Abstract
The aim of this study was to elucidate the expression and functions of interleukin (IL)-24 in C57BL/6 mouse corneas in response to Pseudomonas aeruginosa (PA) infection. Among IL-20R cytokines, only IL-24 was induced at both mRNA and protein levels by infection at early time points. The upregulation of IL-24 was dampened by flagellin pretreatment, which protects the corneas from microbial infection. Time course studies revealed bimodal early and later peaks of IL-24 expression, a pattern shared with SOCS3 but not IL-1β or IL-6. Silencing of IL-24 enhanced S100A8/A9 expression and suppressed SOCS3, IL-1β, IL-1RN, and MMP13 expression at 6 hpi. Downregulation of IL-24 signaling pathway significantly reduced the severity of keratitis; while recombinant IL-24 exacerbated PA-mediated tissue destruction. In vitro, recombinant IL-1β induced the expression of SOCS3, IL-24, IL-1β, and IL-6 in primary cultured human corneal epithelial cells. Recombinant IL-24, on the other hand, stimulated the expression of SOCS3, but not the others. In conclusion, IL-24 promotes PA keratitis through the suppression of early protective mucosal immunity, culminating in increased severity of PA keratitis.
Keywords: bacterial keratitis, interleukin 24, SOCS3, innate immunity
Introduction
Microbial keratitis is a sight-threatening disease that poses a substantial burden to affected individuals. It is reported that corneal infection is a major cause of unilateral blindness around the world (1). P. aeruginosa (PA) is the predominant bacterium isolated in contact lens-related corneal infections (2). The disease is usually manifested by severe inflammation, formation of corneal ulcers, and hypopyon (3). Both the pathogen and the host immune system contribute to the destruction of the cornea (4). Unrestrained inflammation generated by the host immunity can cause scar tissue formation and vascularization of the cornea. If not properly treated, patients eventually suffer from permanent vision loss.
The corneal epithelial layer forms the first line of defense against pathogens. The discovery of Toll-like receptors (TLRs) has elicited keen interest in the innate defensive functions of mucosal epithelial cells (5, 6). Our previous studies showed that mouse corneas pretreated with flagellin, which signals through TLR5, are protective against PA infection (7–9). Flagellin pretreatment has also been demonstrated to be protective in localized infections of the lung (10, 11) and gut (12), radiation pneumonitis (13), and systematically against biological or physical insults (14). We found that this flagellin-induced protection is due to the reprograming of gene expression in epithelial cells to enhance the innate defense against invasive pathogens. In order to better understand the underlying mechanism, we performed a genome-wide complementary DNA (cDNA) microarray to assess the differentially expressed genes in corneal epithelial cells after PA challenge, with or without flagellin pretreatment (15). Among the genes differentially regulated by flagellin pretreatment, the expression of IL-24 and its downstream negative regulator, SOCS3, was notably upregulated in response to infection and significantly suppressed by flagellin pretreatment.
IL-24, along with IL-19, IL-20, IL-22, and IL-26, belongs to the IL-20 subfamily of the IL-10 superfamily of cytokines (16). IL-19, IL-20, and IL-24 (named as “IL-20R cytokines” here) share the IL-20R1 and IL-20R2 heterodimer receptor. IL-20 and IL-24 can also bind to the IL-22R1 and IL-20R2 receptor complex (17). After binding to their receptors, IL-20R cytokines signal through the Janus kinase-signal transducer and activator of transcription 3 (JAK/STAT3) pathway to regulate downstream gene expression (18, 19). The SOCS family of proteins, including eight members, play an important role in the negative regulation of cytokine-JAK-STAT signaling pathway (20). While SOCS1 inhibits STAT1 activation in the IFN-γ signaling cascade (21), SOCS3 is a major negative regulator of the IL-6-STAT3 signaling pathway (22). SOCS3 binds to both JAK and cytokine receptors and targets them for ubiquitination and proteasomal degradation (23). SOCS3 can also directly inhibit JAK activity (24). Increased expression of IL-24 and SOCS3 has been found within inflamed tissues in various diseases, such as skin inflammation (e.g. psoriasis), inflammatory bowel disease (IBD), and rheumatoid arthritis (25–27). IL-24 exerts protective effects against intracellular pathogens, such as Salmonella typhimurium and Mycobacterium tuberculosis (28, 29). Studies in different mouse models have proven the critical importance of SOCS3 in restraining inflammation and promoting optimal levels of protective immunity against the infection (30). On the other hand, some pathogens have evolved to modify host SOCS3 expression to evade the immune response. For example, Epstein-Barr virus and Herpes simplex virus can stimulate SOCS3 expression to suppress type I interferon (IFN) production, thereby subverting the host immune response (31, 32). To date, the functions and mechanism of IL-24 in bacterial keratitis have not been explored.
Given the reported importance of IL-24 in inflammation and host protection against infection, we hypothesized that IL-24 would enhance the host defense against PA keratitis. Surprisingly, we found that IL-24 increased the susceptibility of mouse cornea to PA infection. Our results indicate that PA induces the early expression of IL-24, resulting in a suppression of early protective mucosal immune responses.
Materials and methods
Animals
Wild-type C57BL/6 mice (8 weeks; female) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All animal procedures were performed in compliance with the ARVO Statement for the use of animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of Wayne State University.
Mouse model of P. aeruginosa Keratitis
Mice were anesthetized with an intraperitoneal injection of Ketamine (90 mg/kg) and Xylazine (10 mg/kg) before surgical procedures. Mouse corneas were scratched gently with a sterile 26-gauge needle to create three 1-mm incisions to break the epithelial barrier. Purified flagellin (500ng in 5μl PBS) was applied topically to the injured cornea as an eye drop. PBS was used as control. Twenty-four hours later, the corneas were scratched again and inoculated with 1.0×104 CFUs of ATCC 19660 in 5μl PBS.
Administration of siRNA, recombinant protein, or neutralizing antibody
All the siRNAs used in this study were SMARTpool (a mixture of 4 siRNAs) ON-TARGETplus siRNAs designed by GE Dharmacon Company (Lafayette, CO, USA). Mice were subconjunctivally injected twice with siRNA targeting to a specific gene (50 picomoles in 5μl RNase-free water) over 2 days. Six hours after the second siRNA injection, mouse corneas were inoculated with PA. To administer recombinant protein or neutralizing antibody, mice were subconjunctivally injected with recombinant mouse IL-24 (200ng/5μl, 7807-ML-010; R&D systems, Minneapolis, MN, USA), or anti-IL-20R2 (200ng/5μ l, 14-1206; eBioscience, San Diego, CA, USA) 4 hours before the inoculation with PA on the corneas.
Isolation of mouse corneal epithelial cells
Razor blade was tailored to approximately 5 mm wide in the edge and placed in a Castroviejo razor blade breaker and holder. Mice were euthanized by cervical dislocation. Under the microscope, corneal epithelial cells were surgically scraped off from the basement membrane. Cells were collected to the razor blade from the basement membrane. Liquid nitrogen was used to snap-frozen the cells and cool off the tip of a sharp surgical scalper at the same time. Cells were immediately transferred into a pre-cool, 1.5 ml Eppendorf tubes placed on dry ice by scraping the razor blade with the scalper. Cells were processed for RNA isolation, protein extraction or stored at −80°C freezer for later use.
Clinical examination, quantification of PA colony forming units (CFUs) and determination of myeloperoxidase (MPO) units
Corneas were photographed daily up to 3dpi for the assessment of infection severity. Clinical scores were assigned to the infected corneas in a blinded fashion according to the scale previously reported (33). Whole corneas were excised and placed in 200μl sterile PBS. Tissue was homogenized with TissueLyser II (Qiagen, Valencia, CA, USA). The homogenates were divided into two parts. The first fraction (50μl) was subjected to serial log dilutions for the assessment of viable bacterial number. The remaining homogenates were further lysed for MPO measurement. MPO units were determined according to a previous reported method (34). One MPO unit corresponds to 2.0×105 polymorphonuclear leucocytes (PMNs)
Cell culture
Primary human corneal epithelial cells (HCECs) were obtained from diseased human corneal samples. Corneal epithelial cells were dislodged from underlying basement membrane by dispase and then digested by trypsin. Cells were pelleted, re-suspended and grown in defined keratinocyte serum-free medium (DK-SFM) (Gibco, Waltham, MA, USA) with supplements and antibiotics.
Passage (P) 3 or P4 of the primary HCECs were used for the experiments. Before treatment, cells were starved overnight in growth factor-free and antibiotic-free keratinocyte basic medium (KBM-2, Lonza, Basel, Switzerland). Subsequently, cells were treated with recombinant human IL-1β (50ng/ml, 201-LB; R&D systems, Minneapolis, MN, USA), or recombinant human IL-24 (100ng/ml, 1965-IL-025; R&D systems). At the end of the incubation period, cells were harvested to assess gene expression.
Semi-quantitative and quantitative PCR
The primers used in this study are listed in Table 1. Total RNA was extracted with RNeasy Mini Kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. For semi-quantitative PCR, cDNA was amplified with TaqMan technology (Promega, Madison, WI, USA). PCR products were subjected to electrophoresis on 2% agarose gels containing ethidium bromide. For quantitative PCR, cDNA was amplified using StepOnePlus Real-Time PCR system (Applied Biosystems, University Park, IL, USA) with the SYBR® Green PCR Master Mix (Applied Biosystems). Data were analyzed by using ΔΔCT method with β-actin or GAPDH as the internal control.
Table 1.
PCR primer sequences used in this study.
| Primers | Forward | Reverse |
|---|---|---|
| mIL-19 | aacaacctgctgacattcta | attggtggcttcctgact |
| mIL-20 | tctcagtctgtcattctcac | tatagcatctcctccatcca |
| mIL-24 | gctttcaccaaagcgacttc | gcccagtaaggacaattcca |
| mSOCS3 | attcacccaggtggctacag | gccaatgtcttcccagtgtt |
| mIL-1β | tgccaccttttgacagtgatg | aaggtccacgggaaagacac |
| mIL-1RN | gggaccctacagtcacctaa | ggtccttgtaagtacccagca |
| mIL-6 | gtggctaaggaccaagacca | taacgcactaggtttgccga |
| mCXCL10 | ttaaggagcccttttagaccttt | atgaacccaagtgctgccgtc |
| mMMP13 | tgatgaaacctggacaagc | ctggaccataaagaaactgaa |
| mIL-17A | tttaactcccttggcgcaaaa | ctttccctccgcattgacac |
| mS100A8 | ttcgtgacaatgccgtctga | agggcatggtgatttccttgt |
| mS100A9 | tgggcttacactgctcttacc | ggttatgctgcgctccatct |
| mβ-actin | gtgggccgccctaggcacca | ggttggccttagggttcagg |
| hIL-24 | gtctttcacagcccagaagg | agggccaagaattccacttt |
| hSOCS3 | ggtaccccacatcctctcct | caaatgttgcttccccctta |
| hIL-6 | tttcaccaggcaagtctcct | gaaagcagcaaagaggcact |
| hIL-1β | accaaacctcttcgaggcac | agccatcatttcactggcga |
| hGAPDH | aggggtctacatggcaactg | ggcctccaaggagtaagacc |
m: mouse; h: human.
ELISA
For cytokine measurement, homogenates from mouse cornea samples were sonicated and centrifuged to obtain supernatant. Protein concentration was determined by BCA assay with Thermo Scientific Pierce BCA Protein Assay Kit (Micro BCA, Pierce, Rockford, IL, USA). The amount of cytokines was determined by ELISA assay according to manufacturer’s instructions (mouse IL-1β: DY401; mouse IL-1RA: MRA00; mouse IL-6: DY406; mouse S100A8/9: DY8596-05; mouse CXCL10: MCX100; R&D Systems, Minneapolis, MN, USA).
Western Blot
Mouse corneal samples were lysed with RIPA buffer. The lysates were centrifuged to obtain supernatant. Protein concentration was determined by BCA assay. The protein samples were separated by SDS-PAGE and electrically transferred onto nitrocellulose membranes (Bio-Rad; Hercules, CA, USA). The membranes were blocked with 3% BSA and subsequently incubated with primary and secondary antibodies. Signals were visualized using SuperSignal® West Pico Chemiluminescent Substrate (Thermo Scientific, Pittsburgh, PA, USA). β-actin was used as the loading control. Quantification of protein levels was based on the densitometry of blots by using the software Carestream MI SE (Informer Technologies, Inc. Rochester, NY, USA). Antibodies: anti-IL-24 (ab182567; Abcam, Cambridge, MA, USA); anti-SOCS3 (#516919; R&D Systems, Minneapolis, MN, USA); anti-STAT3 (#4904; Cell signaling); anti-phospho-STAT3 (Tyr705) (#9145; Cell signaling); and anti-β-actin (A1978; Sigma).
Statistical analysis
A nonparametric Mann-Whitney U test was used to compare the clinical scores. Student t-test or one-way ANOVA was used to compare quantitative means. P-value < 0.05 was considered to be significant.
Results
PA infection induces IL-24 and SOCS3 expression in mouse corneal epithelial cells (CECs)
To verify our previously reported cDNA microarray results (15), we infected mouse corneas with 1.0×104 colony forming units (CFUs) of PA stain ATCC 19660. Corneal epithelial cells were collected at 6 hpi and subjected to PCR analysis. As shown in Figure 1A, flagellin challenge alone minimally induced IL-24 expression relative to the naïve cornea. PA infection increased the expression of IL-24, and this elevation of IL-24 expression was dampened by flagellin pretreatment in B6 mouse CECs. As IL-24 shares receptors with IL-19 and IL-20, we explored the induction of these cytokines in response to infection. While low levels of IL-19 were detected in all samples, no IL-20 mRNA was detected. The infection-induced expression of SOCS3 was also dampened by the flagellin-pretreatment (Fig. 1B). The expression of IL-1β, an innate immune and major inflammatory mediator, significantly increased after PA infection, and was attenuated by pretreatment with flagellin (Fig. 1B).
Figure 1. P. aeruginosa.
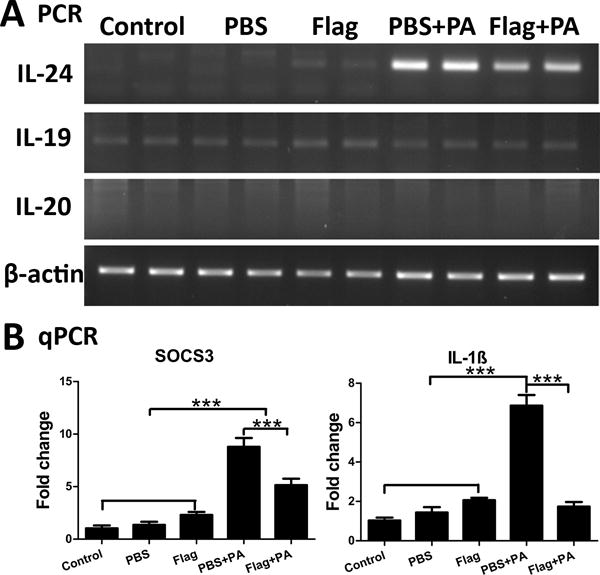
(PA) infection induces IL-24 and SOCS3 expression in B6 mouse corneal epithelial cells; Flagellin pretreatment attenuates their expression. Mouse corneas were scratched with a needle and topically pretreated with 500ng flagellin for 24 hours. PBS was used as control. Then the corneas were scratched again and inoculated with PA (ATCC 19660, CFUs: 1.0×104). Cornea epithelia were collected at 6 hpi for semi-quantitative RT-PCR (A) or quantitative real-time PCR (B) analysis of IL-24, IL-19, and IL-20 (A), SOCS3 and IL-1β (B). Results are presented relative to those of naïve corneas, set as 1. P values were generated by one-way ANOVA. ***P < 0.001. Data are representative of three independent experiments (B: mean + s.e.m.). Flag: flagellin.
IL-24, SOCS3, and IL-1β are early responsive genes during PA infection
Having shown the effects of infection and flagellin-pretreatment on cytokine expression, we then performed a time course study to compare the expression of IL-24 with IL-1β, SOCS3 and IL-6 in response to PA infection. Two peaks of IL-24 up-regulation were observed: a rapid and marked increase at 3 hpi, decreasing by 6 hpi, and then a gradual increase to a higher level at 18hpi. In contrast, IL-1β expression was induced at 3 hpi, and continued to increase as the infection progressed. (Fig. 2A). The expression of SOCS3 followed the observed bimodal expression kinetics of IL-24. IL-6 has been shown to be critical to the host defense of the cornea, including neutrophil infiltration and bacterial clearance, during PA infection (35) as well as in other systems (36). IL-6 expression was first observed at 9 hpi, and reached a significantly higher level at 18 hpi, as compared to the naïve cornea (Fig. 2A). Hence, the infection-induced expression of SOCS3 at 3 hpi is correlated with that of IL-24, but not IL-6, which is also known to activate the JAK/STAT3 pathway.
Figure 2.
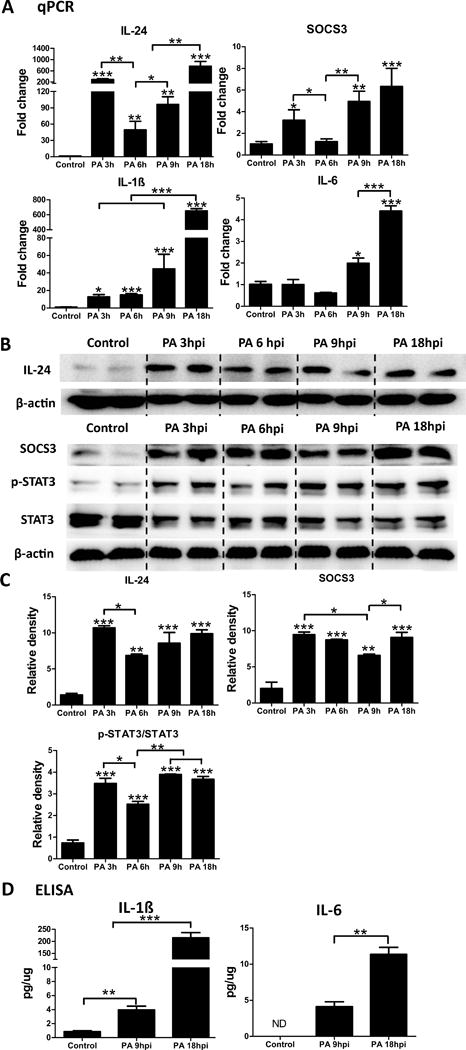
IL-24, SOCS3, and IL-1β are early responsive genes after PA infection in B6 mouse cornea. Mouse corneas were scratched with a needle and inoculated with PA. Whole corneas were collected at 3 hpi, 6 hpi, 9 hpi, and 18 hpi for qPCR analysis of IL-24, IL-1β, SOCS3, and IL-6 (A). Results are presented relative to those of naïve corneas, set as 1. (B) Immunoblot analysis of IL-24, SOCS3, p-STAT3, and STAT3 in cell lysates of whole corneas infected with PA for 3h, 6h, 9 h and 18 h. β-actin serves as the loading control. (C) Quantification of protein levels based on the densitometry of the Western blots in B. (D) ELISA assays of IL-1β and IL-6 in cell lysates of whole corneas infected with PA for 9 h or 18 h. Stars on top of columns are P value results comparing with the control. *P < 0.05, **P < 0.01, and ***P < 0.001 (ANOVA). Data are representative of three independent experiments (A, C and D: mean + s.e.m.). ND: not detected.
The expression of some of these cytokines and the activation of STAT3 were also assessed by using Western blotting or ELISA in whole corneal extracts (Fig. 2B, C, and D). IL-24 expression at the protein level was markedly increased at 3 hpi, reduced at 6 hpi and thereafter. The activation of STAT3 detected by the phospho-STAT3 level followed a similar expression pattern with that of IL-24. SOCS3 protein expression increased at 3 hpi and 6 hpi, down by 9 hpi, followed by an increase at 18 hpi (Fig. 2B and C). The protein levels of IL-1β and IL-6 increased in a time dependent fashion, with peak levels observed at 18 hpi (Fig. 2D).
Silencing of IL-24 improves the outcome of PA keratitis in mouse cornea
To determine whether IL-24 influences the host defense against PA keratitis, we subconjunctivally injected B6 mice with IL-24 siRNA or control siRNA. Western blotting analysis of the whole corneas at 1 dpi revealed an effective knockdown of IL-24 by siRNA, as well as the downregulation of STAT3 activation (Fig. 3A and B).
Figure 3.
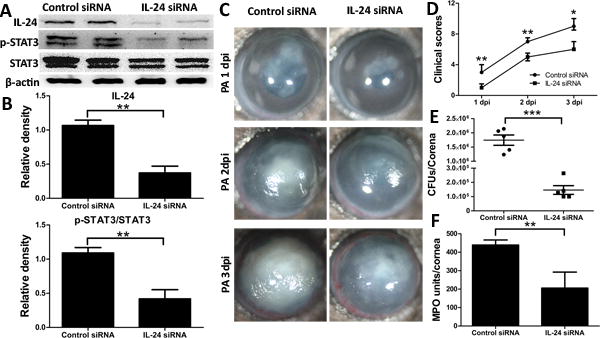
Silencing of IL-24 attenuates the severity of PA infection in mouse cornea. Mice were subconjunctivally injected with IL-24 siRNA (50 picomoles in 5ul RNase free water) twice in two days. Corneas were inoculated with PA 6h after the second injection. Non-specific siRNA serves as control. (A) Immunoblot analysis of IL-24, p-STAT3, and STAT3 in cell lysates of IL-24 siRNA- or control siRNA-treated corneas at 1 dpi. β-actin serves as the loading control. (B) Quantification of protein levels based on the densitometry of the Western blots in A. (C) Mice were monitored and photographed daily up to 3 dpi. (D) Clinical scores were assigned to each cornea daily and plotted as median + interquartile range. At 3 dpi, the corneas were excised and subjected to bacterial plate counting (E) and MPO assay (F). P values were generated by Man-Whitney test (D) or unpaired student t test (B, E and F). *P < 0.05, **P < 0.01 and ***P < 0.001. Data are representative of three independent experiments with five mice per group (B, E and F: mean + s.e.m.).
The progression of PA keratitis in the control siRNA and IL-24 siRNA treated corneas was monitored for up to 3 days post infection. Micrographs taken daily showed that knockdown of IL-24 revealed reduction in the severity of keratitis, as compared to the control group (Fig. 3C). At 3 dpi, the control corneas developed severe keratitis, thick opacity covering the entire corneal surface, and surface irregularity; whereas the corneas injected with IL-24 siRNA had less severe keratitis with lighter opacity located at the center of the cornea. The severity of keratitis was quantitated using a 12-scale clinical score system by evaluating the area and density of opacity and surface irregularity (33). The clinical scores assigned to IL-24 silenced mice were significantly lower than those of their control counterparts at all 3 days examined (Fig. 3D). Mice were euthanized at 3dpi, and whole corneas were subjected to bacterial counting and MPO measurement to evaluate neutrophil infiltration. Silencing of IL-24 resulted in lower bacterial burden and reduced influx of neutrophils compared to the control corneas (Fig. 3E and F).
We next treated the corneas with IL-24 siRNA and assessed its effects on the expression of several innate immune responsive genes at 6 hpi in mouse CECs in vivo. S100A8/A9 and CXCL10 are antimicrobial peptides and chemotactic proteins. The heterodimer of S100A8 and A9, (also referred to as calprotectin), has been shown to prevent the growth of bacteria, fungi, and protozoa by chelating Mn+2 and Zn+2 ions (37–40). CXCL10, also known as interferon-γ-inducible protein 10 (IP-10), can act as a chemoattractant for natural killer cells and an important anti-microbial peptide (AMP) capable of directly killing PA and C. albicans (8, 41, 42). As shown in Fig. 4A, the expression of the AMP S100A8 increased 3-fold (P<0.05, ANOVA) after IL-24 knockdown in uninfected CECs. PA infection induced the expression of both S100A8 and S100A9, and silencing of IL-24 further elevated their expression in the infected corneas. The expression of CXCL10 was also induced by PA infection, and further increased post silencing of IL-24. The expression of SOCS3 was upregulated after PA infection. IL-24 knockdown resulted in the downregulation of SOCS3 expression. IL-1 receptor antagonist (Gene: IL1rn), the natural inhibitor of IL-1 type cytokines, has been demonstrated to ameliorate symptoms in several autoimmune diseases (43, 44). MMP13 (Matrix Metalloproteinase-13) is reported to contribute to basement membrane damage and facilitate PA invasion into the stroma (45). The expression of IL-1β, IL-1RN, and MMP13 was also induced by PA infection. IL-24 knockdown dampened their expression to a level similar to the control siRNA without infection in mouse CECs. The expression of calprotectin and CXCL10 was further confirmed in protein level (Fig. 4B). Basal expression of both calprotectin and CXCL10 was detected in the uninfected corneas. Consistent with the mRNA expression, the protein expression of calprotectin and CXCL10 was induced by PA infection, and further enhanced when knocking down of IL-24.
Figure 4.
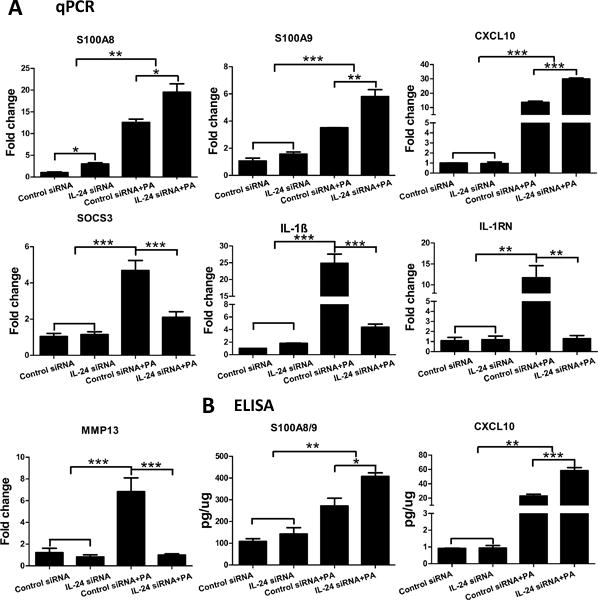
Silencing of IL-24 upregulates the expression of calprotectin and CXCL10 in mouse corneal epithelial cells in response to PA infection. Mouse corneas were treated with IL-24 siRNA or control siRNA and inoculated with PA as in figure 3. Corneal epithelial samples were collected at 6hpi for qPCR (A) or ELISA (B) analysis. For q-PCR, results are presented relative to those of control siRNA treated, uninfected corneas, set as 1. *P < 0.05, **P < 0.01, and ***P < 0.001 (ANOVA). Data are representative of three independent experiments with at least four corneas per group (mean + s.e.m.).
As an early responsive cytokine, IL-24 may induce the expression of other cytokines and/or AMPs in infected corneas. To that end, we used real-time PCR to assess the expression of eight different genes at 1dpi in the whole corneas (Fig. 5A). While most genes were unaffected by the downregulation of IL-24, the expression of IL-1RN (7.2-fold increase, p<0.05), S100A9 (3.3-fold increase, p<0.05), and IL-17A (0.6-fold decrease p>0.05) were altered by IL-24 silencing in non-infected corneas. IL-17A is reported to be secreted by innate γδ T cells to recruit neutrophils (46). Activated neutrophils further produce IL-17A in an autocrine fashion, contributing to bacterial and fungal clearance (47, 48). Infection induced marked upregulation of SOCS3, IL-6, MMP13, IL-17A, CXCL10, IL-1β/IL-1RN, AMP S100A8, and A9. Knocking down of IL-24 resulted in the downregulation of the SOCS3 (1.47 fold), IL-6 (4), MMP13 (1.73), IL-17A (3.3), CXCL10 (5.27), IL-1β (3.0), IL-1RN (5.72), S100A8 (1.98) and A9 (1.45) in infected corneas (Fig. 5A). The expression of IL-6, IL-1β, IL-1Ra (protein encoded by gene IL-1RN), and calprotectin was confirmed at the protein level by ELISA analysis (Fig. 5B). While the basal levels of IL-6 and IL-1β were undetected, basal expression of IL-1RA and S100A8/9 was observed in the control siRNA or IL-24 siRNA treated, non-infected corneas. Similar to the mRNA level, IL-6, IL-1β, IL-1RA, and S100A8/9 were elevated at the protein level by PA infection and significantly attenuated by IL-24 silencing. The downregulation of the cytokines and AMPs after IL-24 silencing at 1dpi in the cornea may be related to the diminished host response to much reduced bacterial burden in the silencing group.
Figure 5.
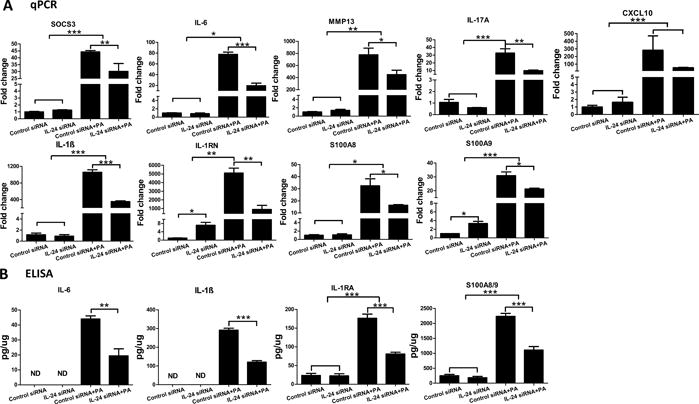
Silencing of IL-24 dampens the expression of cytokines and antimicrobial peptides in mouse cornea in response to PA infection. Mouse corneas were treated with IL-24 siRNA or control siRNA and inoculated with PA as in figure 3. (A) Whole cornea samples were collected at 1dpi for qPCR analysis. Results are presented relative to those of control siRNA treated, uninfected corneas, set as 1. (B) Cell lysates from whole corneal samples at 1dpi were subjected to ELISA analysis. *P < 0.05, **P < 0.01, and ***P < 0.001 (ANOVA or unpaired student T test). Data are representative of three independent experiments with at least four corneas per group (mean + s.e.m.). ND: not detected.
Blockage of IL-20R2 decreases the severity of PA keratitis in mouse cornea
Previous reports based on PCR studies demonstrated that the receptors for IL-20R cytokines are mainly expressed in epithelial cells, but not in immune cells (49, 50). We showed that while equal levels of IL-22R1 were detected in both the naïve and infected CECs at around 95 kDa, the abundance of IL-20R2 proteins was decreased in the infected CECs, comparing with the naïve control (Supplemental figure). We next assessed the effect of blocking IL-20R2, the common receptor chain for IL-19, IL-20, and IL-24, to further verify the effect of the IL-24 signaling pathway on PA keratitis (Fig. 6). While the IgG control corneas developed keratitis gradually within 3 days, the anti-IL-20R2 antibody-treated corneas had slight corneal opacification at 1 dpi, which progressed to cloudy corneas at day 2, and resolved to cloudiness with the outline of the iris and pupil discernable at day 3 (Fig. 6A). Clinical scoring revealed that the anti-IL-20R2 group started with a lower score at day 1 and a slope similar to that observed in the IgG control group at day 2, with scores slightly declining at day 3 (Fig. 6B). The blockage of IL-20R2 reduced the bacterial burden and MPO units in the corneas at 3 dpi as well (Fig. 6C and D).
Figure 6.
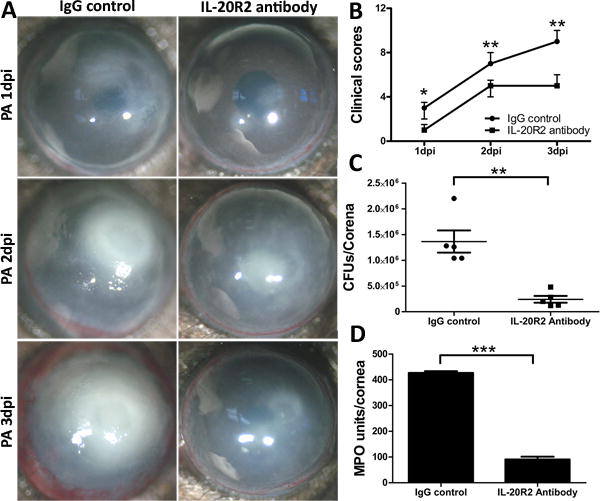
IL-20R2 neutralizing antibody decreases the severity of PA keratitis in mouse cornea. Mice were subconjunctivally injected with IL-20R2 neutralizing antibody (200ng/5ul) 4h before the inoculation with PA. Mouse IgG serves as the control. (A) Mice were monitored and photographed daily up to 3 dpi. (B) Clinical scores were assigned to each cornea daily and plotted as median + interquartile range. At 3 dpi, the corneas were excised and subjected to bacterial plate counting (C) and MPO assay (D). P values were generated by Man-Whitney test (B) or unpaired student t test (C and D). *P < 0.05, **P < 0.01 and ***P < 0.001. Data are representative of three independent experiments with five mice per group (C and D: mean + s.e.m.).
Recombinant IL-24 increases the susceptibility of mouse cornea to PA infection
As a complimentary approach, we assessed the effects of recombinant IL-24 on the severity of PA keratitis. Subconjunctival injection of recombinant mouse IL-24 four hours prior to PA inoculation resulted in more severe keratitis than that of the control with 0.1% BSA injection (Fig. 7A). Recombinant mouse IL-24 was reconstituted with 0.1% BSA in PBS according to manufacturer’s recommendation. BSA, 0.1% in PBS, was used as a vehicle control. The control corneas were covered with opacity by ~30% at day 1, ~80% at day 2, and fully covered at day 3. Surface irregularity was also apparent at day 3. In recombinant IL-24-treated corneas, there was a sign of central melting at day 1, central ring with heavy opacity at day 2, and heavy opacity covered the entire cornea with corneal ulceration and neovascularization present at day 3, pathologies usually seen in the control corneas at day 5 (Fig 7A). The clinical scores revealed significantly higher disease severity in the recombinant IL-24-treated group than that observed in the BSA control group (Fig. 7B). A single dose of recombinant IL-24 resulted in significantly higher bacterial burden and neutrophil infiltration as compared to the BSA control group at 3 dpi (Fig. 7C and D).
Figure 7.
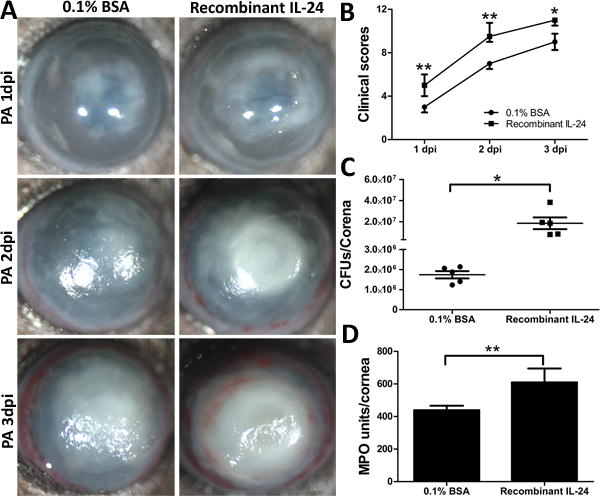
Recombinant IL-24 increases the susceptibility of the mouse cornea to PA infection. Mice were subconjunctivally injected with either recombinant mouse IL-24 (200ng in 5ul 0.1% BSA) or 0.1% BSA as control. Mouse corneas were inoculated with PA 4 h after the injection of recombinant IL-24. (A) The infected corneas were photographed daily. (B) The severity of keratitis was assessed with clinical scores. At 3 dpi, the corneas were excised and subjected to bacterial load (C) and MPO unit determination (D). P values were generated by Man-Whitney test (B) or unpaired student t test (C and D). *P < 0.05 and **P < 0.01. Data are representative of three independent experiments with five mice per group (B: median + interquartile range; C and D: mean + s.e.m.).
The expression of innate immune response genes was also assessed in recombinant IL-24-treated corneas (Fig. 8). In non-infected corneas, recombinant IL-24 stimulated significant upregulation of IL-17A (4.8-fold increase, p<0.01); whereas the expression of IL-1RN (2.5-fold decrease, p<0.01) and S100A8 (0.5-fold decrease, p>0.05) was downregulated, contrasting to those of IL-24 siRNA-treated corneas. The presence of recombinant IL-24 significantly augmented the infection-induced expression of these genes.
Figure 8.
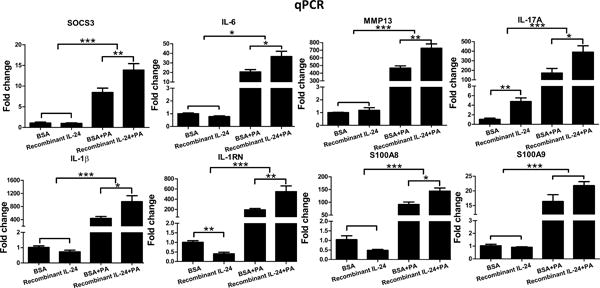
Recombinant IL-24 enhances cytokine expression in mouse cornea in response to PA infection. Mouse corneas were treated with recombinant mouse IL-24 or 0.1%BSA and inoculated with PA as in figure 7. Whole cornea samples were collected at 1dpi for qPCR analysis. Results are presented relative to those of 0.1% BSA treated, uninfected corneas, set as 1. *P < 0.05, **P < 0.01, and ***P < 0.001 (ANOVA). Data are representative of three independent experiments with at least four corneas per group (mean + s.e.m.).
IL-24 specifically induces SOCS3 expression in human corneal epithelial cells
Our in vivo data imply that both IL-1β and IL-24 are the downstream products of toll-like receptor signaling, and that these two cytokines may regulate the expression of each other, as well as other genes involved in the innate defense of the corneas. To that end, we first treated primary HCECs with recombinant human IL-1β and found enhanced expression of SOCS3, IL-24, IL-1β, and IL-6 mRNA (Fig. 9A). The level of SOCS3 mRNA was increased 2.2 fold at hour 1 and further elevated (6.1 fold) at 2 hours post IL-1β stimulation. IL-1β significantly induced the mRNA expression of IL-24, IL-1β, and IL-6 at both hour 1 and hour 2. Conversely, recombinant IL-24 stimulated robust upregulation of SOCS3, peaking at 1 hour post infection, but had no effect on the expression of IL-1β, IL-24, or IL-6 (Fig. 9B).
Figure 9.
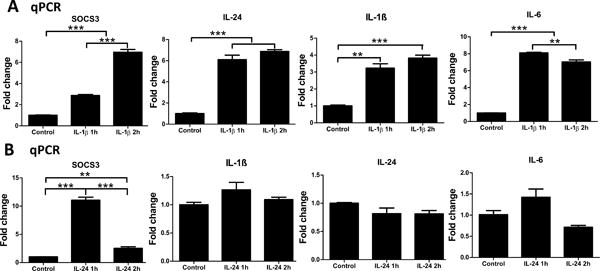
Effects of recombinant human IL-1β or IL-24 on primary human corneal epithelial cells (HCECs). (A) HCECs were treated with recombinant human IL-1β (50ng/ml). Cells were collected at 1h and 2h for qPCR analysis of SOCS3, IL-24, IL-1β, and IL-6. (B) HCECs were treated with recombinant human IL-24 (100ng/ml). Cells were collected at 1h and 2h for qPCR analysis of SOCS3, IL-1β, IL-24, and IL-6. Results are presented relative to those of untreated control cells, set as 1 (mean+s.e.m.). **P < 0.01, and ***P < 0.001 (ANOVA). Data are representative of three independent experiments.
Discussion
The present study demonstrated that among the three mouse IL-20R cytokines, only IL-24 was markedly induced by PA infection. Interestingly, SOCS3 shared a similar expression pattern with IL-24 while the expression of another STAT3 activator, IL-6, was not increased until 9 hpi. Functional analysis revealed that while exogenous IL-24 increased the severity of PA keratitis, the downregulation of IL-24 signaling pathway resulted in reduced clinical scores, less opacity, lower bacterial burden, and reduced infiltration of neutrophils during PA infection. During the initial hours of infection (6 hpi) when most invading pathogens remain at the epithelial layer, the inhibition of IL-24 markedly increased the infection-induced expression of AMPs S100A8 and A9 and CXCL10. At 1 dpi, the expression of the infection response genes (SOCS3, IL-1β, IL-6, IL-17A, MMP13, CXCL10, and S100A8/A9) was all markedly elevated in infected corneas. Induction of these mediators was dampened to different extents by IL-24 knockdown at 1 dpi, correlating with the reduced number of pathogens. Targeting IL-20R2, the common receptor chain in the IL-24 signaling pathway, improved the outcome of PA keratitis. Application of recombinant IL-24, on the other hand, markedly increased the severity of PA keratitis and reversed the expression pattern of infection response genes seen in IL-24 downregulated corneas at 1 dpi. In vitro, while IL-1β induced the expression of IL-1β (self-amplification), IL-24, IL-6, and SOCS3, treatment with IL-24 induced only the expression of SOCS3. Taken together, our data suggest that the anti-inflammatory IL-20R cytokines have immunosuppressive properties and contribute to corneal susceptibility to PA infection.
The host responds to tissue infection by eliciting acute inflammation in an attempt to control invading pathogens (51). These early events are coordinated by several families of pro-inflammatory mediators including cytokines, chemokines, and lipid mediators. The major pro-inflammatory cytokines/chemokines elicited by microbial infection include IL-1β, IL-6, IL-8, and IL-17A, which orchestrate leukocyte recruitment to the sites of infection (52, 53). It is now clear that during the acute inflammatory response, an array of molecules, such as IL-10 and resolvins (51) that function in the resolution of inflammation, are also induced. Among the IL-10 superfamily of cytokines, IL-20 subfamily cytokines, including IL-19, IL-20, and IL-24 (referred as IL-20R cytokines), are known to be produced mainly by myeloid cells and epithelial cells, and to target epithelial cells (54). In this study, we reported that the expression of IL-24, but not IL-19 or IL-20, was induced in mouse corneas by PA infection, a pattern similar to that described for such induction by inflammatory cytokines such as IL-1β and CXCL2 (mouse homolog of human IL-8), but not IL-6. It is reported that the expression of IL-19 and IL-24, but not of IL-20, was induced by infection with methicillin-resistant Staphylococcus aureus (MRSA) in mouse skin (48). Thus, we conclude that IL-24, similar to IL-1β, is an early response gene in response to PA infection in the cornea.
We observed that IL-24 knockdown decreased the expression of IL-1β, IL-6, and MMP13, but increased the expression of S100A8/9 and CXCL10 during the initial hours of infection (6 hpi). In addition, IL-24 alone increased the expression of IL-17A and decreased the expression of IL-1RN, a natural IL-1R antagonist in non-infected corneas (Fig. 8), suggesting a pro-inflammatory role of IL-24 in B6 mouse corneas. It is unlikely that IL-24 directly induces the expression of these cytokines from epithelial cells, since the treatment of recombinant IL-24 alone to B6 mouse corneas (Fig. 8) or cultured human corneal epithelial cells (Fig. 9) did not alter the expression of IL-1β and MMP-13. Importantly, IL-24 was found to inhibit the expression of the antimicrobial peptides S100A8/A9 and CXCL10 in moue corneal epithelial cells (Fig, 4), resulting in impaired mucosal immunity and reduced bacterial clearance at the corneal surface. We postulate that it is the corresponding increase in bacterial burden that drives the enhanced inflammatory responses at the mucosal surface, including increased in vivo expression of inflammatory cytokines (IL-1β, IL-6, and MMP13). Hence, the effects of IL-24 on corneal inflammatory response is likely through an indirect action, such as the induction of SOCS3.
Given well-accepted anti-inflammatory and protective properties of the IL-10 family cytokines (28, 55, 56), we thought that IL-24 would enhance the host innate defense against PA in the corneas. Our data, however, showed that while downregulation of IL-24 or the antibody blockade of the IL-20R2 protected the corneas from PA infection, exogenous IL-24 worsened PA keratitis, suggesting a detrimental role of IL-24 during PA infection of the corneas. While most studies to date in the literature suggest a protective role of IL-20 subfamily cytokines, particularly IL-22 (28, 55–57), a more recent study of MRSA infection of mouse skin revealed that IL-19 and/or 24 play an immunosuppressive role by downregulating IL-1β- and IL-17A-dependent pathways, which mediate host innate immune defense against the infectious agent (48). Our results of IL-24 as a negative mediator in PA keratitis are in line with the MRSA skin infection study, yet by entirely different mechanisms. In MRSA skin infection model, IL-19/IL-24 target IL-1β and IL-17A expression; in our PA keratitis model, IL-24 hampers the expression of innate immune defense molecules, resulting in an increase in bacterial burden which in turn stimulated stronger inflammatory response. Hence, the early induced IL-24 acts as innate immune suppressor in the corneas in response to PA infection.
In IL-24 manipulated B6 mouse corneas, the inflammatory response, measured by the clinical scoring, the levels of pro-inflammatory cytokines, and the intensity of PMN infiltration, is directly correlated to the numbers of recoverable bacteria. This seems paradoxical, given that acute inflammation is known to be required for controlling invading pathogens. This paradox can perhaps be resolved by the locations where bacterial clearance may occur at different times of infection. In our mouse model of microbial keratitis, epithelium-scratched wounds are made with minimal injury of the basement membrane, which serves as a barrier for PA invasion of the stroma (45). During the initial hours of infection (6 hpi), fewer than 1,000 CFUs of PA were detected (1 × 104 inoculated), and most of them were detected within the epithelium. By 24 hpi, the basement membrane was dissolved, and the epithelium was partially disappeared with a large number of PA in the stroma (45). We observed robust expression of potent antimicrobial peptides and bactericidal chemokines, including calprotectin and CXCL10, by PA infection and further enhanced by downregulation of IL-24 signaling in corneal epithelium at 6 hpi. These potent bactericidal peptides participate in innate killing of pathogens within the epithelium (58) and greatly reduced pathogen invasion to the stroma at the initial hours of infection, 3-6 hpi when most invading pathogens remain in the epithelial layer (8, 15, 41). Consistent with the notion that epithelium is the major site of innate defense, we observed that IL-24 receptors were mostly expressed by epithelium in B6 mouse corneas (Supplemental figure), suggesting the effects of IL-20R cytokines on innate defense mechanisms are likely limited to the epithelial layer. It is plausible that the reduction of pathogen burden results in less innate inflammatory response, yet the activated innate immune defense apparatus further eliminates remaining pathogens. Downregulation of IL-24, similar to flagellin administration (9), also greatly reduced the intensity of inflammatory response in the corneas, including marked decrease in the number of PMNs at 1 dpi, giving a marked decrease in the corneal content of calprotectin (60% of total cytosolic proteins in PMN (59)) seen in Figure 5B.
The presence of recombinant IL-24, similar to downregulation of CXCL10 (8), calprotectin (60), or cathelicidin (9), on the other hand, significantly increased bacterial burden due to a decrease in pathogen killing, resulting in much severe keratitis with greatly increased infiltration of PMNs. Hence, the pathogen burden appears to be a determinant factor of the inflammatory response during microbial infection in the cornea. This phenomenon may not be unique for the cornea as the inflammatory response in the mouse ceca was shown to be related to the numbers of Salmonella with or without flagellin administration (61). Moreover, our study using a lethal murine PA model also showed a decrease in PA burden as well as in PMN infiltration in flagellin-pretreated B6 mouse lung at 24 hpi (10).
How might IL-24 suppress innate immune response during tissue infection? We observed that in cultured human primary CECs, while IL-1β stimulated the expression of IL-24, IL-1β (self-amplification), IL-6, as well as SOCS3, IL-24 was only able to induce SOCS3 expression in a robust fashion at 1 h post treatment, with SOCS3 rapidly declined to a lower level at 2 h post treatment (Fig. 9). This early and robust expression of SOCS3 during initial hour of infection may be one of underlying mechanisms for IL-24 to possess immunosuppressive activity in the cornea. Consistent with this notion, cell-specific loss of SOCS3 in keratinocytes has been shown to up-regulate S100A8/A9 expression and to cause skin inflammation and epidermal hyperplasia in an IL-20R cytokine-related manner (27).
In summary, our data demonstrate that IL-24 expression is enhanced at an early time point in mouse corneas after PA infection. IL-24 dampens inflammatory response necessary for mounting an effective defense against microbial infection during the initial hours of infection. This observation highlights the concern for the use of IL-24 as an anticancer agent, as it may render patients more susceptible to opportunistic infection at a time when their immune systems are already comprised. In addition, the IL-20R2 signaling pathway could be therapeutically targeted to alter the susceptibility of the corneas to PA infection.
Supplementary Material
Acknowledgments
The authors thank Sharowynn Smith for proofreading the manuscript, Haijing Sun for technical support, Paul. D. Walker, Ashok Kumar, and Jena Steinle for serving as committee members.
Footnotes
Disclosure: B. Ross, None; N. Gao, None; X. Cui, None; T. J. Standiford, None; J. Xu, none; F. X. Yu, None.
Abbreviations: SOCS3, suppressor of cytokine signaling 3; PA, Pseudomonas aeruginosa; CECs, corneal epithelial cells; CFUs, colony forming units; MPO, myeloperoxidase.
References
- 1.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Stapleton F, Carnt N. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye. 2012;26:185–193. doi: 10.1038/eye.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molina DN, Colon M, Bermudez RH, Ramirez-Ronda CH. Unusual presentation of Pseudomonas aeruginosa infections: a review. Boletin de la Asociacion Medica de Puerto Rico. 1991;83:160–163. [PubMed] [Google Scholar]
- 4.Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optometry and vision science: official publication of the American Academy of Optometry. 2007;84:273–278. doi: 10.1097/OPX.0b013e3180439c3e. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- 6.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Hazlett LD, Yu FS. Flagellin suppresses the inflammatory response and enhances bacterial clearance in a murine model of Pseudomonas aeruginosa keratitis. Infection and immunity. 2008;76:89–96. doi: 10.1128/IAI.01232-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon GS, Dong C, Gao N, Kumar A, Standiford TJ, Yu FS. Interferon regulatory factor-1 in flagellin-induced reprogramming: potential protective role of CXCL10 in cornea innate defense against Pseudomonas aeruginosa infection. Investigative ophthalmology & visual science. 2013;54:7510–7521. doi: 10.1167/iovs.13-12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar A, Gao N, Standiford TJ, Gallo RL, Yu FS. Topical flagellin protects the injured corneas from Pseudomonas aeruginosa infection. Microbes Infect. 2010;12:978–989. doi: 10.1016/j.micinf.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu FS, Cornicelli MD, Kovach MA, Newstead MW, Zeng X, Kumar A, Gao N, Yoon SG, Gallo RL, Standiford TJ. Flagellin stimulates protective lung mucosal immunity: role of cathelicidin-related antimicrobial peptide. Journal of immunology. 2010;185:1142–1149. doi: 10.4049/jimmunol.1000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tolle L, Yu FS, Kovach MA, Ballinger MN, Newstead MW, Zeng X, Nunez G, Standiford TJ. Redundant and cooperative interactions between TLR5 and NLRC4 in protective lung mucosal immunity against Pseudomonas aeruginosa. Journal of innate immunity. 2015;7:177–186. doi: 10.1159/000367790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B, Chassaing B, Shi Z, Uchiyama R, Zhang Z, Denning TL, Crawford SE, Pruijssers AJ, Iskarpatyoti JA, Estes MK, Dermody TS, Ouyang W, Williams IR, Vijay-Kumar M, Gewirtz AT. Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science. 2014;346:861–865. doi: 10.1126/science.1256999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones RM, Sloane VM, Wu H, Luo L, Kumar A, Kumar MV, Gewirtz AT, Neish AS. Flagellin administration protects gut mucosal tissue from irradiation-induced apoptosis via MKP-7 activity. Gut. 2011;60:648–657. doi: 10.1136/gut.2010.223891. [DOI] [PubMed] [Google Scholar]
- 14.Vijay-Kumar M, Aitken JD, Sanders CJ, Frias A, Sloane VM, Xu J, Neish AS, Rojas M, Gewirtz AT. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. Journal of immunology. 2008;180:8280–8285. doi: 10.4049/jimmunol.180.12.8280. [DOI] [PubMed] [Google Scholar]
- 15.Gao N, Sang Yoon G, Liu X, Mi X, Chen W, Standiford TJ, Yu FS. Genome-wide transcriptional analysis of differentially expressed genes in flagellin-pretreated mouse corneal epithelial cells in response to Pseudomonas aeruginosa: involvement of S100A8/A9. Mucosal Immunol. 2013;6:993–1005. doi: 10.1038/mi.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annual review of immunology. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 17.Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. Journal of immunology. 2001;167:3545–3549. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- 18.Chada S, Sutton RB, Ekmekcioglu S, Ellerhorst J, Mumm JB, Leitner WW, Yang HY, Sahin AA, Hunt KK, Fuson KL, Poindexter N, Roth JA, Ramesh R, Grimm EA, Mhashilkar AM. MDA-7/IL-24 is a unique cytokine--tumor suppressor in the IL-10 family. International immunopharmacology. 2004;4:649–667. doi: 10.1016/j.intimp.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. The Journal of biological chemistry. 2002;277:33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nature reviews Immunology. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 21.Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM, Kay TW, Nicola NA, Hertzog PJ, Metcalf D, Hilton DJ. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 22.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. SOCS3 negatively regulates IL-6 signaling in vivo. Nature immunology. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 23.Babon JJ, Sabo JK, Zhang JG, Nicola NA, Norton RS. The SOCS box encodes a hierarchy of affinities for Cullin5: implications for ubiquitin ligase formation and cytokine signalling suppression. Journal of molecular biology. 2009;387:162–174. doi: 10.1016/j.jmb.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babon JJ, Kershaw NJ, Murphy JM, Varghese LN, Laktyushin A, Young SN, Lucet IS, Norton RS, Nicola NA. Suppression of cytokine signaling by SOCS3: characterization of the mode of inhibition and the basis of its specificity. Immunity. 2012;36:239–250. doi: 10.1016/j.immuni.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kragstrup TW, Otkjaer K, Holm C, Jorgensen A, Hokland M, Iversen L, Deleuran B. The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine. 2008;41:16–23. doi: 10.1016/j.cyto.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Andoh A, Shioya M, Nishida A, Bamba S, Tsujikawa T, Kim-Mitsuyama S, Fujiyama Y. Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. Journal of immunology. 2009;183:687–695. doi: 10.4049/jimmunol.0804169. [DOI] [PubMed] [Google Scholar]
- 27.Uto-Konomi A, Miyauchi K, Ozaki N, Motomura Y, Suzuki Y, Yoshimura A, Suzuki S, Cua D, Kubo M. Dysregulation of suppressor of cytokine signaling 3 in keratinocytes causes skin inflammation mediated by interleukin-20 receptor-related cytokines. PloS one. 2012;7:e40343. doi: 10.1371/journal.pone.0040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Y, Chen HD, Wang Y, Wang Q, Li Y, Zhao Y, Zhang XL. Interleukin 24 as a novel potential cytokine immunotherapy for the treatment of Mycobacterium tuberculosis infection. Microbes Infect. 2011;13:1099–1110. doi: 10.1016/j.micinf.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Ma Y, Chen H, Wang Q, Luo F, Yan J, Zhang XL. IL-24 protects against Salmonella typhimurium infection by stimulating early neutrophil Th1 cytokine production, which in turn activates CD8+ T cells. European journal of immunology. 2009;39:3357–3368. doi: 10.1002/eji.200939678. [DOI] [PubMed] [Google Scholar]
- 30.Rottenberg ME, Carow B. SOCS3 and STAT3, major controllers of the outcome of infection with Mycobacterium tuberculosis. Semin Immunol. 2014;26:518–532. doi: 10.1016/j.smim.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Yokota S, Yokosawa N, Okabayashi T, Suzutani T, Fujii N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 confers efficient viral replication. Virology. 2005;338:173–181. doi: 10.1016/j.virol.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 32.Michaud F, Coulombe F, Gaudreault E, Paquet-Bouchard C, Rola-Pleszczynski M, Gosselin J. Epstein-Barr virus interferes with the amplification of IFNalpha secretion by activating suppressor of cytokine signaling 3 in primary human monocytes. PloS one. 2010;5:e11908. doi: 10.1371/journal.pone.0011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model. Investigative ophthalmology & visual science. 2003;44:210–216. doi: 10.1167/iovs.02-0446. [DOI] [PubMed] [Google Scholar]
- 34.Williams RN, Paterson CA, Eakins KE, Bhattacherjee P. Quantification of ocular inflammation: evaluation of polymorphonuclear leucocyte infiltration by measuring myeloperoxidase activity. Current eye research. 1982;2:465–470. doi: 10.3109/02713688208996350. [DOI] [PubMed] [Google Scholar]
- 35.Cole N, Bao S, Stapleton F, Thakur A, Husband AJ, Beagley KW, Willcox MD. Pseudomonas aeruginosa keratitis in IL-6-deficient mice. International archives of allergy and immunology. 2003;130:165–172. doi: 10.1159/000069006. [DOI] [PubMed] [Google Scholar]
- 36.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nature immunology. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 37.Clark HL, Jhingran A, Sun Y, Vareechon C, de Jesus Carrion S, Skaar EP, Chazin WJ, Calera JA, Hohl TM, Pearlman E. Zinc and Manganese Chelation by Neutrophil S100A8/A9 (Calprotectin) Limits Extracellular Aspergillus fumigatus Hyphal Growth and Corneal Infection. Journal of immunology. 2016;196:336–344. doi: 10.4049/jimmunol.1502037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clohessy PA, Golden BE. Calprotectin-mediated zinc chelation as a biostatic mechanism in host defence. Scandinavian journal of immunology. 1995;42:551–556. doi: 10.1111/j.1365-3083.1995.tb03695.x. [DOI] [PubMed] [Google Scholar]
- 39.Sohnle PG, Hunter MJ, Hahn B, Chazin WJ. Zinc-reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor-related proteins 8 and 14) The Journal of infectious diseases. 2000;182:1272–1275. doi: 10.1086/315810. [DOI] [PubMed] [Google Scholar]
- 40.Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, Murphy WJ, Zhang Y, Betz C, Hench L, Fritz G, Skaar EP, Chazin WJ. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3841–3846. doi: 10.1073/pnas.1220341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Gao N, Dong C, Zhou L, Mi QS, Standiford TJ, Yu FS. Flagellin-induced expression of CXCL10 mediates direct fungal killing and recruitment of NK cells to the cornea in response to Candida albicans infection. European journal of immunology. 2014;44:2667–2679. doi: 10.1002/eji.201444490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan C, Gao N, Sun H, Yin J, Lee P, Zhou L, Fan X, Yu FS. Targeting Imbalance between IL-1beta and IL-1 Receptor Antagonist Ameliorates Delayed Epithelium Wound Healing in Diabetic Mouse Corneas. The American journal of pathology. 2016;186:1466–1480. doi: 10.1016/j.ajpath.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Science signaling. 2010;3:cm1. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 44.Cavalli G, Dinarello CA. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatology. 2015;54:2134–2144. doi: 10.1093/rheumatology/kev269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao N, Kumar A, Yu FS. Matrix Metalloproteinase-13 as a Target for Suppressing Corneal Ulceration Caused by Pseudomonas aeruginosa Infection. J Infect Dis. 2015;212:116–127. doi: 10.1093/infdis/jiv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. The Journal of clinical investigation. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor PR, Roy S, Leal SM, Jr, Sun Y, Howell SJ, Cobb BA, Li X, Pearlman E. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nature immunology. 2014;15:143–151. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Myles IA, Fontecilla NM, Valdez PA, Vithayathil PJ, Naik S, Belkaid Y, Ouyang W, Datta SK. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1beta and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nature immunology. 2013;14:804–811. doi: 10.1038/ni.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kunz S, Wolk K, Witte E, Witte K, Doecke WD, Volk HD, Sterry W, Asadullah K, Sabat R. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp Dermatol. 2006;15:991–1004. doi: 10.1111/j.1600-0625.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 50.Nagalakshmi ML, Murphy E, McClanahan T, de Waal Malefyt R. Expression patterns of IL-10 ligand and receptor gene families provide leads for biological characterization. International immunopharmacology. 2004;4:577–592. doi: 10.1016/j.intimp.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16:51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai HC, Velichko S, Hung LY, Wu R. IL-17A and Th17 cells in lung inflammation: an update on the role of Th17 cell differentiation and IL-17R signaling in host defense against infection. Clin Dev Immunol. 2013;2013:267971. doi: 10.1155/2013/267971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Man SM, Karki R, Kanneganti TD. AIM2 inflammasome in infection, cancer, and autoimmunity: Role in DNA sensing, inflammation, and innate immunity. Eur J Immunol. 2016;46:269–280. doi: 10.1002/eji.201545839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rutz S, Wang X, Ouyang W. The IL-20 subfamily of cytokines--from host defence to tissue homeostasis. Nature reviews Immunology. 2014;14:783–795. doi: 10.1038/nri3766. [DOI] [PubMed] [Google Scholar]
- 55.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nature medicine. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 56.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nature medicine. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aujla SJ, Kolls JK. IL-22: a critical mediator in mucosal host defense. J Mol Med. 2009;87:451–454. doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- 58.Evans DJ, Fleiszig SM. Why does the healthy cornea resist Pseudomonas aeruginosa infection? Am J Ophthalmol. 2013;155:961–970 e962. doi: 10.1016/j.ajo.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hessian PA, Edgeworth J, Hogg N. MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J Leukoc Biol. 1993;53:197–204. [PubMed] [Google Scholar]
- 60.Gao N, Sang Yoon G, Liu X, Mi X, Chen W, Standiford TJ, Yu FS. Genome-wide transcriptional analysis of differentially expressed genes in flagellin-pretreated mouse corneal epithelial cells in response to Pseudomonas aeruginosa: involvement of S100A8/A9. Mucosal immunology. 2013;6:993–1005. doi: 10.1038/mi.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vijay-Kumar M, Wu H, Jones R, Grant G, Babbin B, King TP, Kelly D, Gewirtz AT, Neish AS. Flagellin suppresses epithelial apoptosis and limits disease during enteric infection. Am J Pathol. 2006;169:1686–1700. doi: 10.2353/ajpath.2006.060345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


