Abstract
An integrated understanding of therapeutic plasma exchange (TPE) effects on immunoglobulins, autoantibodies, and natural or acquired (vaccine) protective antibodies in patients with autoimmune myasthenia gravis (MG) is lacking. Prior studies measured TPE effects in healthy volunteers or heterogeneous autoimmune diseases populations. We prospectively profiled plasma IgA, IgM, IgG, IgG subclasses (IgG1-4), acetylcholine receptor autoantibodies (AChR+), and protective antibodies in patients with AChR+ MG receiving TPE for an exacerbation. TPE was performed according to institutional practice and patients were profiled for up to 12 weeks. Ten patients were enrolled (median age=72.9 years; baseline MG-Composite=21; median TPE treatments=6 during their first course) and all improved. The maximum decrease in all immunoglobulins, including AChR autoantibodies, was achieved on the final day of the first TPE course (approximately 60–70% reduction). Three weeks post-TPE mean AChR autoantibody, total IgG, IgG1 and IgG2 titers were below the reference range and had not recovered to within 20% of baseline, whereas other measured immunoglobulins approached baseline values. We did not generally observe an “overshoot” of immunoglobulins above pre-TPE levels or accelerated recovery of pathologic AChR autoantibodies. Protective antibody profiles showed similar patterns as other IgGs and were detectable at levels associated with protection from infection. A slow return to baseline for IgGs (except IgG3) was observed, and we did not observe any obvious effect of concomitant medications on this recovery. Collectively, these findings enhance our understanding of the immunological effects of TPE and further supports the concept of rapid immunoglobulin depletion for the treatment of patients with MG.
Keywords: myasthenia gravis, plasma exchange, IgG, plasmapheresis, immunoglobulins
INTRODUCTION
Myasthenia gravis (MG) is a chronic, potentially fatal autoimmune disease characterized by circulating autoantibodies directed against epitopes of the post-synaptic muscle membrane, including nicotinic acetylcholine receptor (AChR). This autoimmune attack causes weakness of voluntary muscles and results in fluctuating weakness that may affect ocular, pharyngeal, respiratory, and limb muscles (1). MG is considered a model of IgG mediated autoimmunity and previous studies have provided invaluable information on the mechanisms of autoimmune disease.
Treatment paradigms for MG include therapies such as intravenous immunoglobulin (IVIg) and therapeutic plasma exchange (TPE) (2, 3). Due to its’ ability to rapidly improve patient weakness, TPE is commonly used in MG to treat disease exacerbations, prepare patients for surgery, and prior to initiating treatment with corticosteroids. The TPE procedure consists of filtering venous blood and removing plasma constituents including normal and pathogenic immunoglobulins. Afterwards, the removed plasma is usually replaced with fresh frozen plasma or albumin (4). While it is known that total autoantibody levels in MG patients drop during TPE, the long term effect of TPE on these parameters or the effect on immunoglobulin (Ig) subtypes and protective autoantibodies have been understudied in patients with MG. Most prior studies have focused on total Ig or autoantibody levels during and immediately after TPE (5). Additionally, no studies have simultaneously evaluated Ig and autoantibody levels in MG patients. Another long-standing controversy is whether TPE therapy elicits an overshoot of Igs or accelerated recovery of pathologic autoantibodies (5, 6).
The goal of this study was to create a more integrated understanding of TPE effects on Ig, autoantibody levels, and protective antibodies. We prospectively profiled the effects of TPE, provided as standard of care to AChR autoantibody positive MG patients (AChR MG), on IgA, IgM, IgG, IgG subclasses, autoantibodies, and selected protective antibodies.
METHODS
Study design
The study was approved by the Institutional Review Board at the respective clinical sites. This study enrolled 10 MG patients who received TPE as standard of care at Duke University Medical Center or The University of North Carolina at Chapel Hill Hospital. All patients were initially treated with TPE due to exacerbations of their disease. Approximately one plasma volume was exchanged during each TPE procedure and colloid replacement was 5% albumin in >90% of procedures. It was expected that most patients would initially receive 5–6 TPE procedures per standard practice at each institution (7). Patients could receive additional TPE procedures if deemed necessary by their treating physician. Any concomitant immunosuppressive medications were kept as constant as possible. Clinical outcomes and immunoglobulins were measured at baseline (Visit 1), prior to the third (V2) and last TPE procedures (V3) and 1, 2, 3, 6, and 12 weeks (V4–8) post-TPE. Data will be presented out to 3 weeks post TPE because at 6 and 12 weeks, differences in the treatment of patients related to changes in immunosuppressive medications and additional courses of TPE introduced variability that made data interpretation difficult. From the screening visit to week 3 post-TPE, there were no treatment changes that would confound the analysis. Clinical outcomes measured in this study include the validated MG-Composite and MG-Manual Muscle Testing (MMT). In these two tests, a change in either score of ≥3 points is considered clinically significant and defined “improvement” (8, 9). Since prior clinical trials of TPE used the change from baseline to 2 weeks post-TPE in clinical scores as primary outcomes, this time point was also used to determine response to TPE (10).
Eligibility criteria
The primary inclusion criteria included age at least 18 years, diagnosis of MG based on clinical features and detectable serum autoantibodies to AChR, and a clinical indication for the use of TPE. Exclusion criteria included a weight <50Kg, a contraindication to treatment with TPE (e.g. clinically significant bleeding disorder), muscle specific tyrosine kinase or low-density lipoprotein receptor-related protein 4 antibody positive MG, prior history of or current thymoma at the time of screening, thymectomy in the past 6 months, and a coexisting autoimmune disease that may preclude accurate study assessments.
Immunoglobulin assays
Quantitative immunoglobulins (IgG, IgA, IgM; Duke Clinical Laboratory) and IgG subclasses (IgG1, IgG2, IgG3, IgG4; Mayo Medical Laboratories) were performed by rate nephelometry and radial immunodiffusion. AChR binding antibody levels (Mayo Medical Laboratories) were determined using a quantitative radioimmunoassay. An ELISA assay (Kansas City Analytical Services) was used to measure the following vaccination induced and natural protective antibodies in serum: anti-tetanus toxoid IgG, anti-diphtheria toxoid IgG, anti-varicella zoster virus IgG and anti-Epstein Barr virus viral capsid antigen IgG. These antibodies were selected since they are part of the recommended vaccination schedule or due to common exposure in the general population.
Data analysis
Demographic data were summarized descriptively as median and range for continuous variables and as counts and percentages for categorical parameters. Total Igs, IgG subclasses, AChR autoantibody, and protective/vaccination antibody levels were summarized as means and standard deviation and plotted graphically by visit. The percentage change from baseline was also calculated for each immunoglobulin measured. A particular interest of the analysis was to determine the point when immunoglobulin, IgG subtypes, and AChR autoantibody levels variables return to pre-TPE levels and whether there is an “overshoot” of immunoglobulin or AChR levels higher than pre-TPE measurements. Return of Igs to within 20% of the pre-TPE level was considered “recovery.” All analyses were performed using SAS® version 9.1 or later (SAS Institute, Cary, NC, USA).
RESULTS
Demographics
Ten AChR MG patients were enrolled. Two patients underwent thymectomy prior to the study, none had a history of thymoma and most were on immunosuppressive therapy with corticosteroids and/or azathioprine or mycophenolate mofetil (Table 1). None had ever received rituximab treatment. Most patients had a moderate severity of weakness at the time of enrollment (11). Nine patients completed the study. One patient was withdrawn after Visit 6 following a stroke that was unrelated to the study procedures.
Table 1.
Baseline demographics of enrolled MG patients (N=10)
| Median Age in years, (range) | 72.9 (20 – 86) |
|
| |
| Male, N (%) | 6 (60%) |
|
| |
| Caucasian, N (%) | 9 (90%) |
|
| |
| Median Weight (kg), (range) | 85.6 (50.0 – 100.1) |
|
| |
| Median BMI (kg/m2), (range) | 28.4 (20.2 – 32.4) |
|
| |
| MG medications | |
| Acetylcholinesterase inhibitor | 80% |
| Corticosteroids | 60% |
| Oral immunosuppressants | 50% |
|
| |
| Median duration of MG in years, (range) | 0.8 (0–38) |
|
| |
| Baseline MGFA Severity Class | IIa = 20% |
| IIIa = 30% | |
| IIIb = 40% | |
| IVa = 10% | |
|
| |
| Median MG-ADL, (range) | 9 (6 – 12) |
|
| |
| Median MG-Composite, (range) | 21 (12 – 27) |
|
| |
| Median MG-MMT, (range) | 23 (11 – 81) |
|
| |
| Median MG-QOL15, (range) | 25 (18 – 43) |
TPE procedures and clinical outcomes
Patients underwent a median of 6 (range 5–27) TPE procedures during the trial. Sixty-nine percent of TPE procedures were performed in the outpatient setting. All patients improved following TPE, and the median change from baseline to 2 weeks post-TPE was −10 points (−3 to −22) for the MG-composite and −10 points (0 to −44) for the MG-MMT.
Three patients required additional TPE due to refractory disease. Two of these received chronic TPE due to an incomplete response to ongoing oral immunosuppressive therapies. The third patient had new onset disease and was found to have a thymoma (WHO Type B1) after enrollment. This patient received additional TPE procedures prior to an uncomplicated thoracoscopic thymectomy. The thymoma patient’s clinical response to TPE was less marked and more transient than other patients. She had a 3 point improvement on MG-composite and 6 point improvement on the MG-MMT 2 weeks after TPE.
IgA, IgM, and total IgG immunoglobulins
All patients showed a reduction following TPE reaching its lowest point on the final day of TPE in all patients (Figure 1A). The mean maximum reduction was 69.1%, 79.1%, and 73.4% for IgA, IgM, and total IgG, respectively (Figure 1B). Recovery of total IgG levels following TPE were slower than IgA or IgM. For example, IgA and IgM levels returned to within approximately 20% of mean baseline values 3 weeks after completing a course of TPE whereas mean total IgG remained 38.5% reduced (Figure 1).
Figure 1. Effect of TPE on Mean IgA, IgM, and total IgG in MG patients.
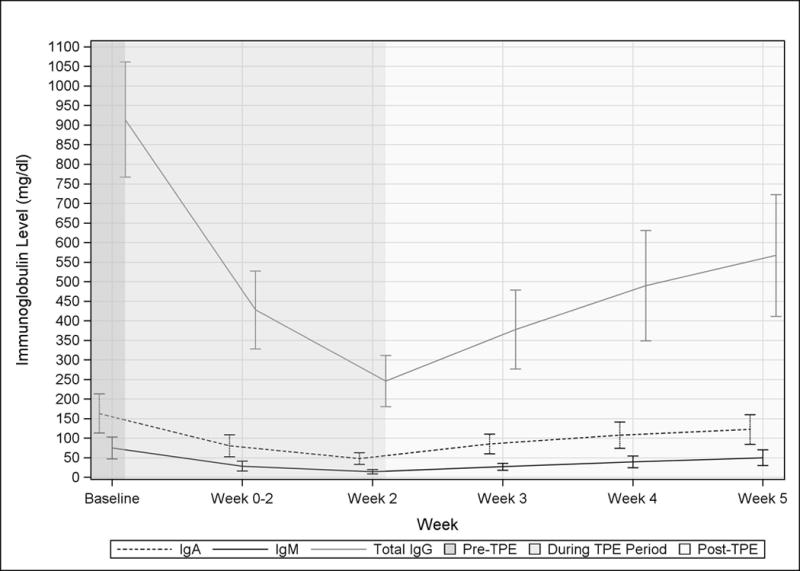
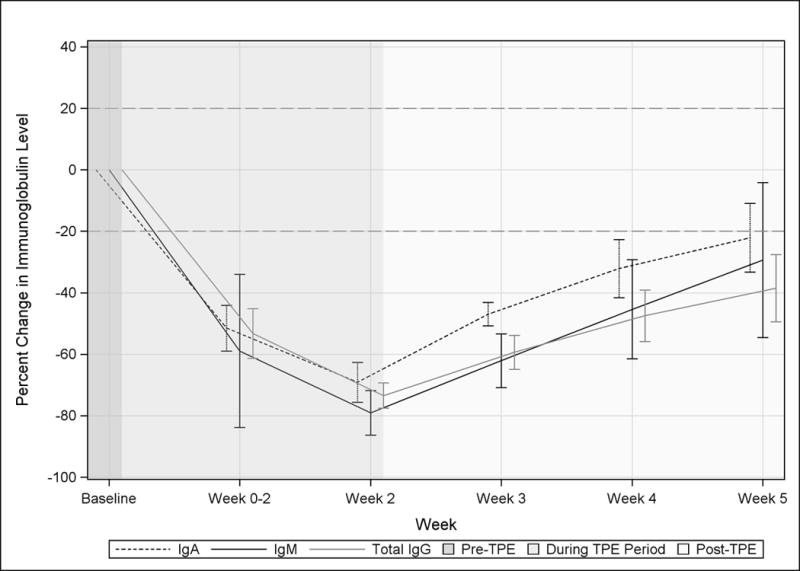
Raw data (A) and percent change from the Baseline (B) are shown by visit. The dotted horizontal line in B indicates return of Igs to within 20% of the pre-TPE level. Visits occurring before during, and after a course of TPE are indicated by background shading.
IgG subclasses
TPE rapidly lowered all IgG subclasses and, similar to total IgG levels, IgG subclasses 1–4 had a maximum change from baseline at visit 3, the final day of TPE (Figure 2). The mean maximum reduction of IgG subclasses was 65.1–74.8%. Amongst the four IgG subclasses return to baseline was fastest for IgG3 which recovered by 3 weeks. IgG 1, 2, and 4 did not recover to within 20% of the mean baseline value by visit 6.
Figure 2. Effect of TPE on IgG subclasses.
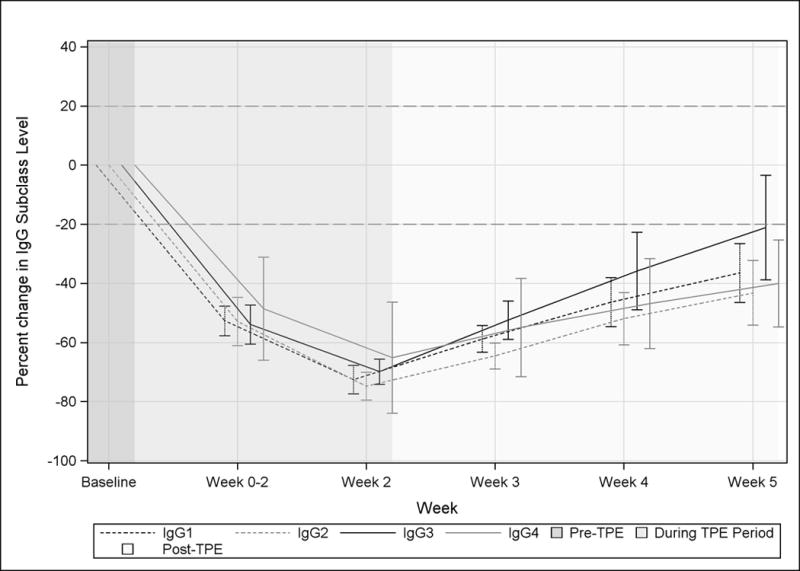
Mean IgG subclasses (IgG1–IgG4) in MG patients are shown in terms of percent change from Baseline. The dotted horizontal line indicates return of IgG subclasses to within 20% of the pre-TPE level. Visits occurring before during, and after a course of TPE are indicated by background shading.
AChR autoantibodies
Titers of AChR autoantibodies demonstrated considerable variability at baseline (median 9.9 nmol/L, range 0.4–42.2). A mean maximum 71.0% reduction in AChR autoantibody titers was observed at visit 3, the final day of TPE (Figure 3). Three weeks post-TPE, the mean titers remained 35.9% reduced from baseline. There was no difference in the mean AChR autoantibody to total immunoglobulin ratio from baseline through visit 6 (stable at 0.01).
Figure 3. Effect of TPE on AChR autoantibodies.
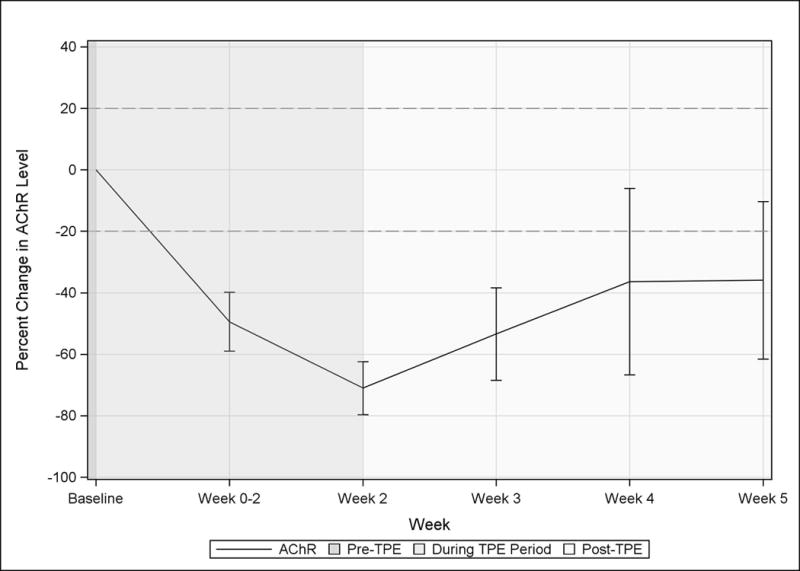
Mean AChR antibody titers in MG patients are shown in terms of percent change from Baseline. The dotted horizontal line indicates return of AChR antibody levels to within 20% of the pre-TPE level. Visits occurring before during, and after a course of TPE are indicated by background shading.
Protective and vaccination antibodies
To determine the effect of TPE of titers on antibodies elicited by vaccination, we measured antibody levels for Varicella Zoster, Epstein Barr virus, Diptheria Toxin, and Tetanus Toxoid (Figure 4). The measured antibodies were detectable in all patients prior to TPE. Similar to the other measured Igs, protective antibody levels reached a nadir on the final day of TPE and most exhibited an equivalent return towards baseline. About half of the patients exhibited a transient drop of diphtheria antibodies below levels associated with increased risk of infection (<0.1 U/mL) and these levels were slower to recover. Tetanus toxoid antibodies remained detectable in nearly all patients throughout the study. Only one patient, who had low titers of nearly all measured protective antibodies prior to TPE, had tetanus toxoid antibodies levels that stayed consistently low post-TPE. One patient had low EBV antibody levels at baseline and these levels remained below the threshold associated with protection throughout the study. The other patients maintained levels above protective thresholds during and after completion of TPE.
Figure 4. Effect of TPE on protective antibodies against diptheria, tetanus, Epstein-Barr virus, and Varicella in MG patients.
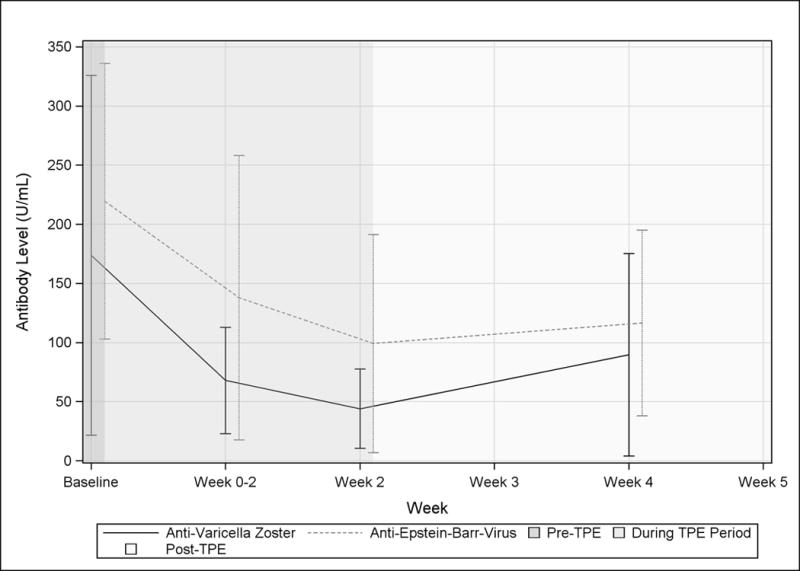
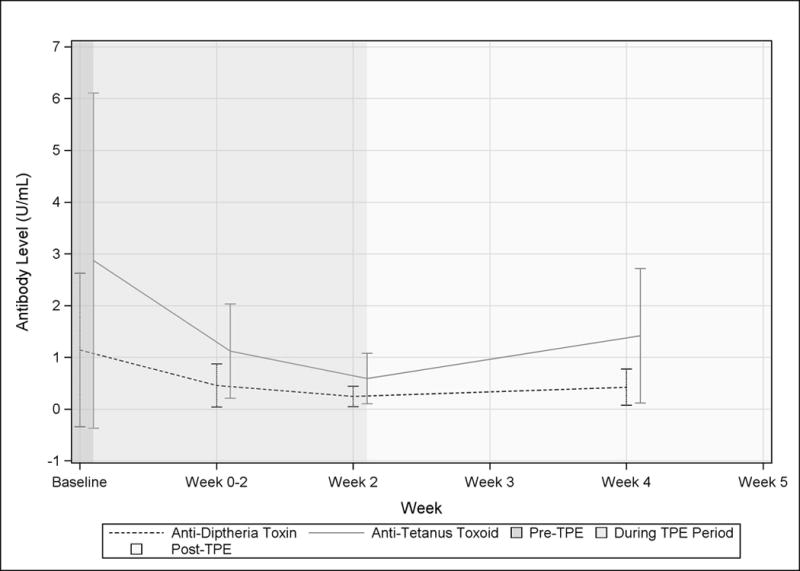
Mean data are shown for anti-Epstein-Barr virus capsid antigen IgG, and anti-varicella IgG (A) and anti-diptheria and tetanus toxoid IgG (B) are shown by visit. Visits occurring before during, and after a course of TPE are indicated by background shading.
DISCUSSION AND CONCLUSIONS
Most of the scientific literature describing the effects of TPE on immunological parameters dates from the 1980s and early 1990s. Those studies typically described total immunoglobulins in healthy subjects following a single TPE procedure or autoantibody levels in heterogeneous patient populations (6, 12). More comprehensive studies evaluating the effects of TPE on total Ig, Ig subclasses, and autoantibody kinetics simultaneously in a patient cohort are lacking. This study addresses this need, demonstrating that TPE reliably reduces all mean Ig levels by approximately 70% in MG patients receiving TPE for an exacerbation of their disease.
The medical literature has somewhat conflicting data on the recovery of Ig levels following TPE. Most studies have reported data in healthy volunteers after a single treatment or in patients using TPE regimens that are not as commonly used in current clinical practice for acute MG exacerbations (e.g., weekly or biweekly exchanges) (5, 6). Animal studies and studies in some patients have reported an increase in Ig above baseline levels, a so-called “overshoot” (13–15). The mechanism of the overshoot has been hypothesized to be attributed to an enhanced synthesis of autoantibodies. Some investigators have suggested that the potential for an overshoot makes TPE a suboptimal acute therapy in autoimmune diseases compared with IVIg due to the potential for worsening of disease related to the overshoot. Alternatively, the addition of an immunosuppressive drug has been suggested as a means to prevent the excessive production of autoantibodies and clinical worsening.
Throughout our study, we did not observe a consistent overshoot phenomenon in pathogenic antibody levels and there was no clear evidence of enhanced autoantibody synthesis as suggested by a stable ratio of AChR autoantibodies to total IgG. Ig levels, including AChR autoantibodies, approached within 20% of baseline levels in most cases after 6 weeks post-TPE without ever fully recovering to the baseline (data not shown). Of note, our patients were also on immunosuppressive medications which may have affected AChR autoantibodies recovery following TPE, though doses remained as stable as possible, particularly through the first 3 weeks. In this setting, there was no suggestion that adding or raising immunosuppressive therapy is needed to prevent overshoot. In current clinical practice, most patients are already on some form of immunosuppressive therapy at the time of an exacerbation or immunosuppressive therapy will be added soon after starting a course of TPE due to the chronic nature of the disease. Thus, the potential for observing a post-TPE “overshoot” in the clinical care of patients not on immunosuppressive therapy is exceedingly uncommon.
The major exception to the observed recovery post TPE is IgG3, which showed a mean reduction of 21% from baseline at 3 weeks and 10% 6 weeks post-TPE. Prior studies in healthy adults suggest that the kinetics of IgG3 are different than the other IgGs. In these past studies the measured half-life of IgG1, IgG2, and IgG3 are approximately 20 days, whereas IgG3 is approximately 7 days (16, 17). Our data in MG patients support these findings and suggests that the faster recovery of IgG3 is related to a shorter half-life and increased fractional turnover of IgG3 that has been described in healthy individuals (17).
The predominant IgG subclass in AChR MG is IgG1 and a smaller fraction of IgG3 (18). In this study AChR autoantibodies did indeed follow the recovery pattern of IgG1. Though not included in this study, patients with muscle specific kinase autoantibody MG (MuSK MG) would be expected to have a recovery of MuSK autoantibodies following TPE similar to AChR MG patients. MuSK autoantibodies are predominantly IgG4 which recovered similar to IgG1 in this study.
Since IgG levels, including AChR autoantibodies, approach baseline levels after 6 weeks post-TPE, excluding other factors, one might expect patient worsening around this time. In our prior personal experience, this often holds true. Also, in a randomized trial of patients with mild to moderate MG, the duration of benefit following TPE was at least four weeks (19). If a patient relapses following TPE it will often occur approximately 5–6 weeks or later after TPE (20). Following a single course of TPE there are patients that remain stable beyond this time. This suggests that factors other than circulating autoantibodies, such as the cause of the exacerbation (e.g., medication related versus idiopathic exacerbation), measurement of plasma versus tissue pathogenic autoantibodies, or other immunopathologic factors, influence clinical status following a disease flare treated with TPE.
One patient in the study with new onset disease was found to have a thymoma. In this case, the patient had the expected response to TPE in terms of the reduction in IgG, AChR autoantibody titers, and clinical response. However, the clinical response was more transient with worsening noted approximately 3 weeks after completing the first TPE course and prior to thymectomy. Some prior studies have shown less favorable responses to treatment in patients with thymoma, including TPE, though usually in malignant cases (20).
A unique aspect of this study was the measurement of protective antibodies. Protective antibodies remained detectable throughout the study and returned towards their pre-TPE level similar to the other measured IgGs. Although titers correlated with protection to varicella have not been established, antibody levels for diphtheria, EBV, and tetanus remained above thresholds associated with protection for most patients (21). Given that TPE is usually administered in discrete courses and the resilience of these protective antibodies, TPE should be safe and not put patients at serious risk of infection. The effect of chronically administered TPE on the risk of these infections is uncertain. Finally, the kinetics of protective antibody recovery was similar to pathogenic AChR autoantibodies, supporting the concept that AChR autoantibodies are produced by long-lived plasma cells that also underlie long term protection from infections.
In this study of patients with AChR MG, we show that TPE rapidly lowers all Igs, including AChR-Ab and protective Abs. Following a course of 5–6 TPE treatments a return to baseline occurs beyond 3 weeks for all IgGs, except IgG3, which recovers faster. There is no apparent need to add immunosuppressive therapy to prevent an overshoot of autoantibodies in MG, but immunosuppressive therapy may be indicated for long term management of a patient’s disease. Longitudinal studies looking for predictors of relapse after 6 weeks post-TPE may be helpful to improve patient management.
Acknowledgments
We thank the apheresis units at Duke University Hospital and The University of North Carolina Hospital for their care of our patients and assistance with this study.
Footnotes
Declaration of interest statement
This study was supported by a research grant from UCB Biosciences. JTG is a consultant for UCB Biosciences. JMM, VJ, JSY and ACA have no relevant discloses. JFH is a non-remunerative consult to Alexion Pharmaceuticals and receives research support from Alexion Pharmaceuticals, Centers for Disease Control, and Prevention, GlaxoSmithKline Pharmaceuticals and NIH. MC receives research support from Alexion Pharmaceuticals, GlaxoSmithKline Pharmaceuticals and NIH. BS, TB, and PA are employees of UCB Biosciences.
References
- 1.Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006;116:2843–2854. doi: 10.1172/JCI29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders DB, Guptill JT. Myasthenia gravis and Lambert-Eaton myasthenic syndrome. Continuum (Minneap Minn) 2014;20:1413–1425. doi: 10.1212/01.CON.0000455873.30438.9b. [DOI] [PubMed] [Google Scholar]
- 3.Lagoumintzis G, Zisimopoulou P, Kordas G, Lazaridis K, Poulas K, Tzartos SJ. Recent approaches to the development of antigen-specific immunotherapies for myasthenia gravis. [Review] Autoimmunity. 2010;43:436–445. doi: 10.3109/08916930903518099. [DOI] [PubMed] [Google Scholar]
- 4.Juel VC, Massey JM. Autoimmune Myasthenia Gravis: Recommendations for Treatment and Immunologic Modulation. Curr Treat Options Neurol. 2005;7:3–14. doi: 10.1007/s11940-005-0001-7. [DOI] [PubMed] [Google Scholar]
- 5.Dau PC. Immunologic rebound. J Clin Apher. 1995;10:210–217. doi: 10.1002/jca.2920100410. [DOI] [PubMed] [Google Scholar]
- 6.Derksen RH, Schuurman HJ, Gmelig Meyling FH, Struyvenberg A, Kater L. Rebound and overshoot after plasma exchange in humans. J Lab Clin Med. 1984;104:35–43. [PubMed] [Google Scholar]
- 7.Dau PC. The fundamental basis for therapeutic plasmapheresis in autoimmune diseases. Transfus Sci. 1996;17(2):235–244. [Google Scholar]
- 8.Burns TM, Conaway M, Sanders DB, Composite MG, M.-Q. S. Group The MG Composite: A valid and reliable outcome measure for myasthenia gravis. Neurology. 2010;74:1434–1440. doi: 10.1212/WNL.0b013e3181dc1b1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders DB, Tucker-Lipscomb B, Massey JM. A simple manual muscle test for myasthenia gravis: validation and comparison with the QMG score. Annals of the New York Academy of Sciences. 2003;998:440–444. doi: 10.1196/annals.1254.057. [DOI] [PubMed] [Google Scholar]
- 10.Barth D, Nabavi Nouri M, Ng E, Nwe P, Bril V. Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology. 2011;76:2017–2023. doi: 10.1212/WNL.0b013e31821e5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaretzki A, 3rd, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, Sanders DB. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. 2000;55:16–23. doi: 10.1212/wnl.55.1.16. [DOI] [PubMed] [Google Scholar]
- 12.Volkin RL, Starz W, Winkelstein A, Shadduck RK, Lewis JH, Hasiba U, Spero JA. Changes in coagulation factors, complement, immunoglobulins, and immune complex concentrations with plasma exchange. Transfusion. 1982;22:54–58. doi: 10.1046/j.1537-2995.1982.22182154218.x. [DOI] [PubMed] [Google Scholar]
- 13.Reverberi R, Reverberi L. Removal kinetics of therapeutic apheresis. Blood Transfus. 2007;5:164–174. doi: 10.2450/2007.0032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dau P, Lindstrom J, Cassel C, Clark E. Plasmapheresis in myasthenia gravis and polymyositis. In: Dau P, editor. Plasmapheresis and the immunobiology of myasthenia gravis. Houghton Mifflin Professional Publishers; Boston, MA: 1978. pp. 229–241. [Google Scholar]
- 15.Keesey J. Indications for thymectomy in myasthenia gravis. In: Dau P, editor. Plasmapheresis and the immunobiology of myasthenia gravis. Houghton Mifflin Professional Publishers; Boston, MA: 1978. pp. 124–134. [Google Scholar]
- 16.Fudenberg HH. The immune globulins. Annu Rev Microbiol. 1965;19:301–338. doi: 10.1146/annurev.mi.19.100165.001505. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan AA. Therapeutic plasma exchange: core curriculum 2008. Am J Kidney Dis. 2008;52:1180–1196. doi: 10.1053/j.ajkd.2008.02.360. [DOI] [PubMed] [Google Scholar]
- 18.Rodgaard A, Nielsen FC, Djurup R, Somnier F, Gammeltoft S. Acetylcholine receptor antibody in myasthenia gravis: predominance of IgG subclasses 1 and 3. Clinical and experimental immunology. 1987;67:82–88. [PMC free article] [PubMed] [Google Scholar]
- 19.Tackenberg B, Kruth J, Bartholomaeus JE, Schlegel K, Oertel WH, Willcox N, Hemmer B, Sommer N. Clonal expansions of CD4+ B helper T cells in autoimmune myasthenia gravis. European journal of immunology. 2007;37:849–863. doi: 10.1002/eji.200636449. [DOI] [PubMed] [Google Scholar]
- 20.Kornfeld P, Ambinder EP, Mittag T, Bender AN, Papatestas AE, Goldberg J, Genkins G. Plasmapheresis in refractory generalized myasthenia gravis. Arch Neurol. 1981;38:478–481. doi: 10.1001/archneur.1981.00510080040003. [DOI] [PubMed] [Google Scholar]
- 21.Advisory Committee on Immunization, P., C. Centers for Disease, and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60:1–45. [PubMed] [Google Scholar]


