SLEEP
There are two independent literatures concerning the fundamental mechanisms of sleep regulation. One is based on neurophysiological methods: this literature has led to the identification of circuits involved in non-rapid eye movement sleep (NREMS) regulation such as corticothalamic projections, ventrolateral preoptic (VLPO), and median preoptic (MnPO) circuits (Lu et al., 2002; McGinty and Szymusiak, 2003). Satisfactory explanations of how these circuits impose sleep on the brain and how they keep track of past sleep—wake activity are not yet available. A second sleep-regulatory literature is based on biochemical methods. This work has its basis in the homeostatic nature of sleep and the 100-year-old finding that the transfer of cerebrospinal fluid from sleep-deprived, but not control, animals enhances sleep in the recipients (Obal and Krueger, 2003). Within the past 20 years several sleep-regulatory substances (SRSs) have been identified and extensively tested in that they have met all the criteria for SRSs (Jouvet, 1984; Borbely and Tobler, 1989: Krueger and Obal, 1994). This literature provides a mechanistic explanation for sleep homeostasis but has only begun to address the issues of the cellular mechanisms leading to sleep. This review discusses SRS that are linked to host defense; we focus on interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and interferons (IFNs). We also briefly discuss how sleep is part of the acute-phase response (APR) induced by viral challenge.
HUMORAL REGULATION OF SLEEP
The accumulation of SRSs in cerebrospinal fluid during prolonged wakefulness (W) provides very strong support of the hypothesis that sleep is regulated, in part, by humoral agents (Borbely and Tobler, 1989; Obal and Krueger, 2003). However, what it is about wakefulness that causes enhanced production of SRSs has not, until recently, been characterized. Sleep is posited to be linked to prior neuronal use via adenosine triphosphate (ATP) released during neurotransmission (Burnstock, 2007; Krueger et al., 2007). ATP, via purine type 2 receptors, in turn releases cytokines from glia (Hide et al., 2000; Solle et al., 2001; Suzuki et al., 2004; Ferrari et al., 2006). Many substances can affect sleep (Figure 15.1). However, only a handful of humoral agents are strongly implicated in sleep regulation. The list includes TNF-α, IL-1β, growth hormone-releasing hormone (GHRH), prostaglandin D2, and adenosine for NREMS and vasoactive intestinal peptide, nitric oxide (NO) (Kapás et al., 1994a, b) and prolactin (Roky et al., 1995) for rapid eye movement sleep (REMS) (Obal and Krueger, 2003). Substantial evidence impheating additional substances in sleep regulation is beginning to accumulate; these molecules include hypocretin (Kilduff and Peyron, 2000), oleamide (Boger et al., 1998), nerve growth factor (NGF) (Yamuy et al., 1995; Takahashi and Krueger, 1999), epidermal growth factor (Kushikata et al., 1998; Foltenyi et al., 2007), and brain-derived neurotrophic factor (BDNF) (Kushikata et al., 1999; Faraguna et al., 2008). It is important to recognize that those agents implicated in NREMS and REMS affect each other’s production and act in concert with each other to affect sleep (Figure 15.1) (Obal and Krueger, 2003). For instance, TNF-α induces IL-1β, NGF, prostaglandin, NO, adenosine, and growth hormone production.
Fig. 15.1.
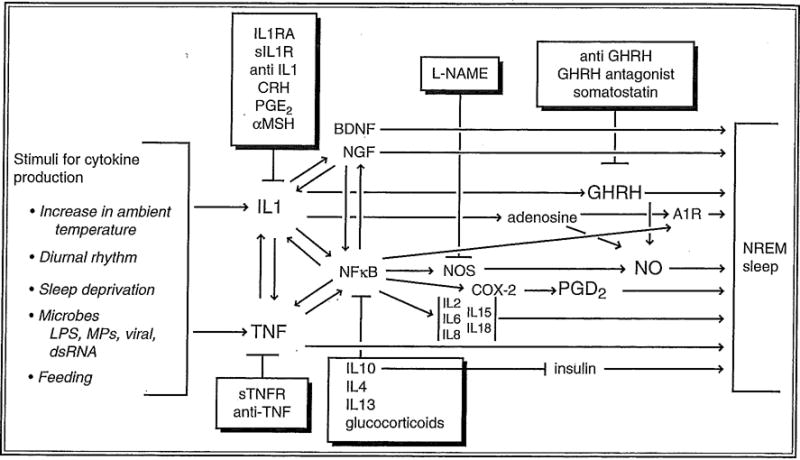
Molecular networks are involved in sleep regulation. Substances in boxes inhibit sleep and inhibit the production or actions of sleep-promoting substances illustrated via feedback mechanisms. Inhibition of one step does not completely block sleep, since parallel sleep-promoting pathways exist. These redundant pathways provide stability to sleep regulation. Our knowledge of the biochemical events involved in sleep regulation is more extensive than that illustrated. The molecular network shown here possesses many of the characteristics of biological networks and engineered systems (this topic is reviewed in several lead articles in Science 2003; 301:5641). Thus, the network is modular in that several proteins (cytokines) are working in “overlapping co-regulated groups” in this pathway. Second, the molecular network is robust in that removal of one of the components does not result in complete sleep loss. Third, the network operates as a recurring circuit element with multiple feedback loops affecting other pathways to the extent that similar networks involving many of the same substances and component network parts are used to regulate body temperature, inflammatory responses, the microcirculation, memory, and food intake, and these systems, to a limited degree, coregulate. Specificity for any one physiological process, such as sleep, results from multiple interacting molecular and cellular circuits, each possessing different, but similar to each other, reactivity. IL-1RA, IL1 receptor antagonist; sIL1R, soluble IL-1 receptor; anti-IL1; anti-IL1 antibodies; CRH, corticotrophin-releasing hormone; PGD2, prostaglandin D2; α-MSH, α-melanocyte-stimulating hormone; sTNFR, soluble TNF receptor; anti-TNF, anti-TNF antibodies; TGFβ, transforming growth factor β; IGF1, insulin-like growth factor; A1R, adenosine A1 receptor; COX2, cyclooxygenase 2; LPS, lipopolysaccharide; MPs, muramyl peptides, BDNF, brain-derived neurotrophic factor; NGF, nerve growth factor; L-NAME, N-nitro-L-arginine methyl ester; GHRH, growth hormone-releasing hormone; NO, nitric oxide; NOS, nitric oxide synthase; NF-κB, nuclear factor kappa B; NREM, nonrapid eye movement.
CYTOKINES IN SLEEP REGULATION
Detailed discussion of the involvement of IL-1β, TNF-α, and other cytokines in sleep regulation has been reviewed (Obal and Krueger, 2003). Briefly, injection of exogenous low doses of IL-1β or TNF-α enhances NREMS (Figure 15.2) (Krueger et al., 1984; Shoham et al, 1987). Conditions that enhance endogenous production of IL-1β or TNF-α, e.g., excessive food intake (Hansen et al., 1998) or infectious disease (Majde and Krueger, 2005), promote NREMS (Figure 15.1). Conversely, inhibition of endogenous IL-1β and TNF-α, using antibodies or endogenous inhibitors such as their soluble receptors, inhibits spontaneous sleep (Opp and Krueger, 1994). These inhibitors of IL-1β and TNF-α also inhibit sleep rebound after sleep deprivation. Brain levels of IL-1β mRNA (Mackiewicz et al., 1996; Taishi et al., 1997) and IL-1 (Lue et al., 1998) and plasma levels of IL-1β (Nguyen et al., 1998) vary with the sleep-wake cycle with highest levels correlating with high sleep propensity. Brain levels of TNF-α (Floyd and Krueger, 1997) and TNF-α mRNA (Bredow et al., 1997) also vary with sleep propensity in a similar fashion. Both IL-1β mRNA and TNF-α mRNA increase in the brain during sleep deprivation (Taishi et al., 1999). Microinjection of TNF-α (Kubota et al., 2002) into the basal forebrain/anterior hypothalamus (BF/AH) enhances NREMS while injection of the TNF soluble receptor into the same area inhibits sleep. IL-1β enhances the firing rate of BF/AH sleep-active neurons while it inhibits wake-active neurons (Alam et al., 2004). Some hypothalamic neurons receptive for GHRH are also receptive for IL-1β (De et al., 2002). These data suggest that this BF/AH NREMS regulatory network is responsive to IL-1β and TNF-α. The IL-1 type I receptor and the TNF 55 kD receptor are responsible for IL-1- and TNF-enhanced NREMS since mice lacking these receptors do not respond to IL-1 or TNF respectively (Fang et al., 1997, 1998). Both IL-1β and TNF-α, affect, or are affected by, several neurotransmittcr systems involved in the activational networks. For example, IL-1β or TNF injected into the locus coeruleus (De Sarro et al., 1997) enhances sleep. IL-1β injected into the dorsal raphe also promotes NREMS (Manfridi et al., 2003).
Fig. 15.2.
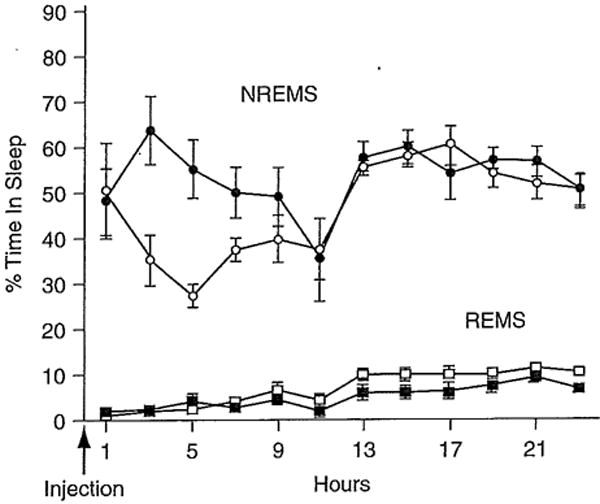
Murine tumor necrosis factor-α (TNF-α) enhances nonrapid eye movement sleep (NREMS) in mice. TNF-α, 3 μg, was injected intraperitoneally at time 0. Mice were kept on a 12-hour light-dark cycle with lights out at 0 hour. Circles are NREMS values ± SE; squares are rapid eye movement sleep (REMS) values ± SE. (Data are from Fang et al. (1997).)
Both IL-1β and TNF-α have been linked to a variety of clinical conditions involving sleep disorders (Obal and Krueger, 2003). For instance, clinical conditions associated with sleepiness correlate with higher blood levels of TNF. TNF-α is elevated in sleep apnea (Entzian et al., 1996; Vgontzas et al., 1997; Minoguchi et al., 2004), chronic fatigue (Moss et al., 1999), acquired immunodeficiency syndrome (AIDS) (Darko et al., 1995), chronic insomnia (Vgontzas et al., 2002), myocardial infarct (Francis et al., 2004), excessive daytime sleepiness (Vgontzas et al., 2003), postdialysis fatigue (Dreisbach et al., 1998), and pre-eclampsia patients (Majde and Krueger, 2002). Cancer patients receiving TNF report fatigue (Eskander et al., 1997). There are TNF-α-associated sleep disturbances in alcoholics (Irwin et al., 2004). There also may be a relationship between TNF and narcolepsy (Okun et al., 2004). Rheumatoid arthritis patients receiving the soluble TNF receptor (sTNFR) report reduced fatigue (Franklin, 1999). Sleep apnea patients treated with the sTNFR have reduced sleepiness (Vgontzas et al., 2004). If obstructive sleep apnea patients are surgically treated, their elevated TNF-α plasma levels return to normal (Kataoka et al., 2004). Systemic TNF, like IL-1, likely signals the brain via multiple mechanisms; one involves vagal afferents since vagotomy attenuates intraperitoneal TNF-α-induced NREMS responses (Kubota et al., 2001b). The effects of systemic bacterial products such as endotoxin may also involve TNF (Mullington et al., 2000). For instance, in humans, endotoxin doses that induce transient increases in sleep also induce concomitant increases in circulating TNF-α (Haack et al., 2001).
There is substantial evidence linking sleep deprivation-enhanced IL-1, and TNF, to symptoms associated with sleep deprivation, such as sensitivity to kindling (Yi et al., 2004) and pain stimuli (Honore et al., 2006; Kawasaki et al., 2008; Kundermann et al., 2008), cognitive (Gambino et al., 2007; Baune et al., 2008; Trompet et al., 2008), and memory (Dantzer, 2004; Banks and Dinges, 2007; Pickering and O’Connor, 2007) impairments, performance impairments (Banks and Dinges, 2007), depression (Anisman and Marali, 2003; Vollmer-Conna et al., 2004), sleepiness (Moldofsky, 1995; Tringall et al., 2000; Krueger et al., 2007), and fatigue (Anisman and Marali, 2003; Omdal and Gunnarsson, 2005; Carmichael et al., 2006), and to chronic sleep loss-associated pathologies such as metabolic syndrome (Hristova and Aloe, 2006; Jager et al., 2007; Larsen et al., 2007), chronic inflammation (Hu et al., 2003; Frey et al, 2007), and cardiovascular disease (Yndestad et al., 2007). These sleep deprivation-associated symptoms can be induced by injection of exogenous IL-1 and/or TNF, (Obal and Krueger, 2003), or in some cases blocked if these cytokines are inhibited (Opp and Krueger, 1991; Depino et al., 2004; Larsen et al., 2007). There is an inhibitor of IL-1 approved for clinical use, anakinra, the IL1-receptor antagonist (IL-1RA), a naturally occurring substance whose levels are altered by sleep loss (Frey et al., 2007) and that inhibits sleep (Takahashi et al., 1996). IL-1RA reduces fatigue (Omdal and Gunnarsson, 2005) and improves pancreatic beta cell function (Larsen et al, 2007).
Cytokines discovered by neurobiologists also promote sleep. For instance, NGF induces NREMS (Takahashi and Krueger, 1999) and REMS (Yamuy et al., 1995). Giant reticular cells and neurons in the mesencephalic trigeminal nucleus are immunoreactive for the p75 and trkA NGF receptors. These neurons may modify NGF-induced REMS (Yamuy et al., 2000). Further, if NGF-receptive basal forebrain cholinergic neurons are selectively removed using an immunotoxin conjugated to an anti-p75 NGF receptor, there is a transient loss of NREMS and a more permanent loss of REMS (Kapás et al, 1996). NGF, in cortical pyramidal cells, upregulates with sleep loss (Brandt et al., 2001). BDNF may also play a role in sleep regulation. BDNF promotes both NREMS and REMS in rabbits, although in rats, only NREMS increases after intracerebroventricular injection (Kushikata et al., 1999; Faraguna et al., 2008). BDNF mRNA upregulates during sleep deprivation and downregulates during sleep (Taishi et al., 2001).
The regulation of cytokines in the brain is complex and not very well understood. Nevertheless, some cytokine-associated substances, such as the IL-1RA and the TNF and IL-1 soluble receptors seem to act as endogenous antagonists, and indeed these substances inhibit spontaneous sleep (Obal and Krueger, 2003). Antisomnogenic cytokines act, in part, by inhibiting production of prosomnogenic cytokines and release of other substances implicated in sleep regulation, e.g., NO (Kasai et al., 1995).
Some of the prosomnogenic cytokines, such as IL-1β and TNF-α, promote inducible nitric oxide synthase (iNOS) activity (Figure 15.1) (Obal and Krueger, 2003). This observation prompted investigations into the role that NO may have in sleep (Kapás et al., 1994a, b; Kapás and Krueger, 1996). There are three types of NOSs: neuronal (nNOS), iNOS, and endothelial (eNOS). nNOS colocalizes with cholinergic neurons in the peduculopontine tegmental nuclei (PPT), the laterodorsal tegmental nucleus (LDT). These neurons project to the thalamus and basal forebrain as well as the medial pontine reticular formation (mPRF), which is crucial in REMS generation (Sakai et al., 2001). The cholinergic cells in the LDT/PPT have ascending projections to thalamic and basal forebrain nuclei that in turn project to the cortex (Jones, 2003). Microinjection of NO donors into the PPT enhances REMS while injection of NOS inhibitors into the, PPT reduces REMS (Datta et al., 1997). Similarly, inhibition of NOS in the mPRF (Leonard and Lydic, 1995, 1997) or dorsal raphe nuclei (Burlet et al., 1999) also results in reduced REMS. The role of NO in NREMS is not as well studied or clear. However, manipulation of NOS does affect NREMS (Kapás and Krueger, 1996). Specific effects depend on route of administration of drugs, time of day they are given, and the specific drugs used (Obal and Krueger, 2003). For example, mice lacking nNOS have less REMS whereas mice lacking iNOS have more REMS, but less NREMS (Chen et al., 2003).
NF-κB and c-Fos (AP-1) are transcription factors that are activated by IL-1β, TNF-α, and NGF (Obal and Krueger, 2003) (Figure 15.1). NF-κB activation promotes the production of several other substances implicated in NREMS regulation, including the adenosine A1 receptor, cyclooxygenase-2, the GHRH receptor as well as several of the prosomnogenic cytokines (Obal and Krueger, 2003). NF-κB is activated within the cortex during sleep deprivation (Chen et al., 1999). Adenosine also elicits NF-κB nuclear translocation in basal forebrain slices (Basheer et al., 2001) and that action is mediated by the A1 receptor. A cell-soluble peptide inhibitor of NF-κB nuclear translocation inhibits NREMS (Kubota et al., 2000).
INTERFERONS AND SLEEP
IFNs fall into two classes, type I and type II (IFN-γ). Type I IFNs, particularly IFN-αs, are commonly associated with viral infections and their primary function is thought to be blockade of viral replication. Type I IFNs are quite distinct structurally and functionally from IFN-γ, as they bind to different receptors and use distinct (but overlapping) signal transduction pathways. Type I IFNs were the first cytokines to be discovered (in 1957), purified, cloned, commercialized, and used clinically. Despite this long history, their physiology and pathophysiology remain somewhat mysterious. This is in part due to the research focus on then-antiviral action, and also to the lack of specificity of the assay used to detect them, i.e., the antiviral activity assay also detects cytokines such as TNF-α that induce IFN-β (Jacobsen et al., 1989). Only recently have specific immunoassays become available for IFN-αs (Hayden et al., 1998).
IFN-α (Hori et al., 1998) and type I IFN receptors (Krueger and Majde, 1994) are found in the brain, and IFN-α and IFN-γ both cross the blood–brain barrier (Pan et al., 1997). Thus circulating IFNs can act directly on the brain, and central nervous system responses such as fever, fatigue, and somnolence are consistently seen with the pharmacological doses commonly employed in the clinic (Smedley et al., 1983). Several studies demonstrate that different subtypes of IFN-α are somnogenic and enhance NREMS (Majde and Krueger, 2005). As is the case with antiviral activity, the somnogenic actions of IFNs are species-specific (Kimura et al., 1994); this specificity is determined by receptor-binding affinities (Uzé et al., 1992). IFN-β has no demonstrable somnogenic activity (De Sarro et al., 1990; Kimura et al., 1994), possibly because it does not circulate (Billiau, 1981) or because it associates with the type I receptor differently than IFN-α (Lewerenz et al., 1998). Mice deficient in a functional type I IFN receptor (IENRI knockouts) have a 30% reduction in spontaneous REMS (Bohnet et al., 2004).
The role of IFN-γ in sleep has received little study. Clinical trials with pharmacological doses of IFN-γ generally report fevers and the “flu syndrome,” and occasionally describe profound somnolence (Sriskandan et al., 1986). IFN-γ is also somnogenic in rabbits, but its effect appears to be due to its interaction with TNF-α (Kubota et al., 2001c). Because IFN-γ is a potent priming agent for cytokine induction, especially IL-12 and somnogenic IL-18 (Kubota et al., 2001a), regulates tryptophan metabolism and thus serotonin metabolism (Werner-Felmayer et al., 1989), stimulates prolactin release (Walton and Cronin, 1990), potentiates the toxicity of double-stranded (ds)RNA (Chapekar and Glazer, 1986) and potentiates NO induction by TNF-α (Zhang et al., 1994), it would seem likely that IFN-γ has an important role in sleep regulation during viral infections. However, analysis of sleep responses to low-dose influenza virus in IFN-γ knockouts did not reveal a substantial role for this cytokine in infection-altered sleep (Toth and Hughes, 2004).
ALTERED SLEEP AS AN ACUTE-PHASE RESPONSE: MEDIATORS AND MECHANISMS
The APR is a complex array of physiological changes that occur within a few days following an acute infection or other systemic inflammatory challenges. The most apparent components of the APR are the behavioral changes, which include body temperature changes, anorexia, immobility, sleepiness, excess sleep, and a feeling of being sick or toxic (malaise). It is well established in bacterial infections that proinflammatory cytokines are largely responsible for these symptoms. Excess NREMS, increased electroencephalogram (EEG) delta-frequency brain waves and often reduced REMS accompany systemic inflammation. SRSs that induce sleep alterations also can induce body temperature changes as well as reduced locomotor activity, though by different mechanisms. The APR is generally considered adaptive (Hart, 1988), particularly the fever response (Kluger et al., 1996). Physiologically regulated hyperthermia enhances a number of immunological functions while inhibiting the replication of heat-sensitive microorganisms (Mackowiak, 1981).
One reason fever has remained the hallmark of systemic inflammation is that it is easy to measure. Excess sleep, being more difficult to measure, has received much less attention, though changes in sleep during disease were noted by Aristotle (Pollmacher et al., 1995). The importance of sleep for health and recovery from disease has been recognized intuitively if not scientifically; few physicians fail to recommend to their infected patients that they should get plenty of rest. However, whether such rest/sleep truly has an adaptive value remains unknown. There are close ties between regulation of the sleep response to diverse microbial products and cytokines (Majde and Krueger, 2002; Krueger and Majde, 2005). A picture is emerging that suggests that sleep, like fever, is a stereotypic response to inflammation that may represent a basic host defense mechanism.
Clinicians have long recognized that it is difficult to distinguish between the APR symptoms induced by bacterial and viral diseases on clinical grounds. In the last 20 years extensive evidence has accumulated that the APR associated with bacterial infections is mediated by proinflammatory cytokines (Krueger and Majde, 2005) released by infected target tissues or invading inflammatory cells. These cytokines activate not only the physiological changes such as fever and sleep, but also the biochemical markers characteristic of the APR, by acting upon the liver, bone marrow, and brain (Steel and Whitehead, 1994). While the mediators of the viral APR are poorly defined, most of the same cytokines induced by bacterial infections are also induced by viruses, along with substantial amounts of type I IFNs (Hayden et al., 1998; Gendrel et al., 1999; Majde, 2000). It is highly likely that these virus-associated cytokines also mediate the viral APR, though minimal direct evidence is available (Kozak et al., 1995, 1997; Kurokawa et al., 1996). Further, pharmacological levels of the IFNs, which are expressed during viral infections; can induce an APR in the absence of other cytokines (Quesada et al., 1986), and can also alter the expression of proinflammatory cytokines known to be essential for the bacterial APR (Reznikov et al., 1998; Begni et al., 2005).
Whether the changes in sleep associated with infectious disease aid the host’s recovery remains to be determined. However, chronic loss of sleep in rats leads to septicemia (Everson and Toth, 2000). Further, sleep loss affects a variety of immune parameters, e.g., the ability of leukocytes to produce IFN (Krueger and Majde, 1994). The strongest evidence that sleep is protective in infections is from observations in rabbits infected experimentally with Staphylococcus aureus; infected rabbits that exhibit reduced NREMS either die or express more severe clinical symptoms than do infected rabbits that exhibit more NREMS in response to infection (Toth et al., 1993). Apart from such correlational studies, the only approach we currently have to assess the role of sleep in infections is to sleep-deprive animals that are subsequently infected and then determine disease outcomes. There are a number of methodological issues associated with such studies, not the least of which is the stress associated with sleep loss and its impact on immune function (Dunn, 2002). Several laboratories have specifically examined the impact of sleep deprivation on influenza, with variable outcomes that appear to depend on subtle changes in experimental parameters. Although it is likely that infection-altered sleep promotes recovery from infection, direct evidence is lacking and the mechanism is unknown.
While toxic mechanisms involved in both triggering and downregulating the bacterial APR have been intensively investigated (Majde and Krueger, 2002), viral toxicity mechanisms have received little attention since the 1940s. Indirect evidence, using both the synthetic dsRNA pI:C and viral dsRNA, supports a major role for viral dsRNA in triggering the viral APR, including sleep responses (Majde, 2000).
Brain-signaling mechanisms underlying the viral APR
From the above discussion it is apparent that many cytokines, hormones, and other inflammatory regulators may play a role in sleep and temperature alterations induced by microbes. Cytokines are posited to activate the central nervous system-regulated APRs via several routes (Dunn, 2002; Banks, 2005; Romanovsky et al., 2005): saturable transendothelial translocation via specific endothelial transporters (Banks, 2005); penetration via circumventricular organs lacking a substantial blood—brain barrier (Banks, 2004); signal transduction via sensory nerves, specifically the vagus (Kubota et al., 2001b; Romanovsky et al., 2005); induction of prostaglandin production in brain endothelial cells (Dunn, 2002; Romanovsky et al., 2005); and diffusion through extracellular spaces (Banks, 2004). To date, the relative contributions of these various mechanisms to signaling the central nervous system from the periphery during infections are unknown.
A THEORY OF THE BRAIN ORGANIZATION OF SLEEP: CYTOKINE INVOLVEMENT IN “LOCAL” SLEEP
Sleep is an unusual physiological process. Until recently (Rector et al., 2005) we did not know exactly what slept and we still do not know with experimental certainty why we sleep. There are several road blocks to understanding whether sleep helps host defense. Regardless, new evidence from several laboratories now suggests that cytokines play an essential role in determining the functional state of cortical assemblies. A theory of brain organization of sleep posits that, as synapses and circuits are used, ATP is released and it in turn induces SRS release from glia (Krueger et al, 2007). The released cytokines, including neurotrophins, are then responsible for synaptic sculpturing. In an autocrine fashion, these activity-dependent SRSs alter synaptic efficacy via nuclear transcription events and translation mechanisms targeted to the specific synapses that were activated. For example, TNF enhances α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor and the adenosine Al receptor; excess expression of either one would change cell sensitivity to glutamate and adenosine respectively. SRSs also act in a paracrine fashion to affect the electrical properties of nearby neurons such that a given input results in a different output (Alam et al., 2004). Within a neuronal assembly, the SRS-induced altered input–output relationships can, by definition, be considered a state shift. It is posited that sleep-regulatory networks modulate and coordinate neuronal assembly state and thereby produce sleepiness and sleep at the whole-organism level. Within a population of neuronal assemblies, as wakefulness becomes prolonged, the fraction of neuronal assemblies into the “sleep” mode would increase. At some point a predicted emergent property of the system (brain) would be a system-wide state shift (Roy et al., 2008). This emergent property would be associated with unconsciousness because a large fraction of the neuronal groups would be in a state where environmental input is divorced from a functional output. Thus, sleep-associated unconsciousness is needed, because output activity would be out of phase with environmental input. Further, it is the consequence of the process itself (Krueger and Obal, 1993, 2003).
There are many ramifications of this theory of brain organization of sleep. Some of the important ones and supporting evidence as it is related to cytokines are:
SRS levels are dependent on prior neural activity and sleep history.
SRSs act locally to affect a sleep-regulatory biochemical network.
Sleep intensity of one part of the brain can be more intense than other parts.
Changes in SRS levels locally within the cortex will activate neural pathways.
Sleep is a fundamental property of neural assemblies.
SRS levels are dependent on prior neural activity and sleep history
Activity-dependent expression of NGF and BDNF by neurons is well known (Brandt et al., 2001). Cellular electrical activity alters the synthesis and actions of these regulatory molecules and, in turn, they directly alter electrical properties of cells containing their receptors and alter the expression of many molecules necessary for synaptic efficacy and plasticity. These mechanisms are posited to be responsible for Hebbian synaptic regulation and collectively form the basis for the neurotrophin hypothesis (Schinder and Poo, 2000). The synthesis of TNF-α (Turrin and Rivest, 2004) and IL-1β (Plata-Salaman et al., 2000; Rizzi et al., 2003) is also enhanced by neural activity. Although the actions of these substances are usually not discussed within the context of Hebbian mechanisms, there are data suggesting TNF-α could influence neuronal connectivity via its actions on AMPA receptors. Thus, TNF-α enhances AMPA receptor expression and cytosolic calcium levels (De et al., 2003). These actions of TNF-α appear to be physiological because an inhibitor of TNF-α inhibits AMPA-induced postsynaptic potentials (Beattie et al, 2002) and AMPA-induced changes in cytosolic Ca2+ (De et al., 2003). AMPA receptors play a key role in EEG synchronization (Bazhenov et al., 2002) and synaptic plasticity. For example, TNF-α plays a role in synaptic scaling (Stellwagen and Melenka, 2006). Further, afferent stimulation of somatosensory cortex neurons is associated with enhanced TNF-α expression (Churchill et al., 2008).
SRSs act locally to affect a sleep-regulatory biochemical network
Unilateral application of TNF-α (Yoshida et al., 2004), IL-1β (Yasuda et al., 2005b), GHRH (Szentirmai et al., 2007), or BDNF (Faraguna et al., 2008) on to the surface of the somatosensory cortex induces unilateral dose-dependent and state-dependent increases in EEG delta wave power. Conversely, the soluble TNF receptor or the soluble IL-1 receptor unilaterally reduces EEG power during the NREMS occurring after sleep deprivation. Associated with the changes in the TNF- or IL1-altered EEG power are enhancements of Fos-IR and IL-1-immunoreactivity unilaterally in the somatosensory cortex and reticular thalamus.
Sleep intensity of one part of the brain can be more intense than other parts
This was the first prediction of the original theory (Krueger and Obal, 1993) that was experimentally tested. Kattler et al. (1994) showed that, using right-hand vibration stimulation, the amplitude of EEG slow waves (indicative of the intensity of sleep) during the first subsequent sleep episode was higher on the contralateral side somatosensory cortex than on the ipsilateral side (the contralateral side receives the input from the stimulated hand). Subsequently similar results were obtained from rats (Vyazovskiy et al., 2000) and mice (Vyazovskiy et al., 2004). Further, Huber et al. (2004) showed that EEG slow-wave power was greater over cortical areas during sleep that were “used” in a prior learning paradigm. Those results confirmed the work of Maquet et al. (2003) and Ferrara et al. (2002), using brain-imaging techniques, concluded that the brain areas activated during sleep were those most active during prior wakefulness. Natural variation in spontaneous activity is also correlated with EEG delta power. Thus, EEG delta power from the visual cortex is relatively higher during daylight hours than that from the somatosensory cortex of rats. Conversely, at night when the rats are using their whiskers for location, EEG delta power is relatively higher in the somatosensory cortex than in the visual cortex (Yasuda et al., 2005a).
Changes in SRS levels locally within the cortex will activate neural pathways
Unilateral injection of either TNF-α (Churchill et al., 2005) or IL-1β on to the surface of the somatosensory cortex activates a pathway unilaterally, as determined by Fos expression, that includes corticoreticular thalamic projections as well as anterior hypothalamic neurons. These circuits are known to be involved in sleep regulation. The results suggest a pathway by which information concerning the state of cortical columns is conveyed to the sleep-regulatory circuits. Such circuits could indeed provide homeostatic input to the hypothalamic “sleep switches.”
Sleep is a fundamental property of neural assemblies
This prediction, although made in 1993 (Krueger and Obal, 1993), was not directly demonstrated until 2005 (Rector et al., 2005). Using either auditory or whisker stimulation to induce cortical evoked response potentials, somatosensory and auditory cortical columns were shown to oscillate between two functional states. During the functional state that correlated with sleep, the amplitude of the evoked response potential is higher than during the wake-like state. Further, the probability of a cortical column being in the sleep-like state was dependent upon prior time in the wake-like state. Finally, in a preliminary study using a conditioning paradigm, the error rate of a learned licking response induced by whisker stimulation is greater if the cortical column receiving the input from the stimulated whisker is in the sleep-like state (Walker et al., 2005). Finally, microinjection of TNF-α on to cortical columns induces the sleep-like functional state (Churchill et al., 2008).
Collectively, the theory and supporting data suggest that sleep begins as a local process fundamental to cortical assemblies. Cytokines play a role in functional state determination and such, states are neuron activity-dependent. Changes in sleep associated with the APR are likely driven by inflammatory mediators such as cytokines, adenosine, NO, and prostaglandins since these molecules are also implicated in physiological sleep regulation. The pathological response likely reflects an amplified physiological sleep mechanism.
Acknowledgments
This work was supported in part by the National Institutes of Health, grant numbers NS25378, NS31453, and HD36520.
References
- Alam MN, McGinty D, Bashir T, et al. Interleukin-1beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: role in sleep regulation. Eur J Neurosci. 2004;20:207–216. doi: 10.1111/j.1460-9568.2004.03469.x. [DOI] [PubMed] [Google Scholar]
- Anisman H, Marali Z. Cytokines, stress and depressive illness: brain–immune interactions. Ann Med. 2003;35:2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- Banks WA. Are the extracellular pathways a conduit for the delivery of therapeutics to the brain? Curr Pharm Des. 2004;10:1365–1370. doi: 10.2174/1381612043384862. [DOI] [PubMed] [Google Scholar]
- Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des. 2005;11:973–984. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- Basheer R, Rainnie DG, Porkka-Heiskanen T, et al. Adenosine, prolonged wakefulness, and A1-activated NF-κB DNA binding in the basal forebrain of the rat. Neuroscience. 2001;104:731–739. doi: 10.1016/s0306-4522(01)00111-7. [DOI] [PubMed] [Google Scholar]
- Baune BT, Ponath G, Rothermundt M, et al. Association between genetic variants of IL-1beta, IL-6 and TNF-alpha cytokines and cognitive performance in the elderly general population of the MEMO-study. Psychoneuroendocrinology. 2008;33:68–76. doi: 10.1016/j.psyneuen.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Bazhenov M, Timofeev I, Steriade M, et al. Model of thalamocortical slow-wave sleep oscillations and transitions to activated states. J Neurosci. 2002;22:8691–8704. doi: 10.1523/JNEUROSCI.22-19-08691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, et al. Control of synaptic strength by glial TNF alpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Begni B, Amadori M, Ritelli M, et al. Effects of IFN-α on the inflammatory response of swine leukocytes to bacterial endotoxin. J Interferon Cytokine Res. 2005;25:202–208. doi: 10.1089/jir.2005.25.202. [DOI] [PubMed] [Google Scholar]
- Billiau A. Pharmacokinetic and pharmacological aspects of interferon therapy in man. Acta Microbiol Acad Sci Hung. 1981;28:257–262. [PubMed] [Google Scholar]
- Boger DL, Henriksen SJ, Cravatt BF. Oleamide: an endogenous sleep-inducing lipid and prototypical member of a new class of biological signaling molecules. Curr Pharm Des. 1998;4:303–314. [PubMed] [Google Scholar]
- Bohnet SG, Traynor TR, Majde JA, et al. Mice deficient in the interferon type I receptor have reduced REM sleep and altered hypothalamic hypocretin, prolactin and 2′,5′-oligoadenylate synthase expression. Brain Res. 2004;1027:117–125. doi: 10.1016/j.brainres.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Borbely AA, Tobler I. Endogenous sleep-promoting substances and sleep regulation. Physiol Rev. 1989;69:605–670. doi: 10.1152/physrev.1989.69.2.605. [DOI] [PubMed] [Google Scholar]
- Brandt J, Churchill L, Guan Z, et al. Sleep deprivation but not a whisker trim increases nerve growth factor within barrel cortical neurons. Brain Res. 2001;898:105–112. doi: 10.1016/s0006-8993(01)02149-7. [DOI] [PubMed] [Google Scholar]
- Bredow S, Taishi P, Guha-Thakurta N, et al. Diurnal variations of tumor necrosis factor-alpha mRNA and alpha-tubulin mRNA in rat brain. Neuroimmunomodulation. 1997;4:84–90. doi: 10.1159/000097325. [DOI] [PubMed] [Google Scholar]
- Burlet S, Leger L, Cespuglio R. Nitric oxide and sleep in the rat: a puzzling relationship. Neuroscience. 1999;92:627–639. doi: 10.1016/s0306-4522(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Carmichael MD, Davis JM, Murphy EA, et al. Role of brain IL-1beta on fatigue after exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1344–R1348. doi: 10.1152/ajpregu.00141.2006. [DOI] [PubMed] [Google Scholar]
- Chapekar MS, Glazer RI. Potentiation of the cytocidal effect of human immune interferon by different synthetic double-stranded RNAs in the refractory human colon carcinoma cell line BE. Cancer Res. 1986;46:1698–1702. [PubMed] [Google Scholar]
- Chen Z, Gardi J, Kushikata T, et al. Nuclear factor kappa B-like activity increases in murine cerebral cortex after sleep deprivation. Am J Physiol. 1999;45:R1812–R1818. doi: 10.1152/ajpregu.1999.276.6.R1812. [DOI] [PubMed] [Google Scholar]
- Chen L, Majde JA, Krueger JM. Spontaneous sleep in mice with targeted disruptions of neuronal or inducible nitric oxide synthase genes. Brain Res. 2003;973:214–222. doi: 10.1016/s0006-8993(03)02484-3. [DOI] [PubMed] [Google Scholar]
- Churchill L, Yasuda K, Yasuda T, et al. Unilateral cortical application of tumor necrosis factor alpha induces asymmetry in Fos- and interleukin-1beta-immunoreactive cells within the corticothalamic projection. Brain Res. 2005;1055:15–24. doi: 10.1016/j.brainres.2005.06.052. [DOI] [PubMed] [Google Scholar]
- Churchill L, Rector DM, Yasuda K, et al. Tumor necrosis factor α: activity dependent expression and promotion of cortical column sleep in rats. Neuroscience. 2008;156:71–80. doi: 10.1016/j.neuroscience.2008.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Darko DF, Miller JC, Gallen C, et al. Sleep electroencephalogram delta-frequency amplitude, night plasma levels of tumor necrosis factor alpha, and human immunodeficiency virus infection. PNAS. 1995;92:12080–12084. doi: 10.1073/pnas.92.26.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Patterson EH, Siwek DF. Endogenous and exogenous nitric oxide in the pedunculopontine tegmentum induces sleep. Synapse. 1997;27:69–78. doi: 10.1002/(SICI)1098-2396(199709)27:1<69::AID-SYN7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- De A, Churchill L, Obal F, et al. GHRH and IL1beta increase cytoplasmic Ca2+ levels in cultured hypothalamic GABAergic neurons. Brain Res. 2002;949:209–212. doi: 10.1016/s0006-8993(02)03157-8. [DOI] [PubMed] [Google Scholar]
- De A, Krueger JM, Simasko SM. Tumor necrosis factor alpha increases cytosolic calcium response AMPA and KCl in primary cultures of rat hippocampal neurons. Brain Res. 2003;981:133–142. doi: 10.1016/s0006-8993(03)02997-4. [DOI] [PubMed] [Google Scholar]
- Depino AM, Alonso M, Ferrari C, et al. Learning modulation by endogenous hippocampal IL-1: blockade of endogenous IL-1 facilitates memory formation. Hippocampus. 2004;14:526–535. doi: 10.1002/hipo.10164. [DOI] [PubMed] [Google Scholar]
- De Sarro GP, Masuda Y, Ascioti C, et al. Behavioral and ECoG spectrum changes by intracerebral infusion of interferons and interleukin 2 in rats are antagonized by naloxone. Neuropharmacology. 1990;29:167–179. doi: 10.1016/0028-3908(90)90057-x. [DOI] [PubMed] [Google Scholar]
- De Sarro G, Gareri P, Sinopoli VA, et al. Comparative, behavioural and electrocortical effects of tumor necrosis factor-alpha and interleukin-1 microinjected into the locus coeruleus of rat. Life Sci. 1997;60:555–564. doi: 10.1016/s0024-3205(96)00692-3. [DOI] [PubMed] [Google Scholar]
- Dreisbach AW, Hendrickson T, Beezhold D, et al. Elevated levels of tumor necrosis factor alpha in postdialysis fatigue. Int J Artif Organs. 1998;21:83–86. [PubMed] [Google Scholar]
- Dunn AJ. Mechanisms by which cytokines signal the brain. Int Rev Neurobiol. 2002;52:43–65. doi: 10.1016/s0074-7742(02)52005-5. [DOI] [PubMed] [Google Scholar]
- Entzian P, Linnemann K, Schlaak M, et al. Obstructive sleep apnea syndrome and circadian rhythms of hormones and cytokines. Am J Respir Crit Care Med. 1996;153:1080–1086. doi: 10.1164/ajrccm.153.3.8630548. [DOI] [PubMed] [Google Scholar]
- Eskander ED, Harvey HA, Givant E, et al. Phase I study combining tumor necrosis factor with interferonalpha and interleukin-2. Am J Clin Oncol. 1997;20:511–514. doi: 10.1097/00000421-199710000-00016. [DOI] [PubMed] [Google Scholar]
- Everson CA, Toth LA. Systemic bacterial invasion induced by sleep deprivation. Am J Physiol Regul Integr Compar Physiol. 2000;278:R905–R916. doi: 10.1152/ajpregu.2000.278.4.R905. [DOI] [PubMed] [Google Scholar]
- Fang J, Wang Y, Krueger JM. Mice lacking the TNF 55 kD receptor fail to sleep more after TNF alpha treatment. J Neurosci. 1997;17:5949–5955. doi: 10.1523/JNEUROSCI.17-15-05949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Wang Y, Krueger JM. The effects of interleukin-1 beta on sleep are mediated by the type I receptor. Am J Physiol. 1998;274:R655–R660. doi: 10.1152/ajpregu.1998.274.3.R655. [DOI] [PubMed] [Google Scholar]
- Faraguna U, Vyazovskiy VV, Nelson AB, et al. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28:4088–4095. doi: 10.1523/JNEUROSCI.5510-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara M, De Gennaro L, Curcio G, et al. Regional differences of the human sleep electroencephalogram in response to selective slow-wave sleep deprivation. Cereb Cortex. 2002;12:737–748. doi: 10.1093/cercor/12.7.737. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Krueger JM. Diurnal variations of TNF alpha in the rat brain. Neuroreport. 1997;8:915–918. doi: 10.1097/00001756-199703030-00020. [DOI] [PubMed] [Google Scholar]
- Foltenyi K, Greenspan RJ, Newport JW. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat Neurosci. 2007;10:1160–1167. doi: 10.1038/nn1957. [DOI] [PubMed] [Google Scholar]
- Francis J, Chu Y, Johnson AK, et al. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol. 2004;286(6):H2264–H2271. doi: 10.1152/ajpheart.01072.2003. [DOI] [PubMed] [Google Scholar]
- Franklin CM. Clinical experience with soluble TNF p75 receptor in rheumatoid arthritis. Semin Arthritis Rheum. 1999;29:171–181. doi: 10.1016/s0049-0172(99)80028-6. [DOI] [PubMed] [Google Scholar]
- Frey DJ, Fleshner M, Wright KP., Jr The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–1057. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Gambino F, Pavlowsky A, Béglé A, et al. IL1-receptor accessory protein-like 1 (IL1RAPL1), a protein involved in cognitive functions, regulates N-type Ca2+-channel and neurite elongation. Proc Natl Acad Sci U S A. 2007;104:9063–9068. doi: 10.1073/pnas.0701133104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrel D, Raymond J, Coste J, et al. Comparison of procalcitonin with C-reactive protein, interleukin 6 and interferon-alpha for differentiation of bacterial vs. viral infections. Pediatr Infect Dis J. 1999;18:875–881. doi: 10.1097/00006454-199910000-00008. [DOI] [PubMed] [Google Scholar]
- Haack M, Schuld A, Kraus T, et al. Effects of sleep on endotoxin-induced host responses in healthy men. Psychosom Med. 2001;63:568–578. doi: 10.1097/00006842-200107000-00008. [DOI] [PubMed] [Google Scholar]
- Hansen MK, Taishi P, Chen Z, et al. Cafeteria-feeding induces interleukin-1 beta mRNA expression in rat liver and brain. Am J Physiol. 1998;43:R1734–R1739. doi: 10.1152/ajpregu.1998.274.6.R1734. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hayden FG, Fritz RS, Lobo MC, et al. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hide I, Tanaka M, Inoue A, et al. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem. 2000;75:965–972. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- Honore P, Donnelly-Roberts D, Namovic MT, et al. A-740003 [N-(1-{[(Cyanoimino)(5-quinolinylamino) methyl] amin)-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl) acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Therap. 2006;319:1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- Hori T, Katafuchi T, Take S, et al. Neuroimmunomodulatory actions of hypothalamic interferon-α. Neuroimmunomodulation. 1998;5:172–177. doi: 10.1159/000026334. [DOI] [PubMed] [Google Scholar]
- Hristova M, Aloe L. Metabolic syndrome-neurotrophic hypothesis. Med Hypotheses. 2006;66:545–549. doi: 10.1016/j.mehy.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, et al. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Hu J, Chen Z, Gorczynski CP, et al. Sleep-deprived mice show altered cytokine production manifest by perturbations in serum IL-1ra, TNFa, and IL-6 levels. Brain Behav Immun. 2003;17:498–504. doi: 10.1016/j.bbi.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Irwin M, Rinetti G, Redwine L, et al. Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain Behav Immun. 2004;18:349–360. doi: 10.1016/j.bbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Jacobsen H, Mestan J, Sibylle M, et al. Beta interferon subtype I induction by tumor necrosis factor. Mol Cell Biol. 1989;9:3037–3042. doi: 10.1128/mcb.9.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager J, Grémeaux T, Cormont M, et al. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148:241–251. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. Arousal systems. Front Biosci. 2003;8:s438–s451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- Jouvet M. Neuromediateurs et facteurs hypnogenes. Rev Neurol (Paris) 1984;140:389–400. [PubMed] [Google Scholar]
- Kapás L, Krueger JM. Nitric oxide donors SIN-1 and SNAP promote nonrapid-eye-movement sleep in rats. Brain Res Bull. 1996;41:293–298. doi: 10.1016/s0361-9230(96)00227-4. [DOI] [PubMed] [Google Scholar]
- Kapás L, Fang J, Krueger JM. Inhibition of nitric oxide synthesis inhibits rat sleep. Brain Res. 1994a;664:189–196. doi: 10.1016/0006-8993(94)91969-0. [DOI] [PubMed] [Google Scholar]
- Kapás L, Shibata M, Kimura M, et al. Inhibition of nitric oxide synthesis suppresses sleep in rabbits. Am J Physiol. 1994b;266:R151–R157. doi: 10.1152/ajpregu.1994.266.1.R151. [DOI] [PubMed] [Google Scholar]
- Kapás L, Obál F, Book AA, et al. The effects of immunolesions of nerve growth factor-receptive neurons by 192 IgG-saporin on sleep. Brain Res. 1996;712:53–59. doi: 10.1016/0006-8993(95)01431-4. [DOI] [PubMed] [Google Scholar]
- Kasai K, Hattori Y, Nakanishi N, et al. Regulation of inducible nitric oxide production by cytokines in human thyrocytes in culture. Endocrinology. 1995;136:4261–4270. doi: 10.1210/endo.136.10.7545102. [DOI] [PubMed] [Google Scholar]
- Kataoka T, Enomoto F, Kim R, et al. The effect of surgical treatment of obstructive sleep apnea syndrome on the plasma TNF-alpha levels. Tohoku J Exp Med. 2004;204:267–272. doi: 10.1620/tjem.204.267. [DOI] [PubMed] [Google Scholar]
- Kattler H, Dijk DJ, Borbely AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–164. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng J-K, et al. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1β, interleukin-6 and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- Kimura M, Majde JA, Toth LA, et al. Somnogenic effects of rabbit and recombinant human interferons in rabbits. Am J Physiol. 1994;267:R53–R61. doi: 10.1152/ajpregu.1994.267.1.R53. [DOI] [PubMed] [Google Scholar]
- Kluger M, Kozak W, Conn CA, et al. The adaptive value of fever. Infect Dis Clin North Am. 1996;10:1–20. doi: 10.1016/s0891-5520(05)70282-8. [DOI] [PubMed] [Google Scholar]
- Kozak W, Zheng H, Conn CA, et al. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1β deficient mice. Am J Physiol. 1995;269:R969–R977. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- Kozak W, Poli V, Soszynski D, et al. Sickness behavior in mice deficient in interleukin-6 during turpentine abscess and influenza pneumonitis. Am J Physiol. 1997;272:R621–R630. doi: 10.1152/ajpregu.1997.272.2.R621. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Obal F., Jr A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Obál F., Jr . Sleep factors. In: Saunders NA, Sullivan CE, editors. Sleep and Breathing. Marcel Dekker; New York: 1994. pp. 79–112. [Google Scholar]
- Krueger JM, Obal F., Jr Sleep function. Front Biosci. 2003;8:511–519. doi: 10.2741/1031. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Majde JA. Microbial products and cytokines in sleep and fever regulation. Crit Rev Immunol. 1994;14:355–379. doi: 10.1615/critrevimmunol.v14.i3-4.70. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Majde JA. Host defense. In: Kryger M, editor. Principles and Practice of Sleep Medicine. 4th. Elsevier Science; Philadelphia, PA: 2005. pp. 256–265. [Google Scholar]
- Krueger JM, Walter J, Dinarello CA, et al. Sleep-promoting effects of endogenous pyrogen (interleukin-1) Am J Physiol. 1984;246:R994–R999. doi: 10.1152/ajpregu.1984.246.6.R994. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Rector DM, Churchill L. Sleep and cytokines. Sleep Med Clinics. 2007;2:161–169. doi: 10.1016/j.jsmc.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Kushikata T, Fang J, et al. A nuclear factor kappa β (NFκB) inhibitor peptide inhibits spontaneous and interleukin-ip-induced sleep. Am J Physiol. 2000;279:R404–R413. doi: 10.1152/ajpregu.2000.279.2.R404. [DOI] [PubMed] [Google Scholar]
- Kubota T, Fang J, Brown RA, et al. Interleukin-18 promotes sleep in rabbits and rats. Am J Physiol Regul Integr Compar Physiol. 2001a;281:R828–R838. doi: 10.1152/ajpregu.2001.281.3.R828. [DOI] [PubMed] [Google Scholar]
- Kubota T, Fang J, Guan Z, et al. Vagotomy attenuates tumor necrosis factor-alpha-induced sleep and EEG delta-activity in rats. Am J Physiol. 2001b;280:R1213–R1220. doi: 10.1152/ajpregu.2001.280.4.R1213. [DOI] [PubMed] [Google Scholar]
- Kubota T, Majde JA, Brown RA, et al. Tumor necrosis factor receptor fragment attenuates interferon-β-induced non-REM sleep in rabbits. J Neuroimmunol. 2001c;119:192–198. doi: 10.1016/s0165-5728(01)00382-4. [DOI] [PubMed] [Google Scholar]
- Kubota T, Li N, Guan Z, et al. Intrapreoptic microinjection of TNF-alpha enhances non-REMS in rats. Brain Res. 2002;932:37–44. doi: 10.1016/s0006-8993(02)02262-x. [DOI] [PubMed] [Google Scholar]
- Kundermann B, Hemmeter-Spemal J, Huber MT, et al. Effects of total sleep deprivation in major depression: overnight improvement of mood is accompanied by increased pain sensitivity and augmented pain complaints. Psychosom Med. 2008;70:92–101. doi: 10.1097/PSY.0b013e31815c1b5d. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Imakita M, Kumeda CA, et al. Cascade of fever production in mice infected with influenza virus. J Med Virol. 1996;50:152–158. doi: 10.1002/(SICI)1096-9071(199610)50:2<152::AID-JMV8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Kushikata T, Fang J, Chen Z, et al. Epidermal growth factor enhances spontaneous sleep in rabbits. Am J Physiol. 1998;275:R509–R514. doi: 10.1152/ajpregu.1998.275.2.R509. [DOI] [PubMed] [Google Scholar]
- Kushikata T, Fang J, Krueger JM. Brain-derived neurotrophic factor enhances spontaneous sleep in rats and rabbits. Am J Physiol. 1999;276:R1334–R1338. doi: 10.1152/ajpregu.1999.276.5.R1334. [DOI] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- Leonard TO, Lydic R. Nitric oxide synthase inhibition decreases pontine acetylcholine release. Neuroreport. 1995;6:1525–1529. doi: 10.1097/00001756-199507310-00015. [DOI] [PubMed] [Google Scholar]
- Leonard TO, Lydic R. Pontine nitric oxide modulates acetylcholine release, rapid eye movement sleep generation, and respiratory rate. J Neurosci. 1997;17:774–785. doi: 10.1523/JNEUROSCI.17-02-00774.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz M, Mogensen KE, Uzé G. Shared receptor components but distinct complexes for alpha and beta interferons. J Mol Biol. 1998;282:585–599. doi: 10.1006/jmbi.1998.2026. [DOI] [PubMed] [Google Scholar]
- Lu J, Bjorkum AA, Xu M, et al. Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci. 2002;22:4568–4576. doi: 10.1523/JNEUROSCI.22-11-04568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue FA, Bail FA, Jephthah-Ocholo J, et al. Sleep and cerebrospinal fluid interleukin-1 like activity in the cat. Int J Neurosci. 1998;42:179–183. doi: 10.3109/00207458808991595. [DOI] [PubMed] [Google Scholar]
- McGinty D, Szymusiak R. Hypothalamic regulation of sleep and arousal. Front Biosci. 2003;8:d1074–d1083. doi: 10.2741/1159. [DOI] [PubMed] [Google Scholar]
- Mackiewicz M, Sollars PJ, Ogilvie MD, et al. Modulation of IL-1beta gene expression in the rat CNS during sleep deprivation. Neuroreport. 1996;7:529–533. doi: 10.1097/00001756-199601310-00037. [DOI] [PubMed] [Google Scholar]
- Mackowiak PA. Direct effects of hyperthermia on pathogenic microorganisms: teleologic implications with regard to fever. Rev Infect Dis. 1981;3:508–520. doi: 10.1093/clinids/3.3.508. [DOI] [PubMed] [Google Scholar]
- Majde JA. Viral double-stranded RNA, cytokines and the flu. J Interferon Cytokine Res. 2000;20:259–272. doi: 10.1089/107999000312397. [DOI] [PubMed] [Google Scholar]
- Majde JA, Krueger JM. Neuroimmunology of sleep. In: D’haenen H, den Boer JA, Willner P, editors. Biological Psychiatry. John Wiley; London: 2002. pp. 1247–1257. [Google Scholar]
- Majde JA, Krueger JM. Links between the innate immune system and sleep. In: Shearer WT, Rosenwasser LJ, Bochner BS, editors. Molecular Mechanisms in Allergy and Clinical Immunology. 2005. [DOI] [PubMed] [Google Scholar]
- Manfridi A, Brambilla D, Bianchi S, et al. Interleukin-1 beta enhances non-rapid eye movement sleep when microinjected into the dorsal raphe nucleus and inhibits serotonergic neurons in vitro. Eur J Neurosci. 2003;18:1041–1049. doi: 10.1046/j.1460-9568.2003.02836.x. [DOI] [PubMed] [Google Scholar]
- Maquet P, Peigneux P, Laureys S, et al. Memory processing during human sleep as assessed by functional neuroimaging. Rev Neurol (Paris) 2003;159:6S27–6S29. [PubMed] [Google Scholar]
- Minoguchi K, Tazaki T, Yokoe T, et al. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–1479. doi: 10.1378/chest.126.5.1473. [DOI] [PubMed] [Google Scholar]
- Moldofsky H. Sleep, neuroimmune and neuroendocrine functions in fibromyalgia and chronic fatigue syndrome. Adv Neuroimmunol. 1995;5:39–56. doi: 10.1016/0960-5428(94)00048-s. [DOI] [PubMed] [Google Scholar]
- Moss RB, Mercandetti A, Vojdani A. TNF-alpha and chronic fatigue syndrome. J Clin Immunol. 1999;19:314–316. doi: 10.1023/a:1020595709352. [DOI] [PubMed] [Google Scholar]
- Mullington J, Korth C, Hermann DM, et al. Dose-dependent effects of endotoxin on human sleep. Am J Physiol Regul Integr Comp Physiol. 2000;278:R947–R955. doi: 10.1152/ajpregu.2000.278.4.R947. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, et al. Exposure to acute stress induces brain interleukin-1 beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obal F, Jr, Krueger JM. Biochemical regulation of sleep. Front Biosci. 2003;8:520–550. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- Okun ML, Giese S, Lin L, et al. Exploring the cytokine and endocrine involvement in narcolepsy. Brain Behav Immun. 2004;18:326–332. doi: 10.1016/j.bbi.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Omdal R, Gunnarsson R. The effect of interleukin-1 blockade on fatigue in rheumatoid arthritis – a pilot study. Rheumatol Int. 2005;25:481–484. doi: 10.1007/s00296-004-0463-z. [DOI] [PubMed] [Google Scholar]
- Opp MR, Krueger JM. Interleukin 1 receptor antagonist blocks interleukin 1-induced sleep and fever. Am J Physiol. 1991;260:R453–R457. doi: 10.1152/ajpregu.1991.260.2.R453. [DOI] [PubMed] [Google Scholar]
- Opp MR, Krueger JM. Anti-interleukin-1 beta reduces sleep and sleep rebound after sleep deprivation in rats. Am J Physiol. 1994;266:R688–R695. doi: 10.1152/ajpregu.1994.266.3.R688. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Kastin AJ. Permeability of the blood-brain and blood-spinal cord barriers to interferons. J Neuroimmunol. 1997;76:105–111. doi: 10.1016/s0165-5728(97)00034-9. [DOI] [PubMed] [Google Scholar]
- Pickering M, O’Connor JJ. Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog Brain Res. 2007;163:339–354. doi: 10.1016/S0079-6123(07)63020-9. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Ilyin SE, Turrin NP, et al. Kindling modulates the IL1 beta system, TNF-alpha, TGF-beta 1, and neuropeptide mRNAs in specific brain regions. Mol Brain Res. 2000;75:248–258. doi: 10.1016/s0169-328x(99)00306-x. [DOI] [PubMed] [Google Scholar]
- Pollmacher T, Mullington J, Korth C, et al. Influence of host defense activation on sleep in humans. Adv Neuroimmunol. 1995;5:155–169. doi: 10.1016/0960-5428(95)00006-n. [DOI] [PubMed] [Google Scholar]
- Quesada JR, Talpaz M, Rios A, et al. Clinical toxicity of interferons in cancer patients: a review. J Clin Oncol. 1986;4:234–243. doi: 10.1200/JCO.1986.4.2.234. [DOI] [PubMed] [Google Scholar]
- Rector DM, Topchiy LA, Carter KM, et al. Local functional state differences between rat cortical columns. Brain Res. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Reznikov LL, Puren AJ, Fantuzzi G, et al. Spontaneous and inducible cytokine responses in healthy humans receiving a single dose of IFN-alpha2b: increased production of interleukin-1 receptor antagonist and suppression of IL-l-induced IL-8. J Interferon Cytokine Res. 1998;18:897–903. doi: 10.1089/jir.1998.18.897. [DOI] [PubMed] [Google Scholar]
- Rizzi M, Perego C, Aliprandi M, et al. Glia activation and cytokine increase in rat hippocampus by kainic acid-induced status epilepticus during postnatal development. Neurobiol Dis. 2003;14:494–503. doi: 10.1016/j.nbd.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Roky R, Obal F, Valatx JL, et al. Prolactin and rapid eye movement. Sleep. 1995;18:536–542. [PubMed] [Google Scholar]
- Romanovsky A, Almeida MC, Aronoff DM, et al. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci. 2005;10:2193–2216. doi: 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- Roy S, Krueger JM, Rector DM, et al. A network model for activity-dependent sleep regulation. J Theor Biol. 2008;253:462–478. doi: 10.1016/j.jtbi.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Crochet S, Onoe H. Pontine structures and mechanisms involved in the generation of paradoxical (REM) sleep. Arch Ital Biol. 2001;139:93–107. [PubMed] [Google Scholar]
- Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- Shoham S, Davenne D, Cady AB, et al. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am J Physiol. 1987;253:R142–R149. doi: 10.1152/ajpregu.1987.253.1.R142. [DOI] [PubMed] [Google Scholar]
- Smedley H, Katrak M, Sikola K, et al. Neurological effects of recombinant human interferon. Brit Med J. 1983;286:262–264. doi: 10.1136/bmj.286.6361.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solle M, Labsi J, Perragaux DG, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Sriskandan K, Gamer P, Watkinson J, et al. A toxicity study of recombinant interferon-gamma given by intravenous infusion to patients with advanced cancer. Cancer Chemother Pharmacol. 1986;18:63–68. doi: 10.1007/BF00253067. [DOI] [PubMed] [Google Scholar]
- Steel DM, Whitehead AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994;15:81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Ido K, et al. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai E, Yasuda T, Taishi P, et al. Growth hormone-releasing hormone: cerebral cortical sleep-related EEG actions and expression. Am J Physiol Regul Integr Comp Physiol. 2007;293:R922–R930. doi: 10.1152/ajpregu.00237.2007. [DOI] [PubMed] [Google Scholar]
- Taishi P, Bredow S, Guha-Thakurta N, et al. Diurnal variations of interleukin-1 beta mRNA and beta-actin mRNA in rat brain. J Neuroimmunol. 1997;75:69–74. doi: 10.1016/s0165-5728(97)00002-7. [DOI] [PubMed] [Google Scholar]
- Taishi P, Gardi J, Chen Z, et al. Sleep deprivation increases the expression of TNF alpha mRNA and TNF 55kD receptor mRNA in rat brain. Physiologist. 1999;42:A4. [Google Scholar]
- Taishi P, Sanchez C, Wang Y, et al. Conditions that affect sleep alter the expression of molecules associated with synaptic plasticity. Am J Physiol. 2001;281:R839–R845. doi: 10.1152/ajpregu.2001.281.3.R839. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Krueger JM. Nerve growth factor enhances sleep in rabbits. Neurosci Lett. 1999;264:149–152. doi: 10.1016/s0304-3940(99)00196-2. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kapás L, Fang J, et al. An interleukin-1 receptor fragment inhibits spontaneous sleep and muramyl dipeptide-induced sleep in rabbits. Am J Physiol. 1996;271:R101–R108. doi: 10.1152/ajpregu.1996.271.1.R101. [DOI] [PubMed] [Google Scholar]
- Toth LA, Hughes LF. Macrophage participation in influenza-induced sleep enhancement in C57BL/6J mice. Brain Behav Immun. 2004;18:375–389. doi: 10.1016/j.bbi.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Toth LA, Tolley EA, Krueger JM. Sleep as a prognostic indicator during infectious disease in rabbits. Proc Soc Exp Biol Med. 1993;203:179–192. doi: 10.3181/00379727-203-43590. [DOI] [PubMed] [Google Scholar]
- Tringall G, Dello Russo C, Preziosi P, et al. Interleukin-1 in the central nervous system: from physiology to pathology. Therapie. 2000;55:171–175. [PubMed] [Google Scholar]
- Trompet S, de Craen AJ, Slagboom P, et al. Genetic variation in the interleukin-1 beta-converting enzyme associates with cognitive function. The PROSPER study. Brain. 2008;131:1069–1077. doi: 10.1093/brain/awn023. [DOI] [PubMed] [Google Scholar]
- Turrin NP, Rivest S. Innate immune reaction in response to seizures: implications for the neuropathology associated with epilepsy. Neurobiol Dis. 2004;16:321–334. doi: 10.1016/j.nbd.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Uzé G, Lutfalla G, Bandu M-T, et al. Behavior of a cloned murine interferon α/β receptor expressed in homospecific or heterospecific background. Proc Natl Acad Sci U S A. 1992;89:4774–4778. doi: 10.1073/pnas.89.10.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis M, Papanicolaou DA, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. Metabolism. 2002;51:887–892. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Intern Med. 2003;254:32–44. doi: 10.1046/j.1365-2796.2003.01177.x. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Lin HM, et al. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-α antagonist. J Clin Endocrinol Metab. 2004;89:4409–1413. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- Vollmer-Conna U, Fazou C, Cameron B, et al. Production of pro-inflammatory cytokines correlateswith the symptoms of acute sickness behavior in humans. Psychol Med. 2004;34:128–1297. doi: 10.1017/s0033291704001953. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy V, Borbely AA, Tobler I. Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. Sleep Res. 2000;9:367–371. doi: 10.1046/j.1365-2869.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy V, Welker E, Fritschy J, et al. Regional cortical metabolism and dynamics of slow wave activity during sleep after unilateral whisker stimulation and sleep deprivation in mice. Sleep. 2004;27:A5. [Google Scholar]
- Walker AJ, Topchiy I, Kouptsou K, et al. ERP differences during conditioned lick response in the rat. Sleep. 2005;28:A15. [Google Scholar]
- Walton PE, Cronin MJ. Tumor necrosis factor-α and interferon-γ reduce prolactin release in vitro. Am J Physiol. 1990;259:E672–E676. doi: 10.1152/ajpendo.1990.259.5.E672. [DOI] [PubMed] [Google Scholar]
- Wemer-Felmayer G, Werner ER, Fuchs D, et al. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochem Biophys Acta. 1989;1012:140–147. doi: 10.1016/0167-4889(89)90087-6. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Morales FR, Chase MH. Induction of rapid eye movement sleep by microinjection of nerve growth factor into the pontine reticular formation of the cat. Neuroscience. 1995;66:9–13. doi: 10.1016/0306-4522(95)00052-k. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Sampogna S, Chase MH. Neurotrophin-receptor immunoreactive neurons in mesopontine regions involved in the control of behavioral states. Brain Res. 2000;866:1–14. doi: 10.1016/s0006-8993(00)02204-6. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Yasuda K, Brown RA, et al. State-dependent effects of light–dark cycle on somatosensory and visual cortex EEG in rats. Am J Physiol Regul Integr Comp Physiol. 2005a;289:R1083–R1089. doi: 10.1152/ajpregu.00112.2005. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Yoshida H, Garcia-Garcia F, et al. Interleukin-1β has a role in cerebral cortical state-dependent electroencephalographic slow-wave activity. Sleep. 2005b;28:177–184. doi: 10.1093/sleep/28.2.177. [DOI] [PubMed] [Google Scholar]
- Yi PL, Tsai CH, Lin JG, et al. Kindling stimuli delivered at different times in the sleep-wake cycle. Sleep. 2004;27:203–212. doi: 10.1093/sleep/27.2.203. [DOI] [PubMed] [Google Scholar]
- Yndestad A, Damås JK, Øie E, et al. Role of inflammation in the progression of heart failure. Curr Cardiol Rep. 2007;9:236–241. doi: 10.1007/BF02938356. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Peterfi Z, Garcia-Garcia F, et al. State-specific asymmetries in EEG slow wave activity induced by local application of TNF alpha. Brain Res. 2004;1009:129–136. doi: 10.1016/j.brainres.2004.02.055. [DOI] [PubMed] [Google Scholar]
- Zhang X, Alley EW, Russell SW, et al. Necessity and sufficiency of beta interferon for nitric oxide production in mouse peritoneal macrophages. Infect Immun. 1994;62:33–40. doi: 10.1128/iai.62.1.33-40.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


