Abstract
Introduction
The aim of this study was to describe the clinical characteristics and radiological findings in patients with a previous history of malignancy who underwent CT screening for lung cancer.
Methods
Patients with a previous history of malignancy with a life expectancy of at least 5 years referred for lung cancer screening between 5/2/2011 and 9/24/2014 were included. CT features assessed included nodule size, morphology and number. The lung-RADs scoring system was retrospectively applied to all studies.
Results
139 patients were studied (mean age 66 years, median pack year smoking history of 50). All had a previous history of cancer, most commonly breast 60 (43%), head/neck 26 (19%) and lung 16 (12%). 42 (30%) patients had a positive screening study. 7 (5%) patients were diagnosed with lung cancer. 1 (1%) patient was diagnosed with a radiation induced chest wall sarcoma. 42 (30%) patients had a positive chest CT as per NCCN Lung Cancer Screening nodule follow-up algorithm.
Conclusion
The rate of lung cancer diagnosis in our patient population is higher than in several previously published studies. Smokers with a prior history of malignancy may be in a particularly high risk group for the development of subsequent lung cancer.
Introduction
Lung cancer is the leading cause of cancer death in the United States. The American Cancer Society estimates there will be 224,390 new cases of lung cancer in 2016 and 158,080 lung cancer related deaths [1]. Data from the in the National Lung Screening Trial (NLST) provided the first level 1 evidence that low-dose screening computed tomography (CT) can reduce lung cancer mortality, demonstrating a 20% relative reduction in mortality from lung cancer in individuals aged 55–74 with a 30 or more pack- year smoking history, undergoing annual low-dose CT screening [2]. In recent years numerous national and international groups have recommended the use of low-dose CT for lung cancer screening [3, 4, 5, 6, 7].
Although there is no evidence that lung cancer screening reduces mortality outside the setting of the NLST, it has been suggested that some high-risk patients not meeting the NLST inclusion criteria may benefit from lung cancer screening [8]. The National Comprehensive Cancer Network (NCCN) guidelines have expanded to include a second high-risk group for whom lung cancer screening is recommended, comprised of smokers ≥ 50 years old, with a pack year history ≥20 pack years, who have additional risk factors. In particular, a previous history of thoracic radiation exposure and a previous history of certain malignancies are both recognized as risk factors which merit inclusion in this cohort [2].
To date there has been an inconsistent approach to the inclusion of patients with a previous history of malignancy in lung cancer screening studies, some studies excluding them [9, 10], and others placing limits on their participation [11, 12]. To our knowledge there has been no previous systematic report of the radiological findings of a cohort of patients with a previous history of malignancy undergoing lung cancer screening. We reviewed our experience of lung cancer screening in patients with a previous history of malignancy, who underwent low-dose screening chest CT in our institution, a tertiary cancer referral center.
Methods
This was a retrospective, single-center study of our experience with clinical CT lung screening in a population of patients with a previous history of malignancy. The institutional review board approved this study.
Patients
Patients were referred for lung cancer screening with low dose CT between 5/2/2011 and 9/24/2014. All were considered by referring clinicians to have a life expectancy of greater than 5 years. All had a smoking history, except for 1 patient with a previous history of lung cancer.
Image Acquisition and Interpretation
CT lung screening examination were performed without intravenous contrast on either 16-row or 64- row multidetector CT scanners [LightSpeed 16, LightSpeed VCT, LightSpeed CT750 (GE Medical Systems, Milwaukee, Wisconsin)] at 120 kVp and either 40 or 80mA [mA was selected depending on chest diameter on the anteroposterior scout view (if ≤ 36cm = 40mA; if >36cm = 80mA)]. Axial images were obtained from the lung apices through the lung bases at 1.25mm thickness.
Clinical image interpretation was performed by one of 6 thoracic radiologists on the institutional Picture Archive and Communication System (PACS) (GE Healthcare, Milwaukee, Wisconsin). Lung window settings (width, 1500 HU; level, -500 HU) and soft tissue window settings (width, 400 HU; level, 30 HU) were used. As part of this study, all exams were also retrospectively reviewed by 1 chest radiologist who recorded the clinical interpretation and retrospectively applied the Lung-RADS scoring system. CT features recorded were nodule size, morphology and number, in addition to any incidental thoracic findings. A thoracic radiologist with 20 years experience adjudicated in cases of disagreement with the clinical interpretation, which occurred in 5 cases.
For the study period clinical CT reports were issued using a structured reporting system based on National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Lung Cancer Screening (version 1.2012) nodule follow-up algorithms [13]. A screening examination was considered positive if a solid nodule without benign features was >4mm, or a ground glass nodule was ≥5mm, requiring follow up imaging within less than 1 year. When the Lung-RADs scoring system was retrospectively applied, a CT was considered positive if: (i) A solid nodule was ≥ 6mm, or was new and 6mm, or was new and >4mm, or was growing; (ii) A ground glass nodule was ≥ 20mm; (iii) A part solid nodule was new, or had a growing solid component, or was ≥ 6mm.
Results
Demographics
Between 5/2/2011 and 9/24/2014 139 patients with a previous history of malignancy underwent lung cancer screening. 60 (43%) were male and 79 (56%) female. Mean age was 66 years (range 40-80). All patients had a previous history of cancer, most commonly breast 60 (43%), head and neck 26 (19%) and lung 16 (12%) (Table 1). 67 (48%) patients had a history of radiation to either the thorax or head/neck, and 87 (63%) patients had received chemotherapy. Mean time between the date of cancer diagnosis and screening chest CT was 83.1 months (range 1.6 - 426.4 months). 138 patients (99%) had a smoking history [median pack years 50 (range 0-120)]. 5 patients had a pack year history of <20 years. Of these patients, the pack year history ranged from 0 years (1 patient) to 16 years, with a median of 15 years. All patients without a >20 pack year smoking history had a prior history of lung cancer which was diagnosed between 5 and 18 years prior to the date of screening.
Table 1.
Subtype of cancer in patients undergoing lung cancer screening (percentages are ≤100% as 15 patients had more than 1 cancer diagnosis).
| Type of cancer | Number of patients |
|---|---|
| Breast Cancer | 60(43%) |
| Head and Neck Cancer | 26 (19%) |
| Lung Cancer | 16(11%) |
| Prostate | 14 (10%) |
| Renal | 9 (6%) |
| Melanoma | 4 (3%) |
| Uterine | 3 (2%) |
| Testicular | 3 (2%) |
| Colorectal | 3 (2%) |
| Anal, Cervical, Lymphoma, Leukemia, Bladder | 2 (1%)each |
| Myeloma , Small bowel neuroendocrine, Thyroid, Fallopian tube carcinoma, Esophageal, Sarcoma 1 | 1 (1%) each |
Cancer diagnoses
7 (5%) patients were diagnosed with lung cancer on screening CT [6 adenocarcinoma, 1 squamous cell carcinoma (SCC)]. 1 (1%) patient was diagnosed with radiation induced high-grade undifferentiated pleomorphic chest wall sarcoma [9 years following radiation for head and neck SCC (HNSCC)]. None of the lesions identified represented metastatic disease.
The imaging characteristics of the malignant pulmonary nodules are summarized in Table 2. None of the patients diagnosed with cancer had a previous history of lung cancer (3 patients had breast cancer, 1 had head and neck cancer, 1 had prostate cancer and 1 had cervical cancer). Of note, the patient with lung SCC had a history of breast cancer, not head and neck cancer. 2 (1%) patients had a biopsy or surgery for non-malignant lesions (1 atypical pneumocyte hyperplasia, 1 nodular scar).
Table 2.
Characteristics of malignant pulmonary nodules identified on screening chest CT
| Size on baseline screening CT | Nodule consistency | LUNG-RADS category prior to surgery/biopsy | Pathology |
|---|---|---|---|
| 8 mm – grew to 12 mm on follow up (Figure 1) | Solid | 4B | Adenocarcinoma |
| 22 mm | Solid | 4B | Squamous cell carcinoma |
| 15 mm | Pure Ground Glass | 2 | Adenocarcinoma |
| 5mm – grew to 8 mm on follow-up | Solid | 4B | Adenocarcinoma |
| 10 mm | Sub-solid | 4A | Adenocarcinoma |
| 10 mm | Solid | 4B | Adenocarcinoma |
| 30 mm | Sub-solid | 4B | Adenocarcinoma |
All patients diagnosed with lung cancer had the lesions surgically resected. 5 were stage 1A and 2 were stage 1B. 1 patient died from lung cancer 11 months following diagnosis.
Additional Imaging Findings
139 patients had at least 1 chest CT, 71 had at least 2 CTs, 30 had at least 3 CTs and 8 had 4 CTs. The mean duration of imaging follow up was 14.7 months (range 2.7 – 34.1). The mean dose length product (DLP) was 90.7 mGy·cm (range 41-548 mGy·cm).
37 (27%) patients had no nodules. 14 (10%) patients only had definitively benign calcified nodules. 88 (63%) patients had at least 1 non-calcified pulmonary nodule. The number of positive chest CTs is summarized in Table 3 and the Lung- RADS™ categories after each CT are summarized in Table 4. Overall 42 (30%) patients had a positive chest CT as per NCCN Lung Cancer Screening nodule follow-up algorithm [13]. 2 patients who had positive screening studies were lost to follow-up.
Table 3.
A summary of the number of positive chest CTs, both overall and after each chest CT.
| Number of positive Chest CTs | |
|---|---|
| Overall | 42 (30% of patients) |
| After 1st CT | 26 (18% of scans) |
| After 2nd CT | 11 (15% of scans) |
| After 3rd CT | 4 (13% of scans) |
| After 4th CT | 1 (13% of scans) |
Table 4.
A summary of the Lung-RADS™ assessment categories after each chest CT.
| After 1st chest CT | After 2nd chest CT | After 3rd chest CT | After 4th chest CT | Expected population prevalence | |
|---|---|---|---|---|---|
| Lung- RADS ≤ 2 | 117 (84%) | 69 (97%) | 27 (90%) | 8 (100%) | 90% |
| Lung- RADS 3 | 13 (9%) | 0 (0%) | 0 (0%) | 0 (0%) | 5% |
| Lung- RADS 4 | 9 (7%) | 2 (3%) | 3 (10%) | 0(0%) | 4% |
3 patients had lesions that enlarged during the period of screening. One solid nodule grew from 8mm to 15mm over a 7 month period and one solid nodule grew from 5mm to 8mm over a 12 month period. Both were adenocarcinoma. One additional lesion, a ground glass opacity with a growing 7mm solid component, is awaiting follow-up.
Discussion
This study describes the clinical characteristics and radiologic findings in a cohort of patients with a prior history of malignancy undergoing lung cancer screening. 7 (5%) of participants were diagnosed with lung cancer. Although our cohort is small, the 5% rate of lung cancer diagnosis is high compared to several previous lung cancer screening studies. In the NLST, 2.4% of participants in the low dose CT arm were diagnosed with lung cancer after a positive screening study [2]. A Canadian population based study demonstrated screen detected malignancy rates of 3.9 % [12], while 3 European studies, based in the Netherlands, Denmark and Italy, detected malignancy in 3.0% [14], 0.7% [10] and 2.0% [11] of participants respectively.
There has been an inconsistent approach to the inclusion of patients with a prior cancer history in previous lung cancer screening trials. For example in the studies referenced above, 1 excluded patients with any history of cancer [10], 1 included patients with a history of smoking related cancers [8], 1 excluded patients with a history of cancer within 5 years, 1 excluded patients with a history of renal cancer, melanoma or breast cancer [14], while 2 excluded most cancer patients with exception of non-melanomatous skin cancer, and in-situ carcinoma of the bladder [2], prostate or cervix, [12]. Despite these inconsistencies, the clinical reality is that lung cancer occurring as a second primary malignancy currently accounts for between 8-14% of all lung cancer diagnoses [15], [16], [17], [18], [19]. As lung cancer screening becomes more widely used, the potential inclusion of certain cancer survivors in lung cancer screening programs needs to be more systematically addressed.
Cancer survivorship is a growing public health issue. On January 1, 2014 14.5 million people with a history of cancer were alive in the USA, a number projected to increase to 19 million by 2024 [20], [21]. This unique patient group has a number of well documented potential health issues. In particular, many cancer survivors are at risk of developing a second primary malignancy, including lung cancer. In some cases this reflects the carcinogenic effects of cancer treatment, for example radiotherapy for breast cancer has been linked to a low, but increased risk of lung cancer [22]. In other cases the increased risk results from historical exposure to a carcinogen such as cigarette smoke [23,24]. In many such cases the diagnosis of lung cancer is the primary driver of future life expectancy, for example accounting for 12% of deaths in (HNSCC) patients in the Surveillance Epidemiology and End Results (SEER) database [24] and for 100% of cancer related deaths reported in a recently reported small cohort of early stage bladder cancer patients with a second primary lung malignancy [25].
It should be emphasized that no study has been performed to demonstrate a survival benefit for patients with a previous malignancy screened for lung cancer. Indeed, despite the NCCN guideline recommending screening in high-risk groups outside the NLST inclusion criteria, no data exists to support screening any patient outside the NLST population. The available retrospective literature analyzing the pattern of second primary lung cancers in cancer survivors emphasizes the complex and heterogenous nature of this patient population. For example Milano et al demonstrated that breast cancer patients with a second primary lung cancer tend to be diagnosed at an earlier stage and have a slightly increased overall survival, when compared to lung cancer patients without breast cancer [26]. Conversely patients with a history of Hodgkin’s lymphoma or chronic lymphocytic leukemia and second primary lung cancer have a significantly worse survival when compared to patients with de-novo lung cancer [27, 28]. In a small study by Pagedar et al the median survival of HNSCC patients with a second primary lung cancer was significantly lower than lung cancer patients without HNSCC [29]. Another important point is that simply detecting a lung cancer in a screening program doesn’t prove that screening is beneficial. The cancers reported in this paper, were all prevalence cancers, 86% were adenocarcinomas and 50% were sub-solid lesions. These factors raise the possibility that at least some of the lesions may have had an indolent biology, and emphasize the fact that further study is needed to prove a benefit to screening in this scenario.
A particular concern in a complex group of patients such as this undergoing any screening test is the potential for a high false positive rate. Our overall 30% rate of positive chest CT, although high, compares to both the 24% rate in the NLST [2] and the 25% rate in the recent study by McKee et al assessing high risk patients falling outside the NLST guidelines [8]. Our 9% rate of ACR Lung-RADS category 3 lesions on initial screening CT is higher than ACR guidelines suggest the background population prevalence should be. This probably reflects the high rate of thoracic surgery or radiation in our cohort, with consequent parenchymal abnormalities. An additional concern in any screened population is the risk of expose to ionizing radiation. This is particularly the case in cancer survivors, many of whom have a high cumulative exposure to ionizing radiation from both diagnostic and therapeutic sources. In our cohort the mean DLP for screening chest CT was low at 90.7 mGy·cm (1.3mSv), and comparable to the NLST in which the mean dose was 1.5 mSv [2].
The primary limitations of this study are its retrospective nature and its small cohort size, both of which could be overcome by future prospectively performed, larger studies to confirm our results and to assess any impact on survival that lung cancer screening could have in cancer survivors. Several of the screening CTs were performed with a short interval of the patient’s initial cancer diagnosis. These cases reflected local referral policy in patients with early stage or indolent neoplasms, whose life expectancy was greater than 5 years.
In conclusion, we report the radiological findings in a group of patients with a prior history of malignancy undergoing lung cancer screening. Although our patient cohort is small, the rate of cancer diagnoses is higher than in several previously published studies which did not specifically assess patients with a prior history of malignancy. To date, no study has demonstrated a benefit for screening outside of the NLST population, and further study is required to define the role screening has to play in this complex patient population.
Figure 1.

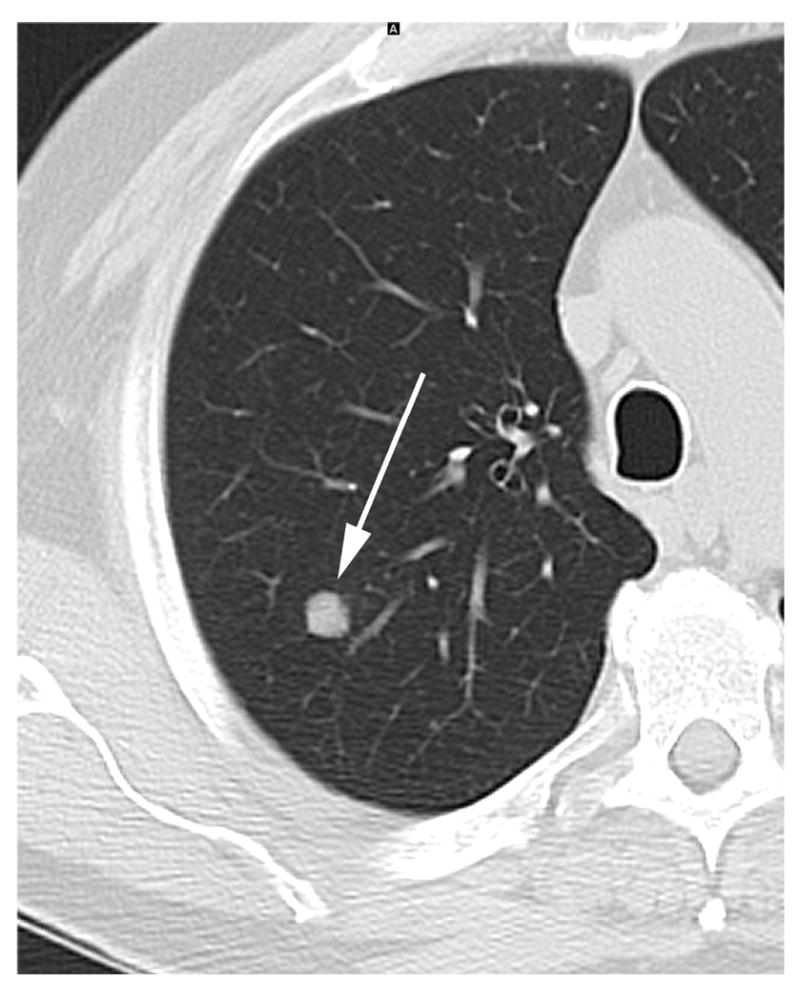
a – 64 year old male with a previous history of head and neck cancer. Baseline screening Chest CT on lung windows demonstrates an 8 mm solid right upper lobe node (arrow).
b – CT chest on lung windows in the same patient performed 15 weeks later demonstrating slight growth in the right upper lobe nodule (arrow). The lesion was resected and pathology revealed lung adenocarcinoma.
Table 5.
A summary of incidental thoracic findings
| Incidental thoracic finding | Number of patients (%) |
|---|---|
| Emphysema | 37 (26%) |
| Post-radiation change | 22 (16%) |
| Post-surgical change | 11 (8%) |
| Calcified pleural plaque | 2 (1%) |
| Pleural effusion, Pericardial effusion, Axilla seroma, Clavicular osteomyelitis, Bronchiectasis | 1 (1%) each |
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Disclosures:
Darragh F Halpenny – Nothing to disclose, Jane D Cunningham – Nothing to disclose, Niamh M Long – Nothing to disclose, Ramon E Sosa – Nothing to disclose, Michelle S Ginsberg – Nothing to disclose
IRB statement:
This study was performed under a waiver from the IRB.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics 2016. CA Cancer J Clin. 2016 Jan;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Aberle DR, Adams AM, Berg CD, et al. N Engl J Med. 5. Vol. 365. National Lung Screening Trial Research Team; 2011. Aug 4, Reduced lung-cancer mortality with low-dose computed tomographic screening; pp. 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyer VA on behalf of the US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–8. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 4.Wender R, Fontham ET, Barrera E, Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63:107–17. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American College of Radiology. ACR statement on USPSTF draft recommendation for CT lung cancer screening. [7/22/2015]; Available at: http://www.acr.org/About-Us/Media-Center/Press-Releases/2013-Press-Releases/20130729-ACR-Statement-on-USPSTF-Draft-Recommendation-for-CTLung-Cancer-Screening.
- 6.American Lung Association. Guidance on CT lung cancer screening. [7/22/2015]; Available at: http://www.lung.org/about-us/our-impact/top-stories/guidance-on-ct-lung-cancer.html.
- 7.American Society of Clinical Oncology. Statement from the American Society of Clinical Oncology and the American College of Chest Physicians on the joint systematic review and clinical practice guideline on the role of CT screening for lung cancer (endorsed by the American Thoracic Society) [7/22/2015]; Available at: http://www.asco.org/press-center/statement-american-society-clinical-oncology-and-american-college-chestphysicians.
- 8.McKee BJ, Hashim JA, French RJ, et al. Experience with a CT screening program for individuals at high risk for developing lung cancer. J Am Coll Radiol. 2015 Feb;12(2):192–7. doi: 10.1016/j.jacr.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Infante M, Cavuto S, Lutman FR, et al. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009 Sep 1;180(5):445–53. doi: 10.1164/rccm.200901-0076OC. [DOI] [PubMed] [Google Scholar]
- 10.Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax. 2012 Apr;67(4):296–301. doi: 10.1136/thoraxjnl-2011-200736. [DOI] [PubMed] [Google Scholar]
- 11.Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev. 2012 May;21(3):308–15. doi: 10.1097/CEJ.0b013e328351e1b6. [DOI] [PubMed] [Google Scholar]
- 12.McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013 Sep 5;369(10):910–9. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network. The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Lung Cancer Screening (version 1.2012) [7/22/2015]; Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 14.Horeweg N, Scholten ET, de Jong PA, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol. 2014 Nov;15(12):1342–50. doi: 10.1016/S1470-2045(14)70387-0. [DOI] [PubMed] [Google Scholar]
- 15.Reinmuth N, Stumpf P, Stumpf A, et al. Characteristics of lung cancer after a previous malignancy. Respir Med. 2014 Jun;108(6):910–7. doi: 10.1016/j.rmed.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Haraguchi S, Hioki M, Koizumi K, et al. Characteristics of multiple primary malignancies associated with lung cancer by gender. Respiration. 2007;74:192e5. doi: 10.1159/000093324. [DOI] [PubMed] [Google Scholar]
- 17.Quadrelli S, Lyons G, Colt H, Chimondeguy D, Silva C. Lung cancer as a second primary malignancy: increasing prevalence and its influence on survival. Ann Surg Oncol. 2009;16:1033e8. doi: 10.1245/s10434-008-0296-1. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann HS, Neef H, Schmidt P. Primary lung cancer and extrapulmonary malignancy. Eur J Cardiothorac Surg. 2007;32:653e8. doi: 10.1016/j.ejcts.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Massard G, Ducrocq X, Beaufigeau M, et al. Lung cancer following previous extrapulmonary malignancy. Eur J Cardiothorac Surg. 2000;18:524e8. doi: 10.1016/s1010-7940(00)00571-6. [DOI] [PubMed] [Google Scholar]
- 20.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014 Jul-Aug;64(4):252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 21.Altekruse SF, Kosary CL, Krapcho M, et al., editors. National Cancer Institute; Bethesda, MD: 2010. [7/22/2015]. SEER Cancer Statistics Review, 1975-2007. http://seer.cancer.gov/csr/1975_2007. [Google Scholar]
- 22.Salminen E, Pukkala E, Teppo L. Bladder cancer and the risk of smoking-related cancers during followup. J Urol. 1994 Nov;152(5 Pt 1):1420–3. doi: 10.1016/s0022-5347(17)32435-7. [DOI] [PubMed] [Google Scholar]
- 23.Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long-term survivors of head and neck cancer. Cancer. 2014 May 15;120(10):1507–13. doi: 10.1002/cncr.28588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Rey J, Placer J, Vallmanya F, et al. Are patients with non-muscle-invasive bladder cancer a suitable population for a lung cancer screening trial? BJU Int. 2010 Jul;106(1):49–52. doi: 10.1111/j.1464-410X.2009.09081.x. [DOI] [PubMed] [Google Scholar]
- 25.Milano MT, Strawderman RL, Venigalla S, Ng K, Travis LB. Non-small-cell lung cancer after breast cancer: a population-based study of clinicopathologic characteristics and survival outcomes in 3529 women. J Thorac Oncol. 2014 Aug;9(8):1081–90. doi: 10.1097/JTO.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 26.Milano MT, Li H, Constine LS, Travis LB. Survival after second primary lung cancer: a population- based study of 187 Hodgkin lymphoma patients. Cancer. 2011;117:5538–5547. doi: 10.1002/cncr.26257. [DOI] [PubMed] [Google Scholar]
- 27.Solomon BM, Rabe KG, Slager SL, Brewer JD, Cerhan JR, Shanafelt TD. Overall and cancer-specific survival of patients with breast, colon, kidney, and lung cancers with and without chronic lymphocytic leukemia: a SEER population-based study. J Clin Oncol. 2013;31:930–937. doi: 10.1200/JCO.2012.43.4449. [DOI] [PubMed] [Google Scholar]
- 28.Pagedar NA, Jayawardena A, Charlton ME, Hoffman HT. Second Primary Lung Cancer After Head and Neck Cancer: Implications for Screening Computed Tomography. Ann Otol Rhinol Laryngol. 2015 Apr 16; doi: 10.1177/0003489415582259. [DOI] [PMC free article] [PubMed] [Google Scholar]


