Abstract
A cell model of primary macrophages isolated from the peritoneal cavity of flavivirus-susceptible and congenic resistant mice has been used to study the extent and kinetics of antiviral effects against West Nile virus upon priming with alpha/beta interferon (IFN-α/β) or poly(I-C) (pIC). The pattern of flavivirus resistance expressed after priming of cells in this model was in good agreement with the pattern of flavivirus resistance described in the brains of the corresponding mouse strains. While priming with either IFN-α/β or pIC completely blocked flavivirus replication in macrophages from resistant mice, it only transiently reduced flavivirus replication in macrophages from susceptible mice. It was only the combined pretreatment with IFN-α/β and pIC that elicited strong antiviral responses that completely prevented flavivirus replication in macrophages from susceptible mice. Primary macrophages isolated from the blood of healthy human donors expressed a similar need for double-stranded RNA (dsRNA) cofactor in developing efficient antiviral responses against West Nile virus. These findings reveal that the inefficient IFN-α/β-induced antiviral effects against flaviviruses in cells from susceptible hosts could be successfully complemented by an external dsRNA factor leading to the complete eradication of the virus. This treatment appears to compensate for the lack of an inborn resistance mechanism in cells from the susceptible host. Furthermore, it may also provide useful clues for the prevention and treatment of flavivirus infections.
Together with hepatitis C virus and Pestivirus genera, flaviviruses belong to the Flaviviridae family of positive single-stranded RNA viruses. The genus Flavivirus consists of a number of medically important viruses such as yellow fever, West Nile (WN), Japanese encephalitis (JE), Murray Valley encephalitis (MVE), and dengue (DEN) viruses (25). While yellow and dengue fevers may deteriorate into life-threatening conditions, an acute, apparent infection with encephalitogenic flaviviruses rapidly progresses to lethal flavivirus-induced encephalitis in almost one-quarter of infected patients (21). The recent emergence of the flavivirus WN virus in New York State and its rapid spread to surrounding states in North America is an alarming event (29), in addition to the long-standing risk imposed by JE and DEN viruses in other parts of world. There are almost 20 serologically unrelated flaviviruses implicated in human diseases for which there is no vaccine or antiviral treatment available. The majority of these viruses are arthropod borne with an amplifying vertebrate host serving as a natural reservoir and a mosquito or tick vector needed for further dissemination of the virus in nature. Although humans often represent an accidental dead-end host for the virus (21), the incidence of flavivirus-mediated diseases in human populations may expand to either seasonal outbreaks or even localized epidemics causing a major concern to public health. In the absence of an adequate vaccination program, the host natural resistance to flaviviruses as well as development of appropriate antiviral therapies gain importance as alternative approaches for the prevention of diseases caused by flaviviruses (3, 44).
Involvement of genetic factors in the resistance to flavivirus infection is difficult to estimate in humans. In contrast, mice carry a genetic trait conferring resistance to flaviviruses that is widely distributed among wild mice populations and rare among laboratory mouse strains (34). Only a few strains of laboratory mice have preserved this trait; among these are PRI, CAST/Ei, CASA/Rk, and MOLD/Rk (34). Some of these strains have been used for backcross breeding to flavivirus-susceptible C3H/He mice, resulting in the development of new congenic flavivirus-resistant mouse strains: C3H.PRI-Flvr mice, which carry the resistance allele from outbred PRI mice (12); C3H.M. domesticus-Flvr-like mice, which are derived from flavivirus-resistant wild Mus musculus domesticus; and C3H.MOLD-Flvmr mice, which carry the resistance allele from MOLD/Rk mice (38, 45).
Flavivirus resistance in mice is controlled by a single locus, flavivirus resistance (Flv), on mouse chromosome 5 (31, 44). Although we have defined a narrow region on mouse chromosome 5 that carries the Flv locus by low- and high-resolution genetic and physical mapping, those studies have not clearly identified the critical boundaries of the Flv segment within a larger genomic region of ∼31 centimorgans that is polymorphic between susceptible C3H/He and resistant C3H.PRI-Flvr mice (42, 43, 45). We have recently determined that a differential sensitivity of C3H/He and C3H.PRI-Flvr mice to dopamine-induced hypothermia, previously assumed to be controlled by Flv (19), is conferred by a novel chromosome 5-linked locus, Diht, that was mapped distal to Flv to the same polymorphic region (39). At present, it is difficult to predict how many additional genes that reside at this large genomic interval are different between He and RV mice and whether some of these genes may also influence flavivirus replication in various cell types and tissues.
Recently, a gene family of eight members, all coding for a variant of oligoadenylate synthetase 1 (OAS1, A to H), has been characterized and mapped to a narrow chromosomal region in the vicinity of Flv (8, 15). Concurrently, a gene family member, Oas1b, which carries a premature stop codon in the fourth exon in susceptible mouse strains only, has been identified as a possible Flv candidate (3, 24, 27). While in vitro transfection of the intact Oas1b gene from resistant RV mice into mouse embryo fibroblasts derived from susceptible C3H/He mice caused only a partial inhibition of WN virus replication (27), the same gene obtained from wild flavivirus-resistant mice exerted a slightly greater inhibition of virus replication upon transfection into a neuronal cell line (20). Consistently, both sets of experiments showed a much more limited resistance effect than that conferred by Flv in vivo (45).
Extensive genetic and mechanistic studies suggested that a discrete intracellular mechanism interferes with either flavivirus replication at the level of viral single-stranded RNA synthesis or virion maturation during viral RNA packaging (46). It is well established that the genes of the Oas1 family are both alpha/beta interferon (IFN-α/β) and poly(I-C) (pIC) inducible, potentially contributing to the cellular antiviral effects via the ubiquitous OAS1/RNase L pathway (8, 15). Although genetically determined host resistance to flaviviruses was shown to be IFN-α/β independent following IFN-α/β depletion (2), pretreatment with IFN-α/β in a separate study has on the contrary induced a significant enhancement of flavivirus resistance both in vivo and in vitro (13). These two findings taken together indicate that IFN-α/β may be an amplifying rather than a critical factor of flavivirus-specific resistance (41).
Although IFN-α/β involvement in flavivirus resistance has remained controversial for a long time, here we provide a novel insight into this issue. In the present study, we have used primary macrophage cultures derived from both susceptible and resistant mice to investigate the kinetics and magnitude of IFN-α/β-induced antiviral effects in parallel to the induction of Oas1 genes as determined by Northern blot analysis and reverse transcription (RT)-PCR. In addition, we have studied the extent of the antiviral effect of the synthetic double-stranded RNA (dsRNA) pIC, alone or in combination with IFN-α/β. The findings presented here indicated that single, independent pretreatments with either IFN-α/β or pIC provoked a strong induction of the antiviral effect against WN virus in cells from resistant mice, while in cells from congenic susceptible mice, a similarly intensive antiviral effect against WN virus was induced only upon combined pretreatments with both agents.
MATERIALS AND METHODS
Materials.
Universal human-mouse IFN-α/β and mouse IFN-β as well as rabbit polyclonal antibodies (Ab) to mouse IFN-α and -β were purchased from PBL Biomedical Laboratories (Piscataway, N.J.); protein kinase R (PKR) inhibitor 2-aminopurine (2-AP); inducible nitric oxide synthase (iNOS) inhibitor NG-methyl-l-arginine (NMMA), synthetic polyinosinic-polycitidylic acid (pIC), 3,3′-diaminobenzidine tetrahydrochloride (DAB) with a Metal Enhancer tablet set, and monoclonal rabbit-raised anti-phospho-PKR (pThr451) were purchased from Sigma (St. Louis, Mo.). A secondary antibody, horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG), was purchased from Seemed Laboratory Inc. (San Francisco, Calif.). Radioactive compound [α-32P]dCTP and a GIGAprime Random Labeling kit were purchased from GeneWorks (Adelaide, Australia).
Viruses.
Human flavivirus WN virus strain Sarafend and encephalomyocarditis virus (EMCV) were obtained from our in-house collection. WN virus stock was derived from a single propagation through suckling mouse brains and double Vero cell culture propagations, while EMCV was maintained in the mouse fibroblast cell line L929.
Mice.
Four inbred mouse strains, flavivirus-susceptible C3H/HeJARC and congenic flavivirus-resistant strains C3H.PRI-Flvr, C3H.M. domesticus-Flvr-like, and C3H.MOLD-Flvmr (here designated He, RV, DUB, and MLD), were all obtained from specific pathogen-free stocks maintained at the Animal Resources Centre, Murdoch, Western Australia, and kept under barrier conditions. Adult mice 8 weeks of age and older of both sexes were inoculated intraperitoneally with 1 ml of 6% thioglycolate 3 days before peritoneal washouts were collected.
Primary cells.
Peritoneal macrophages obtained from thioglycolate-treated mice were seeded on 6-well plates at a density of 106 cells per well in 10% fetal calf serum (FCS)-RPMI medium overnight, and nonadherent cells were washed off on the following day as described previously (37). The adherent cells were shown to be 90 to 95% CD11b/CD18 positive (35). In order to achieve optimal infection with viruses, the cells were further maintained for 5 days under similar culture conditions to allow for the clearance of cytokines. No IFN-α/β and very low tumor necrosis factor alpha (TNF-α) levels were detected by IFN-α/β bioassay or TNF-α immunoassay, respectively, in these control noninfected macrophage cultures.
Peripheral blood mononuclear cells obtained from healthy donors (Red Cross), were suspended in RPMI medium-10% FCS and seeded in 80-cm2 tissue culture flasks to adhere for 1 h. Nonadherent cells were removed by several washings, while adherent cells were removed by cell scrapers and transferred to Teflon pots at a concentration of 106 cells/ml in RPMI medium supplemented with 10% FCS and 5% autologous human serum. After 6 to 7 days in culture, the cells exhibited macrophage-like morphology and tested positive for two macrophage-specific markers, CD14 and CD68, by flow cytometry (FACScalibur; Becton-Dickinson, San Jose, Calif.). At this stage, cells were plated on 6-well plates at 106 cells/ml in RPMI medium-10% FCS and used for infection with WN virus in the absence or presence of various inhibitors.
Cell priming, virus infection, and inhibitors.
Virus infection was conducted for 1 h at 37°C following a single pretreatment with either IFN-α/β for 24 h or pIC for 1 h. Alternatively, a combination of agents was used: IFN-α/β for 24 h followed by a 1-h treatment with pIC or antibodies to IFN-α/β for 5 days followed by a 1-h treatment with pIC with or without 2-AP and NMMA. Antibodies to IFN-α/β (200 IU of each antibody) and inhibitors 2-AP (2 mM) and NMMA (100 μM) were omitted only for 1 h during virus inoculation and were then present for the remainder of the experiment.
Pretreatment of primary macrophages with synthetic pIC was done for 1 h at concentrations of 1 to 100 μg of serum-free RPMI medium/ml in the absence of dextran-sulfate or any other transfection reagent. At various times postinfection (p.i.), cell culture supernatants were collected, aliquoted, and stored at −80°C to be later used for both virus titration and IFN-α/β bioassay. Biochemical responses of primary mouse macrophages to pIC and IFN-α/β priming were determined by either Western blot analysis for monitoring the levels of PKR phosphorylation (see below) or RT-PCR for monitoring the induction of Oas1 genes, respectively.
The concentration of IFN-α/β Ab was estimated by its inhibitory effect against an IFN-α/β standard of known concentration in the IFN-α/β bioassay (as described below). The optimal concentration of NMMA that inhibits nitric oxide (NO) production (100 μM) was determined on L929 cells primed to produce NO upon transfection with 25 μg of pIC/ml in the presence of dextran-sulfate.
Western blot analysis.
The extent of inhibition of PKR phosphorylation by 2 mM 2-AP on primary mouse macrophages was analyzed by Western blot analysis using rabbit anti-phospho-PKR antibody (1:500 dilution), specific for both human and mouse phosphorylated PKR (Sigma), as a primary antibody. As a secondary antibody, HRP-conjugated goat anti-rabbit IgG (Zymed Laboratory Inc., San Francisco, Calif.) was used at a dilution of 1:1,000. The cell monolayers were harvested in Laemmli sample buffer (106 cells/100 μl) and heated for 5 min at 95°C, and equal amounts were loaded onto 10% polyacrylamide gels in parallel with prestained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) protein standards (Broad Range; Bio-Rad, Hercules, Calif.) (18). Upon SDS-PAGE electrophoresis, the samples were transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.), and the membrane was blocked in 3% skim milk-phosphate-buffered saline for 60 min at room temperature before incubation with the primary antibody. Following incubation with the secondary antibody, blots were washed in phosphate-buffered saline and then soaked in DAB with a Metal Enhancer solution (Sigma) until a positive signal was developed (2 to 5 min).
Virus titration.
WN virus titers in cell culture supernatants were determined by 50% tissue culture infectivity dose (TCID50) bioassay on Vero cells as described previously (37). Similarly, EMCV was titrated by TCID50 in L929 cells. The sensitivity of the TCID50 assay for detection of accurate virus titers was at a threshold value of 2.0 log10 TCID50 units corresponding to 0.7 × 102 viral infectious particles per 100 μl. The virus detection was less accurate below 2.0 log10 TCID50, while below 0.1 × 102 infectious particles/100 μl, virus was not detectable at all by this assay.
IFN-α/β bioassay.
Interferon levels in cell culture supernatants were determined by a bioassay on mouse L929 cells as previously described (36). The principle of the assay is based on the ability of IFN-α/β to prevent a cytopathic effect (CPE) caused by EMCV on L929 cells. Serial double dilutions of IFN-α/β standard (PBL Biomedical Laboratories) and cell culture supernatants were added in parallel to the cell monolayers on 96-well plates for 20 h followed by infection with EMCV. Positive and negative controls were either noninfected cells or cells that were infected with the virus in the absence of any protection, respectively. The CPE induced by EMCV in the absence of any protection was observed as early as 24 h p.i., resulting in a complete detachment of cells in contrast to the healthy monolayers of noninfected cells. The 50% cell protection endpoints for samples containing either IFN-α/β standard of known concentration or macrophage supernatants having unknown amounts of IFN-α/β were determined in parallel by microscopic examination of cell monolayers following staining with 1% methylene blue in 10% formaldehyde, a modification of a method described previously (30). The IFN-α/β levels in cell supernatants were determined by plotting the observed 50% protection dose relative to the 50% protection dose of IFN-α/β standard of known concentration and expressed in international units (IU) per milliliter. The threshold dose of IFN-α/β determined by this assay was 2.5 IU.
In addition to IFN-α/β production, TNF-α levels were also monitored in cell culture supernatants following WN virus infection on day 3 p.i. by the Quantikine M mouse TNF-α immunoassay (R&D Systems, Minneapolis, Minn.). No rise in TNF-α levels was detected upon WN virus infection over the control values of noninfected cell cultures that were well below 10 pg/ml in macrophages from both resistant and susceptible mice, respectively.
Nitric oxide assay.
The NO levels in cell culture supernatants were determined by indirect measurement of its stable nonvolatile breakdown product, nitrite (NO2−), using the Griess reagent (Promega, Madison, Wis.) as previously described (37).
Total cellular RNA isolation and RT-PCR.
Total primary macrophage RNA isolated by a Total RNA Isolation reagent (FisherBiotec, Perth, Australia) and checked on native agarose gels in 1× Tris-boric acid-EDTA (TBE) buffer, pH 8.0, was used for RT-PCR. Equal amounts of 0.5 μg of total cellular RNA were subjected to RT-PCR in parallel by using a Platinum Quantitative RT-PCR ThermoScript One-Step system (Life Technologies, Carlsbad, Calif.) for a single RT cycle (50°C for 30 min and 94°C for 5 min) followed by a variable number of PCR cycles, all at 94°C for 30 s, 52 or 56°C for 30 s, and 68°C for 2 min as previously described (36). In brief, transcripts of the Oas1b gene were amplified by 35 PCR cycles at the annealing temperature of 52°C, while the remaining Oas1a mRNA and WN virus RNA were amplified at the annealing temperature of 56°C by 30 and 28 PCR cycles, respectively. Oligonucleotide primers for the Oas genes are as follows: for Oas1a, (GenBank accession no. X04958) sense primer (position 48) 5′-CTCAGGAGCATCCCAGCCTG-3′ and antisense primer (position 261) 5′-CTGACCTGCCCTTGAGTGTG-3′; for Oas1b (GenBank accession no. X55982), sense primer (position 380) 5′-GCTTTATGGGGCTTCAGAAG-3′ and antisense primer (position 564) 5′-GAATTTAGTTACTCGACTCCC-3′ (36); and for WN virus (GenBank accession no. NC_001563), sense primer (position 1182) 5′-TGCGTGTCCAACCATGGGTGAAGC-3′ and antisense primer (position ∼1790) 5′-GCGTGTGGTTCTCAAACTCCA-3′. Native 2% agarose gels were used for separation of PCR products in 1× TBE running buffer. The gels were stained with 0.1 μg of ethidium bromide/ml in 1× TBE and visualized under UV light. The gels were documented by using the Kodak (New Haven, Conn.) Electrophoresis Documentation and Analysis System 290.
Northern blot hybridization.
Total macrophage RNA (4 μg/lane) was separated under denaturing conditions on 1.2% agarose, 2.2 M formaldehyde gels for 3 h in 1× MOPS electrode buffer (20 mM morpholinopropanesulfonic acid, 8 mM sodium acetate, 1 mM EDTA [pH 7.0]) under constant voltage of 100 V. After capillary transfer to a Hybond NX membrane in 50 mM NaOH was done overnight, RNA was fixed to the membrane by baking at 65°C. Prehybridization and hybridization were performed in a Hybaid hybridization oven at 42°C for several hours without a labeled probe (prehybridization) or overnight with 32P-labeled corresponding cDNA probes (hybridization). Hybridization probes for Oas genes were obtained by RT-PCR from total mouse brain RNA by using a SuperScript One-Step RT-PCR system (Life Technologies) with primers as described above, gel purified, and labeled with [α-32P]dCTP and a GIGAprime Random Labeling kit (GeneWorks). Unincorporated label was removed by Sephadex G-50 columns. The most stringent wash used posthybridization was done with 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% SDS twice for 20 min at 65°C. The membranes were exposed to Fuji X-ray film with an intensifying screen for up to 5 days at −80°C.
Statistics.
Student's t test was used to determine the significance of differences in virus titers and IFN-α/β production between cell cultures derived from susceptible and resistant mouse strains during infection with WN virus as well as following various pretreatments.
RESULTS
Peritoneal macrophages as a cell model of flavivirus resistance.
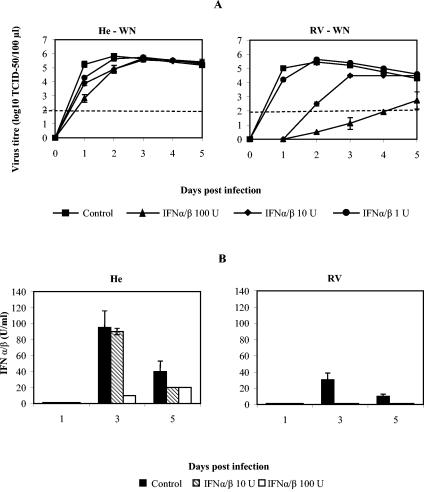
When primary peritoneal macrophages derived from mice either susceptible or resistant to flaviviruses were infected with WN virus, slightly higher virus titers were detected in supernatants of macrophages derived from susceptible He mice than from those of congenic resistant RV (Fig. 1A) and DUB mouse strains (unpublished data). This difference became more pronounced at later time points, especially on day 5 p.i. (5.0 log10 TCID50/105 cells from He versus 3.9 to 4.1 log10 TCID50/105 cells from RV and DUB mice, respectively [0.001 < P < 0.002; Student's t test), indicating only limited expression of flavivirus resistance similar in pattern, although much lower in magnitude, to that previously reported for the brains of the corresponding mouse strains (36, 45). WN virus infection did not affect cell viability of macrophages from both susceptible and resistant mice at any time point up to day 8 p.i., as determined by both the trypan blue exclusion assay and CPE monitoring (35; data not shown).
FIG. 1.
Dose-dependent effects of IFN-α/β on replication of WN virus and induction of IFN-α/β in macrophages from flavivirus-susceptible and congenic resistant mice. (A) Macrophages from susceptible He and resistant RV mice were exposed to various amounts of mouse IFN-α/β of 1, 10, and 100 IU 24 h before being infected with WN virus at an MOI of 1. Average values for virus titers were derived from four experiments with 9 to 12 replicas per treatment and time point. Student's t test indicated a significant difference between control values and 10 and 100 IU of IFN-α/β for He samples at days 1 and 2 p.i. (0.01 < P < 0.05 and 0.001 < P < 0.01). IFN-α/β in a 10-IU dose had a significant effect on virus replication at days 1 to 3 (P < 0.001), and IFN-α/β in a 100-IU dose had a very significant effect for all time points in all samples from resistant RV mice (P ≪ 0.001). Standard error bars are included where appropriate. A dashed line denotes a threshold value of 2.0 log10 TCID50 units for accurate detection of virus titers, corresponding to 0.7 × 102 infectious particles per 100 μl. Virus was detectable below the 2.0 log10 TCID50 threshold as presented by two arbitrary values: one at 1.0 log10 TCID50 units, corresponding to viral titers between 0.70 × 102 and 0.35 × 102 infectious particles, and the other at 0.5 log10 TCID50 units, corresponding to viral titers between 0.35 × 102 and 0.10 × 102 infectious particles inclusive, all per 100 μl of cell supernatant. Virus was not detectable below 0.1 × 102 infectious particles/100 μl by this assay. (B) IFN-α/β production following WN virus infection in the presence or absence of prior treatment with IFN-α/β. The effect of 24 h of pretreatment with various doses of IFN-α/β on IFN-α/β production in WN virus-infected macrophages from susceptible He and resistant RV mice is shown. IFN-α/β levels were determined by the interferon bioassay in L929 cells. Supernatant collected from four experiments and eight replicas were combined and tested in four replicas by this assay and used for calculation of average values. Standard error bars are included where appropriate. A threshold dose of IFN-α/β that was determined by this assay was 2.5 IU/100 μl of supernatant.
In addition, although equal numbers of macrophages from resistant and susceptible mice were infected as determined by an infectious center assay (35), fewer infected cells (2 to 5%) were found to express the viral NS1 protein in cell cultures derived from resistant mice than those derived from susceptible mice (5 to 8%) at days 2 and 3 p.i. by immunostaining (35) and flow cytometric analysis (data not shown), respectively. The proportion of virus-infected macrophages was not increased following infection at a higher multiplicity of infection (MOI) of 50, although the lower MOI of 0.1 resulted in a lower infection rate and decreased virus production in both cell cultures (35).
In contrast to the flavivirus WN virus, a nonflavivirus EMCV that also replicated well in the same macrophage model and infected the same mice did not show decreased virus replication in either macrophages or brains of the same flavivirus-resistant mice (data not shown). This finding confirms the previous findings that peritoneal macrophages from flavivirus-resistant mice express flavivirus-specific resistance, although of a much lower magnitude than that observed in brains in vivo (11, 37).
Effect of IFN-α/β pretreatment on WN replication in primary macrophages.
IFN-α/β is the major intracellular antiviral factor induced by the virus that in turn inhibits viral replication by either “autocrine” or “paracrine” action. To evaluate its effects on WN virus replication in macrophages from all four congenic mouse strains, the cells were primed by treatment overnight with various amounts of IFN-α/β (1, 10, or 100 IU per ml of cell medium) prior to infection with WN virus. While 1 IU of IFN-α/β had little effect, 10 and 100 IU had a significantly greater effect, especially on WN virus replication in macrophages from resistant RV mice, causing a dose-dependent inhibition of flavivirus replication (Fig. 1A). When a single dose of 100 IU of IFN-α/β was used to prime macrophages from MLD and DUB mice prior to infection with WN virus, a very strong antiviral response that has blocked viral replication on day 1 in cells from moderately resistant MLD mice and up to day 5 p.i. in cells from resistant DUB mice was observed (unpublished data). These findings indicated a very significant enhancement of flavivirus resistance in macrophages derived from two resistant mouse strains, RV and DUB, upon priming with IFN-α/β that has reached the levels of resistance observed in brains of the corresponding mouse strains (45).
It is possible that the relative resistance of macrophages from susceptible He mice to the IFN-α/β-induced antiviral effect against WN virus is caused by an inability of these cells to respond to IFN-α/β priming. Accordingly, we compared the effect of IFN-α/β priming on the induction of antiviral responses to EMCV in macrophages from susceptible He mice and resistant RV mice (unpublished data). Indeed, IFN-α/β elicited equally strong dose-dependent antiviral effects to EMCV in macrophage cultures derived from both susceptible and resistant mice (unpublished data). These data demonstrated that macrophages from He and RV mice possess equal potential to develop IFN-α/β-induced responses.
Although priming with IFN-α/β had a very profound effect on flavivirus resistance, flavivirus replication was also shown to be a strong inducer of IFN-α/β in vivo (36). Here, we used the IFN-α/β bioassay to monitor the extent and duration of IFN-α/β induction in primary macrophages from flavivirus-resistant RV and susceptible He mice upon infection with WN virus following pretreatments with either control RPMI medium or 10 and 100 IU of IFN-α/β (Fig. 1B). Infection with WN virus induced much stronger IFN-α/β synthesis in macrophages from susceptible He mice than in macrophages from resistant RV mice (Fig. 1B). This result agrees with our earlier findings on IFN-α/β synthesis in the brains of the corresponding mouse strains following infection with MVE or Kunjin virus (36; R. H. Shueb and N. Urosevic, unpublished data). The strong induction of IFN-α/β by WN virus in macrophages from susceptible He mice that commenced on day 2 (data not shown) and was detected until day 5 p.i. (Fig. 1B) appeared to be a result of steady WN virus replication, since no IFN-α/β synthesis was detected in parallel samples of noninfected cells at any time point during the cultivation period (data not shown). Priming with both IFN-α/β doses, 10 and 100 IU, prevented induction of IFN-α/β synthesis in macrophages from resistant RV mice upon WN virus infection (Fig. 1B), probably by blocking WN virus replication (Fig. 1A). In He macrophages, only priming with 100 IU of IFN-α/β affected IFN-α/β synthesis on day 5 p.i. (Fig. 1B), probably by indirectly reducing virus titers since this IFN-α/β dose had the greatest inhibitory effect on flavivirus replication (Fig. 1A).
Effect of pIC pretreatment on WN replication in primary macrophages.
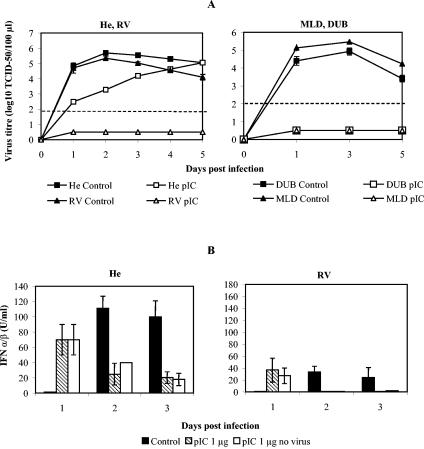
In order to obtain further insight into the ability of mouse macrophages from flavivirus-susceptible and -resistant mice to develop an antiviral state, we also used priming with the synthetic double-stranded RNA pIC. Priming with pIC exerts pleiotropic effects on the cell by activating parallel Toll-like receptor 3-mediated antiviral pathways in addition to IFN-α/β induction (7). Pretreatment of mouse macrophages with 1 μg of pIC/ml prior to infection with WN virus induced very strong antiviral responses in cells from all three flavivirus-resistant mouse strains that almost completely prevented WN virus replication at any time point for up to day 5 p.i. (Fig. 2A). The antiviral effect against WN virus in macrophages from flavivirus-susceptible He mice was very pronounced on days 1 and 2 p.i. but was lost by day 5 p.i. (Fig. 2A).
FIG. 2.
Effect of pIC on virus replication and IFN-α/β production in peritoneal macrophages derived from congenic mouse strains. A dose of 1 μg of pIC/ml was used to pretreat the cells for 1 h at 37°C followed by infection with WN virus for 1 h at an MOI of 1. (A) Effect of pIC on WN virus replication in cells from susceptible He and resistant RV mice and moderately resistant MLD and highly resistant DUB mice. Average values for WN virus titers were derived from four experiments with eight replicas per treatment and time point for He and RV samples, while data for DUB and MLD were derived from one experiment with two to four replicas per pretreatment and time point. Student's t test indicated significant changes upon pIC treatment in He samples up to day 4 p.i. (P < 0.001), while in samples from RV, DUB, and MLD mice, these changes were very significant for all time points (P ≪ 0.001). A dashed line denotes a threshold value of 2.0 log10 TCID50 units for accurate detection of virus titers, while virus present below the threshold value was detected as described in the legend for Fig. 1A. (B) Effect of a 1-h pretreatment with pIC on IFN-α/β production in macrophages from susceptible He and resistant RV mice in the presence and absence of infection with WN virus. IFN-α/β levels were determined by the interferon bioassay in L929 cells. Supernatant collected from four experiments and eight replicas were combined and tested in four replicas by this assay and used for the calculation of average values. Standard error bars are included where appropriate. A sensitivity of the assay was at a threshold dose of 2.5 IU for IFN-α/β per 100 μl of sample supernatant.
To estimate to what extent the pIC effect was mediated by IFN-α/β, IFN-α/β induction was monitored in macrophage culture supernatants from susceptible and resistant mice following priming with pIC in the presence or absence of WN virus infection (Fig. 2B). One microgram of pIC per milliliter served as an early and strong inducer of IFN-α/β synthesis in both He and RV macrophages as early as 6 h (data not shown) and up to 24 h postpriming, regardless of the presence of infection (Fig. 2B). In contrast, WN virus infection alone induced much higher IFN-α/β synthesis than pIC priming, although as late as day 2 p.i. (Fig. 2B). When pIC priming was combined with WN virus infection, IFN-α/β induction was similar in kinetics and magnitude to that induced by pIC priming alone (Fig. 2B), suggesting that this early IFN-α/β induction possibly contributed to the direct inhibitory effect of pIC on WN virus replication (Fig. 2A) and to its indirect effect on the IFN-α/β synthesis at later time points p.i. (Fig. 2B). The fact that pIC in combination with WN virus infection induced much lower IFN-α/β levels in RV macrophages than in He macrophages was not surprising in terms of previous findings related to the effect of WN virus infection alone on IFN-α/β induction. However, it was unexpected that pIC alone would induce different IFN-α/β levels in macrophages from susceptible and resistant mice even in the absence of virus infection (Fig. 2B).
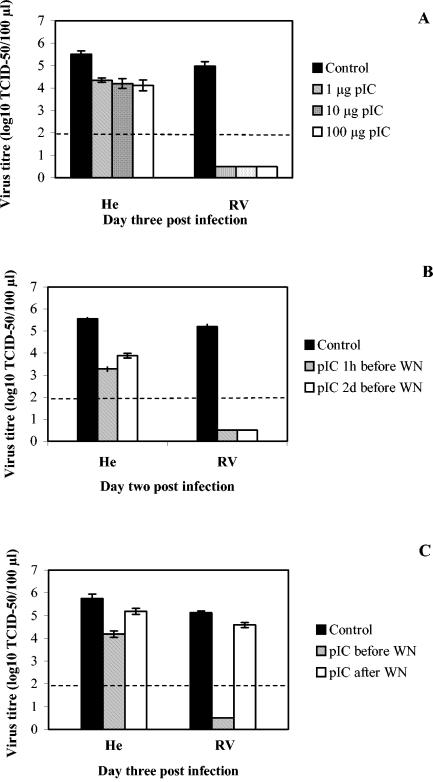
To further characterize the antiviral effect of pIC against WN virus, we used different doses or regimens of treatments. Doses of 1, 10, and 100 μg/ml had a similar effect on WN virus replication in cell cultures from both resistant and susceptible mice (Fig. 3A). A dose of pIC as little as 0.1 μg/ml was sufficient to elicit the maximal antiviral response against WN virus (data not shown). Poly(I-C) did not induce NO production or any CPE in macrophages upon WN virus infection at any concentration (data not shown), as previously reported for noncytopathic pestiviruses (33). In addition, there was no dose-dependent difference in the amounts of IFN-α/β induced upon pIC priming with different doses of pIC (data not shown). Priming with 1 μg of pIC/ml for 1 h had induced a similarly strong antiviral effect against WN virus infection in cells from resistant RV mice both immediately upon priming and 2 days postpriming, while this effect was reduced in cells from susceptible mice when infection was postponed for 2 days (Fig. 3B). In contrast, application of pIC treatment following WN infection had a much smaller antiviral effect than priming the cells with pIC (Fig. 3C). Taken together, these findings suggest that the antiviral effect elicited by pIC is IFN-α/β dependent and long lasting and targets early steps in flavivirus replication.
FIG. 3.
Dose dependence, timing, and duration of pIC antiviral effect on replication of WN virus in macrophages from both susceptible He and resistant RV mice. (A) Three different doses of pIC, 1, 10, and 100 μg/ml, were used to treat macrophages for 1 h prior to infection with WN virus. Virus titers were determined by TCID50 at day 2 p.i. Average virus titers values were derived from two experiments with five replicas. Student's t test indicated significant changes for He samples for all pIC doses (0.001 < P < 0.01) and very highly significant changes for all RV samples (P ≪ 0.001). (B) One microgram of pIC per milliliter was applied 1 h or 2 days (2d) prior to infection with WN virus for 1 h. Virus titers were determined by TCID50 at day 2 p.i. Average values for virus titers were derived from one experiment with two replicas. (C) One microgram of pIC per milliliter was applied 1 h prior to infection or 1 day postinfection with WN virus for 1 h. Virus titers were determined by TCID50 at day 3 p.i. Average virus titer values were derived from three experiments with seven replicas indicating very significant changes for all treatments (P < 0.001; Student's t test). Standard error bars are included where appropriate. A dashed line denotes a threshold value of 2.0 log10 TCID50 units for accurate detection of virus titers, while virus present below the threshold value was detected as described in the legend for Fig. 1A.
Effect of inhibitors on antiviral responses induced by IFN-α/β and pIC.
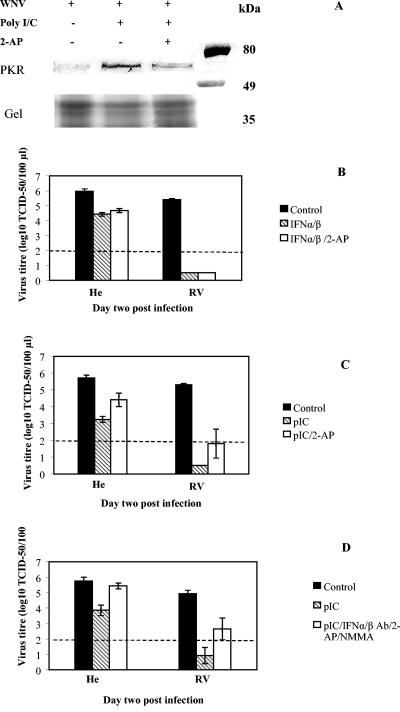
The inhibitory effect of IFN-α/β and pIC on virus replication is mediated by a number of intracellular effectors including PKR and OAS1/RNase L. Since 2-AP was shown to be a strong PKR inhibitor (28, 40), we used this inhibitor prior to WN virus infection and concomitant with IFN-α/β and pIC priming. The concentration of 2-AP used was not toxic to the cells. As shown in Fig. 4A, 2-AP inhibited PKR phosphorylation induced by pIC in macrophages 24 h postpriming in the absence of WN virus infection. This was determined by the Western blot analysis with rabbit anti-phospho-PKR (pThr451) antibody on nylon membranes containing equal amounts of cellular proteins from control cells and cells treated with either pIC alone or pIC in the presence of 2-AP (Fig. 4A). When applied simultaneously with IFN-α/β, it did not significantly affect the antiviral effect of IFN-α/β in cell cultures derived from either susceptible He or resistant RV mice, suggesting that PKR did not contribute to IFN-α/β-induced antiviral responses against WN virus (Fig. 4B).
FIG. 4.
PKR contribution to antiviral responses against WN virus upon priming with either IFN-α/β or pIC in primary mouse macrophages from susceptible and resistant mice. (A) Western blot analysis of PKR activity. The levels of phosphorylated PKR were analyzed by Western blot in macrophage lysates obtained from susceptible mice. Cells were isolated and cultivated on 6-well tissue culture plates as described in Materials and Methods. Priming with 1 μg of pIC/ml was performed for 1 h at 37°C in the absence or presence of 2 mM 2-AP. Control cells did not have any treatment. Twenty-four hours later, the cells were lysed in 100 μl of Laemmli sample buffer, and equal amounts were loaded onto SDS-10% PAGE gel in parallel with protein standards (Prestained Broad Range; Bio-Rad). The molecular weights in kilodaltons of the protein standards bovine serum albumin (80 kDa), ovalbumin (49 kDa), and carbonic anhydrase (35 kDa) are shown on the right. The amount of cellular proteins applied was additionally estimated by Coomassie blue staining of the gel below the ovalbumin protein standard. Monoclonal rabbit anti-phospho-PKR (pThr451) antibody (Sigma) was used as a primary antibody, while HRP-conjugated goat anti-rabbit IgG was used as a secondary antibody (Zymed Laboratory). (B and C) Effects of 2-AP inhibitor on IFN-α/β-induced (B) and pIC-induced (C) antiviral responses were determined on day 2 p.i. Average virus titer values were derived from two experiments with four replicas. Student's t test indicated significant differences in virus titers between control samples and each of the treatments (P < 0.001), while no significant difference was estimatedbetween other treatments (P > 0.1). (D) Effect of IFN-α/β Ab in the absence or presence of 2-AP and NMMA inhibitors on the antiviral responses induced by pIC was determined on day 2 p.i. Average values are derived from three experiments and a minimum of six replicas per treatment. Standard error bars are included where appropriate. Student's t test indicated significant difference between virus titers in control infections and those derived from pIC-primed cells for both susceptible and resistant cell cultures (0.001 < P < 0.01 and P < 0.001, respectively), while simultaneous treatments with IFN-α/β Ab, 2-AP, and NMMA did not restore pIC-inhibited WN virus replication back to the control values in cells from resistant mice (P = 0.02). A dashed line denotes a threshold value of 2.0 log10 TCID50 units for accurate detection of virus titers, while virus present below the threshold value was detected as described in the legend for Fig. 1A.
We also used 2-AP to determine the contribution of PKR to pIC-induced antiviral responses against WN virus. It is well established that pIC has numerous effects in the cell such as the induction of IFN-α/β synthesis via Toll-like receptor 3 signaling, activation of both PKR and OAS enzymes by serving as their dsRNA cofactor, and induction of iNOS activity and NO synthesis in cooperation with IFN-γ (1, 22). Due to these various effects, dsRNA may impose its antiviral effect via several pathways. We expected that application of the 2-AP and iNOS inhibitor NMMA would prevent PKR and iNOS contributing to the antiviral state against WN virus elicited by pIC. 2-AP caused an increase in virus titers in both cell cultures upon treatment (Fig. 4C), indicating that the antiviral effect of pIC is partially inhibited in the presence of the PKR inhibitor, suggesting a significant contribution of PKR to the overall pIC-induced antiviral effect (0.001 < P < 0.01; Student's t test [Fig. 4C]). Single treatment with NMMA did not have any significant effect on either the control or the reduced infection with WN virus following priming with either IFN-α/β or pIC, further arguing against any involvement of NO in the control of flavivirus infection and resistance, confirming our previous findings (37).
In addition to direct activation of PKR and OAS, pIC also induces IFN-α/β synthesis (Fig. 2B) that may further amplify its antiviral effect. To dissect a direct antiviral effect of pIC from the indirect one mediated by IFN-α/β, we have used polyclonal antibodies to murine IFN-α and -β in combination with pIC to block IFN-α/β effects. Surprisingly, the combined treatment with pIC and IFN-α/β Ab caused significant induction of iNOS enzyme and high production of NO in macrophages from both susceptible and resistant mice (data not shown). The high NO production caused an immediate inhibition of WN replication (data not shown) that masked parallel effects elicited by the treatments with pIC and IFN-α/β Ab. To circumvent this, we have used 2-AP and NMMA together with pIC and IFN-α/β Ab (Fig. 4D). As a result of this combined pretreatment, inhibition of WN virus replication by pIC was completely inhibited in cells from susceptible He mice, resulting in a full restoration of virus replication back to the control values (Fig. 4D). This result indicated that the antiviral effect of pIC against WN virus in susceptible cells was mediated by both PKR and IFN-α/β. In contrast, in parallel macrophage cultures derived from resistant RV mice, pretreatment with the same set of inhibitors and IFN-α/β Ab in the presence of pIC only partially restored WN replication (Fig. 4D) (P = 0.02; Student's t test). These findings suggested that in addition to PKR and IFN-α/β-regulated pathways, pIC activates an IFN-α/β-independent antiviral mechanism(s) that cannot be inhibited by 2-AP, NMMA, or IFN-α/β Ab in cells from resistant mice.
OAS1 gene induction.
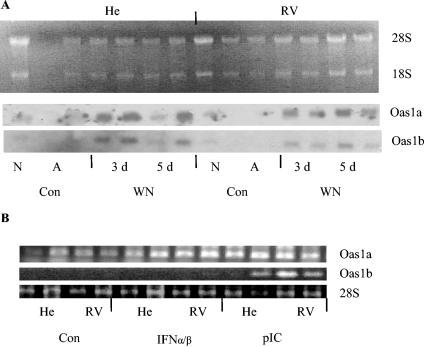
To estimate the effect of WN virus infection on the induction of Oas1 genes, we performed a Northern blot analysis of total cellular RNA isolated from macrophages from susceptible and resistant mice. Samples from nonadherent macrophages immediately upon isolation (Fig. 5A, lanes N), adherent macrophages 24 h postcultivation (Fig. 5A, lanes A), and WN-infected macrophages at either 3 or 5 days p.i. (Fig. 5A, lanes 3d and 5d, respectively) were analyzed in parallel. The hybridization probes were obtained by RT-PCR and were specific to either Oas1a or Oas1b genes. Following hybridization, these probes revealed similar patterns of gene induction for both genes upon virus infection in cells from both susceptible and resistant mice (Fig. 5A).
FIG. 5.
Northern blot and RT-PCR analyses of Oas1a and Oas1b gene transcripts during WN virus infection and following priming with IFN-α/β and pIC. (A) Total cellular RNA (4 μg) from noninfected, nonadherent macrophages (N) (one lane); noninfected, adherent macrophages (A) (two lanes); and WN virus-infected macrophages at days 3 (3d) (two lanes) and 5 (5d) (two lanes) p.i. was separated on a 1.2% agarose denaturing gel in the presence of 2.2 M formaldehyde. After capillary transfer to Hybond NX membrane, the blot was hybridized to 32P-labeled cDNA probes for either Oas1a or Oas1b genes, respectively. The same blot was reused after stringent stripping of the probe and testing for the absence of remaining activity by extended autoradiography. The cells isolated from susceptible He and resistant RV mice were either infected (WN) or not infected (Con) with WN virus. (B) Effects of priming with 100 IU of IFN-α/β for 24 h or with 1 μg of pIC/ml for 1 h on Oas1a and Oas1b gene induction prior to WN virus infection. Total cell RNA from two parallel replica samples (106 cells each) was isolated 24 h posttreatment and used in RT-PCR. Reaction products were analyzed by agarose gel electrophoresis. The total cellular RNA was also analyzed by agarose gel electrophoresis, and the amount of 28S rRNA was used as an internal control.
Since Oas1a and Oas1b genes carry an extensive homology of up to 70% which may result in cross-hybridization during Northern blot analysis, we used RT-PCR with unique 20-nucleotide primers to monitor the specific gene induction following IFN-α/β or pIC priming before and after infection with WN virus. Transcriptional induction of cellular genes Oas1a and Oas1b upon priming with IFN-α/β and pIC in the absence of WN virus infection revealed an unexpected pattern: while Oas1a transcripts were strongly induced by both IFN-α/β and pIC, Oas1b transcripts were induced only by pIC in both macrophage cultures (Fig. 5B).
Effect of combined IFN-α/β-pIC pretreatments on WN virus replication in macrophages from susceptible mice and humans.
Since single treatments with IFN-α/β and pIC were not efficient in the prevention of flavivirus replication in cells from susceptible He mice, alternative strategies including priming with increased doses of IFN-α/β or pIC were explored. However, complete prevention of flavivirus replication in susceptible cells was not achieved even with doses as high as 100 μg of pIC/ml (Fig. 3A) or 500 IU of IFN-α/β in a single treatment (data not shown).
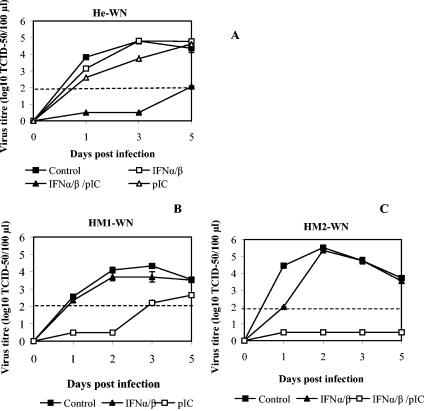
Alternatively, combined pretreatments with lower doses of IFN-α/β and pIC were also considered. For this, we used both IFN-α/β and pIC for priming of He macrophages prior to WN infection, since those agents are believed to induce parallel antiviral pathways that could complement each other and provide better protection than pathways induced by a single agent. As shown in Fig. 6A, when cells from susceptible He mice were primed for 24 h with 10 IU of IFN-α/β followed by a 1-h treatment with 1 μg of pIC/ml, very low virus growth below the 2.0 log10 TCID50 threshold value was observed up to day 5 p.i. This result confirmed our expectations that a combined effect of IFN-α/β and pIC is significantly stronger than the effect of single treatments (P < 0.001; Student's t test).
FIG. 6.
Prevention of viral replication in cells from susceptible He mice and human donors by various antiviral agent combinations. (A) Mouse peritoneal macrophages from susceptible He mice were treated with either IFN-α/β or pIC alone or in combination. Virus titers were monitored up to day 5 p.i. by TCID50 assay. Average values for all treatments for day 2 p.i. were obtained from three experiments with six replicas, while data for the remaining time points were derived from one experiment with three replicas. (B and C) Human macrophages maintained as primary cultures were pretreated with either 10 IU of human IFN-α/β for 24 h or 1 μg of pIC/ml for 1 h prior to infection with WN virus (B), or primary macrophages obtained from another healthy donor were pretreated with 100 IU of human IFN-α/β for 24 h followed by incubation with either pIC or RPMI medium for 1 h prior to infection with WN virus (C). Virus titers were determined by TCID50 at various times p.i. Average values for human macrophages were obtained from single experiments with two replicas each. The type and number of pretreatments on human macrophages were affected by the limited availability of human cells. A dashed line denotes a threshold value of 2.0 log10 TCID50 units for accurate detection of virus titers, while virus present below the threshold value was detected as described in the legend for Fig. 1A.
In addition to primary mouse macrophages from susceptible mice, we have also studied the requirements of primary human macrophages for antiviral stimuli sufficient to elicit strong antiviral responses to WN virus. We have obtained monocyte-derived human macrophage cultures from two independent healthy volunteer donors and infected them in culture with the same WN virus under experimental conditions similar to those used for mouse macrophages. After 5 days in culture, cells were pretreated with either IFN-α/β or pIC alone or in combination prior to infection with WN virus. While nontreated, control macrophages from both donors supported WN replication, IFN-α/β pretreatment at a low dose of 10 IU/ml had only a minor inhibitory effect on virus growth in both human macrophage cultures (Fig. 6B and C). Surprisingly, in macrophages derived from one of the donors, a single pIC pretreatment had a strong antiviral effect on WN virus replication (Fig. 6B) similar to the effect elicited by the same pIC dose in macrophages from resistant mice. In macrophages from the other donor, simultaneous priming with a low IFN-α/β dose (10 IU) and pIC had a very strong inhibitory effect (Fig. 6C) similar to the one observed in cells from susceptible mice.
DISCUSSION
Here, we describe a cell model of primary peritoneal macrophages from flavivirus-susceptible and congenic resistant mice that was used to study the effect of priming with IFN-α/β or pIC on natural resistance to flaviviruses. The ability of mouse peritoneal macrophages to express natural flavivirus resistance has been previously described in flavivirus-resistant BC-8 backcross mice generated during RV strain development (11), as well as in RV and DUB mice carrying the allelic variants Flvr and Flvr-like, respectively (37). In the present study, we have determined a significant amplification of flavivirus-specific resistance against a flavivirus WN virus following both IFN-α/β and pIC priming in peritoneal macrophages from flavivirus-resistant RV mice, in contrast to the induction of equal antiviral responses against a picornavirus, EMCV, by the same agents in macrophages from both mouse strains.
The life cycle of flaviviruses is prolonged in comparison to other positive-strand RNA viruses including EMCV, resulting in a much slower rate of the replicative RNA formation and induction of dsRNA pathways in the infected cell than with other viruses. Indeed, IFN-α/β synthesis in macrophages from susceptible and resistant mice induced by WN virus infection was first detected as late as 2 days p.i., unlike EMCV, which induced IFN-α/β as early as 14 h p.i. (data not shown). Such a late induction of IFN-α/β synthesis during flavivirus infection suggests very little contribution of IFN-α/β to autocrine control of WN virus infection in vivo, especially in light of the recent findings of Diamond and colleagues indicating that the effect of IFN-α/β treatment on DEN virus infection in different cell cultures occurs very early in the virus life cycle (6). The same authors also suggested that the IFN-α/β-induced antiviral effect most probably occurs during viral polyprotein synthesis, preventing virus negative-strand RNA accumulation by the mechanism independent of PKR and RNase L (5). A similar antiviral effect of IFN-α/β has been recently reported to restrict hepatitis C virus replicon synthesis by reducing the translational efficiency of viral RNA, although this effect was mediated by PKR and cytosolic protein p56 (47).
In our macrophage model, WN virus infection is restricted to only a small population of permissive cells with a very limited spread (35). WN virus-induced IFN-α/β synthesis occurs very late, allowing for a segregation of autocrine from paracrine IFN-α/β effects. While the autocrine effects take part in the control of WN virus replication in unprimed infected macrophages, the paracrine effects are mimicked by IFN-α/β priming prior to infection. In agreement with previous findings (6), we observed the greatest effect of IFN-α/β on flavivirus replication when it was applied before infection, showing at the same time the greatest potentiation of the resistance conferred by Flv. This observation suggests that IFN-α/β is indeed an amplifying factor of flavivirus resistance, inducing a significantly stronger flavivirus-specific antiviral effect in resistant RV mice as well as cell cultures derived from them, in contrast to induction of an inefficient flavivirus-specific antiviral effect in mice and cells susceptible to flaviviruses (2, 13, 41). Furthermore, this finding highlights the role of IFN-α/β produced by the virus-infected cells in amplifying the natural resistance in vivo by a paracrine action on noninfected neighboring cells. The PKR inhibitor 2-AP did not interfere with either the paracrine or autocrine effects of IFN-α/β on WN virus replication in macrophages from susceptible and resistant mice, indicating that PKR plays only a minor role in the IFN-α/β-induced antiviral effects against WN virus, as previously reported for DEN and classical swine fever viruses (6, 16).
While both the WN virus and pIC induced a lower level of IFN-α/β synthesis in macrophages from resistant RV mice than in cells from susceptible He mice, the Oas1a/Oas1b gene induction was similar in both cell cultures, suggesting alternative mechanisms of gene regulation for these genes. A possible explanation for this discrepancy could be that either different transcription factors are involved in the induction of Oas1 (23) and IFN-α/β genes (48) or the transcripts of these genes show different stability in cells from susceptible and resistant mice.
Similar inducibility of Oas1 genes by pIC in cells from susceptible and resistant mice presented here is supported by the recent analysis of the Oas1b promoter region in genomic DNA from various WN virus-resistant and -susceptible mouse strains (23). This analysis has revealed the absence of any particular sequence difference within the Oas1b promoter that segregated between WN virus-susceptible and -resistant mouse strains (23), suggesting similar inducibility and usage of similar transcription factors during induction of this gene in cells from flavivirus-resistant and -susceptible mouse strains. In contrast, unequal induction of IFN-α/β gene expression by pIC in cells from susceptible and resistant mice may result from either an asymmetric transcriptional induction (14) or from an unequal stability and availability of IFN-α/β mRNA for translation.
It is well established from microarray analyses that IFN-α/β and dsRNA, in addition to commonly induced genes, may also induce separate sets of genes (4, 10). Interestingly, in the experiments in which pIC-specific antiviral effects were dissected from IFN-α/β-specific antiviral effects by using IFN-α/β Ab to block effects of IFN-α/β, an excessive production of NO and a great reduction in WN virus replication occurred, suggesting activation of iNOS that may have masked the effect of IFN-α/β Ab (data not shown). The antiviral effect of high levels of NO against a broad spectrum of viruses, including flaviviruses MVE and JE in cell culture (32, 37), has been documented. Induction of NO production in response to dsRNA is mediated by active PKR in macrophage cultures (22), while IFN-α/β was shown to down-regulate NO induction (17). The application of IFN-α/β Ab to deplete IFN-α/β in primary macrophage cultures from both mouse strains elicited an unwanted NO synthesis that was prevented by the PKR inhibitor 2-AP and iNOS inhibitor NMMA. The inclusion of these inhibitors in the presence of IFN-α/β Ab completely restored WN virus replication in cells from susceptible He mice. However, in cells from resistant RV mice, residual resistance against WN virus remained following this pretreatment, suggesting the existence of a unique pIC-inducible antiviral factor that is active only in RV macrophages. This factor may well be OAS1B based on the results of the gene induction analyses described here as well as on the previously reported data on the Oas1b gene promoter analyses (23). Since 2-AP, which failed to restore flavivirus replication in resistant macrophages, is known to partially inhibit the OAS enzymes by preventing ATP binding to their active site rendering them inactive, similar to inactivation of PKR (28), an alternative explanation could be that the residual antiviral activity observed in macrophages from resistant RV mice upon induction with pIC may have resulted from OAS1B activity other than the synthesis of oligoadenylates. Alternatively, it could also be that another gene from the large chromosomal region polymorphic between He and RV mice may have contributed to this effect (41, 45).
During the preparation of the manuscript, IFN regulatory factor 3 (IRF-3), another dsRNA-related protein, was reported to have an effect on WN virus spread between cells in culture (9). Although IRF-3 was efficient in limiting the virus spread during acute infection of various mouse and human cell lines with WN virus NY strain, it did not affect virus titers up until day 4 p.i (9). It would be very interesting to investigate IRF-3 activation in macrophages from resistant and susceptible mice following pIC priming and WN virus infection, especially in light of uneven IFN-α/β induction and virus replication between these cell cultures.
The most important finding in this study is that the combined treatment with IFN-α/β and pIC completely prevented WN virus replication in macrophages from susceptible He mice, in direct contrast to the outcomes of individual treatments in the same cell model. This result may suggest that a dsRNA cofactor is required following IFN-α/β priming of He macrophages, a constraint which is not observed in macrophages from resistant mice. The reason for this is unknown, especially in light of the fact that the virus replicates in susceptible He cells to higher titers. At present, we could only speculate that in resistant cells, there is a great accumulation of viral double-stranded replicative-form RNA not actively involved in replication, as we previously reported for the brains of resistant mice (45). It remains to be resolved by future experiments whether an unequal accessibility of viral dsRNA in macrophages from susceptible and resistant mice determines their different abilities to express various levels of resistance to flaviviruses.
In macrophages from healthy human donors, we have observed resistance to the IFN-α/β antiviral effect similar to that in macrophages from susceptible mice. While the combined IFN-α/β-pIC treatment of cells from one donor induced a drop in virus replication that was similar to that in macrophages from susceptible mice, a single treatment with pIC of cells from another donor was sufficient to induce a response similar to that in cells from resistant mice. This result may reflect a different extent of inborn susceptibility to flaviviruses in these two human donors. This approach to testing susceptibility to flavivirus infection in humans may have great potential, although it would be necessary to extend this study further and to include a greater number of human donors and regimens of the in vitro treatments before complete evaluation of the reproducibility and reliance of this macrophage assay for prediction of the levels of inborn susceptibility to flavivirus infection in humans is achieved.
In conclusion, results presented here indicate complex interactions between antiviral stimuli, intracellular factors, and virus replication in macrophages from resistant and susceptible mouse strains. Comparative analyses of flavivirus replication, IFN-α/β production, and Oas1 gene induction between these parallel cell culture models revealed that (i) macrophages from all four congenic mouse strains supported WN virus replication, although at various levels that were greatly influenced by the pretreatments with either IFN-α/β or pIC, resulting in the expression of resistance-susceptibility phenotypes similar to those observed in the brains; (ii) WN virus replication caused a prominent, although delayed, induction of IFN-α/β that was more abundant in cells supporting greater virus replication carrying Flvs; (iii) pIC induced early IFN-α/β synthesis that was unexpectedly lower in cells from resistant mice even in the absence of WN virus infection; (iv) both IFN-α/β and pIC completely prevented WN virus replication in cells from resistant (Flvr) mice when applied individually, while the same effect was obtained by the combined IFN-α/β-pIC treatment in cells from susceptible (Flvs) mice; (v) the major Flv candidate, Oas1b, shows IFN-α/β-independent but WN virus- and pIC-dependent induction; and (vi) the other gene candidate, Oas1a, which is intact in both susceptible and resistant mice, shows strong IFN-α/β-dependent induction.
These findings are very important for a better understanding of flavivirus infection and its prevention at the cellular level, providing a clue to successful antiviral therapy. In brief, despite an active IFN-α/β-inducible antiviral mechanism that is capable of preventing infections with other viruses, flavivirus-susceptible hosts lack an intrinsic ability to resist infection with flaviviruses that is controlled by the host genetic locus Flv. In addition, flaviviruses have adopted a strategy to down-regulate IFN-α/β-stimulated gene expression by the viral proteins NS4B, NS2A, and NS4A (26). The data presented here clearly indicate that an additional pretreatment with dsRNA combined with IFN-α/β priming may result in a more efficient usage of IFN-α/β-induced antiviral pathways leading to full protection from flavivirus infection of the susceptible host in the absence of the host resistance factor Flvr, despite the possible inhibitory effect of several viral protein factors.
Acknowledgments
We thank M. W. Beilharz for advice and assistance with the interferon bioassay and for the provision of murine IFN-α/β and Helen Currie for kindly providing human monocyte-derived macrophages. We are also grateful to G. R. Shellam, M. W. Beilharz, and A. Redwood for the critical reviews of the manuscript.
N.U. is a Research Fellow of the Western Australian Institute of Medical Research (WAIMR).
REFERENCES
- 1.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 2.Brinton, M. A., H. Arnheiter, and O. Haller. 1982. Interferon independence of genetically controlled resistance to flaviviruses. Infect. Immun. 36:284-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinton, M. A., and A. A. Perelygin. 2003. Genetic resistance to flaviviruses. Adv. Virus Res. 60:43-85. [DOI] [PubMed] [Google Scholar]
- 4.Der, S. D., A. Zhou, B. R. G. Williams, and R. H. Silverman. 1998. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:15623-15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamond, M. S., and E. Harris. 2001. Interferon inhibits dengue virus infection by preventing translation of viral RNA through a PKR-independent mechanism. Virology 289:297-311. [DOI] [PubMed] [Google Scholar]
- 6.Diamond, M. S., T. G. Roberts, D. Edgil, B. Lu, J. Ernst, and E. Harris. 2000. Modulation of dengue virus infection in human cells by alpha, beta, and gamma interferons. J. Virol. 74:4957-4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle, S. E., S. A. Vaidya, R. O'Connell, H. Dadgostar, P. W. Dempsey, T. T. Wu, G. Rao, R. Sun, M. E. Haberland, R. L. Modlin, and G. Cheng. 2002. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17:251-263. [DOI] [PubMed] [Google Scholar]
- 8.Eskildsen, S., R. Hartmann, N. O. Kjeldgaard, and J. Justesen. 2002. Gene structure of the murine 2′-5′-oligoadenylate synthetase family. Cell. Mol. Life Sci. 59:1212-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredericksen, B. L., M. Smith, M. G. Katze, P. Y. Shi, and M. Gale, Jr. 2004. The host response to West Nile virus infection limits viral spread through the activation of the interferon regulatory factor 3 pathway. J. Virol. 78:7737-7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geiss, G., G. Jin, J. Guo, R. Bumgarner, M. G. Katze, and G. C. Sen. 2001. A comprehensive view of regulation of gene expression by double-stranded RNA-mediated cell signalling. J. Biol. Chem. 276:30178-30182. [DOI] [PubMed] [Google Scholar]
- 11.Goodman, G. T., and H. Koprowski. 1961. Macrophages as a cellular expression of inherited natural resistance. Proc. Natl. Acad. Sci. USA 48:160-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groschel, D., and H. Koprowski. 1965. Development of a virus-resistant inbred mouse strain for the study of innate resistance to Arbo B viruses. Arch. Gesam. Virusf. 17:379-391. [DOI] [PubMed] [Google Scholar]
- 13.Hanson, B., H. Koprowski, S. Baron, and C. E. Buckler. 1969. Interferon-mediated natural resistance of mice to arboB virus infection. Microbios 1B:51-68. [Google Scholar]
- 14.Horvai, A. E., L. Xu, E. Korzus, G. Brard, D. Kalafus, T. M. Mullen, D. W. Rose, M. G. Rosenfeld, and C. K. Glass. 1997. Nuclear integration of JAK/STAT and Ras/AP-1 signaling by CBP and p300. Proc. Natl. Acad. Sci. USA 94:1074-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kakuta, S., S. Shibata, and Y. Iwakura. 2002. Genomic structure of the mouse 2′,5′-oligoadenylate synthetase gene family. J. Interf. Cytok. Res. 22:981-993. [DOI] [PubMed] [Google Scholar]
- 16.Knoetig, S. M., K. C. McCullough, and A. Summerfield. 2002. Lipopolysaccharide-induced impairment of classical swine fever virus infection in monocytic cells is sensitive to 2-aminopurine. Antivir. Res. 53:75-81. [DOI] [PubMed] [Google Scholar]
- 17.Kreil, T. R., and M. M. Eibl. 1995. Viral infection of macrophages profoundly alters requirements for induction of nitric oxide synthesis. Virology 212:174-178. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Lagerspetz, K. Y. H., H. Koprowski, M. Darnell, and H. Tarkkonen. 1973. Thermoregulation in group B arbovirus-resistant and group B arbovirus-susceptible mice. Am. J. Physiol. 225:532-537. [DOI] [PubMed] [Google Scholar]
- 20.Lucas, M., T. Mashimo, M.-P. Frenkiel, D. Simon-Chazottes, X. Montagutelli, P.-E. Ceccaldi, J.-L. Guénet, and P. Desprès. 2003. Infection of mouse neurones by West Nile virus is modulated by the interferon-inducible 2′-5′ oligoadenylate synthetase 1b protein. Immunol. Cell Biol. 81:230-236. [DOI] [PubMed] [Google Scholar]
- 21.Mackenzie, J. S., M. D. Lindsay, R. J. Coelen, A. K. Broom, R. A. Hall, and D. W. Smith. 1994. Arboviruses causing human disease in the Australasian zoogeographic region. Arch. Virol. 136:447-467. [DOI] [PubMed] [Google Scholar]
- 22.Maggi, L. B., Jr., M. R. Heitmeier, D. Scheuner, R. J. Kaufman, R. M. L. Buller, and J. A. Corbett. 2000. Potential role of PKR in double-stranded RNA-induced macrophage activation. EMBO J. 19:3630-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mashimo, T., P. Glaser, M. Lucas, D. Simon-Chazottes, P. E. Ceccaldi, X. Montagutelli, P. Desprès, and J.-L. Guénet. 2003. Structural and functional genomics and evolutionary relationship in the cluster of genes encoding murine 2′,5′-oligoadenylate synthetases. Genomics 82:537-552. [DOI] [PubMed] [Google Scholar]
- 24.Mashimo, T., M. Lucas, D. Simon-Chazottes, M.-P. Frenkiel, X. Montagutelli, P.-E. Ceccaldi, V. Deubel, J.-L. Guénet, and P. Desprès. 2002. A nonsense mutation in the gene encoding 2′-5′-oligoadenylate synthetase/L1 isoform is associated with West Nile virus susceptibility in laboratory mice. Proc. Natl. Acad. Sci. USA 99:11311-11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monath, T. P., and F. X. Heinz. 1996. Flaviviruses, p. 961-1034. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 26.Munoz-Jordan, J. L., G. G. Sanchez-Burgos, M. Laurent-Rolle, and A. Garcia-Sastre. 2003. Inhibition of interferon signalling by dengue virus. Proc. Natl. Acad. Sci. USA 100:14333-14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perelygin, A. A., S. V. Scherbik, I. B. Zhulin, B. M. Stockman, Y. Li, and M. A. Brinton. 2002. Positional cloning of the murine flavivirus resistance gene. Proc. Natl. Acad. Sci. USA 99:9322-9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyo, S. 1994. The mechanism of poly I:C-induced antiviral activity in peritoneal macrophage. Arch. Pharm. Res. 17:93-99. [DOI] [PubMed] [Google Scholar]
- 29.Roehrig, J. T., M. Layton, P. Smith, G. L. Campbell, R. Nasci, and R. S. Lanciotti. 2002. The emergence of West Nile virus in North America: ecology, epidemiology, and surveillance. Curr. Top. Microbiol. Immunol. 267:223-240. [DOI] [PubMed] [Google Scholar]
- 30.Rubinstein, S., P. C. Familletti, and S. Pestka. 1981. Convenient assay for interferons. J. Virol. 37:755-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sangster, M. Y., N. Urosevic, J. P. Mansfield, J. S. Mackenzie, and G. R. Shellam. 1994. Mapping the Flv locus controlling resistance to flaviviruses on mouse chromosome 5. J. Virol. 68:448-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saxena, S. K., A. Mathur, and R. C. Srivastava. 2001. Induction of nitric oxide synthase during Japanese encephalitis virus infection: evidence of protective role. Arch. Biochem. Biophys. 391:1-7. [DOI] [PubMed] [Google Scholar]
- 33.Schweizer, M., and E. Peterhans. 2001. Noncytopathic bovine viral diarrhea virus inhibits double-stranded RNA-induced apoptosis and interferon synthesis. J. Virol. 75:4692-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shellam, G. R., M. Y. Sangster, and N. Urosevic. 1998. Genetic control of host resistance to flavivirus infection in animals. Rev. Sci. Technol. Office Int. Epiz. 17:231-248. [DOI] [PubMed] [Google Scholar]
- 35.Silvia, O. J. 2000. The analysis of natural resistance to flaviviruses in mice and different cell culture models. Ph.D. thesis. University of Western Australia, Perth, Australia.
- 36.Silvia, O. J., L. Pantelic, J. S. Mackenzie, G. R. Shellam, J. Papadimitriou, and N. Urosevic. 2003. Virus spread, tissue inflammation and antiviral response in brains of flavivirus susceptible and resistant mice acutely infected with Murray Valley encephalitis virus. Arch. Virol. 149:447-464. [DOI] [PubMed] [Google Scholar]
- 37.Silvia, O. J., G. R. Shellam, and N. Urosevic. 2001. Innate resistance to flavivirus infection in mice controlled by Flv is nitric oxide-independent. J. Gen. Virol. 82:603-607. [DOI] [PubMed] [Google Scholar]
- 38.Silvia, O. J., and N. Urosevic. 1999. Variations in LPS responsiveness among different mouse substrains of C3H lineage and their congenic derivative sublines. Immunogenetics 50:354-357. [DOI] [PubMed] [Google Scholar]
- 39.Stoddart, C. W., M. T. Martin-Iverson, A. Jablensky, and N. Urosevic. 2004. A novel mouse Chr5 locus Diht controls dopamine-induced hypothermia. Mamm. Genome 15:901-913. [DOI] [PubMed] [Google Scholar]
- 40.Thomis, D. C., and C. E. Samuel. 1993. Mechanism of interferon action: evidence for intermolecular autophosphorylation and autoactivation of the interferon-induced, RNA-dependent protein kinase PKR. J. Virol. 67:7695-7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urosevic, N. 2003. Is flavivirus resistance interferon type I-independent? Immunol. Cell Biol. 81:224-229. [DOI] [PubMed] [Google Scholar]
- 42.Urosevic, N., K. Mann, S. I. Hodgetts, and G. R. Shellam. 1997. High-resolution genetic mapping of the chromosomal region around the mouse flavivirus resistance locus (Flv). Arbovir. Res. Aust. 7:296-299. [Google Scholar]
- 43.Urosevic, N., J. P. Mansfield, J. S. Mackenzie, and G. R. Shellam. 1995. Low resolution mapping around the flavivirus resistance locus (Flv) on mouse chromosome 5. Mamm. Genome 6:454-458. [DOI] [PubMed] [Google Scholar]
- 44.Urosevic, N., and G. R. Shellam. 2002. Host genetic resistance to Japanese encephalitis group viruses. Curr. Top. Microbiol. Immunol. 267:153-170. [DOI] [PubMed] [Google Scholar]
- 45.Urosevic, N., O. J. Silvia, J. P. Mansfield, M. Y. Sangster, S. I. Hodgetts, and G. R. Shellam. 1999. Development and characterisation of new flavivirus resistant mouse strains bearing Flvr-like and Flvmr alleles from wild or wild-derived mice. J. Gen. Virol. 80:897-906. [DOI] [PubMed] [Google Scholar]
- 46.Urosevic, N., M. van Maanen, J. P. Mansfield, J. S. Mackenzie, and G. R. Shellam. 1997. Molecular characterisation of virus specific RNA produced in the brains of flavivirus susceptible and resistant mice after challenge with Murray Valley encephalitis virus. J. Gen. Virol. 78:23-29. [DOI] [PubMed] [Google Scholar]
- 47.Wang, C., J. Pflugheber, R. Sumpter, Jr., D. L. Sodora, D. Hui, G. C. Sen, and M. Gale, Jr. 2003. Alpha interferon induces distinct translational control programs to suppress hepatitis C virus RNA replication. J. Virol. 77:3898-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wathelet, M. G., C. H. Lin, B. S. Parekh, L. V. Ronco, P. M. Howley, and T. Maniatis. 1998. Virus infection induces the assembly of co-ordinately activated transcription factors on the IFN-β enhancer in vivo. Mol. Cell 1:507-518. [DOI] [PubMed] [Google Scholar]