Abstract
Liver metastases from gastric cancer (LMGC) is a non-curable, fatal disease with a 5-year survival rate of <10%. Although various local treatments have been applied, their clinical utility has not been established. The purpose of this study was to investigate the safety and effectiveness of proton beam therapy (PBT) for the treatment of patients with LMGC. A total of nine patients (seven men, two women; aged 56–78 years) with LMGC who received PBT between 2002 and 2012 were retrospectively reviewed. Patients who had tumors confined to the liver were investigated, and patients who had extrahepatic tumors were excluded. Six of the patients had solitary tumors, and three patients had multiple tumors. The total irradiation dose was 64–77 Gy relative biological effectiveness (RBE), and three patients received concurrent chemotherapy. The overall and progression-free survival (OS and PFS) rates, local control (LC) rate, and adverse effects were investigated. All patients completed treatment without interruption, and late adverse effects of higher than Grade 3 were not observed. The OS rates at 1, 3 and 5 years were 100%, 78% and 56%, respectively (median, 5.5 years); the PFS rates were 67%, 40% and 40% (median, 2.6 years); and the LC rates were 89%, 71% and 71%. PBT was demonstrated to be a safe treatment, and the OS and PFS rates were not inferior to those for other types of local treatment. Therefore, PBT should be considered as an effective local treatment option for patients with LMGC.
Keywords: proton beam therapy, metastatic liver tumor, gastric cancer, survival rate
INTRODUCTION
Gastric cancer is one of the most common malignancies worldwide. Liver metastasis can be found in 5–14% of patients with gastric cancer [1–4]. Most patients with liver metastases from gastric cancer (LMGC) have bilobar multinodular tumors, and the cancer recurrence and metastatic patterns are associated with locoregional peritoneal dissemination and with diffuse metastases to the lymph nodes. The vast majority of patients with LMGC may in fact have systemic disease. Only a very small number of patients with LMGC are candidates for local treatment [5].
Although systemic chemotherapy with new molecular targeting agents has been developed, LMGC is a non-curable, fatal disease with a 5-year survival rate of <10%, and the management of liver metastasis remains challenging. Thus, the treatment of patients with LMGC is regarded as palliative. To improve the treatment outcome, various local treatments such as surgery, radiofrequent ablation (RFA), and transcatheter arterial chemoembolization (TACE) have been applied, in combination with chemotherapy or as an alternative to chemotherapy [6]. However, the clinical utility of local treatment for LMGC has not been established.
Proton beam therapy (PBT) has the physical characteristic of precisely delivering a high dose of radiation to the target tumor, while greatly limiting the exposure to the regions beyond the target. It is well known that PBT for primary liver cancer achieves excellent local control (LC) rates with few adverse effects [7–11]. Moreover, we previously investigated the effectiveness of PBT to metastatic liver tumors although that study included various primary cancers and disease conditions [12]. The adaptations in the use of PBT for each disease type were yet to be clarified, because radiosensitivity and the aim of treatment differs among various types of primary cancers and disease conditions. In this study we reviewed patients with LMGC who received PBT, and investigated the safety and effectiveness of the PBT.
MATERIALS AND METHODS
Patients
We retrospectively investigated nine patients with LMGC who received PBT at the University of Tsukuba between 2002 and 2012. They comprised seven men and two women and had a median age of 71 years (range, 56–78 years). The patients’ tumors could be categorized as solitary tumors or as multiple tumors that could be included within a few irradiation fields. Patients who had extrahepatic tumors were excluded.
All patients had previously received curative surgery for the primary tumors. The duration from gastric surgery to PBT was 7–113 months (median, 36 months), and that from onset of liver metastasis to PBT was 1–30 months (median, 6 months). Of the nine patients, six had solitary tumors, and the remaining three, multiple tumors (2, 4 and >10). The maximal diameter of the tumors was 2–6 cm (median, 3 cm). Eight of the patients had received another form of treatment before the PBT, such as chemotherapy and TACE. Three patients received concurrent chemotherapy (cisplatin/5-fluorouracil (5-FU), 5-FU, or tegafur/gimeracil/oteracil). According to the Eastern Cooperative Oncology Group Performance Status (PS) scale, all patients had a PS of 0 to 1 and a Child–Pugh score of 5 (class A). The follow-up period after PBT was 1.2–11 years (median, 4.5 years). The treatment strategy was discussed with surgeons or medical oncologists on an individual basis, considering the patient's PS, tumor location, and tumor size, and was approved at the in-hospital conference. The reasons for selection of PBT were as follows: incurability of the cancer by chemotherapy (eight patients), inoperability of the cancer (three of those eight patients), and strong demand for PBT by the patient (the one remaining patient). Written informed consent was obtained from all the patients before the PBT, and analyses were carried out with the approval of the Institutional Review Board. The characteristics of the patients and of the tumors are shown in Table 1.
Table 1.
Summary of patients
| Case | Age/sex | Number | Distribution | Size (cm) | Dose Gy (RBE)/fr | Precedent therapy | Concurrent therapy | Adjuvant therapy | Survival period (years) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 69/F | S | Uni | 2 | 72.6/22 | C | N | N | 11.0 |
| 2 | 76/F | S | Uni | 4 | 72.6/22 | C | C | N | 8.4 |
| 3 | 61/M | M | Uni | 6 | 70/35 | C | N | N | 6.4 |
| 66/10 | |||||||||
| 4 | 77/M | S | Uni | 2 | 66/10 | N | C | C | 5.5 |
| 5 | 77/M | S | Uni | 4 | 72.6/22 | C | N | C | 4.5 |
| 6 | 71/M | M | Bi | 5 | 72.6/22 | C | N | N | 3.8 |
| 72.6/22 | |||||||||
| 7 | 78/M | s | Uni | 2 | 64/32 | C | N | C | 3.0 |
| 8 | 56/M | S | Uni | 3 | 72.6/22 | C | N | N | 2.3 |
| 9 | 59/M | M | Bi | 2 | 77/35 | C | C | C | 1.2 |
| 66/10 |
S = solitary, M = multiple, Uni = unilateral lobe, Bi = bilateral lobes, C = chemotherapy, N = none. Underlining indicates the cases who are still alive at the final follow-up (April–June 2016).
Proton beam therapy
Each patient's body was immobilized using a custom-made body cast. Computed tomography (CT) images were taken at 5-mm intervals during the expiratory phase under a respiratory gating system [13]. At the treatment planning stage, an aperture margin of 5–10 mm, a depth margin of 5–10 mm, and a margin of 5 mm on the caudal axes were added to cover the entire clinical target volume to compensate for uncertainty resulting from respiration-induced hepatic movements. These margins included the field margins. A bolus was fabricated for the smearing process. Proton beams from 155–250 MeV, generated through a linear accelerator and synchrotron, were spread out and shaped with ridge filters, double-scattering sheets, multicollimators, and custom-made boluses to ensure that the beams conformed to the treatment planning data.
The proton beam therapy dose and fractionation were decided according to the tumor location and the treatment strategy. As many tumors as possible were included within the same irradiation field, and all three patients with multiple tumors (one: unilateral, two: bilateral lobes) were treated with two irradiation fields. The total irradiation dose was 64–77 (median, 72.6) Gy relative biological effectiveness (RBE). The most frequent dosage was 72.6 Gy (RBE) in 22 fractions, used in six patients, followed by 66 Gy (RBE) in 10 fractions, used in three patients. The maximum cumulative dose was set below 50 Gy (RBE) for the spinal cord, stomach and duodenum and below 60 Gy (RBE) for the colon. The RBE of the PBT was assumed to be 1.1 [14].
Treatment after PBT
Four patients received adjuvant chemotherapy after PBT. Moreover, four patients received additional treatment to new or recurrent tumors (PBT in two patients and chemotherapy in two patients).
Follow-up procedures and evaluation criteria
During the treatment sessions, acute treatment-related toxicities were assessed weekly in all patients. After completion of the PBT, the patients were evaluated by means of physical examinations, blood tests, and CT or magnetic resonance imaging scans. Assessment of response was evaluated according to the Response Evaluation Criteria in Solid Tumors (version 1.1) [15]. We defined local failure as an increase in the maximal diameter of the treated target tumors of >20% and of >5 mm. Adverse events were assessed after every procedure according to the Common Terminology Criteria for Adverse Effects (CTCAE; version 4.03) [16]. The patients treated before 2010 were also retrospectively reviewed using the CTCAE.
For examination of safety, the treatment completion rate, liver toxicity, and late adverse effects were examined. For examination of the treatment effect, the overall survival (OS), progression-free survival (PFS) and LC rates were calculated using the Kaplan–Meier method.
RESULTS
Three of eight patients already had multiple tumors in the liver and thus were obliged to undergo two-field irradiation, and five patients were treated with a single irradiation field. All patients completed the treatment without interruption. The biologically effective dose for the liver (α/β = 3) was minimum: 10.0, maximum: 29.8 and median: 12.5 Gy (RBE), and the volume that received >30 Gy (RBE) (V30) was minimum: 12%, maximum: 42% and median: 20.7% of the liver. Late adverse effects of higher than Grade 3 were not observed.
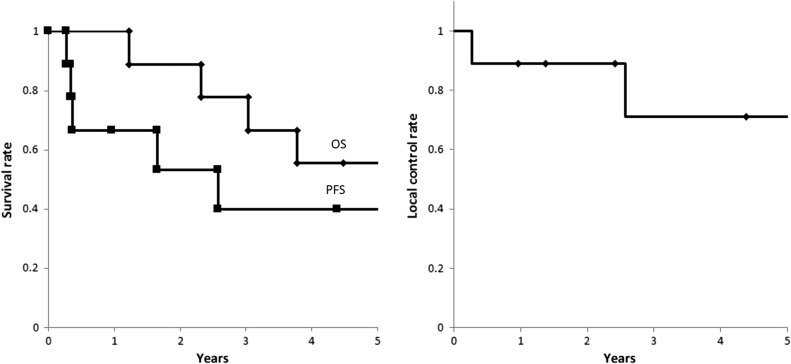
Of the nine patients, two were still alive at the final follow-up between April and June 2016. Local recurrence was observed in two patients who had multiple tumors (two and four, respectively) in bilateral lobes. In the former patient, local recurrence was observed 2.6 years later, and the patient received additional PBT to the recurrent tumor; he then survived 3.8 years from the first PBT (Case 6 in Table 1). In the latter patient, local recurrence and para aortic lymph node metastases were observed 3.3 months later; he then died 1.2 years later from the PBT (Case 9). The OS rates at 1, 3 and 5 years were 100%, 78% and 56%, respectively, with a median of 5.5 years; the PFS rates were 67%, 40% and 40%, with a median of 2.6 years; and the LC rates were 89%, 71% and 71% (Fig. 1). Acute dermatitis was Grade 1–2 in all patients. Radiation-induced liver damage was not observed; nor were severe adverse effects of more than Grade 3. No patient showed a Child–Pugh score elevation of >2 during the follow-up.
Fig. 1.
(a) OS and PFS rates for all patients. (b) LC rates for all patients.
Figure 2 shows a 77-year-old male patient with LMGC. He had a solitary tumor in the S2 region. He received PBT at a dose of 72.6 Gy (RBE) in 22 fractions. CT 3.8 years after the PBT showed a scar and atrophic change without recurrence. As at April 2016, he is still alive 4.5 years after the PBT.
Fig. 2.
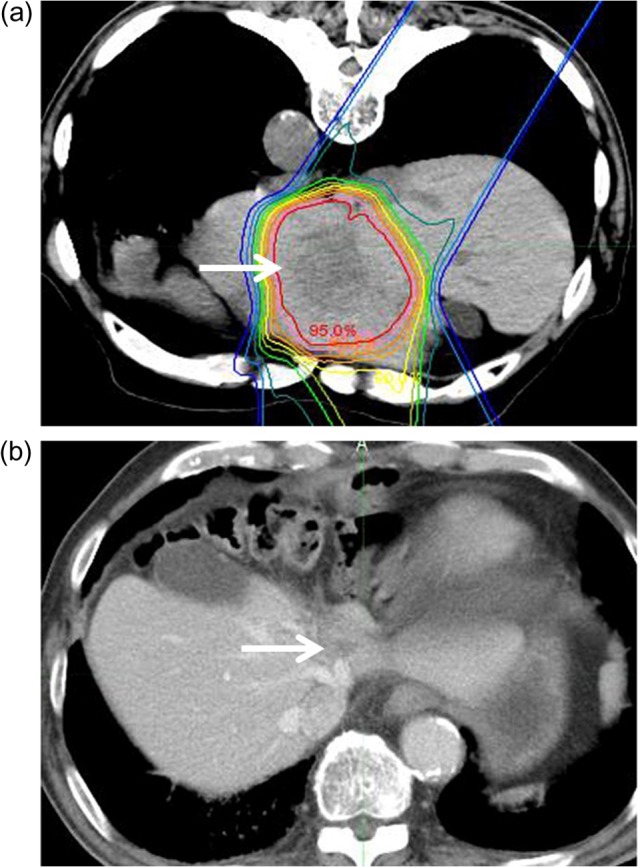
A 77-year-old male patient with LMGC. (a) Solitary tumor with a 4-cm diameter in S2 (arrow) was irradiated with PBT at a dose of 72.6 Gy (RBE)/22 fractions with the patient in the prone position. Isodose lines represent, from the inside to the outside, 95% to 10% of the dose at the isocenter. (b) CT 3.8 years after PBT. The arrow indicates the scar and atrophic change.
DISCUSSION
Reports on the local treatment of patients with LMGC are extremely rare. For surgically treated patients, the OS rates at 1, 3 and 5 years were 62–79, 17–41 and 10–39%, with a median of 0.9–2.6 years [17–20]. For RFA-treated patients, the OS rates were 70–73, 5–43 and 3–34% [20, 21]. Moreover, some researchers reported using combined therapy such as surgery, RFA and microwave coagulation therapy or various local treatment methods. The outcomes of the local treatment for patients with LMGC in terms of the OS rates were 62–87, 17–51 and 10–42% at 1, 3 and 5 years, respectively, with a median of 0.4–3.4 years, and in terms of the PFS rates were 48–57, 25–37 and 14–33%, with a median of 0.8–1 year [5, 17–23] (Table 2). Although severe adverse effects are rare, Gunner et al. reported that 12 of 68 patients (18%) suffered severe adverse effects of more than Grade 3 and that one patient died of hepatic insufficiency on postoperative Day 19 [20]. The only case study in which PBT combined with concurrent chemotherapy was used was that reported by Gohongi et al.: their patient survived >2 years without severe adverse effects [24].
Table 2.
Local treatment outcome of LMGC (review and current study)
| Author | Population | Number (S/M) | Distribution (Uni/Bi) | Size (cm) | Treatment | 1/3/5-yearOS (years) | 1/3/5YPFS (Y) |
|---|---|---|---|---|---|---|---|
| Makino et al. [17] | 16 | 9/7 | 11/5 | <3/≥3 = 8/8 | S | 62/17/10% (1.3) | |
| Garancini et al. [18] | 21 | 12/9 | 16/5 | <5/≥5 = 14/7 | S | 66/31/19% (0.9) | 51/25/14% |
| Baek et al. [19] | 12 | 11/1 | <4/≥4 = 8/4 | S | 65//39% (2.6) | ||
| Guner et al. [20] | 68 | 45/23 | 60/8 | 0.6–10 (2.7) | S | 79/41/30% (2) | 49/30/26% |
| Chen et al. [21] | 21 | 12/9 | 16/5 | <3/≥3 = 9/12 (3.8) | R | 70/5/3% | |
| Guner et al. [20] | 30 | 22/8 | 24/6 | 0.5–5.8 (2.2) | R | 73/43/34% (1.9) | 57/37/33% |
| Oki et al. [23] | 94 | 54/38 | <3/≥3 = 41/51 | S/S+M, R = 69/25 | 87/51/42% (3.4) | 48/29/28% (1) | |
| Hwang et al. [22] | 27 | R/R+C | (1.7) | (0.8) | |||
| Hwang et al. [5] | 38 | R, C, T, etc. | (0.4) | ||||
| Current study | 9 | 6/3 | 7/2 | 2–6 (3.3) | P/P+C = 6/3 | 100/78/56% (5.5) | 67/40/40% (2.6) |
S = solitary, M = multiple, Uni = unilateral lobe, Bi = bilateral lobes, OS = overall survival, PFS = progression-free survival, S = surgery, M = microwave coagulation therapy, R = radiofrequent ablation, C = chemotherapy, T = transcatheter arterial chemoembolization, P = proton beam therapy. Number in parenthesis is median value.
As shown above, the OS rates at 1, 3 and 5 years in our study using PBT were 100%, 78% and 56%, respectively, with a median of 5.5 years; the PFS rates were 67%, 40% and 40%, with a median of 2.6 years. In our study, seven of nine patients had oligometastasis, whereas in the previous studies mentioned above, only one report treated oligometastasis [19]; others were treated regardless of the number of tumors. It was difficult to compare our results with those of previous studies unless the number of tumors corresponded. Therefore, we compared our data with that of all previous reports in which the tumor number, size and distribution were comparable with those in our study. Our OS and PFS rates were not inferior to those of the previous studies. Only four patients received regular chemotherapy after PBT (paclitaxel: two, tegafur/gimeracil/osteracil: two), and no patients received advanced chemotherapy because most of the patients were initially chemo-resistant. In addition, local recurrence was observed in two of the five patients who did not receive adjuvant chemotherapy and also in two of the four patients who received adjuvant chemotherapy. PFS includes both local PFS and distant PFS. We consider that a high LC rate could directly avoid a PFS rate reduction. Severe acute or late adverse effects or radiation-induced liver damage was not observed. In view of the poor outcome for the patients with LMGC (a 5-year OS rate of <10% after chemotherapy), PBT is a safe and effective treatment option if the metastasis is confined to the liver.
The advantages of PBT for LMGC are as follows: (i) few adverse effects, (ii) high LC rates, (iii) repeatable treatment and (iv) the possibility of treatment for large tumors.
First, the tolerance doses for treatment of the liver have been well documented. Austin-Seymour et al. reported the tolerance dose as 30–35 Gy to one-third of the liver volume [25], and Emami et al. reported that the 5% risk at 5 years was 30 Gy to the entire liver [26]. The V30 was 12–42% in our patients. Even in the three patients who had multiple tumors, the V30 was 42%, 20% and 21% (which are tolerable values according to Emami's criteria), and the changes in the Child–Pugh score were 5 to 5, 5 to 6, and 5 to 5 during follow-up, respectively (Cases 3, 5, 6). It is well established that PBT has the distinct advantage of causing relatively little damage to the healthy liver tissue in the treatment of primary liver cancers [7, 9, 27, 28]. Complicated hepatitis or liver cirrhosis is obviously less frequent in patients with gastric cancer than in those with primary liver cancer. From the data obtained in our study, in which no patients showed a Child–Pugh score elevation of >2 during the follow-up, and from the data regarding the safety obtained in the previous studies of primary liver cancers [7, 9, 27, 28], we consider that the amount of liver toxicity following PBT was extremely small when used for the treatment of LMGC. We concluded that the low level of adverse effects is the best advantage of PBT for LMGC, compared with surgery or systematic chemotherapy.
Second, we previously reported that the LC rates at 1, 3 and 5 years after PBT for primary liver cancers were 98%, 87% and 81%, respectively. It seems that the LC rate for LMGC is not as high as that for primary liver cancers because gastric cancer is not particularly radiosensitive. However, in situations for which no other useful treatment exists, we consider that PBT can play a role in local treatment, given our data of LC rates of 71% at 5 years.
Third, gastric cancer can cause additional metastatic tumors, and some of them might occur in the liver. Kakeji et al. reported that the high recurrence rate within 2 years of surgery might suggest the presence of occult intrahepatic metastases at the surgery and that recurrence tumors usually develop in the liver after surgery (62–79%) [6]. It is highly possible that additional local treatment will be required for new metastatic tumors in gastric cancer patients. In our study, three patients received concurrent two-field PBT and two patients received additional PBT when new liver metastases appeared. Repeated PBT to the liver is sometimes experienced in primary liver cancers, and its safety is sufficiently proven [29]. The most important factors for determining repeated PBT are the original liver function and the accumulated irradiation dose. We try to manage proton beam delivery so as not to overlap the previous proton beam paths and to leave >500 ml of the liver volume unirradiated as much as possible in daily clinical practice. Surgery is one of the options for local treatment. However, repeated surgery can be difficult because of adhesions or complications, and it can be unacceptable in many patients. Considering the success of repeated PBT for primary liver cancers and the lower frequency of complicated liver disease mentioned above, PBT should be an effective and safe option in repeated treatment.
Fourth, RFA, which is another local treatment option in terms of safety and repeatability, is limited to tumors of <5 cm in diameter. PBT can be used to treat much larger tumors without severe adverse effects. In our institute, the maximal tumor size that can be treated is 15 × 15 cm length × width, 12 cm depth. In our study, two patients had tumors >5 cm. Those patients survived 3.8 and 6.4 years, respectively. While smaller tumors can be controlled as effectively using PBT as using RFA, larger tumors can be treated with far less unnecessary irradiation to the liver.
For patients with LMGC, chemotherapy is the first choice. However, the treatment outcome for chemotherapy is not satisfactory. If the metastatic tumors are confined to the liver, several treatment options have been performed, and some of them can achieve higher OS rates than can conventional chemotherapy, even though evidence-rich data for such higher OS rates do not exist. Our study was a retrospective one, and the number of patients was small. To determine the total dose and fractionation for each disease type was difficult; thus, we used the same irradiation protocol as that used for primary liver cancers. The equivalent dose (α/β = 10) of the two tumors that showed recurrence was 80.1 Gy (RBE) [77 Gy (RBE) in 35 fractions] and 91.5 Gy (RBE) [72.6 Gy (RBE) in 22 fractions]. On the other hand, three tumors that were irradiated with 66 Gy (RBE) in 10 fractions [equivalent dose: 126.7 Gy (RBE)] were controlled. Considering the conventional irradiation dose is ~70 Gy (RBE) for most cancers, an equivalent dose >90 Gy (RBE) would be necessary for radio-resistant gastric cancers. However, the number of the patients in this study was not large enough to determine the adequate dose fractionation; further study with a larger number of patients is necessary. Moreover, concurrent therapy could improve the treatment effect of radio-resistant gastric cancer.
To the best of our knowledge, only one similar case report has been published; ours is the first report of multiple patients with LMGC who have undergone PBT. The OS and PFS rates were not inferior to those of other local treatments, and these data are similar to those of previous studies of liver tumors [7–12, 27]. We concluded that PBT is an effective local treatment option for LMGC. High OS and PFS and LC rates comparable with that of surgery indicate it would be a good second choice when inoperable tumors are confined to the liver. Unfortunately, we only have the data for nine patients. We are considering further investigation with a great number of patients to provide more detailed information on PBT for patients with LMGC.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1. D'Angelica M, Gonen M, Brennan MF et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 2004;240:808–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheon SH, Rha SY, Jeung HC et al. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol 2008;19:1146–53. [DOI] [PubMed] [Google Scholar]
- 3. Sakamoto Y, Sano T, Shimada K et al. Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J Surg Oncol 2007;95:534–9. [DOI] [PubMed] [Google Scholar]
- 4. Okano K, Maeba T, Ishimura K et al. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg 2002;235:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hwang SE, Yang DH, Kim CY. Prognostic factors for survival in patients with hepatic recurrence after curative resection of gastric cancer. World J Surg 2009;33:1468–72. [DOI] [PubMed] [Google Scholar]
- 6. Kakeji Y, Morita M, Maehara Y. Strategies for treating liver metastasis from gastric cancer. Surg Today 2010;40:287–94. [DOI] [PubMed] [Google Scholar]
- 7. Bush DA, Hillebrand DJ, Slater JM et al. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology 2004;127(5 Suppl 1):S189–93. [DOI] [PubMed] [Google Scholar]
- 8. Kawashima M, Furuse J, Nishio T et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol 2005;23:1839–46. [DOI] [PubMed] [Google Scholar]
- 9. Fukumitsu N, Sugahara S, Nakayama H et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2009;74:831–6. [DOI] [PubMed] [Google Scholar]
- 10. Mizumoto M, Okumura T, Hashimoto T et al. Proton beam therapy for hepatocellular carcinoma: a comparison of three treatment protocols. Int J Radiat Oncol Biol Phys 2011;81:1039–45. [DOI] [PubMed] [Google Scholar]
- 11. Qi WX, Fu S, Zhang Q, Guo XM. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol 2015;114:289–95. [DOI] [PubMed] [Google Scholar]
- 12. Fukumitsu N, Okumura T, Takizawa D et al. Proton beam therapy for metastatic liver tumors. Radiother Oncol 2015;117:322–7. [DOI] [PubMed] [Google Scholar]
- 13. Fukumitsu N, Hashimoto T, Okumura T et al. Investigation of the geometric accuracy of proton beam irradiation in the liver. Int J Radiat Oncol Biol Phys 2012;82:826–33. [DOI] [PubMed] [Google Scholar]
- 14. Paganetti H, Niemierko A, Ancukiewicz M et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys 2002;53:407–21. [DOI] [PubMed] [Google Scholar]
- 15. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 16. National Cancer Institute Common terminology criteria for adverse events (CTCAE). US Department of Health and Human Services, National Institutes of Health,2010. [Google Scholar]
- 17. Makino H, Kunisaki C, Izumisawa Y et al. Indication for hepatic resection in the treatment of liver metastasis from gastric cancer. Anticancer Res 2010;30:2367–76. [PubMed] [Google Scholar]
- 18. Garancini M, Uggeri F, Degrate L et al. Surgical treatment of liver metastases of gastric cancer: is local treatment in a systemic disease worthwhile. HPB 2012;14:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baek HU, Kim SB, Cho EH et al. Hepatic resection for hepatic metastases from gastric adenocarcinoma. J Gastric Cancer 2013;13:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guner A, Son T, Cho I et al. Liver-directed treatments for liver metastasis from gastric adenocarcinoma: comparison between liver resection and radiofrequency ablation. Gastric Cancer 2016;19:951–60. [DOI] [PubMed] [Google Scholar]
- 21. Chen J, Tang Z, Dong X et al. Radiofrequency ablation for liver metastasis from gastric cancer. Eur J Surg Oncol 2013;39:701–6. [DOI] [PubMed] [Google Scholar]
- 22. Hwang JE, Kim SH, Jin J et al. Combination of percutaneous radiofrequency ablation and systemic chemotherapy are effective treatment modalities for metachronous liver metastases from gastric cancer. Clin Exp Metastasis 2014;31:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oki E, Tokunaga S, Emi Y et al. Surgical treatment of liver metastasis of gastric cancer: a retrospective multicenter cohort study (KSCC1302). Gastric Cancer 2016;19:968–76. [DOI] [PubMed] [Google Scholar]
- 24. Gohongi T, Tokuuye K, Iida H et al. Concurrent proton beam radiotherapy and systemic chemotherapy for the metastatic liver tumor of gastric carcinoma: a case report. Jpn J Clin Oncol 2005;35:40–4. [DOI] [PubMed] [Google Scholar]
- 25. Austin-Seymour MM, Chen GT, Castro JR et al. Dose volume histogram analysis of liver radiation tolerance. Int J Radiat Oncol Biol Phys 1986;12:31–5. [DOI] [PubMed] [Google Scholar]
- 26. Emami B, Lyman J, Brown A et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109–22. [DOI] [PubMed] [Google Scholar]
- 27. Mizumoto M, Tokuuye K, Sugahara S et al. Proton beam therapy for hepatocellular carcinoma adjacent to the porta hepatis. Int J Radiat Oncol Biol Phys 2008;71:462–7. [DOI] [PubMed] [Google Scholar]
- 28. Mizumoto M, Okumura T, Hashimoto T et al. Evaluation of liver function after proton beam therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2012;82:e529–35. [DOI] [PubMed] [Google Scholar]
- 29. Hashimoto T, Tokuuye K, Fukumitsu N et al. Repeated proton beam therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2006;65:196–202. [DOI] [PubMed] [Google Scholar]