Abstract
This research sought to determine whether smoking influences affect by means other than withdrawal reduction. Little previous evidence suggests such an effect. We surmised that such an effect would be especially apparent in posttraumatic stress disorder (PTSD) and major depressive disorder (MDD), two disorders that are frequently comorbid with smoking and that involve dysregulated affect. Participants were US veterans who were regular smokers (N=159): 52 with PTSD (58% with comorbid MDD); 51 with MDD, and 56 controls with no psychiatric disorder. During three positive and three negative mood induction trials (scheduled over two sessions), non-withdrawn participants smoked either a nicotine-containing cigarette (NIC+), a nicotine-free cigarette (NIC−), or held a pen. Positive and negative affect were each measured before and after mood induction. Results showed a significant 2-way interaction of smoking condition x time on negative affect during the negative mood induction [F(6, 576)=2.41, p=.03] in those with PTSD and controls. In these groups, both NIC+ and NIC−, relative to pen, produced lower negative affect ratings following the negative mood induction. There was also a 2-way interaction of smoking condition x time on positive affect response to the positive mood induction amongst those with PTSD and controls F(6, 564)=3.17, p= .005] and amongst MDD and controls [F(6, 564)=2.27, p= .036]. Amongst all smokers, NIC+ enhanced the magnitude and duration of positive affect more than did NIC−. Results revealed affect modulation outside the context of withdrawal relief; such effects may motivate smoking among those with psychiatric diagnoses, and among smokers in general.
Keywords: PTSD, MDD, Depression, psychiatric comorbidity, tobacco dependence, smoking, experimental cigarettes, mood manipulation
As smoking prevalence continues to decline in the general population, smoking rates remain intractably high in those with mental health disorders (e.g., Steinberg, Williams, & Li, 2015). For instance, both posttraumatic stress disorder (PTSD) and major depressive disorder (MDD) are characterized by especially high rates of smoking prevalence, heavy smoking, and low quit rates (Feldner, Babson, & Zvolensky, 2007; Lasser et al., 2000; McClave et al., 2009; Weinberger, Mazure, Morlett, & McKee, 2013). Tobacco smoking amongst those with PTSD and with MDD is an important clinical and public health concern since both disorders are fairly prevalent (Breslau, Davis, & Schultz, 2003; Breslau, Johnson, Hiripi, & Kessler, 2001; Kessler et al., 2005) and because smoking contributes to disproportionate morbidity and mortality in these populations (Druss et al., 2011). To date, the processes that motivate smoking in persons with PTSD and MDD remain poorly understood. The current experiment arises, in part, from the notion that improved understanding of the psychological mechanisms underlying smoking in these populations could inform treatment development and ultimately improve quitting success for smokers with PTSD and MDD.
PTSD and MDD are both associated with severe elevations in negative affect and deficits in positive affect (Beckham et al., 2000; Joormann & Stanton, 2016). We posited that to the extent that smoking regulates affect, this effect might be especially important for persons with those disorders. It has been proposed that the higher-order affective dimensions (i.e., high negative affect and low positive affect) that span different mental health disorders might broadly underlie the relation between smoking and mental illness (Ameringer & Leventhal, 2010). This suggests that smoking would produce the same pattern of affect modulation across those with either disorder. Alternatively, if smoking’s effects on affect are influenced by factors that are unique to each disorder, then different patterns of affective modulation by nicotine would be observed. To date, the effects of smoking on affective responding captured in real time have not been directly compared for different psychiatric disorders. Thus, the transdiagnostic effects of smoking on affect remain unknown.
The results of the present study were compared with the predictions of two motivational models. One model was the negative reinforcement model, which has received considerable empirical and experimental support (e.g., Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Eissenberg, 2004; Pang, Khoddam, Guillot, & Leventhal, 2014; Robinson et al., 2011). This model holds that avoidance and escape from distress powerfully motivates addictive drug use, including smoking (Baker et al., 2004). For instance, it is clear that nicotine ameliorates withdrawal induced negative affect (e.g., Gloria et al., 2009; Perkins et al., 2008; Perkins & Karelitz, 2015; Perkins, Karelitz, Conklin, Sayette, & Giedgowd, 2010; Piper & Curtin, 2006; Shahab, McEwen, & West, 2011; Strong et al., 2011; Zinser, Baker, Sherman, & Cannon, 1992) and that affective distress serves as a powerful prod to smoking lapses and relapse (Baker et al., 2004; Conklin & Perkins, 2005; Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996). It is less clear, however, that nicotine mitigates stress-induced negative affect or distress (Kassel, Stroud, & Paronis, 2003; Perkins et al., 2010). Some laboratory studies found that smoking mitigates stressor-induced negative affect (Gilbert, Robinson, Chamberlin, & Spielberger, 1989; Juliano & Brandon, 2002), but others have not (Conklin & Perkins, 2005; Herbert, Foulds, & Fife-Schaw, 2001; Willner & Jones, 1996). Thus, the question of whether nicotine reduces stress-induced negative affect remains unresolved (Kassel et al., 2003; Perkins et al., 2010) despite its relevance to theoretical models of addiction (Baker et al., 2004).
The negative reinforcement model may be especially relevant to PTSD and MDD since both disorders are associated with high levels of negative affect and reactivity to negative stimuli (Beckham et al., 2000; Cook, McFall, Calhoun, & Beckham, 2007; Joormann & Stanton, 2016; Orr, Lasko, Shalev, & Pitman, 1995; Orsillo, Batten, Plumb, Luterek, & Roessner, 2004). Also, smokers with PTSD and/or current MDD strongly endorse the notion that smoking reduces distress (Beckham et al., 1997; Currie, Hodgins, el-Guebaly, & Campbell, 2001). In smokers with PTSD, ad lib smoking tends to be more frequently preceded by anxiety and stress than it is in smokers without the disorder (Beckham et al., 2008). Further, negative affect likely spurs smoking lapse (Beckham, Calhoun, Dennis, Wilson, & Dedert, 2013) and relapse (Brodbeck, Bachmann, Brown, & Znoj, 2014), in persons with either PTSD or depression. One experiment showed that nicotinized and denicotinized cigarettes similarly reduce negative affect (although these smoking manipulations were not compared with a neutral stimulus) in both smokers with PTSD and nondiagnosed smokers (Beckham et al., 2007). Another study found that depression was positively associated with more puffs and longer smoking in response to a sad stimulus (Fucito & Juliano, 2009). In sum, there is evidence that negative affect influences smoking in those with PTSD or MDD, but, as with smokers in general, it is unclear whether smoking reduces negative affect by means other than withdrawal relief. Thus, we believed it was vital to examine whether 1) smoking ameliorates stressor-induced negative affect amongst smokers in general, and, 2) whether this effect is magnified amongst smokers with either PTSD or MDD.
This experiment also derived hypotheses from the reward enhancement model. This model posits that smoking has the potential to enhance pleasurable responses to rewarding events that smokers encounter in their daily lives. In support of this model, the administration of nicotine to rodents increases their rate of responding for non-drug rewards (e.g., Caggiula et al., 2009; Chaudhri et al., 2006; Donny et al., 2003). This effect appears to be due to the direct, agonist effects of nicotine as it occurs regardless of prior conditioning contingencies (Chaudhri et al., 2006). Human research similarly suggests that nicotine increases the value of non-drug rewards as reflected by increased responding for exposure to auditory (music) or visual (video) rewards (Perkins, Karelitz, Jao, & Stratton, 2013).
The reward enhancement model might be especially relevant in smokers with PTSD and MDD since both disorders are associated with blunted responsivity to environmental rewards (i.e., anhedonia) (Hasler, Drevets, Manji, & Charney, 2004; Litz & Gray, 2002). In addition, anhedonia/low positive affect are positively related to heavier smoking in those with PTSD (Cook et al., 2007; Joseph et al., 2012). Among smokers with a history of depression, anhedonia increases risk for relapse, even when accounting for depressive symptoms (Cook et al., 2010). In one experimental study, history of MDD (relative to no history) enhanced nicotine’s effects on positive affect in response to a positive autobiographical memory. However, nicotine’s mood regulating effects were not moderated by current MDD (Spring et al., 2008). Clearly, additional laboratory data are needed to assess the magnitude of smoking induced reward enhancement in smokers and whether it differs with diagnostic status. Thus, we examined whether 1) smoking enhances positive affective response to non-drug rewarding stimuli, and 2) whether this effect is magnified amongst smokers with either PTSD or MDD. An assumption here is that the extent to which smoking enhances the pleasure ratings made with regard to a stimulus indexes both the reward value of the stimulus and the strength of a reward enhancement motive to smoke.
In order to characterize smoking motivational processes (i.e., negative reinforcement, reward enhancement) amongst those with PTSD and with MDD, we: 1) exposed participants to personalized negative and positive auditory scripts in order to manipulate their positive and negative affect; 2) allowed participants to smoke nicotine-containing cigarettes, nicotine-free cigarettes, or handle a neutral object during the affectively valenced scripts, and 3) compared the affective responses of persons with PTSD and with MDD to those with no psychiatric diagnosis. The following strategies were used to enhance the external and internal validity of this research:
Route of nicotine administration
Ad libitum cigarette smoking was used as the route of nicotine administration because our goal was to examine the affective impact of smoking as it would occur in non-experimental contexts. Non-smoking nicotine delivery strategies, such as nicotine nasal spray, may distort the orosensory and pharmacologic effects of smoking, potentially influencing the affective impact of the experience (Perkins, et al., 2008). Also, smoking was ad libitum since prior research suggests that restricted or controlled puffing may produce attenuated motivational effects (Perkins et al., 2008).
Nonpharmacologic control
We attempted to control for the nonpharmacologic (motoric, orosensory) and expectancy effects of smoking by using denicotinized cigarettes and manipulation of a neutral object (a pen).
Non-withdrawal state
This experiment used smokers who were minimally deprived of nicotine (27 minutes) in order to analyze the effects of smoking and affective cues on mood, relatively unaffected by withdrawal relief.
Personally relevant affect manipulations
Finally, we used personally relevant and tailored affect manipulations versus standardized stressors (e.g., unpleasant photographic slides) to manipulate affect. Script-elicited imagery is well validated and reliable across PTSD populations (e.g., Orr, Pitman, Lasko, & Herz, 1993; Pitman, Orr, Forgue, de Jong, & Claiborn, 1987) and tends to produce stronger effects than do generic mood prompts (Pitman et al., 1987).
Manipulation timing
In this research, participants smoked (or held a pen in a control condition) during exposure to the affectively valenced scripts. In most prior research, affectively valenced stimuli such as stressors were presented prior to smoking or nicotine administration (Conklin & Perkins, 2005; Herbert et al., 2001; Willner & Jones, 1996), or interspersed, with them (Perkins et al., 2010). We used a concurrent smoking-script exposure strategy for several reasons. First, alcohol’s stress dampening effects depend upon alcohol effects early in the stress appraisal process (Sayette, 1993); a similar effect might be present for nicotine. Also, concurrent exposure might be externally valid since smokers respond to stress by smoking (Conklin & Perkins, 2005). Third, prohibiting smoking during stress may produce behavioral withdrawal effects (Baker, Japuntich, Hogle, McCarthy, & Curtin, 2006).
In sum, this research was intended to elucidate the motivational bases of smoking in those with either PTSD or MDD, as well as in smokers in general. We hypothesized that relative to nondiagnosed controls, smokers with either PTSD or MDD would report greater negative affect in response to a stressor, and less positive affect in response to an appetitive stimulus. We also hypothesized that smoking a nicotine-containing cigarette would produce greater affect modulation (negative affect reduction and positive affect enhancement) than would smoking a denicotinized cigarette or holding a neutral stimulus. Further, we believed that nicotine induced affect modulation would be magnified amongst those with either PTSD or MDD, relative to controls. Finally, we hypothesized that smoking a denicotinized cigarette would produce greater affect modulation than holding a neutral object, and that this effect would be magnified amongst smokers with either PTSD or MDD.
Methods
Participants
Participants were 159 US veterans from southern Wisconsin who were recruited from a VA hospital via fliers. All participants were US veterans (enrolled at a VA hospital) ranging in age from 18–65 who had smoked at least 10 cigarettes per day for the past year and produced a carbon monoxide (CO) value > 8 ppm. This research was advertised to veterans as a study examining the effect of smoking on mood states. Eligible participants met DSM-IV criteria for one of the following categories: PTSD (n=52), MDD (n=51), or no current psychiatric disorder (controls) (n=56). In the PTSD group, 30 (57.7%) met criteria for comorbid MDD, consistent with population based comorbidity estimates (Elhai, Grubaugh, Kashdan, & Frueh, 2008). Exclusion criteria included current use of nicotine replacement therapy, bupropion or varenicline; current Axis I disorders other than tobacco dependence, MDD, and PTSD. This study was approved by the University of Wisconsin Health Sciences Institutional Review Board.
Screening
Following a brief telephone screen, study candidates were scheduled for an in-person screening session that involved CO assessment, a diagnostic interview, and other baseline measures. Those who were eligible and provided consent were scheduled for two experimental sessions for Study 1 and two experimental sessions for Study 2; only results from Study 1 are described in this manuscript.
Experimental Sessions
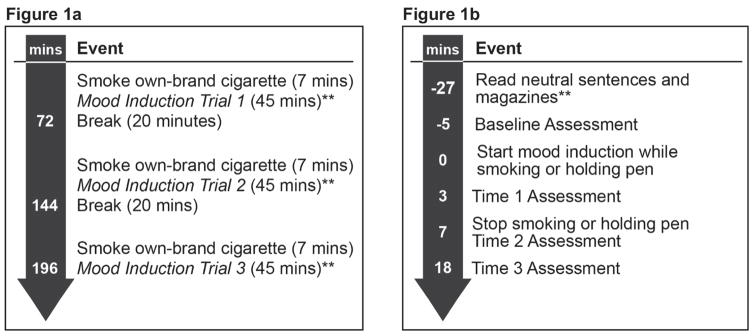
Participants participated in two, 3.5-hour laboratory sessions (see Figure 1a for Session Overview). During each of the sessions, participants underwent three mood induction trials (positive or negative), with the order to the two types being counterbalanced between participants over the two days of testing.1 During each of the three negative and three positive mood induction trials, participants either smoked a cigarette containing nicotine (NIC+), a nicotine-free cigarette (NIC−), or held a pen, with order of smoking condition counterbalanced amongst participants across the mood induction trials. On testing days, participants were asked to avoid caffeine for two hours before testing to prevent acute stimulating effects of caffeine on mood and were asked to avoid drinking any alcohol on the day of testing. Participants who reported any same-day alcohol consumption or caffeine consumption within the prior 2 hours were rescheduled for a make-up session to occur at least 24 hours later. CO was assessed at the beginning of each session to assure carbon monoxide (CO) value of at least 8 ppm.
Figure 1.
Figure 1a. Session Overview*
*Three mood induction trials occurred during each of the two sessions, which were scheduled on two different testing days (for a total of 6 trials). Negative and positive mood induction trials were counterbalanced across the six mood induction trials. **See Mood Induction Trial details in Figure 1b.
Figure 1b. Mood Induction Trial Schedule*
*The schedule for each of the 6 mood induction trials. Smoking condition (NIC+, NIC−, or Pen) was counter-balanced across trials.
**Participants read neutral sentences immediately after smoking their own-brand cigarette. Therefore, the mood induction while smoking or holding pen occurred 27 minutes after finishing own-brand smoking.
At the beginning of each of the three mood induction trials within each session, participants smoked one of their own-brand cigarettes to standardize nicotine exposure and ensure that they were not in withdrawal (see Figure 1a). Use of the smokers’ own cigarette brand permitted variability in cigarette nicotine levels, but was thought to produce a smoking experience that was externally valid for each smoker (Spring et al., 2008). After smoking their own-brand cigarette, participants first read neutral sentences and were then allowed to read magazines provided by the study (see Figure 1b). Following the 22-minute rest period, participants completed self-report baseline affect measures for 5 minutes. Participants then underwent the mood induction while smoking an experimental cigarette (either NIC+ or NIC−) or holding a pen (minute 0 on Figure 1b timeline: 27 minutes after finishing own-brand cigarette). We designed the experiment so that the latency between own-brand smoking at the beginning of each trial and the smoking manipulation would be less than 30 minutes, a time period too brief to produce, on average, significant increases in withdrawal symptoms (Bujarski et al., 2015; Hendricks, Ditre, Drobes, & Brandon, 2006). Mood was assessed immediately after mood induction (Time 1), and then again at 7 minutes (Time 2, immediately after smoking NIC+, NIC− or holding a pen) and at 18 minutes after the start of mood induction (Time 3: see Figure 1b).
Mood Induction
Mood inductions involved imagining three positive and three negative autobiographical memories (Spring et al., 2008). During the screening session, participants reported positive and negative memories via a semi-structured interview format. Participants rated on 1–5 Likert scales how happy or distressed each positive or negative memory, respectively, made them feel and how vividly they recalled each memory. Research staff assisted participants in selecting memories for the mood induction trials that were rated as ≥ 4 for both valence (happy or distressed) and vividness (although ratings of = 3 were accepted if more elevated ratings could not be obtained). In order to increase comparability of the negative memories across diagnostic groups, trauma memories were not used2. For the selected negative memories, the average level of distress was 4.58 (SD=.54) and vividness was 4.66 (SD=.46). For the positive memories, the average level of happiness was 4.46 (SD=.41) and vividness was 4.69 (SD=.46)3.
The selected positive and negative memories were recorded and then delivered via a headset during trials. Participants were instructed to close their eyes and remember the memory in detail, while trying intensely to re-experience the happy or sad/distressing feelings of the memory.
Nicotinized/Denicotinized Cigarettes
Nicotinized (containing .6 mg nicotine) and denicotinized cigarettes (containing .05 mg nicotine) that were smoked during the mood induction trials were produced by Quest and matched on tar and carbon monoxide content. Participants were not informed which cigarette they were smoking.
Assessments
Structured Clinical Interviews were conducted by a trained diagnostician to assess MDD, PTSD, and other Axis I Disorders. The Structured Clinical Interview for DSM-IV, non-patient version (SCID: Spitzer, Williams, Gibbon, & First, 1992) was used to assess MDD and other Axis I Disorders that were excluded from the study. The Clinician-Administered PTSD Scale (CAPS: Blake et al., 1990) was used to assess PTSD. A total CAPS score ≥ 50 was required for inclusion in the PTSD group (Weathers, Keane, & Davidson, 2001). The diagnostician was supervised by a licensed clinical psychologist.
Smoking Status was assessed via self-report and CO test (via an Ecolyzer, Bedfont). To be included in the study, subjects had to report smoking ≥ 10 cigarettes daily for at least the past year and have a CO score ≥ 8.
Positive and Negative Affect was assessed via the Positive and Negative Affect Schedule (PANAS: Watson, Clark, & Tellegen, 1988). The PANAS is a self-report state mood questionnaire comprising 10 adjectives describing positive mood states and 10 adjectives describing negative mood states that are rated on a 5-point scale.
Dependence. The Fagerström Test for Nicotine Dependence (FTND: Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), was used to assess tobacco dependence (α=.61).
Analytic Plan
The PTSD, MDD, and control groups were compared on demographic, baseline mood, and smoking variables using analyses of variance (ANOVA) for continuously scaled variables and chi-square tests for dichotomous variables. We also examined the effects of mood induction (positive, negative) and smoking manipulation (NIC+, NIC−, Pen) order on positive and negative affective outcomes using ANOVA. Mean negative affect was examined across the Pen condition of the positive mood induction trials to evaluate the degree to which participants experienced increases in withdrawal-related affective changes during the experiment independent of the negative mood induction.
Repeated measures ANOVAs were used to examine the effect of nicotine condition (NIC+, NIC−, Pen), diagnostic group (PTSD vs control; MDD vs control), and Time (Baseline, Time 1, Time 2, Time 3) on change in mood ratings in response to positive and negative mood scripts. Negative affect was the dependent measure for the negative mood induction analyses and positive affect was the dependent measure for the positive mood induction analyses (see Supplement Figures for positive affective response to the negative mood induction and negative affective response to the positive mood induction). Based on a priori hypotheses we conducted focused comparisons (Rosenthal, Rosnow, & Rubin, 2000; Tabachnick & Fidell, 2007) that contrasted each diagnostic group with the controls; i.e., via the following orthogonal a priori contrasts: (1) PTSD versus controls (b) MDD versus controls (Tabachnick & Fidell, 2007). Thus, both negative affect and positive affect ANOVAs were run for both the PTSD vs. control comparison and for the MDD vs. control comparison (4 models). For the within-subjects condition factor, the following a priori contrasts were used: 1) NIC+ vs. NIC−; 2) NIC+ vs. Pen; 3) NIC− vs. Pen. For the time factor, orthogonal polynomial contrasts were tested to characterize the temporal pattern of mood responses in terms of their linear and quadratic trend components, with the former indicating linear trend (slope) from Baseline values forward and the latter indicating effects captured by time-squared. Finally, we conducted an exploratory ANOVA that contrasted the MDD and PTSD groups. The Benjamini-Hochberg procedure was used to control experimentwise error in these exploratory analyses (Benjamini & Hochberg, 1995; Keselman, Cribbie, & Holland, 2002).
With respect to the hypothesized effects, a significant two-way interaction between diagnostic group and time would indicate that diagnostic group affected the temporal pattern of positive and negative affective response to the mood inductions. A significant two-way interaction between smoking condition and time would indicate that the smoking manipulation (NIC+, NIC−, or Pen) affected the temporal pattern of affective responses to the mood inductions. A significant three-way interaction amongst diagnostic group, smoking condition, and time would indicate that the temporal pattern of affective response differed significantly as a function of diagnostic group (PTSD, MDD, and controls) and smoking condition. Analyses were conducted using SPSS (IBM Corporation, 2013). We report partial eta squared for effects size with confidence intervals computed via SPSS scripts (Smithson, 2003).
Results
Preliminary analyses
Group differences between PTSD and controls were found for gender and dependence (see Table 1). Analyses with and without covariates produced the same pattern of results (covariates were gender, tobacco dependence, mood induction and nicotine condition order, or MDD-PTSD comorbidity); thus, unadjusted models are presented below.
Table 1.
Participant demographic and smoking characteristics by group
| Full sample (n=159) | PTSD (n=52) | MDD (n=51) | Control (n=56) | |
|---|---|---|---|---|
| Women % (n) | 6.3 (10) | 15.4 (8)a | 2.0 (1)a,b | 1.8 (1)b |
| White % (n) | 71.7 (114) | 71.2 (37) | 78.4 (40) | 66.1 (37) |
| High School Diploma/GED or less % (n) | 34.6 (55) | 28.8 (15) | 35.3 (18) | 39.3 (22) |
| Age (M, SD) | 52.43 (9.7) | 49.4 (11.05) | 55.0 (8.8) | 52.89 (8.45) |
| CPD (M, SD) | 19.8 (8.4) | 21.1 (6.95) | 18.21 (8.61) | 19.82 (9.50) |
| FTND (M, SD) | 5.7 (2.1) | 6.3 (2.01)a | 5.8 (2.06)a,b | 5.05 (2.22)b |
| Baseline NA (Negative Mood Induction | 14.37 (5.36) | 16.26 (6.56)a | 15.52 (5.00)a | 11.41 (2.11)b |
| Baseline NA (Positive Mood Induction) | 14.63 (5.95) | 16.43 (7.57)a | 16.27 (5.43)a | 11.26 (1.91)b |
| Baseline PA (Negative Mood Induction) | 29.40 (8.33) | 29.93 (9.91)a,b | 27.62 (7.08)a | 30.72 (8.19)b |
| Baseline PA (Positive Mood Induction) | 29.40 (8.32) | 29.42 (9.60)a,b | 28.14 (7.00)a | 30.59 (8.13)b |
Note: Within each column, numbers with different superscript letters differ significantly with a p value < .05. GED = General Educational Development; CPD=cigarettes per day; FTND= Fagerstrom Test for Nicotine Dependence; NA=Negative Affect; PA= Positive affect
Missing Data
A total of four participants initially enrolled in the study but did not attend any sessions. These participants were therefore excluded from analysis. For the negative mood inductions, 153 (96%) completed all three mood inductions trials, 3 (2%) completed two trials, and 3 (2%) completed 1 trial. For the positive mood inductions, 148 (94%) completed three trials, 6 (4%) completed wo trials, and 4 (3%) completed one experimental trial.4 There were no group differences in missing data. All available data were used in the analyses.
Withdrawal Check
Mean negative affect was examined during the positive mood induction trials to evaluate whether participants experienced increases in withdrawal-related negative affect over the course of a trial independent of negative mood induction. We assumed that if withdrawal were increasing over the course of the trial we would see evidence of steadily increasing negative affect. We examined negative affect during the Pen condition because we wished to avoid smoking or smoking cue effects on withdrawal. Mean negative affect was stable across the trial (B= 14.48 [6.57]; T1= 14.15 [6.16]; T2 = 14.73 [6.65]; T3=14.46 [6.32]) and was highly similar to negative affect assessed during the screening session, which occurred when smokers had been smoking ad lib (14.43 [6.57]). Thus, these data show no evidence that negative affect increased over the course of a trial or that it was greater than when participants had been smoking ad libitum.
Negative Mood Induction Outcomes
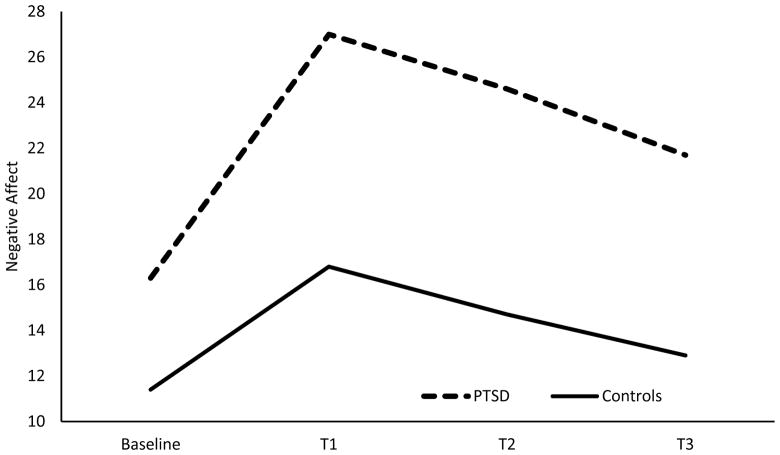
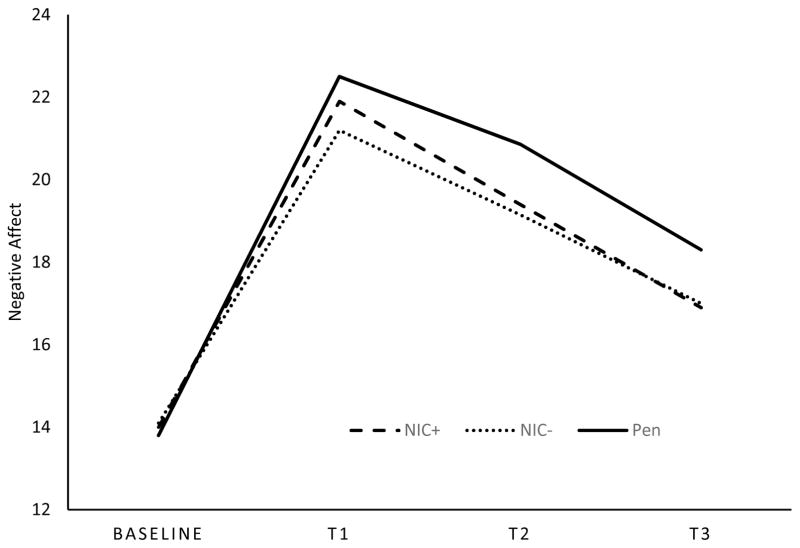
Mean negative affective responses to the negative mood induction for the diagnostic groups are presented in Table 2. In the PTSD model (PTSD versus controls), there was a main effect of PTSD on negative affect [F(1, 98)=38.24, p=.00], suggesting that those with PTSD reported higher levels of negative affect, averaged across time, relative to controls. There was also a significant PTSD x time effect [F(3, 576)=9.10, p=.00; ηp2 =.045, 90% CI(.019, .072)] for negative affective responses to the negative mood induction with significant linear [F(1, 96)=9.56, p=.00; ηp2 =.091, 90% CI(.020, .188)] and quadratic trends [F(1, 96)=9.91, p=.00 ; ηp2 =.094, 90% CI(.021, .192)]. As shown in Figure 2, relative to controls, those with PTSD experienced a greater rise in negative affect in response to the stressor and showed reduced affective recovery (i.e., less of a decrease in negative mood) following the stressor. There was also a significant smoking condition x time effect [F(6, 576)=2.41, p=.03; ηp2 =.024, 90% CI(.001, .039)] for negative affective response. Both NIC+ and NIC− significantly differed from Pen with regard to the linear trend [F(1, 96)=5.40, p=.02; ηp2 =.053, 90% CI(.004, .139); F(1, 96)=7.53, p=.01; ηp2 =.073, 90% CI(.011, .166) respectively]. As shown in Figure 3, the NIC+ and NIC− conditions produced lower negative affect ratings across T1–T3 than did the Pen condition for controls and those with PTSD (also see Table 2).5
Table 2.
Mean affective responses to negative and positive mood inductions by smoking condition and diagnostic group.
| PTSD | MDD | Control | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| NA | PA | NA | PA | NA | PA | |
| Baseline | ||||||
| NIC+ | 16.71(8.19) | 29.16(10.78) | 15.71(5.98) | 27.31(7.69) | 11.31(2.42) | 30.42(8.62) |
| NIC− | 16.49(7.54) | 29.55(9.25) | 15.48(5.56) | 28.02(7.90) | 11.95(3.48) | 31.02(8.69) |
| Pen | 16.00(6.74) | 30.94(11.16) | 15.36(5.61) | 27.62(8.10) | 11.59(3.30) | 30.75(8.31) |
| Time 1 | ||||||
| NIC+ | 27.10(10.52) | 32.52(11.11) | 23.98(9.04) | 31.80(8.37) | 16.87(7.37) | 33.80(9.06) |
| NIC− | 25.61(12.02) | 32.43(10.27) | 24.26(9.92) | 30.86(8.30) | 16.78(7.49) | 32.15(9.12) |
| Pen | 27.94(10.70) | 32.85(10.64) | 22.06(8.82) | 31.18(9.64) | 17.07(8.64) | 33.27(8.35) |
| Time 2 | ||||||
| NIC+ | 24.55(10.86) | 32.06(12.01) | 22.80(9.59) | 29.92(8.63) | 14.42(5.51) | 32.64(9.28) |
| NIC− | 23.78(11.48) | 31.35(11.26) | 22.06(9.33) | 29.29(8.85) | 14.53(6.29) | 31.54(9.06) |
| Pen | 26.24(12.17) | 30.54(10.99) | 21.10(8.89) | 29.60(9.55) | 15.48(7.60) | 31.65(9.30) |
| Time 3 | ||||||
| NIC+ | 21.24(10.93) | 30.64(11.55) | 19.43(9.22) | 28.33(8.22) | 12.50(3.63) | 31.76(9.47) |
| NIC− | 21.24(11.21) | 30.29(10.72) | 19.62(8.27) | 28.92(8.32) | 12.87(5.49) | 31.06(9.41) |
| Pen | 23.13(11.68) | 30.50(11.48) | 19.42(7.46) | 27.72(9.35) | 13.59(6.11) | 31.32(9.38) |
Note: NA = negative affect measured during the negative mood induction trial; PA= positive affect measured during the positive mood induction trial; NIC+ = nicotine-containing cigarettes; NIC− = denicotinized cigarettes
Figure 2.
This figure depicts the negative affective response to the negative mood induction of those with PTSD (n=52) and controls (n=56) averaged across the three smoking conditions.
Figure 3.
This figure depicts the negative affect response to negative mood induction averaged across those with PTSD (n=52) and controls (n=56). NIC+ indicates nicotinized cigarette and NIC− indicates denicotinized cigarette.
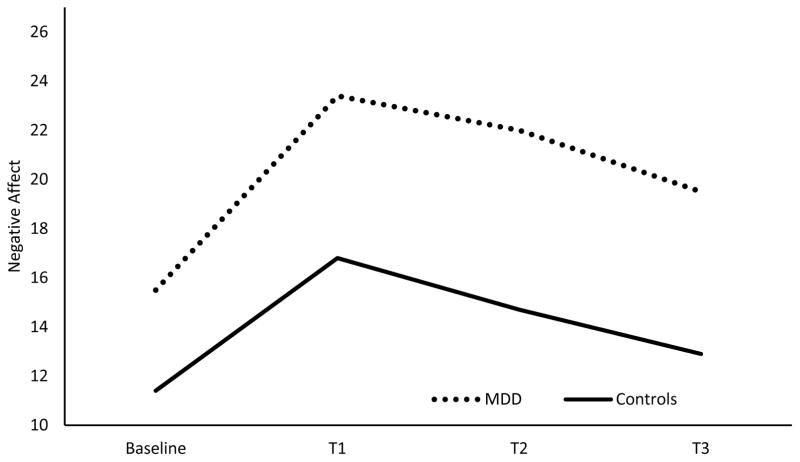
Within the MDD model (MDD vs. controls), there was a main effect of MDD on affective responding [F(1, 100)=30.72, p=.00], as those with MDD reported higher levels of negative affect averaged across all time points relative to controls. There was also a significant MDD x time effect [F(3, 582)=4.33, p=.01; ηp2 =.022, 90% CI(.004, .041)] for negative affective response to the negative mood induction, reflecting a significant linear trend [F(1, 97)= 8.4, p=.01; ηp2 =.080, 90% CI(.015, .174)]. Smokers with MDD reported greater negative affect across T1 – T3, relative to Baseline, than did controls (see Figure 4). As opposed to the analysis comprising the PTSD-control comparison, there was no significant smoking condition x time interaction for negative affect in the MDD analysis.
Figure 4.
This figure depicts the negative affective response to the negative mood induction of those with MDD (n=51) and controls (n=56) averaged across the three smoking conditions.
Positive Mood Induction Outcomes
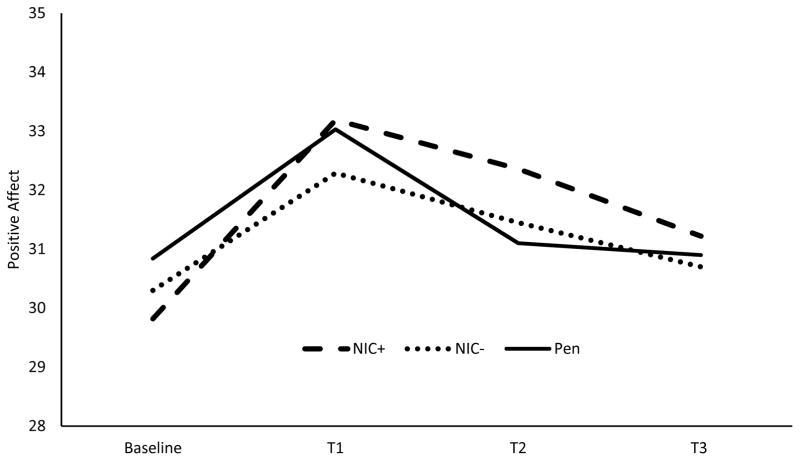
Mean positive affective responses to the positive mood induction for the diagnostic groups are presented in Table 2. In the PTSD model, there was no main effect of PTSD on positive affect (p = .112). However, there was a significant 2-way interaction of smoking condition x time on positive affect response to the positive mood induction [F(6, 564)=3.17, p= .005.; ηp2 =.032, 90% CI(.006, .050)]. That is, for both the PTSD and control participants, NIC+ differed significantly from Pen with regard to the linear trend [F(1, 94)=5.47, p=.021; ηp2 =.055, 90% CI(.004, .143)]. As shown in Figure 5, NIC+ produced greater increases in positive affect than did the Pen condition moving from Baseline across the subsequent assessment time points (T1 – T3). NIC− did not differ from Pen in linear (p=.29) or quadratic trends (p=.65). The difference between NIC+ and NIC− with regard to the linear trend approached but did not achieve statistical significance [F(1, 94)=3.58, p=.06; ηp2 =.037, 90% CI(.000, .116)].
Figure 5.
This figure depicts the positive affect response to positive mood induction averaged across those with PTSD (n=52) and controls (n=56). NIC+ indicates nicotine-containing cigarette and NIC− indicates denicotinized cigarette.
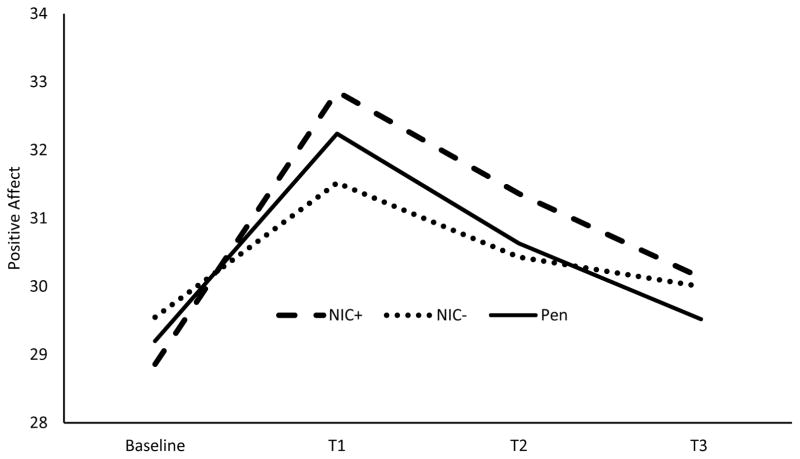
In the MDD model, MDD did not produce a main effect on positive affect response (p = .108). However, there was a significant 2-way interaction of smoking condition x time on positive affect response to the positive mood induction [F(6, 564)=2.27, p= .036; ηp2 =.024, 90% CI(.001, .038)]. NIC+ differed significantly from NIC− with regard to the quadratic trend [F(1, 94)=7.94, p=.006; ηp2 =.078, 90% CI(.013, .174)]. As shown in Figure 6, the NIC+ condition had a steeper rise from Baseline to T1 relative to the NIC− condition.
Figure 6.
This figure depicts the positive affect response to positive mood induction averaged across those with MDD (n=51) and controls (n=56). NIC+ indicates nicotine-containing cigarette and NIC− indicates denicotinized cigarette.
Exploratory Analyses
Exploratory Negative Mood Induction Outcomes
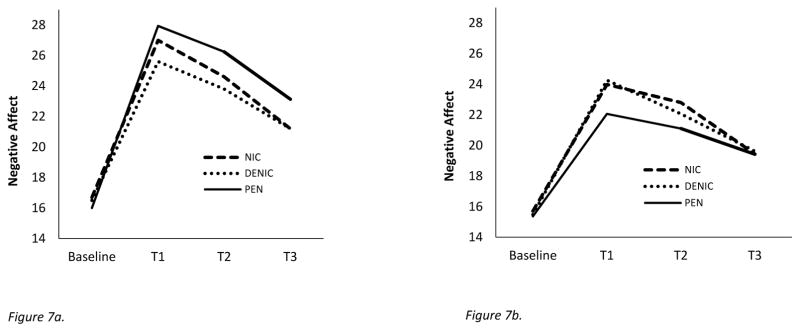
Exploratory analyses directly contrasted the PTSD group with the MDD group6. Negative affective response to the negative mood induction showed a significant three-way interaction of diagnostic group (PTSD vs. MDD) x smoking condition x time [F(6, 882)=2.80, p = .028; ηp2 =.019, 90% CI(.002, .030)]. The Pen condition significantly differed from both the NIC+ and NIC− conditions on quadratic trends [F(1, 147)=4.44, p=.042; ηp2 =.029, 90% CI(.001, .087); F(1, 147)=8.86, p=.015; ηp2 =.057, 90% CI(.011, .126) respectively]. This appears to be due, in part, to Pen’s producing an exaggerated quadratic trend relative to NIC+ and NIC− in those with PTSD, but a relatively diminished quadratic pattern (relative to NIC+ and NIC−) in those with MDD (see Figures 7a and 7b). Whereas Pen exposure tended to produce an elevated negative affective response across T1–T3 in those with PTSD, it was associated with diminished negative affect relative to NIC+ and NIC− in those with MDD (see Figure 7a and 7b).
Figure 7.
Figure 7a. This figure depicts the negative affect response to negative mood induction amongst those with PTSD (n=52). NIC+ indicates nicotine-containing cigarette and NIC− indicates denicotinized cigarette.
Figure 7b. This figure depicts the negative affect response to negative mood induction amongst those with MDD (n=51). NIC+ indicates nicotine-containing cigarette and NIC− indicates denicotinized cigarette.
Exploratory Positive Mood Induction Outcomes
There were no significant interactions involving diagnostic group (PTSD vs MDD) for positive affect.
Discussion
Results showed that the PTSD and MDD groups reported greater negative affect in response to the negative mood induction than did the controls. Results also showed a significant smoking condition x time effect on negative affect in those with PTSD and in controls. Specifically, both NIC+ and NIC−, relative to Pen, blunted the negative affective response to the stressor and enhanced affective recovery. Thus, both for those with PTSD and for nondiagnosed controls, smoking a cigarette with or without nicotine mitigated the negative affect response to the stressor when the smoker was in a non-withdrawn state. In contrast to these findings, the NIC+ and NIC− conditions produced higher negative affect ratings across time in the MDD group; significantly higher than the ratings produced by the PTSD group. No diagnostic group differences were observed with regard to positive affective response to the positive mood induction. However, there was a smoking condition x time effect on positive affective response to the positive mood induction in PTSD vs controls and in MDD vs controls. Amongst all smokers, NIC+ enhanced experimentally induced positive affect and maintained it relative to NIC−. NIC+ also elevated positive affect over time relative to the Pen condition in both the PTSD group and controls. This suggests that amongst those with mental illness (PTSD, MDD) as well as in non-diagnosed controls, smoking a nicotine-containing cigarette enhanced the effects of positive mood induction in non-withdrawn smokers.
Negative Reinforcement Hypothesis
Mixed evidence for the effects of smoking on stressor-induced negative affect (Conklin & Perkins, 2005; Gilbert et al., 1989; Herbert et al., 2001; Juliano & Brandon, 2002; Willner & Jones, 1996) has led some to posit that smoking might relieve negative affect primarily or only under withdrawal conditions (Parrott & Kaye, 1999; Schachter, 1978) cf. (Baker et al., 2004). The fact that smokers in this research were minimally deprived of nicotine (< 30 minutes) suggests that withdrawal relief was not a significant influence on smoking’s effects on stress related negative affect (Hendricks et al., 2006). Several factors might contribute to the prior inconsistent findings regarding the acute effects of smoking on stressor-induced distress. For instance, previous research typically examined the effects of smoking after exposure to a stressor (e.g., Conklin & Perkins, 2005; Herbert et al., 2001; Willner & Jones, 1996); this study required participants to smoke during the stressor. Smoking early in the course of stress induction might allow smoking to influence stressor appraisal; a stressor might be construed as less aversive when an effective coping response (i.e., smoking) is present (Lazarus & Folkman, 1984). Moreover, the prevention of smoking until after exposure to the stressor may have exacerbated the stressor effects due to the inability to practice a highly mapped coping response (Curtin, McCarthy, Piper, & Baker, 2006). Finally, the use of autobiographic scripts, as opposed to the standardized stressors used in most smoking research (Conklin & Perkins, 2005; Herbert et al., 2001; Willner & Jones, 1996), may have produced a more intense or meaningful affective experience (Pitman et al., 1987), potentially creating greater opportunity for negative reinforcement.
Both NIC+ and NIC− relative to the Pen condition resulted in alleviation of negative affect in smokers with PTSD and in controls, and did so in the absence of significant tobacco withdrawal (cf. Beckham et al., 2007; Donny, Houtsmuller, & Stitzer, 2007; Perkins et al., 2008; Perkins et al., 2010). Further, the equivalence of the effects of NIC+ and NIC− suggests that it was produced, at least in part, through associative processes. The greater negative affect suppression by NIC− relative to the Pen condition could be due to any of multiple candidate mechanisms; e.g., the execution of the motor elements of smoking, the orosensory elements (Perkins et al., 2008), attentional effects, expectancy effects, conditioned incentive effects, and conditioned reinforcement. The NIC+ versus Pen condition differences may reflect the effects of the same mechanism(s). We think that the effects of NIC+ and NIC− on stress induced negative affect may reflect a combination of processes, but posit that conditioned reinforcement occurring via cues associated with the iterative self-administration ritual is a leading candidate. This is supported by a wealth of evidence of the acquisition of strong conditioned reinforcement effects using addictive drug unconditioned stimuli (UCSs) (Everitt & Robbins, 2005; Taylor & Robbins, 1984). Further, the instatement of associative drug motivational effects might be enhanced by stressors and negative affect (Bossert, Marchant, Calu, & Shaham, 2013). Also, Perkins and his colleagues have shown that the orosensory cues produced by smoking elicit significant suppression of stress-related negative affect (in withdrawn smokers), even when the smokers knew that smoking would deliver no nicotine. This accords with considerable evidence that interoceptive cues of drug action may be powerful elicitors of drug motivational processes (Baker et al., 2004). In other words, the effects of denicotinized cigarettes on negative affect may depend strongly on the experience of smoking-related sensory cues (versus general expectations of nicotine effects). This account, of course, suggests that the effects of denicotinized cigarettes on stressor related negative affect reflect the residue of prior unconditioned effects of nicotine on stress reactivity. It is also possible that the conditioned effects of smoking observed in this study reflect smoking’s prior withdrawal-reducing effects; that is, such conditioned effects may have arisen from learning where the unconditioned response was withdrawal reduction, not stress reduction. Finally, it is important to note though, that while the effects of NIC+ and NIC− on stressor induced distress may reflect the history of nicotine’s pharmacologic actions, nicotine’s contemporaneous, acute pharmacologic actions appeared to have exerted little additional effect on negative affect in this study.
Reward Enhancement Hypothesis
With regard to the reward enhancement hypothesis, we found that for all diagnostic groups NIC+ enhanced experimentally induced positive affect and maintained it relative to NIC−. NIC+ also elevated positive affect over time relative to Pen exposure in the PTSD group and in controls. These findings are consistent with research showing that nicotine can enhance the rewarding properties of nonpharmacologic stimuli (Cook, Spring, & McCharugue, 2007; Perkins & Karelitz, 2014). Similar to the current experiment, Perkins and his colleagues (e.g., Perkins & Karelitz, 2014) found that smoking nicotine-containing cigarettes, versus denicotinized cigarettes, enhanced the rewarding effects of appetitive stimuli. However, it is important to recognize key differences between the current experiment and the Perkin’s research. First, the current experiment did not measure the effects of smoking on conditioned responding (i.e., on its ability to modulate reinforcement) as did the Perkin’s research. Thus, we are inferring that the same processes that cause nicotine’s modulation of reinforcement value also affected subjective ratings of reward value. Second, Perkin’s research involved withdrawn smokers. Perkin’s and colleagues argued that withdrawal relief was unlikely to have affected their results (e.g., results with dependent and nondependent smokers were similar; Perkins & Karelitz, 2013). Through the use of non-withdrawn smokers, the current results support that assertion. Further, the current research shows that across all diagnostic groups, smoking enhanced the pleasure induced by imaginal re-experiencing, rather than by an external, sensory stimulus per se (vs. Perkins & Karelitz, 2013). This considerably broadens the range of experiences whose affective consequences might be modulated by nicotine. Since this effect occurred regardless of withdrawal status (Perkins & Karelitz, 2013, 2014), and in response to imaginal processing, it may be perpetually available via smoking.
Effects of Diagnostic Status
Smokers with PTSD and MDD reported greater negative affect in response to stressors than did the controls. Smokers with these diagnoses, therefore, might have greater opportunity for negative reinforcement from smoking given the severity of their distress. However, the effects of smoking on affect were fairly similar when each diagnostic group was compared with the controls. Even though the pattern of affect modulation by smoking was similar between the diagnostic groups and the controls, it could be that the greater affective response magnitudes in those with PTSD and MDD render negative reinforcement motives more appealing in those groups. It is also possible that diagnostic group differences in affect modulation via cigarette smoking might be more apparent in the presence of tobacco withdrawal. Nicotine effectively mitigates withdrawal-related affective distress (e.g., Baker et al., 2004; Gloria et al., 2009), which is especially severe in smokers with PTSD and MDD (Dedert et al., 2012; Weinberger, Desai, & McKee, 2010).
One notable diagnostic difference found in this research was that only amongst those with MDD did smoking NIC+ and NIC− cigarettes magnify negative affective responding to the stressor. In contrast, smoking suppressed affective reactions to the stressor in both the PTSD and control groups. It is unclear why smoking might have uniquely inflated distress amongst smokers with MDD. One possibility is that smoking exerts some of its effects by channeling attentional resources (Kassel, 1997) and this has paradoxical effects in the depressed. Kassel’s attention allocation model posits that smoking reduces attentional resources so that attention is fixed on current activities or salient stimuli (e.g., smoking), and is less likely to be diverted to intrusive, negative thoughts; presumably avoidance of such thoughts should reduce negative affect. Those with MDD, however, attend to self-referential negative cognitions preferentially when distractors are present (Nolen-Hoeksema, 1991). Thus, amongst those with MDD, smoking might channel attentional resources to the negative memories that are elicited by stressors, and thus magnify the resulting distress (Richmond, Spring, Sommerfeld, & McChargue, 2001). In any event, the current results suggest that smokers with MDD only may smoke for reasons other than stress relief; e.g., reward enhancement. It is also the case that such smokers may merely believe that smoking reduces stress effects because it so effectively reduces withdrawal-related distress.
It is important to note that MDD comorbidity did not appear to meaningfully influence affective reactions of participants with PTSD; i.e., PTSD participants performed similarly regardless of MDD status (see Supplemental Figures 4 and 5). Although comorbidity between PTSD and MDD is extremely common (Ginzburg et al., 2010), possibly due to the overlap in symptoms across the two disorders (i.e., numbing/dysphoria; Gootzeit and Markon, 2011: Elhai et al., 2015), we believe that it was the anxiety dimension of PTSD that led to the observed smoking related suppression of negative affect in this experiment. PTSD manifests with strong anxiety related symptoms that are associated with its re-experiencing, hyperarousal, and avoidance dimensions (Byllesby et al., 2016; Wang et al., 2011). Further, research shows that persons with PTSD show especially heightened anxiety in response to stressors and smoking appears to reduce such distress (Beckham et al., 2005, 2007). We believe that smoking may reduce heightened anxiety amongst persons with PTSD either directly (Hogle et al., 2010), or via its effects on attentional processing or by mitigating sensorimotor gating deficits (Baschnagel & Hawk, 2008; Calhoun et al., 2011; Vrana et al., 2013). Some research fails to show that smoking reduces acoustically elicited startle in smokers with PTSD, casting doubt on the notion that smoking exerts anxiolytic effects. However, those results may be challenged on the bases of the timing of the smoking experience in those studies and the fact that the startle paradigm may have primarily activated fear and not anxiety (Calhoun et al., 2011; Vrana et al., 2013), with the latter likely being more sensitive to smoking effects (Hogle et al., 2010; Jonkman et al., 2008). In short, we believe that smokers with PTSD are likely to show especially strong anxiety reactions to stressful stimuli, and that nicotine can quell such reactions (Beckman et al., 2005, 2007). Importantly, anxiety disorder diagnosis was exclusionary for the MDD condition, reducing the likelihood that heightened anxiety would affect responding of those with MDD.
Treatment Implications
If the effects of smoking on negative affect reflect conditioned reinforcement, then interventions aimed at smoking cue extinction or avoidance strategies might be helpful. Such strategies might focus on exposure to orosensory cues contingent with smoking (e.g., via extended use of denicotinized cigarettes) or even strategies that imaginally induce distress. Our results also underscore the importance of targeting reward reactivity following quitting. If smokers strategically use cigarettes to enhance pleasure in response to non-drug rewards, then quitting could lead to a decrement in the experience of such reward (i.e., anhedonia). Indeed, we have found that quitting smoking leads to anhedonia, and that this loss in post-cessation pleasure is a significant barrier to quitting (Cook et al., 2015). Helping smokers prepare for withdrawal-related anhedonia by encouraging their pursuit of alternative sources of non-smoking reinforcement might buffer the loss in reward functioning that follows quitting.
Limitations
In order to enhance both the study’s ecological validity and the effects of smoking (Perkins et al., 2008), participants smoked nicotinized and denicotinized cigarettes ad lib, rather than using a controlled form of nicotine administration. Thus, we relinquished control over the handling and dosing of the cigarettes as well as the ability to detect group differences in smoking patterns. Although such variability likely affected the effects of the smoking experience, it better characterizes participants’ smoking as it occurs in nonexperimental contexts. The smoking of three own-brand cigarettes over the course of each session could be viewed as both a strength and a limitation. It is a strength in that it virtually ensures that participants did not experience significant withdrawal during the study; it is a weakness in that the amount of smoking may have blunted affective reinforcement because of mild, undetected aversive effects (this may have been mitigated because smoking was ad libitum). In addition, smoking own-brand cigarettes may have reduced the smoking and nicotine yield of the experimental cigarettes, potentially reducing affective response to them. However, it is important to note that the significant effects of the nicotine containing cigarettes on positive affect suggests that they still possessed the capacity to produce meaningful affective impact. Also, participants did not rate the subjective experience of smoking their own-brand versus the experimental cigarettes; thus, perceived cigarette strength or other qualities could not be compared. Although evidence suggests that participants were minimally deprived of nicotine, the assessment of withdrawal was limited by the absence of a measure of craving. In addition, withdrawal may have been associatively elicited by external or internal cues. We also could not determine the exact processes through which NIC+ and NIC− cigarettes produced affect modulation. For instance, we do not know if the effects of smoking on reactions to the imaginal scripts were caused by affective modulation per se, or instead because smoking directly affected the ability to access and focus on the relevant imaginal material. In addition, because this veteran sample included primarily men, results may not generalize to women who smoke. Further, no neutral scripts were used in this study (due to concerns regarding experimental burden); thus we can determine the magnitude of affective responses relative to a baseline, but not relative to a neutral condition. Finally, although positive and negative memories were matched on dimensions of vividness and valence, they were not matched on arousal.
Conclusions
This research may shed light on the motivational bases of smoking in those with and without comorbid psychopathology. First, there was evidence that smoking reduced negative affective response to a stressor in non-withdrawn smokers. Specifically, smoking either nicotine-containing or denicotinized cigarettes (relative to holding a pen) mitigated negative affective responses to a stressor amongst smokers with PTSD and amongst non-diagnosed controls but not for those with MDD. Because denicotinized cigarettes produced this effect, it is likely that the effect is primarily associative in nature. While the mechanism is unknown, this finding is significant since much prior research has failed to find that smoking alleviates stress induced negative affect in non-withdrawn smokers. Second, smoking a nicotine-containing cigarette (relative to smoking a denicotinized cigarette or holding a Pen) enhanced positive affective ratings of an appetitive stimulus. Third, diagnostic status appeared to affect stress reactivity. Thus, relative to non-diagnosed controls, smokers with comorbid PTSD and MDD reported greater affective distress to the stressor; such heightened stress reactivity could certainly affect the strength of smoking motivation. In addition, only amongst smokers with MDD did smoking exacerbate stress induced distress relative to a neutral control condition. Such findings encourage further research on mechanisms that may account for the heightened persistence and prevalence of smoking amongst those with PTSD and MDD. In addition, more research is needed to elucidate the nature of the smoking related conditioned stimuli that elicit associative effects on affective responding to stressors.
Supplementary Material
Scientific Summary.
This research sought to determine whether smoking influences affect by means other than withdrawal reduction among smokers with posttraumatic stress disorder (PTSD), major depressive disorder (MDD), and in controls with no psychiatric disorder. Results revealed that smoking modulated positive and negative affect outside the context of withdrawal relief; such effects may motivate smoking among those with psychiatric diagnoses, and among smokers in general.
Acknowledgments
This research was supported by grants K08DA02131 and K05CA139871 from NIH and by VA Merit Review Award 101CX00056 from the US Department of Veterans Affairs, and by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Science Research and Development.
Footnotes
Affective responses to the three positive and three negative mood inductions were stable across trials.
Typical negative memories included divorce, death of loved one, job loss; typical positive memories involved the birth of a child/grandchild, marriage, graduations, and special vacations.
There were no diagnostic group differences for negative memory ratings of distress or vividness, nor were there differences for positive memory ratings of happiness. However, participants with PTSD rated positive memories as significantly less vivid (M=4.47, SD=.61) than controls (M=4.83, SD=.25; p=.02) and participants with MDD rated the positive mood induction memories as less happy (M=4.54, SD=.53) than controls (M=4.79, SD=.22; p=.04).
One participant participated in only negative mood induction trials and did not complete any positive mood induction trials. Thus, there was one less participant included in the positive mood induction analyses.
The negative mood response in those with PTSD as a sole diagnosis was highly similar to the mood response in those with PTSD+MDD (see Supplement).
We report only effects that involved the diagnostic group effect (PTSD vs MDD) in these analyses in order to avoid repeating analyses of nicotine condition effects per se.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ameringer KJ, Leventhal AM. Applying the tripartite model of anxiety and depression to cigarette smoking: an integrative review. Nicotine & Tobacco Research. 2010;12(12):1183–1194. doi: 10.1093/ntr/ntq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Japuntich SJ, Hogle JM, McCarthy DE, Curtin JJ. Pharmocologic and behavioral withdrawal from addictive drugs. Current Directions in Psychological Science. 2006;15(5):232–236. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baschnagel JS, Hawk LW., Jr The effects of nicotine on the attentional modification of the acoustic startle response in nonsmokers. Psychopharmacology (Berl) 2008;198(1):93–101. doi: 10.1007/s00213-008-1094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Calhoun PS, Dennis MF, Wilson SM, Dedert EA. Predictors of lapse in first week of smoking abstinence in PTSD and non-PTSD smokers. Nicotine & Tobacco Research. 2013;15(6):1122–1129. doi: 10.1093/ntr/nts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Dennis MF, McClernon FJ, Mozley SL, Collie CF, Vrana SR. The effects of cigarette smoking on script-driven imagery in smokers with and without posttraumatic stress disorder. Addictive Behaviors. 2007;32(12):2900–2915. doi: 10.1016/j.addbeh.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Feldman ME, Barefoot JC, Fairbank JA, Helms MJ, Haney TL, … Davidson JR. Ambulatory cardiovascular activity in Vietnam combat veterans with and without posttraumatic stress disorder. Journal of Consulting and Clinical Psychology. 2000;68(2):269–276. doi: 10.1037//0022-006x.68.2.269. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Feldman ME, Vrana SR, Mozley SL, Erkanli A, Clancy CP, Rose JE. Immediate antecedents of cigarette smoking in smokers with and without posttraumatic stress disorder: a preliminary study. Exp Clin Psychopharmacol. 2005;13(3):219–228. doi: 10.1037/1064-1297.13.3.219. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Kirby AC, Feldman ME, Hertzberg MA, Moore SD, Crawford AL, … Fairbank JA. Prevalence and correlates of heavy smoking in Vietnam veterans with chronic posttraumatic stress disorder. Addictive Behaviors. 1997;22(5):637–647. doi: 10.1016/s0306-4603(96)00071-8. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Wiley MT, Miller SC, Dennis MF, Wilson SM, McClernon FJ, Calhoun PS. Ad lib smoking in post-traumatic stress disorder: an electronic diary study. Nicotine & Tobacco Research. 2008;10(7):1149–1157. doi: 10.1080/14622200802123302. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B. 1995;57:289–300. [Google Scholar]
- Blake DD, Weathers RW, Nagy LM, Kaloupek DG, Klauminzer G, Charney DS, Keane TM. A clinician rating scale for assessing current and lifetime PTSD: The CAPS-1. Behavior Therapist. 1990;13:187–188. [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229(3):453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Archives of General Psychiatry. 2003;60(3):289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- Breslau N, Johnson EO, Hiripi E, Kessler R. Nicotine dependence in the United States: prevalence, trends, and smoking persistence. Archives of General Psychiatry. 2001;58(9):810–816. doi: 10.1001/archpsyc.58.9.810. [DOI] [PubMed] [Google Scholar]
- Brodbeck J, Bachmann MS, Brown A, Znoj HJ. Effects of depressive symptoms on antecedents of lapses during a smoking cessation attempt: an ecological momentary assessment study. Addiction. 2014;109(8):1363–1370. doi: 10.1111/add.12563. [DOI] [PubMed] [Google Scholar]
- Bujarski S, Roche DJ, Sheets ES, Krull JL, Guzman I, Ray LA. Modeling naturalistic craving, withdrawal, and affect during early nicotine abstinence: A pilot ecological momentary assessment study. Experimental and Cliical Psychopharmacology. 2015;23(2):81–89. doi: 10.1037/a0038861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byllesby BM, Durham TA, Forbes D, Armour C, Elhai JD. An investigation of PTSD's core dimensions and relations with anxiety and depression. Psychological Trauma. 2016;8(2):214–217. doi: 10.1037/tra0000081. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebraska Symposium on Motivation. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun PS, Wagner HR, McClernon FJ, Lee S, Dennis MF, Vrana SR, Clancy CP, Collie CF, Johnson YC, Beckham JC. The effect of nicotine and trauma context on acoustic startle in smokers with and without posttraumatic stress disorder. Psychopharmacology (Berl) 2011;215(2):379–389. doi: 10.1007/s00213-010-2144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, … Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 2006;189(1):27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Perkins KA. Subjective and reinforcing effects of smoking during negative mood induction. Journal of Abnormal Psychology. 2005;114(1):153–164. doi: 10.1037/0021-843X.114.1.153. [DOI] [PubMed] [Google Scholar]
- Cook JW, McFall MM, Calhoun PS, Beckham JC. Posttraumatic stress disorder and smoking relapse: A theoretical model. Journal of Traumatic Stress. 2007;20(6):989–998. doi: 10.1002/jts.20275. [DOI] [PubMed] [Google Scholar]
- Cook JW, Piper ME, Leventhal AM, Schlam TR, Fiore MC, Baker TB. Anhedonia as a component of the tobacco withdrawal syndrome. Journal of Abnormal Psychology. 2015;124(1):215–225. doi: 10.1037/abn0000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology. 2007;192(1):87–95. doi: 10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- Cook J, Spring B, McChargue D, Doran N. Effects of anhedonia on days to relapse among smokers with a history of depression. Nicotine and Tobacco Research. 2010;12(9):978–982. doi: 10.1093/ntr/ntq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SR, Hodgins DC, el-Guebaly N, Campbell Influence of depression and gender on smoking expectancies and temptations in alcoholics in early recovery. Journal of Substance Abuse. 2001;13:443–458. doi: 10.1016/s0899-3289(01)00090-6. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, McCarthy DE, Piper ME, Baker TB. Implicit and explicit drug motivational processes: A model of boundary conditions. In: Weirs RW, Stacy AW, editors. Handbook of implicit cognition and addiction. Thousand Oaks, CA: Sage; 2006. pp. 233–250. [Google Scholar]
- Dedert EA, Calhoun PS, Harper LA, Dutton CE, McClernon FJ, Beckham JC. Smoking withdrawal in smokers with and without posttraumatic stress disorder. Nicotine & Tobacco Research. 2012;14(3):372–376. doi: 10.1093/ntr/ntr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, … Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169(1):68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102(2):324–334. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Druss BG, Zhao L, Von Esenwein S, Morrato EH, Marcus SC. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Medical Care. 2011;49(6):599–604. doi: 10.1097/MLR.0b013e31820bf86e. [DOI] [PubMed] [Google Scholar]
- Eissenberg T. Measuring the emergence of tobacco dependence: the contribution of negative reinforcement models. Addiction. 2004;99(Suppl 1):5–29. doi: 10.1111/j.1360-0443.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Elhai JD, Contractor AA, Tamburrino M, Fine TH, Cohen G, Shirley E, Chan PK, Liberzon I, Calabrese JR, Galea S. Structural relations between DSM-5 PTSD and major depression symptoms in military soldiers. Journal of Affective Disorders. 2015;175:373–378. doi: 10.1016/j.jad.2015.01.034. [DOI] [PubMed] [Google Scholar]
- Elhai JD, Grubaugh AL, Kashdan TB, Frueh BC. Empirical examination of a proposed refinement to DSM-IV posttraumatic stress disorder symptom criteria using the National Comorbidity Survey Replication data. Journal of Clinical Psychiatry. 2008;69(4):597–602. doi: 10.4088/jcp.v69n0411. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ. Smoking, traumatic event exposure, and post-traumatic stress: a critical review of the empirical literature. Clinical Psychology Review. 2007;27(1):14–45. doi: 10.1016/j.cpr.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, Juliano LM. Depression moderates smoking behavior in response to a sad mood induction. Psychology of Addictive Behaviors. 2009;23(3):546–551. doi: 10.1037/a0016529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DG, Robinson JH, Chamberlin CL, Spielberger CD. Effects of smoking/nicotine on anxiety, heart rate, and lateralization of EEG during a stressful movie. Psychophysiology. 1989;26(3):311–320. doi: 10.1111/j.1469-8986.1989.tb01924.x. [DOI] [PubMed] [Google Scholar]
- Ginzburg K, Ein-Dor T, Solomon Z. Comorbidity of posttraumatic stress disorder, anxiety and depression: a 20-year longitudinal study of war veterans. Journal of Affective Disorders. 2010;123(1–3):249–257. doi: 10.1016/j.jad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Gloria R, Angelos L, Schaefer HS, Davis JM, Majeskie M, Richmond BS, … Baker TB. An fMRI investigation of the impact of withdrawal on regional brain activity during nicotine anticipation. Psychophysiology. 2009;46:681–693. doi: 10.1111/j.1469-8986.2009.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootzeit J, Markon K. Factors of PTSD: differential specificity and external correlates. Clinical Psychology Review. 2011;31(6):993–1003. doi: 10.1016/j.cpr.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology (Berl) 2006;187(3):385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Herbert M, Foulds J, Fife-Schaw C. No effect of cigarette smoking on attention or mood in non-deprived smokers. Addiction. 2001;96(9):1349–1356. doi: 10.1046/j.1360-0443.2001.969134914.x. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Kaye JT, Curtin JJ. Nicotine withdrawal increases threat-induced anxiety but not fear: neuroadaptation in human addiction. Biological Psychiatry. 2010;68(8):719–725. doi: 10.1016/j.biopsych.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corporation. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corporation; 2013. [Google Scholar]
- Jonkman S, Risbrough VB, Geyer MA, Markou A. Spontaneous nicotine withdrawal potentiates the effects of stress in rats. Neuropsychopharmacology. 2008;33(9):2131–2138. doi: 10.1038/sj.npp.1301607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Stanton CH. Examining emotion regulation in depression: A review and future directions. Behaviour Research and Therapy. 2016 doi: 10.1016/j.brat.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Joseph AM, McFall M, Saxon AJ, Chow BK, Leskela J, Dieperink ME, … Beckham JC. Smoking intensity and severity of specific symptom clusters in posttraumatic stress disorder. Journal of Traumatic Stress. 2012;25(1):10–16. doi: 10.1002/jts.21670. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Effects of nicotine dose, instructional set, and outcome expectancies on the subjective effects of smoking in the presence of a stressor. Journal of Abnormal Psychology. 2002;111(1):88–97. doi: 10.1037//0021-843x.111.1.88. [DOI] [PubMed] [Google Scholar]
- Kassel JD. Smoking and attention: a review and reformulation of the stimulus-filter hypothesis. Clinical Psychology Review. 1997;17(5):451–478. doi: 10.1016/s0272-7358(97)00032-9. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Keselman HJ, Cribbie R, Holland B. Controlling the rate of Type I error over a large set of statistical tests. British Journal of Mathematical and Statistical Psychology. 2002;55:27–40. doi: 10.1348/000711002159680. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York, NY: Springer Publishing Company; 1984. [Google Scholar]
- Litz BT, Gray MJ. Emotional numbing in posttraumatic stress disorder: current and future research directions. Australian and New Zealand Journal of Psychiatry. 2002;36(2):198–204. doi: 10.1046/j.1440-1614.2002.01002.x. [DOI] [PubMed] [Google Scholar]
- McClave AK, Dube SR, Strine TW, Kroenke K, Caraballo RS, Mokdad AH. Associations between smoking cessation and anxiety and depression among U.S. adults. Addictive Behaviors. 2009;34(6–7):491–497. doi: 10.1016/j.addbeh.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100(4):569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Shalev AY, Pitman RK. Physiologic responses to loud tones in Vietnam veterans with posttraumatic stress disorder. Journal of Abnoral Psychology. 1995;104(1):75–82. doi: 10.1037//0021-843x.104.1.75. [DOI] [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, Herz LR. Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. Journal of Abnormal Psychology. 1993;102(1):152–159. doi: 10.1037//0021-843x.102.1.152. [DOI] [PubMed] [Google Scholar]
- Orsillo SM, Batten SV, Plumb JC, Luterek JA, Roessner BM. An experimental study of emotional responding in women with posttraumatic stress disorder related to interpersonal violence. Journal of Traumatic Stress. 2004;17(3):241–248. doi: 10.1023/B:JOTS.0000029267.61240.94. [DOI] [PubMed] [Google Scholar]
- Pang RD, Khoddam R, Guillot CR, Leventhal AM. Depression and anxiety symptoms moderate the relation between negative reinforcement smoking outcome expectancies and nicotine dependence. Journal of Studies on Alcohol and Drugs. 2014;75(5):775–780. doi: 10.15288/jsad.2014.75.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC, Kaye FJ. Daily uplifts, hassles, stresses and cognitive failures: in cigarette smokers, abstaining smokers, and non-smokers. Behavioural Pharmacology. 1999;10(6–7):639–646. doi: 10.1097/00008877-199911000-00010. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Ciccocioppo M, Conklin CA, Milanak ME, Grottenthaler A, Sayette MA. Mood influences on acute smoking responses are independent of nicotine intake and dose expectancy. Journal of Abnormal Psychology. 2008;117(1):79–93. doi: 10.1037/0021-843X.117.1.79. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology (Berl) 2013;228(3):479–486. doi: 10.1007/s00213-013-3054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Sensory reinforcement-enhancing effects of nicotine via smoking. Experimental and Clinical Psychopharmacology. 2014;22(6):511–516. doi: 10.1037/a0037823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Sex differences in acute relief of abstinence-induced withdrawal and negative affect due to nicotine content in cigarettes. Nicotine Tob Res. 2015;17(4):443–448. doi: 10.1093/ntr/ntu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Conklin CA, Sayette MA, Giedgowd GE. Acute negative affect relief from smoking depends on the affect situation and measure but not on nicotine. Biological Psychiatry. 2010;67(8):707–714. doi: 10.1016/j.biopsych.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Jao NC, Stratton E. Possible reinforcement enhancing effects of bupropion during initial smoking abstinence. Nicotine & Tobacco Research. 2013;15(6):1141–1145. doi: 10.1093/ntr/nts224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Curtin JJ. Tobacco withdrawal and negative affect: an analysis of initial emotional response intensity and voluntary emotion regulation. Journal of Abnormal Psychology. 2006;115(1):96–102. doi: 10.1037/0021-843X.115.1.96. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Archives of Geeraln Psychiatry. 1987;44(11):970–975. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- Richmond M, Spring B, Sommerfeld BK, MeChargue D. Rumination and cigarette smoking: a bad combination for depressive outcomes? Journal of Consulting and Clinical Psychology. 2001;69(5):836–840. doi: 10.1037//0022-006x.69.5.836. [DOI] [PubMed] [Google Scholar]
- Robinson JD, Lam CY, Carter BL, Minnix JA, Cui Y, Versace F, … Cinciripini PM. A multimodal approach to assessing the impact of nicotine dependence, nicotine abstinence, and craving on negative affect in smokers. Experimental and Clinical Psychopharmacology. 2011;19(1):40–52. doi: 10.1037/a0022114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow RL, Rubin DB. Contrasts and effect sizes in behavioral research: a correlational approach. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- Sayette MA. An appraisal-disruption model of alcohol's effects on stress responses in social drinkers. Psychological Bulletin. 1993;114(3):459–476. doi: 10.1037/0033-2909.114.3.459. [DOI] [PubMed] [Google Scholar]
- Schachter S. Pharmacological and psychological determinants of smoking. In: Thornton RE, editor. Smoking behaviour, physiological and psychological influences. Edinburgh: Churchill-Livingstone; 1978. [Google Scholar]
- Shahab L, McEwen A, West R. Acceptability and effectiveness for withdrawal symptom relief of a novel oral nicotine delivery device: a randomised crossover trial. Psychopharmacology. 2011;216(2):187–196. doi: 10.1007/s00213-011-2204-9. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64(2):366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Smithson M. Confidence Intervals. London: Sage Publications; 2003. [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). History, rationale, and description. Archives of General Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Spring B, Cook JW, Appelhans B, Maloney A, Richmond M, Vaughn J, … Hedeker D. Nicotine effects on affective response in depression-prone smokers. Psychopharmacology (Berl) 2008;196(3):461–471. doi: 10.1007/s00213-007-0977-7. [DOI] [PubMed] [Google Scholar]
- Steinberg ML, Williams JM, Li Y. Poor mental health and reduced decline in smoking prevalence. American Journal of Prevtive Medicine. 2015;49(3):362–369. doi: 10.1016/j.amepre.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong DR, Leventhal AM, Evatt DP, Haber S, Greenberg BD, Abrams D, Niaura R. Positive reactions to tobacco predict relapse after cessation. Journal of Abnormal Psychology. 2011;120(4):999–1005. doi: 10.1037/a0023666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick GG, Fidell LS. Experimental designs using ANOVA. Belmont, CA: Duxbury; 2007. [Google Scholar]
- Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology (Berl) 1984;84(3):405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Calhoun PS, McClernon FJ, Dennis MF, Lee ST, Beckham JC. Effects of smoking on the acoustic startle response and prepulse inhibition in smokers with and without posttraumatic stress disorder. Psychopharmacology (Berl) 2013;230(3):477–485. doi: 10.1007/s00213-013-3181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li Z, Shi Z, Zhang J, Zhang K, Liu Z, Elhai JD. Testing the dimensionality of posttraumatic stress responses in young Chinese adult earthquake survivors: further evidence for “dysphoric arousal” as a unique PTSD construct. Depression and Anxiety. 2011;28(12):1097–1104. doi: 10.1002/da.20823. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depression and Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Desai RA, McKee SA. Nicotine withdrawal in U.S. smokers with current mood, anxiety, alcohol use, and substance use disorders. Drug and Alcohol Dependence. 2010;108(1–2):7–12. doi: 10.1016/j.drugalcdep.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Mazure CM, Morlett A, McKee SA. Two decades of smoking cessation treatment research on smokers with depression: 1990–2010. Nicotine & Tobacco Research. 2013;15(6):1014–1031. doi: 10.1093/ntr/nts213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Jones C. Effects of mood manipulation on subjective and behavioural measures of cigarette craving. Behavioural Pharmacology. 1996;7(4):355–363. doi: 10.1097/00008877-199608000-00007. [DOI] [PubMed] [Google Scholar]
- Zinser MC, Baker TB, Sherman JE, Cannon DS. Relation between self-reported affect and drug urges and cravings in continuing and withdrawing smokers. Journal of Abnormal Psychology. 1992;101(4):617–629. doi: 10.1037//0021-843x.101.4.617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.