5, p1.3HBcl-3, and p1.3HBcl-5/3) was examined and compared with that of wild-type HBV. Three days after transient transfection, the viral replicative intermediates were extracted for Southern blot analysis. (B) (Left and center) The chromosome-integrated features of 1.3.ES2 and 1.3.ES11 cells were determined by restriction enzyme digestion. The symbol “a” indicates the restriction enzyme digestion of genomic DNA extracted from 1.3ES2 cells, while “b” indicates genomic DNA prepared from 1.3ES11 cells. Bands marked with asterisks represent the predicted HBV restriction fragments after hybridization with an HBV-specific probe. (Right) Restriction enzyme digestion revealed the chromosome-integrated features of 1.3.ES8 cells. (C) (Left) Cytoplasmic viral replicative intermediates of BclI-bearing HBV lines were extracted to analyze their viral replication ability by Southern blotting. (Right) Total RNAs of various lines were extracted, and viral transcript expression profiles were determined by Northern blotting after probing with an HBV-specific probe. The expression of GAPDH was monitored as a loading control.
Determination of the viral transcriptional ability of cccDNA in stable HBV transfectants with an embedded BclI marker.
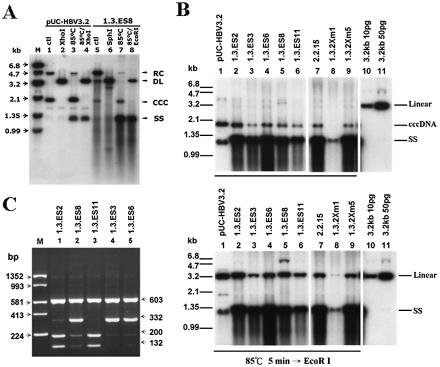
To demonstrate whether these HBV-producing cells could synthesize cccDNA in their nuclei, DNA was prepared from Hirt supernatants (without proteinase K digestion) and subjected to Southern blot analysis. A 3,182-bp control plasmid, pUC3.2-HBV, was constructed and served as a reference for cccDNA (supercoiled plasmid) and RC DNA (nicked plasmid). As shown in Fig. 4A, the pUC3.2-HBV plasmids (lane 1 to 4) and DNA prepared from Hirt supernatants of 1.3.ES8 cells (lane 5 to 8) could be separated on an agarose gel and detected by the HBV-specific probe. The upper band, migrating as 4.7 kb, represents the RC form of DNA, while the lower band, migrating as 2.0 kb, is the supercoiled form, or cccDNA. Both the RC DNA and cccDNA molecules shifted to the 3.2-kb position of DL DNA upon digestion with the single-cutting-site enzyme XhoI or SphI (Fig. 4A, lanes 2 and 6), indicating that RC DNA and cccDNA are the same length but have different conformations. Upon heating of the sample to 85°C for 5 min in 0.1× TE buffer, the majority of the RC DNA was shifted down to the position of single-stranded DNA, while the cccDNA was not affected (Fig. 4A, lanes 3 and 7). To further confirm that the 2.0-kb DNA species from the Hirt extraction was indeed cccDNA, the 85°C heat-denatured DNA sample was digested with XhoI or EcoRI (XhoI, SphI, and EcoRI are the single-cutting-site enzymes within the HBV genome) before electrophoresis, and the band predicted to be cccDNA was shifted to the position of the linear form of DNA (Fig. 4A, lanes 4 and 8). These alterations in gel mobility thus established the supercoiled nature of the 2.0-kb DNA species, a result that strongly suggested that the HBV-producing cell lines are able to produce cccDNA (Fig. 4B). Based on the signal intensity compared to that of input linear HBV DNA (with full-length 3,182-bp HBV sequence, which was eluted from pHBV1.3), we divided the intensity of the linearized cccDNA by the cell number and estimated that there were 2 to 6 copies of cccDNA within each hepatocyte of our stable cell lines (Fig. 4B, lower panel, lanes 2 to 6).
FIG. 4.
Determination of the amount and transcriptional ability of cccDNA in each stable transfectant. (A) The presence of the supercoiled plasmid as well as cccDNA was defined by restriction enzyme digestion, thermal denaturation, and thermal denaturation followed by restriction enzyme digestion. Changes in the mobilities of the supercoiled plasmid and cccDNA were revealed by 1.2% agarose gel electrophoresis. (B) Hirt extracts from each stable line were thermally denatured at 85°C for 5 min (upper panel) or subjected to thermal denaturation combined with restriction enzyme digestion (lower panel) before electrophoresis on a 1.2% agarose gel. The 3,182-bp control plasmid pUC3.2-HBV served as a reference for the mobility of cccDNA (supercoiled plasmid). The copy number of cccDNA in each HBV line was calculated by judging the signal intensity compared to 10 or 50 pg of input linear HBV DNA (with full-length 3,182-bp HBV sequence, which was eluted from pHBV1.3). (C) The transcriptional activity of cccDNA was estimated by the RT-PCR-BclI method. RNA extracts from each BclI-containing line were amplified by RT-PCR, followed by SspI/BclI double digestion, and the resulting restricted PCR products were separated by 2% agarose gel electrophoresis. The relative intensities of restricted RT-PCR fragments were used to monitor the transcription efficiencies of the integrated viral genome and cccDNA.
To verify the transcriptional ability of cccDNA in those HBV-producing cell lines, we next performed an RT-PCR-BclI restriction assay and measured the relative intensity of each restricted fragment (Fig. 4C). In the case of 1.3.ES6 (as well as 1.3.ES3 and 1.3.ES8), the RT-PCR product was restricted with SspI and BclI, which generated fragments of 603, 332, 200, and 132 bp. The 332-bp product that lacks the BclI site represents the RNA transcribed from the integrated viral genome, whereas the appearance of two fragments of 200 and 132 bp indicates that transcripts were derived from cccDNA. Significantly, the relative intensities of the two fragments derived from the cccDNA RNAs were only 10 to 20% of those from the integrated genome (Fig. 4C, lanes 2, 4, and 5). A similar conclusion was drawn from the other HBV-producing cell line, 1.3.ES2 (Fig. 4C, lane 1). Since the BclI restriction site was located primarily within the 3′-terminal redundant region of the viral genome in 1.3.ES2, transcripts from the integrated viral genome would contain the BclI genetic marker and the RT-PCR product could be further divided into two fragments of 200 and 132 bp after restriction. Since the BclI genetic marker would disappear after cccDNA formation, the presence of a single 332-bp fragment after BclI restriction indicated that the transcripts were from cccDNA. Taking these results together, we observed that fewer transcripts were derived from cccDNA than from the integrated genome. This is highly significant, as there are 2 to 6 copies of cccDNA present in a single cell and only 1 to 2 integrated HBV copies (Fig. 4B and 3B). Our results conclusively showed that the transcriptional ability of cccDNA was much poorer than that of the integrated HBV genome.
The regulatory role of the transcriptional activator HBx in the transcriptional activity of cccDNA.
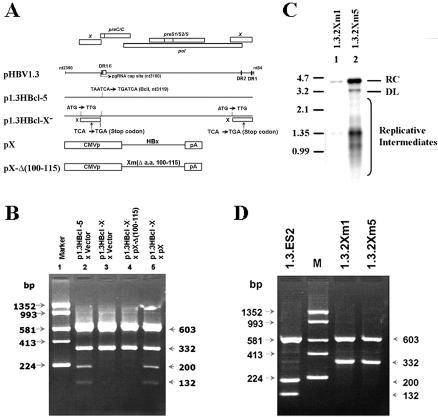
To clarify whether the trans-activation function of HBx is involved in the transcriptional regulation of cccDNA, we modified p1.3HBcl-5, removing HBx production by an AUG mutation and a stop codon. The resulting plasmid (1.3HBclI-X−) was cotransfected with either wild-type HBx (pX) or mutated HBx (pX-Δ(100-115) which has a defect in its trans-activation ability (2, 38) (Fig. 5A). Southern blot analysis suggested that the replication pattern of 1.3HBclI-X− was similar to that of wild-type HBV after transient transfection (data not shown). The viral transcripts were extracted for RT-PCR-BclI analysis, and the disappearance of BclI-restricted fragments (200 and 132 bp) indicated the abolition of cccDNA transcription in the 1.3HBclI-X− transfectant (Fig. 5B, lanes 2 and 3). The reduction in the amount of cccDNA-derived transcripts in the HBx null-mutant could be trans-complemented by cotransfection with a plasmid expressing wild-type HBx (Fig. 5B, lane 5) but not with a plasmid expressing truncated HBx (Fig. 5B, lane 4). This suggests that the trans-activation function of HBx is required for the production of viral RNA from cccDNA.
FIG. 5.
The trans-activation function of HBx is required for cccDNA transcription. (A) Scheme for construction of the HBx− construct derived from p1.3HBcl-5 and an HBx mutant with the trans-activating domain truncated [pX-Δ(100-115)]. (B) HepG2 cells were cotransfected with the indicated plasmids. Three days posttransfection, total RNAs were extracted for RT-PCR with the primer pair HBV2338/F-T20-Taq/HBV5. After SspI/BclI double digestion and electrophoresis on a 2% agarose gel, the transcriptional ability of the cccDNA was measured by the relative intensities of restricted PCR products. (C) Cytoplasm replicative intermediates of 1.3.2Xm1 and 1.3.2Xm5 were extracted for determination of their replicative capacities by Southern blot analysis. (D) Total RNAs of stable HBx-null lines were extracted, and the expression profiles of viral transcripts were determined by RT-PCR combined with SspI/BclI double digestion. The electrophoresis results showed no transcriptional activity from cccDNA in HBx-null mutants 1.3.2Xm1 and 1.3.2Xm5.
Although the transcripts from cccDNA were eliminated after removal of HBx, there are two scenarios that can explain this phenomenon. First, the functionally inactive HBx protein may fail to support cccDNA formation. Second, the trans-activation function of the X protein may be responsible for the transcriptional regulation of cccDNA. To exclude the first possibility, two HBx-null mutants were obtained by stably transfecting cells with an HBx mutant 1.3-fold HBV genome. The cytoplasmic replicative intermediates of 1.3.2Xm1 and 1.3.2Xm5 were extracted and analyzed by Southern blotting (Fig. 5C). Hirt extracts from cells of our HBx-null lines, 1.3.2Xm1 and 1.3.2Xm5, revealed a DNA molecule that migrated as cccDNA on a Southern blot (Fig. 4B, lanes 8 and 9). The smaller amount of cccDNA production in 1.3.2Xm1 cells seems to be due to the clonal variation of inefficient viral replication (Fig. 4B and 5C). Thus, these HBx-null lines are able to produce cccDNA molecules at the same level as Hep2.2.15, a well-established HBV-producing cell line (35) (Fig. 4B, lane 7). Results from an RT-PCR-BclI restriction assay of these HBx-null lines further confirm our previous observation that the transcripts from cccDNA were down-regulated in the absence of HBx (Fig. 5D). Additionally, we have demonstrated that coexpression of wild-type HBx can improve the signal level of transcripts from cccDNA in the transient transfection system (Fig. 5B, lane 5). Taken together, our results suggest that HBx is a fundamental molecule necessary for optimal transcriptional activity of cccDNA.
DISCUSSION
We have designed a strategy to detect HBV cccDNA-derived transcripts by taking advantage of a modified HBV genome with an inserted BclI genetic marker. During the HBV replication cycle, the BclI site embedded in the 5′ end is retained and incorporated into the cccDNA molecule. The cccDNA-derived transcripts duplicate the BclI genetic marker onto both the 5′- and 3′-end redundant regions. First, we used RT-PCR to amplify the 3′-end regions of transcripts and specifically examined the cccDNA-derived transcripts by BclI restriction analysis. Viral transcripts were terminated and polyadenylated at 12 to 19 nt behind the unique polyadenylation signal (TATAAA) (37). This prompted us to believe that T20-Taq/HBV5 should work well to monitor those viral transcripts. Second, to verify that our RT-PCR method could specifically detect transcripts from cccDNA, we first excluded the possibility of mismatch amplification of the transfected plasmid by our primer pair (HBV2338/F-T20-Taq/HBV5). There were no detectable PCR products when plasmid p1.3HBclI was used as the PCR template (data not shown), whereas large amounts of PCR products were amplified by using cDNA prepared from transfectants as the PCR template. The RT-PCR results indicate that contamination of cDNA with a plasmid is not a significant problem in our transient transfection assay. To exclude the possibility that jumping PCR might generate PCR fragments containing a BclI site derived from the 5′ end of the 3.5-kb HBV RNA carrying the BclI sequence, the reverse primer was designed to contain an extremely short HBV sequence with only 5 nt. Under this condition, plasmid p1.3SspI, which produces transcripts only with a BclI site at its 5′ end, verified that jumping PCR did not occur during RT-PCR. Third, the expected size of the PCR product containing the BclI marker was detected only in cDNAs prepared from p1.3HBcl-transfected cells, not from p1.3HBcl-P2- or p1.3SspI-transfected cells, which lack cccDNA. Taken together, these results established that our RT-PCR method is able to specifically detect the BclI marker present in the 3′ end of HBV mRNAs, which allows us to identify distinctive transcripts from cccDNA.
Based on relative intensity, the copy number of cccDNA that we calculated was in the range of 2 to 6 copies per cell in our established HBV lines (Fig. 4B). For instance, there are 4 to 6 copies of cccDNA per cell in the 1.3.ES-2, -6, and -8 cell lines and about 2 to 3 copies in the 1.3.ES-3 and -11 cell lines and in 1.3.2Xm5 cells (as well as in 2.2.15 cells). In contrast, the calculated number of integrated genomes is between 1 and 2 copies per cell. By comparing the relative intensities of cccDNA-derived and integrated-genome-derived transcripts, our results showed that the proportion of transcripts from cccDNA relative to the amount from the integrated HBV genome was 10 to 20%. Thus, compared to the integrated genome, cccDNA is dramatically inefficient at transcription in our established cell lines. The degree of inefficiency would seem to range from 10% (2 cccDNA copies, 1 integrated-genome copy) to as low as 2% (6 cccDNA copies, 1 integrated-genome copy) relative to the integrated genome. cccDNA has been reported to form a compact minichromosome-like structure within the hepatocyte (6), and this inactive form of the cccDNA molecule may partially explain why the transcription rate of cccDNA is so low. One cannot exclude the possibility that other regulatory factors may participate in cccDNA-associated transcriptional activity. To date, many transcriptional factor binding elements within the viral genome and cccDNA have been located and investigated. These transcriptional factors, including AP-1, AP-2, NF-κB, C/EBP, ATP/CREB, SRF, and SP1, could interact with the viral genome or cccDNA and participate in the regulation of HBV gene transcription (9, 16, 19, 21). Moreover, these cellular transcriptional factors have been reported to be activated by HBx (9, 16, 19, 21), suggesting that HBx may alter the transcriptional ability of cccDNA by elevating the expression of cellular transcription factors. In agreement with the above possibility, our results for the first time provide evidence that HBx is involved in the transcriptional regulation of cccDNA. We have shown that the loss of X protein expression results in a reduction in the number viral transcripts from cccDNA, whereas overexpression of wild-type HBx in the HBx-null transient transfectant was sufficient to support transcription from cccDNA. However, the exact mechanism by which HBx and the cellular transcription factors interact with the cccDNA molecule to activate transcription remains to be elucidated.
On the basis of the current model of the HBV life cycle, the cccDNA molecule is the most pivotal of the DNA intermediates in the establishment of the infection cycle in vivo. It has been reported that a woodchuck hepatitis virus genome harboring an X mutation could not establish an infection cycle after being delivered into a woodchuck liver (10, 44). Our preliminary results, showing HBx as contributing to the transcriptional regulation of cccDNA, strongly imply that the inability to establish an infection cycle in X-mutated woodchuck hepatitis virus may be due to inefficient transcription from cccDNA preceding virus production. Since the expression of viral pgRNA is down-regulated in the absence of X protein, the reentry of the viral genome into the nucleus and the amplification of the cccDNA pool may then be abolished indirectly. In this scenario, the viral trans-acting protein, HBx, may thus play a critical role in the accumulation of cccDNA in a natural infection and also in the establishment of viral infection. However, this hypothesis needs to be further investigated.
Acknowledgments
We thank Tony C. T. Liang and Ralph Kirby for helping us to correct grammatical and spelling errors and improve the quality of our manuscript.
This work was supported by intramural research grants MG-090-PP-04 and MG-091-PP-04 from the National Health Research Institutes of the Republic of China.
Footnotes
This article is dedicated to the memory of Tung-Yuan Shih in appreciation of the experimental design and research discussions.
REFERENCES
- 1.Abdelhamed, A. M., C. M. Kelley, T. G. Miller, P. A. Furman, and H. C. Isom. 2002. Rebound of hepatitis B virus replication in HepG2 cells after cessation of antiviral treatment. J. Virol. 76:8148-8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arii, M., S. Takada, and K. Koike. 1992. Identification of three essential regions of hepatitis B virus X protein for trans-activation function. Oncogene 7:397-403. [PubMed] [Google Scholar]
- 3.Ben-Levy, R., O. Faktor, I. Berger, and Y. Shaul. 1989. Cellular factors that interact with the hepatitis B virus enhancer. Mol. Cell. Biol. 9:1804-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benn, J., and R. J. Schneider. 1994. Hepatitis B virus HBx protein activates Ras-GTP complex formation and establishes a Ras, Raf, MAP kinase signaling cascade. Proc. Natl. Acad. Sci. USA 91:10350-10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benn, J., F. Su, M. Doria, and R. J. Schneider. 1996. Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-regulated and c-Jun N-terminal mitogen-activated protein kinases. J. Virol. 70:4978-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bock, C. T., S. Schwinn, S. Locarnini, J. Fyfe, M. P. Manns, C. Trautwein, and H. Zentgraf. 2001. Structural organization of the hepatitis B virus minichromosome. J. Mol. Biol. 307:183-196. [DOI] [PubMed] [Google Scholar]
- 7.Bouchard, M., S. Giannakopoulos, E. H. Wang, N. Tanese, and R. J. Schneider. 2001. Hepatitis B virus HBx protein activation of cyclin A-cyclin-dependent kinase 2 complexes and G1 transit via a Src kinase pathway. J. Virol. 75:4247-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buendia, M. A. 1998. Hepatitis B viruses and cancerogenesis. Biomed. Pharmacother. 52:34-43. [DOI] [PubMed] [Google Scholar]
- 9.Caselmann, W. H. 1995. Transactivation of cellular gene expression by hepatitis B viral proteins: a possible molecular mechanism of hepatocarcinogenesis. J. Hepatol. 22:34-37. [PubMed] [Google Scholar]
- 10.Chen, H. S., S. Kaneko, R. Girones, R. W. Anderson, W. E. Hornbuckle, B. C. Tennant, P. J. Cote, J. L. Gerin, R. H. Purcell, and R. H. Miller. 1993. The woodchuck hepatitis virus X gene is important for establishment of virus infection in woodchucks. J. Virol. 67:1218-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiaramonte, M., T. Stroffolini, A. Vian, M. A. Stazi, A. Floreani, U. Lorenzoni, S. Lobello, F. Farinati, and R. Naccarato. 1999. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer 85:2132-2137. [PubMed] [Google Scholar]
- 12.Chisari, F. V. 2000. Viruses, immunity, and cancer: lessons from hepatitis B. Am. J. Pathol. 156:1117-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi, B. H., G. T. Park, and H. M. Rho. 1999. Interaction of hepatitis B viral X protein and CCAAT/enhancer-binding protein alpha synergistically activates the hepatitis B viral enhancer II/pregenomic promoter. J. Biol. Chem. 274:2858-2865. [DOI] [PubMed] [Google Scholar]
- 14.Choi, C. Y., B. H. Choi, G. T. Park, and H. M. Rho. 1997. Activating transcription factor 2 (ATF2) down-regulates hepatitis B virus X promoter activity by the competition for the activating protein 1 binding site and the formation of the ATF2-Jun heterodimer. J. Biol. Chem. 272:16934-16939. [DOI] [PubMed] [Google Scholar]
- 15.Chou, H. W., D. Harrell, R. Forough, and K. Watabe. 1988. Binding of tissue-specific factors to the enhancer sequence of hepatitis B virus. FEBS Lett. 229:349-354. [DOI] [PubMed] [Google Scholar]
- 16.Doria, M., N. Klein, R. Lucito, and R. J. Schneider. 1995. The hepatitis B virus HBx protein is a dual specificity cytoplasmic activator of Ras and nuclear activator of transcription factors. EMBO J. 14:4747-4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galibert, F., E. Mandart, F. Fitoussi, P. Tiollais, and P. Charnay. 1979. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature 281:646-650. [DOI] [PubMed] [Google Scholar]
- 18.Ganem, D., J. R. Pollack, and J. Tavis. 1994. Hepatitis B virus reverse transcriptase and its many roles in hepadnaviral genomic replication. Infect. Agents Dis. 3:85-93. [PubMed] [Google Scholar]
- 19.Henkler, F. F., and R. Koshy. 1996. Hepatitis B virus transcriptional activators: mechanisms and possible role in oncogenesis. J. Viral Hepat. 3:109-121. [DOI] [PubMed] [Google Scholar]
- 20.Kao, J. H., and D. S. Chen. 2002. Global control of hepatitis B virus infection. Lancet Infect. Dis. 2:395-403. [DOI] [PubMed] [Google Scholar]
- 21.Kekule, A. S., U. Lauer, L. Weiss, B. Luber, and P. H. Hofschneider. 1993. Hepatitis B virus transactivator HBx uses a tumour promoter signalling pathway. Nature 361:742-745. [DOI] [PubMed] [Google Scholar]
- 22.Kock, J., T. F. Baumert, W. E. Delaney IV, H. E. Blum, and F. von Weizsacker. 2003. Inhibitory effect of adefovir and lamivudine on the initiation of hepatitis B virus infection in primary tupaia hepatocytes. Hepatology 38:1410-1418. [DOI] [PubMed] [Google Scholar]
- 23.Kong, H. J., S. H. Hong, M. Y. Lee, H. D. Kim, J. W. Lee, and J. Cheong. 2000. Direct binding of hepatitis B virus X protein and retinoid X receptor contributes to phosphoenolpyruvate carboxykinase gene transactivation. FEBS Lett. 483:114-118. [DOI] [PubMed] [Google Scholar]
- 24.Lau, D. T., M. F. Khokhar, E. Doo, M. G. Ghany, D. Herion, Y. Park, D. E. Kleiner, P. Schmid, L. D. Condreay, J. Gauthier, M. C. Kuhns, T. J. Liang, and J. H. Hoofnagle. 2000. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology 32:828-834. [DOI] [PubMed] [Google Scholar]
- 25.Lin, Y., T. Nomura, J. Cheong, D. Dorjsuren, K. Iida, and S. Murakami. 1997. Hepatitis B virus X protein is a transcriptional modulator that communicates with transcription factor IIB and the RNA polymerase II subunit 5. J. Biol. Chem. 272:7132-7139. [DOI] [PubMed] [Google Scholar]
- 26.Lin, Y., H. Tang, T. Nomura, D. Dorjsuren, N. Hayashi, W. Wei, T. Ohta, R. Roeder, and S. Murakami. 1998. The hepatitis B virus X protein is a co-activator of activated transcription that modulates the transcription machinery and distal binding activators. J. Biol. Chem. 273:27097-27103. [DOI] [PubMed] [Google Scholar]
- 27.Mason, W. S., J. Cullen, G. Moraleda, J. Saputelli, C. E. Aldrich, D. S. Miller, B. Tennant, L. Frick, D. Averett, L. D. Condreay, and A. R. Jilbert. 1998. Lamivudine therapy of WHV-infected woodchucks. Virology 245:18-32. [DOI] [PubMed] [Google Scholar]
- 28.Moraleda, G., J. Saputelli, C. E. Aldrich, D. Averett, L. Condreay, and W. S. Mason. 1997. Lack of effect of antiviral therapy in nondividing hepatocyte cultures on the closed circular DNA of woodchuck hepatitis virus. J. Virol. 71:9392-9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosley, J. W., W. Huang, D. O. Stram, M. J. Nowicki, F. B. Hollinger, R. D. Aach, C. E. Stevens, L. H. Barbosa, and G. J. Nemo. 1996. Donor levels of serum alanine aminotransferase activity and antibody to hepatitis B core antigen associated with recipient hepatitis C and non-B, non-C outcomes. Transfusion 36:776-781. [DOI] [PubMed] [Google Scholar]
- 30.Murakami, S. 2001. Hepatitis B virus X protein: a multifunctional viral regulator. J. Gastroenterol. 36:651-660. [DOI] [PubMed] [Google Scholar]
- 31.Qadri, I., J. W. Conaway, R. C. Conaway, J. Schaack, and A. Siddiqui. 1996. Hepatitis B virus transactivator protein, HBx, associates with the components of TFIIH and stimulates the DNA helicase activity of TFIIH. Proc. Natl. Acad. Sci. USA 93:10578-10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radziwill, G., W. Tucker, and H. Schaller. 1990. Mutational analysis of the hepatitis B virus P gene product: domain structure and RNase H activity. J. Virol. 64:613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seeger, C., D. Ganem, and H. E. Varmus. 1986. Biochemical and genetic evidence for the hepatitis B virus replication strategy. Science 232:477-484. [DOI] [PubMed] [Google Scholar]
- 34.Seeger, C., and W. S. Mason. 2000. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 64:51-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sells, M. A., M. L. Chen, and G. Acs. 1987. Production of hepatitis B virus particles in HepG2 cells transfected with cloned hepatitis B virus DNA. Proc. Natl. Acad. Sci. USA 84:1005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sells, M. A., A. Z. Zelent, M. Shvartsman, and G. Acs. 1988. Replicative intermediates of hepatitis B virus in HepG2 cells that produce infectious virions. J. Virol. 62:2836-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonsen, C. C., and A. D. Levinson. 1983. Analysis of processing and polyadenylation signals of the hepatitis B virus surface antigen gene by using simian virus 40-hepatitis B virus chimeric plasmids. Mol. Cell. Biol. 3:2250-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takada, S., and K. Koike. 1994. Three sites of the hepatitis B virus X protein cooperatively interact with cellular proteins. Virology 205:503-510. [DOI] [PubMed] [Google Scholar]
- 39.Truant, R., J. Antunovic, J. Greenblatt, C. Prives, and J. A. Cromlish. 1995. Direct interaction of the hepatitis B virus HBx protein with p53 leads to inhibition by HBx of p53 response element-directed transactivation. J. Virol. 69:1851-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuttleman, J. S., C. Pourcel, and J. Summers. 1986. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell 47:451-460. [DOI] [PubMed] [Google Scholar]
- 41.Unger, T., and Y. Shaul. 1990. The X protein of the hepatitis B virus acts as a transcription factor when targeted to its responsive element. EMBO J. 9:1889-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu, T. T., L. Coates, C. E. Aldrich, J. Summers, and W. S. Mason. 1990. In hepatocytes infected with duck hepatitis B virus, the template for viral RNA synthesis is amplified by an intracellular pathway. Virology 175:255-261. [DOI] [PubMed] [Google Scholar]
- 43.Yeh, C. T., H. T. Chiu, C. M. Chu, and Y. F. Liaw. 1998. G1 phase dependent nuclear localization of relaxed-circular hepatitis B virus DNA and aphidicolin-induced accumulation of covalently closed circular DNA. J. Med. Virol. 55:42-50. [DOI] [PubMed] [Google Scholar]
- 44.Zoulim, F., J. Saputelli, and C. Seeger. 1994. Woodchuck hepatitis virus X protein is required for viral infection in vivo. J. Virol. 68:2026-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]