Abstract
The purpose of this study was to evaluate the feasibility and efficacy of radiotherapy (RT) using intensity-modulated radiotherapy (IMRT) boosts after hyperbaric oxygen (HBO) therapy with chemotherapy in patients with glioblastoma. Twenty-four patients with glioblastoma were treated with the combined therapy, which was RT using IMRT boosts after HBO with chemotherapy, and were retrospectively analyzed. The RT protocol was as follows: first, 3D conformal RT [40 Gy/20 fractions (fr)] was delivered to the gross tumor volume (GTV) and the surrounding edema, including an additional 1.5–2.0 cm. The IMRT boost doses were then continuously delivered to the GTV plus 5 mm (28 Gy/8 fr) and the surrounding edema (16 Gy/8 fr). Each IMRT boost session was performed immediately after HBO to achieve radiosensitization. The planned RT dose was completed in all patients, while HBO therapy was terminated in one patient (4%) due to Grade 2 aural pain. The toxicities were mild, no non-hematological toxicity of Grade 3–5 was observed. The 2-year overall survival (OS) and progression-free survival rates in all patients were 46.5% and 35.4%, respectively. The median OS time was 22.1 months. In conclusion, the combined therapy of RT using IMRT boosts after HBO with chemotherapy was a feasible and promising treatment modality for patients with glioblastoma. The results justify further evaluation to clarify the benefits of this therapy.
Keywords: glioblastoma, hyperbaric oxygen, intensity-modulated radiotherapy, high-grade glioma, radiosensitization
INTRODUCTION
The prognosis for patients with glioblastoma remains poor, and the use of conventional radiotherapy (RT) at doses beyond 60 Gy has not led to a survival benefit. Modern RT planning techniques such as intensity-modulated radiotherapy (IMRT) allow for more accurate delivery of the radiation dose and the possibility of escalating the dose without increasing morbidity. Recently, several prospective Phase II studies have demonstrated that IMRT can be administered in combination with chemotherapy without increasing the toxicity, and that it is a feasible method of achieving a dose escalation in patients with high-grade glioma, although the overall survival (OS) rates in those studies were comparable with those of patients treated with conventional RT with chemotherapy [1–3]. Other previous experimental and clinical studies have indicated that hyperbaric oxygen (HBO) therapy could enhance the antitumor effect of RT due to the increased supply of oxygen to hypoxic tumor cells [4–6]. High-grade glioma commonly shows an extremely low oxygen tension [7]. Some Phase II trials in patients with high-grade glioma have indicated that conventional RT immediately after HBO therapy with chemotherapy was safe and that it seemed to be effective, although the combined therapy was time-consuming and complex [8, 9]. In this context, we administered a combination therapy of RT using IMRT boosts after HBO therapy with chemotherapy to improve the clinical outcome of patients with glioblastoma. The total dose of RT in the current study was based on some previous data for high-dose radiotherapy in patients with glioblastoma [10–12]. The number of HBO sessions was chosen in consideration of the capacity for the human resource and machine time in our institutions. The purpose of this study was to evaluate the feasibility and efficacy of the combination therapy.
MATERIALS AND METHODS
Patients
From April 2008 and September 2013, 40 consecutive patients who were newly diagnosed primary glioblastoma were treated with postoperative RT or definitive RT. Postoperative RT was performed after a tumor resection, and definitive RT was done after a tumor biopsy. Of these, the treatment responses of 24 consecutive patients, who were treated with chemoradiotherapy using IMRT boosts in combination with HBO therapy, were retrospectively evaluated. The exclusion criteria for the combined therapy was as follows; Karnofsky performance status (≤30), advanced age (≥85), brainstem invasion, and gliomatosis cerebri. The remaining 16 patients did not receive the combination therapy due to the following reasons: poor performance status (n = 5), advanced age (n = 4), brainstem invasion (n = 3) and gliomatosis cerebri (n = 4).
The patient characteristics are listed in Table 1. Before the administration of the combined therapy, a tumor resection was performed in 20 (83%) patients, and a tumor biopsy was performed in 4 (17%) patients. The extent of resection was determined based on the findings of preoperative MR imaging, 5-aminolevulinic acid fluorescence navigation and/or motor-evoked potential monitoring. The recursive partitioning analysis (RPA) classes for malignant glioma were evaluated [13, 14]. Neurologic functional classification was as follows; Class 1 was able to work, Class 2 was able to be at home, and Class 3 was hospitalized [15]. Neurologic symptoms contained the following; cerebral deficit, cranial nerve deficit, memory lag, personality change, seizure history, sensory deficit, motor deficit, papilledema, somnolence, speech impairment, headache, and mental status changes [15]. Duration of symptoms of >3 months meant that each neurologic symptom persisted for more than 3 months. Written informed consent for treatment was obtained from all patients. The study was approved by the authors’ Institutional Review Boards.
Table 1.
Patient characteristics
| Variable | n (%) |
|---|---|
| Age, median (range) | 65 (24–84) |
| Gender | |
| Male | 15 (63) |
| Female | 9 (37) |
| KPS (%), median (range) | 90 (40–90) |
| Tumor size (cm), median (range) | 4.0 (1.5–7.0) |
| Tumor lacation | |
| Frontal | 16 (67) |
| Other | 8 (33) |
| Mental status | |
| Normal | 22 (92) |
| Abnormal | 2 (8) |
| Neurological function | |
| Work | 12 (50) |
| Other | 12 (50) |
| Duration of symptoms (months) | |
| ≤3 | 21 (88) |
| >3 | 3 (12) |
| Surgery | |
| Gross total | 8 (33) |
| Partial | 12 (50) |
| Biopsy | 4 (17) |
| RPA classes* | |
| III | 2 (8) |
| IV | 9 (38) |
| V | 12 (50) |
| VI | 1 (4) |
Radiotherapy
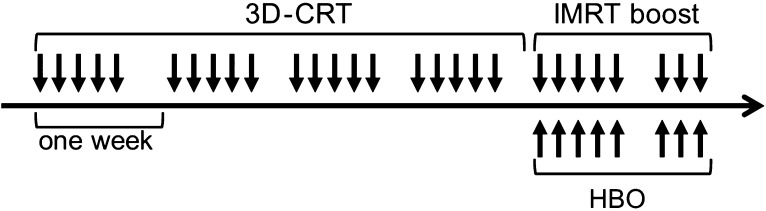
Three-dimensional conformal RT (3D-CRT) [40 Gy/20 fractions (fr)] was first delivered with a 6 or 10 MV linear accelerator. CT-assisted 3D treatment planning (Xio; Elekta, Tokyo, Japan) was used to determine the radiation fields in all patients. Continuously, IMRT boosts were started after completion of the 3D-CRT (Fig. 1); a CyberKnife system (CyberKnife II, Accuray; a lightweight 6 MV X-band linear accelerator mounted on a fully articulated robotic arm) was used. The patients were non-invasively immobilized in the supine position using a custom-made thermoplastic mask, and were treated under a skull-tracking system to determine the real-time target location. The planning CT images were fused with T1-weighted magnetic resonance imaging (MRI) with gadolinium to determine the extent of the lesions more precisely.
Fig. 1.
Timing of 3D-CRT, IMRT boost and HBO therapy.
The gross tumor volume (GTV) was defined as the contrast-enhancing residual tumor plus the entire surgical cavity on CT and MRI. The clinical target volume extended (CTV1) was the GTV and the surrounding edema plus 1.5—2.0 cm. The clinical target volume annulus (CTV2) was the surrounding edema. The clinical target volume gross (CTV3) was the GTV plus 5 mm.
The RT protocol was as follows: first, 3D-CRT (40 Gy in 2-Gy fractions) was delivered to the CTV1 using a four-field box technique. The IMRT boosts were continuously delivered by the CyberKnife system to the CTV3 (28 Gy in 3.5-Gy fractions) and the CTV2 (16 Gy in 2.0-Gy fractions) (Figs 1 and 2). An inverse planning method with a non-isocenteric technique was used. Our dose-prescription policies for the IMRT boost were based on the D95 (the percentage of the prescribed dose covering 95% of the volume) of the CTV. The biologically effective dose (BED) can be used to compare the efficacy of various dose-fractionation regimens in providing tumor control [16, 17]. The sum of the BED in the CTV3 was 85.8 Gy10, while that in the CTV2 was 67.2 Gy10. The sums of the maximum doses to the critical structures in the 3D-CRT and IMRT boost for the lens, the retina, the optic nerve, the optic chiasm and the brainstem were 10 Gy, 50 Gy, 55 Gy, 55 Gy and 55 Gy, respectively. The maximum doses for the lens, retina, optic nerve, optic chiasm and brain stem were in the ranges of 2.6–17.3 Gy (median: 6.6 Gy), 13.6–50.0 Gy (median: 29.3 Gy), 7.8–55.2 Gy (median: 34.4 Gy), 8.2–49.4 Gy (median: 36.0 Gy) and 7.1–56.0 Gy (median: 40.0 Gy), respectively. Time to finish all IMRT treatment was ~20–40 min.
Fig. 2.
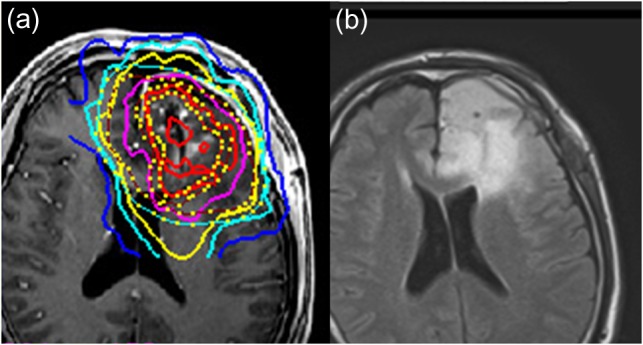
A magnetic resonance T1-weighted contrast-enhanced image with isodose lines of the IMRT boosts (a) and fluid-attenuated inversion recovery (FLAIR) image sequences (b) in a patient. The red line represents a dose of 28 Gy/8 fr, daily, 3.5 Gy to the CTV3. The yellow line indicates a dose of 16 Gy/8 fr, daily, 2.0 Gy to the CTV2.
HBO therapy
HBO therapy was performed in conjunction with each IMRT boost session to achieve radiosensitization. The patients underwent a single treatment for 60–90 min in a monoplace HBO chamber (Sechrist Industries Inc., Model 2800 J, Anaheim, CA) pressurized with 100% oxygen to 2.0 atmospheres absolute. After each session of HBO, the patient was promptly moved to the treatment room to receive the IMRT. The interval between the completion of HBO and the start of the IMRT was <15 min for each session. All patients wore non-flammable pajamas, and no patients underwent sedation for these sessions.
Chemotherapy
Temozolomide (as a concurrent and/or adjuvant chemotherapy) was administered in 24 (100%) patients. Twenty-two (92%) patients were treated with concomitant systemic chemotherapy during the course of RT as follows: temozolomide (n = 19), and ranimustine (MCNU) in combination with interferon-beta (IFN-beta) (n = 3). Adjuvant chemotherapy using temozolomide after the RT was performed in 21 patients. In the early days, MCNU and IFN-beta were chosen, when temozolomide could not be widely used in clinical practice. Typically, the patients were administered temozolomide with a concurrent RT at a dose of 75 mg/m2 per day from the first day of conventional 3D-CRT until the last day of the IMRT boost, and patients generally received six cycles or more of adjuvant temozolomide on a 5-day schedule of 150 mg/m2 every 28 days.
Evaluation and follow-up
The follow-up evaluations were performed by MRI at 1-to 4-month intervals during the first 2 years and at 3- to 6-month intervals thereafter, even in the absence of clinical symptoms. The treatment response was evaluated according to the response criteria for the RANO criteria [18]. The first site of disease progression was defined as follows: a local recurrence was a failure within the CTV1, which could include a contiguous progression of contrast enhancement beyond the CTV1, and a new lesion was a non-contiguous failure outside the CTV1, which could include the development of leptomeningeal seeding or new parenchymal disease [19]. The disease-progression was evaluated based on the response assessment of the Neuro-Oncology Working Group to resolve the difficulty of differentiating true progression from pseudoprogression; progressive disease (<12 weeks after completion of chemoradiotherapy) can only be defined using diagnostic imaging if there is new enhancement outside of the radiation field or if there is unequivocal evidence of viable tumor on histopathologic sampling [18].
The OS and progression-free survival (PFS) rates were calculated from the start of the RT using the Kaplan–Meier method. Common Terminology Criteria for Adverse Events (CTCAE) version 4 was used to score patient toxicity. The highest toxicity grade obtained for each patient was used for the toxicity analysis. The toxicity was defined as acute (during therapy and up to 3 months after the combination therapy) or late (over 3 months after completion of the combination therapy).
RESULTS
The planned RT dose was completed in all patients. Following the completion of the 3D-CRT, the IMRT boost was completed without a treatment break, while HBO therapy was terminated in one (4%) patient due to Grade 2 aural pain in the first session, the remaining 23 patients completed the eight planned HBO sessions. The observed toxicities were mild. Acute toxicities of ≥Grade 2 occurred in 11 patients (46%); Grade 3 bone marrow suppression occurred in 1 patient, Grade 2 alopecia occurred in 5 patients, Grade 2 bone marrow suppression occurred in 2 patients, and Grade 2 appetite loss occurred in 1 patient. During HBO treatment, 3 of 24 (13%) patients experienced Grade 2 aural pain, but they recovered with conservative management. No late toxicities of ≥Grade 3 were observed. Grade 2 radiation necrosis occurred in 2 patients and Grade 1 radiation necrosis occurred in 6 patients. No other late toxicities were recognized.
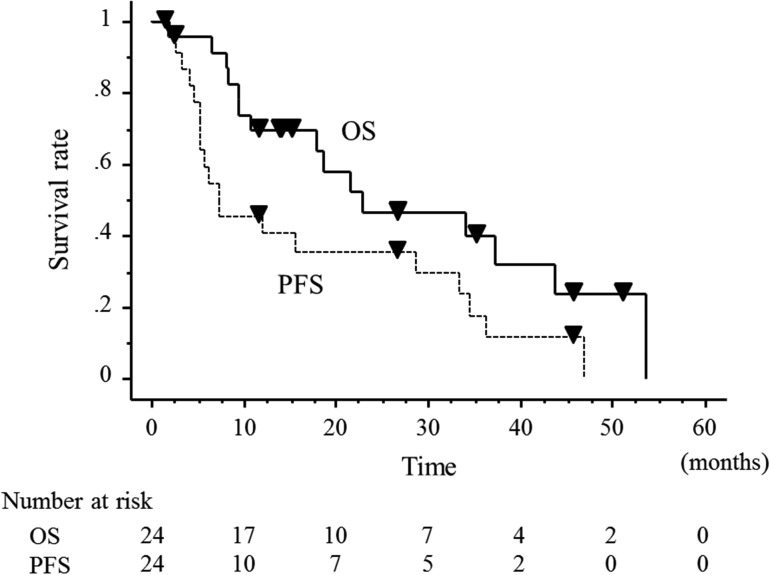
The median follow-up duration was 16.7 months. During the follow-up, death from other diseases was seen in 1 patient at 1.9 months after the start of the RT, and death from the glioblastoma was recognized in 14 patients. The 2-year OS and PFS rates were 46.5% and 35.4%, respectively (Fig. 3). The 3-year OS and PFS rates were 39.8% and 17.7%, respectively. The median survival times (MSTs) with regard to the OS and PFS rates were 22.1 and 7.4 months, respectively. Disease progression was seen in 19 (79%) of 24 patients during the follow-up. Table 2 presents the first sites of disease progression. Ten patients underwent re-operation to treat the local failure. The median period between initial operation and re-operation was 5 months (range: 2–29 months). Chemotherapy was selected for recurrent disease in 11 patients. Re-irradiation for recurrent disease was performed in 1 patient.
Fig. 3.
The overall survival and progression-free survival of all patients.
Table 2.
The first site of disease progression
| Patterns of failure | No. of patients (%) (n = 24) |
|---|---|
| Local failure | 14 (58) |
| Regional failure | |
| Leptomeningeal seeding | 2 (8) |
| New parenchymal disease | 0 (0) |
| Local failure and leptomeningeal seeding | 1 (4) |
| No failure | 7 (29) |
Table 3 lists for comparison the OS rates (according to the RPA classes) for Radiation Therapy Oncology Group (RTOG) 90-06, two representative clinical studies using IMRT and temozolomide, and the current study [2, 14, 15].
Table 3.
Comparison of overall survival in the previous representative studies and in the current study, based on RPA
| RTOG 90-06*60 Gy/30 fr or 72 Gy/60 fr and BCNU | Paravati (2011)** IMRT and TMZ |
Iuchi (2014)*** IMRT (high-dose) and TMZ |
Current study | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 2-year OS rate (%) | MST (mo) | n | 2-year OS rate (%) | MST (mo) | n | 2-year OS rate (%) | MST (mo) | n | 2-year OS rate (%) | MST (mo) | |
| RPA | ||||||||||||
| III | 105 | 30 | 17.5 | 8 | 67 | 25.0 | 2 | N/A | N/A | 2 | 100 | N/A |
| IV | 240 | 17 | 11.5 | 40 | 31 | 17.7 | 17 | 51 | 25.1 | 9 | 71 | 38.3 |
| V | 150 | 8 | 7.4 | 62 | 12 | 10.8 | 16 | 44 | 21.4 | 12 | 28 | 14.9 |
| VI | 23 | 0 | 2.7 | 17 | 6 | 6.4 | 11 | N/A | N/A | 1 | 0 | 6.5 |
MST = median survival time, mo = months, RTOG = radiation therapy oncology group, RPA = recursive partitioning analysis, BCNU = bis-chlorethyl nitrosourea, TMZ = temozolomide, N/A = not applicable. *Previously reported data from RTOG 90-06 [14]. **Previously reported data from Paravati et al. [15]. ***Previously reported data from Iuchi et al. [2].
DISCUSSION
The present study is, to the best of our knowledge, the first study to evaluate the feasibility and efficacy of RT using IMRT boosts immediately after HBO therapy with chemotherapy in patients with glioblastoma. In the 1970s and 1980s, several Phase III clinical trials were conducted to estimate the clinical benefits of HBO therapy in achieving radiosensitization. The local control and OS rates were significantly improved in the head and neck cancer patients who were treated with RT plus simultaneous HBO therapy in comparison with patients who were treated with RT alone. However, in those clinical trials, the delivery of simultaneous HBO therapy and RT was very complex and time-consuming. In addition, some trials indicated that the combined therapy increased side effects. Recently, the ability of RT delivered immediately after HBO therapy to achieve radiosensitization has been investigated because the high oxygen concentration of normal brain tissue decreases quickly after decompression, whereas that in tumor tissue falls more slowly after decompression [5, 6]. This combined method of administering RT and HBO therapy is simple and may be suitable for incorporation into multimodality therapies including modern RT. We also confirmed that the combined therapy is feasible and without severe toxicity because, in the current study, the planned RT (using IMRT boosts immediately after HBO) was completed in 96% of the patients.
The current standard treatment for glioblastoma is the maximal safe surgical resection followed by conventional RT (60 Gy/30 fr) and concurrent temozolomide, followed by adjuvant temozolomide; the MST is 14.6 months [20]. Some Phase II clinical trials for conventional RT immediately after HBO therapy have shown promising results in patients with high-grade glioma [8, 9]. Ogawa et al. reported the long-term results of a Phase II trial for conventional RT (60 Gy/30 fr) immediately after HBO with a multi-agent chemotherapy (consisting of procarbazine and nimustine) in patients with high-grade gliomas: all patients were able to complete the RT immediately after HBO, the median OS of 39 patients with glioblastoma was 17.2 months, and no serious toxicities were observed [9]. In the current study on RT (using IMRT boosts immediately after HBO therapy with chemotherapy, with temozolomide as the main chemotherapy agent) the median OS of 22.1 months in patients with glioblastoma was very promising. Comparing the results (according to the RPA classes) for RTOG 90-06, previous clinical studies using IMRT and temozolomide, and the current study indicated that the OS rates in the current study were favorable in most of the RPA classes (Table 3). Our results justify further evaluations with detailed treatment protocols in a prospective study to clarify whether our combined therapy including IMRT boosts immediately after HBO could improve survival in patients with glioblastoma.
Previous studies have reported that dose escalation of RT for glioblastoma above 60 Gy using conventional RT has resulted in limited success (although some studies have suggested it to be of benefit) [10–12, 21]. Recently, several Phase I dose escalation trials for glioblastoma using hypofractionated IMRT with temozolomide were conducted to determine the maximal tolerated dose of RT [22–24]. Massaccesi et al. reported that a radiation dose of 70 Gy in 25 fr (BED of 92.8 Gy10) using IMRT can be delivered with concurrent and sequential standard dose temozolomide, without unacceptable toxicity [22]. Chen et al. also conducted a Phase I dose escalation trial, and found that 60 Gy in 10 fr (BED of 96.0 Gy10) with concurrent and adjuvant temozolomide is tolerable in selected patients with a T1-weighted enhancing tumor of <6 cm [23]. Iuchi et al. reported the results of a Phase II trial for hypofractionated high-dose IMRT (68 Gy/8 fr, BED of 125 Gy10) with concurrent and adjuvant temozolomide [2]. The high-dose IMRT with temozolomide altered the dominant failure pattern from local failure to dissemination and prolonged the survival; the median OS was 20.0 months. In the current study, the total RT dose, including the IMRT boosts (BED of 85.8 Gy10) was relatively low in comparison with the above-mentioned trials. The first site of disease progression was local in 58% of the patients with glioblastoma, and ≥Grade 3 toxicities, such as radiation necrosis of the brain, were not observed. Therefore, a further dose escalation trial for IMRT boosts immediately after HBO is warranted to evaluate the maximum tolerated RT dose under the current combination therapy in patients with glioblastoma.
There are some limitations associated with the present study. First, the current study was a retrospective study with heterogeneous treatment, especially with regard to the chemotherapeutic regimen. However, the protocols of RT with IMRT boosts after HBO were uniform. Second, the possibility of some selection bias, with regard to the prognostic factors could not be ruled out, because 16 patients with some poor prognostic factors were excluded from this combined therapy. Comparison of the results (according to the RPA classes) for RTOG 90-06 and the current study showed that the OS rates in the current study were better than those in RTOG 90-06 in every RPA class. Therefore, we assumed that the selection bias might be low. However, a formal prospective trial is needed to determine the efficacy and prognostic factors of this combined therapy in patients with glioblastoma.
CONCLUSION
In summary, this is the first study to assess the feasibility and efficacy of RT using IMRT boosts immediately after HBO therapy with chemotherapy (mainly temozolomide), in patients with glioblastoma. The combined therapy was feasible without severe toxicities and proved to be a promising modality with longer OS times. However, local disease progression was recognized in more than half of the patients. The results justify further evaluation in prospective trials including a dose escalation of IMRT to clarify the benefits of this combined treatment in patients with glioblastoma.
CONFLICT OF INTEREST
Potential conflicts of interest do not exist in this study.
REFERENCES
- 1. Reddy K, Damek D, Gaspar LE, et al. Phase II trial of hypofractionated IMRT with temozolomide for patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys 2012;84:655–60. [DOI] [PubMed] [Google Scholar]
- 2. Iuchi T, Hatano K, Kodama T, et al. Phase 2 trial of hypofractionated high-dose intensity modulated radiation therapy with concurrent and adjuvant temozolomide for newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys 2014;88:793–800. [DOI] [PubMed] [Google Scholar]
- 3. Piroth MD, Pinkawa M, Holy R, et al. Integrated boost IMRT with FET-PET–adapted local dose escalation in glioblastomas. Results of a prospective phase II study. Strahlenther Onkol 2012;188:334–9. [DOI] [PubMed] [Google Scholar]
- 4. Daruwalla J, Christophi C.. Hyperbaric oxygen therapy for malignancy: a review. World J Surg 2006;30:2112–31. [DOI] [PubMed] [Google Scholar]
- 5. Kohshi K, Beppu T, Tanaka K, et al. Potential roles of hyperbaric oxygenation in the treatments of brain tumors. Undersea Hyperb Med 2013;40:351–62. [PubMed] [Google Scholar]
- 6. Ogawa K, Kohshi K, Ishiuchi S, et al. Old but new methods in radiation oncology: hyperbaric oxygen therapy. Int J Clin Oncol 2013;18:364–70. [DOI] [PubMed] [Google Scholar]
- 7. Collingridge DR, Piepmeier JM, Rockwell S, et al. Polarographic measurements of oxygen tension in human glioma and surrounding peritumoural brain tissue. Radiother Oncol 1999;53:127–31. [DOI] [PubMed] [Google Scholar]
- 8. Beppu T, Kamada K, Nakamura R, et al. A phase II study of radiotherapy after hyperbaric oxygenation combined with interferon-beta and nimustine hydrochloride to treat supratentorial malignant gliomas. J Neurooncol 2003;61:161–70. [DOI] [PubMed] [Google Scholar]
- 9. Ogawa K, Ishiuchi S, Inoue O, et al. Phase II trial of radiotherapy after hyperbaric oxygenation with multiagent chemotherapy (procarbazine, nimustine, and vincristine) for high-grade gliomas: long-term results. Int J Radiat Oncol Biol Phys 2012;82:732–8. [DOI] [PubMed] [Google Scholar]
- 10. Werner-Wasik M, Scott CB, Nelson DF, et al. Final report of a phase I/II trial of hyperfractionated and accelerated hyperfractionated radiation therapy with carmustine for adults with supratentorial malignant gliomas. Radiation Therapy Oncology Group Study 83-02. Cancer 1996;77:1535–43. [DOI] [PubMed] [Google Scholar]
- 11. Nakagawa K, Aoki Y, Fujimaki T, et al. High-dose conformal radiotherapy influenced the pattern of failure but did not improve survival in glioblastoma multiforme. Int J Radiat Oncol Biol Phys 1998;40:1141–9. [DOI] [PubMed] [Google Scholar]
- 12. Nieder C, Andratschke N, Wiedenmann N, et al. Radiotherapy for high-grade gliomas. Does altered fractionation improve the outcome. Strahlenther Onkol 2004;180:401–7. [DOI] [PubMed] [Google Scholar]
- 13. Curran WJ Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 1993;85:704–10. [DOI] [PubMed] [Google Scholar]
- 14. Scott CB, Scarantino C, Urtasun R, et al. Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: a report using RTOG 90-06. Int J Radiat Oncol Biol Phys 1998;40:51–5. [DOI] [PubMed] [Google Scholar]
- 15. Paravati AJ, Heron DE, Landsittel D, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma and anaplastic astrocytoma: validation of Radiation Therapy Oncology Group-Recursive Partitioning Analysis in the IMRT and temozolomide era. J Neurooncol 2011;104:339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 1989;62:679–94. [DOI] [PubMed] [Google Scholar]
- 17. Fowler JF. Biological factors influencing optimum fractionation in radiation therapy. Acta Oncol 2001;40:712–7. [DOI] [PubMed] [Google Scholar]
- 18. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010;28:1963–72. [DOI] [PubMed] [Google Scholar]
- 19. Cho KH, Kim JY, Lee SH, et al. Simultaneous integrated boost intensity-modulated radiotherapy in patients with high-grade gliomas. Int J Radiat Oncol Biol Phys 2010;78:390–7. [DOI] [PubMed] [Google Scholar]
- 20. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- 21. Nieder C, Adam M, Grosu AL. Combined modality treatment of glioblastoma multiforme: the role of temozolomide. Rev Recent Clin Trials 2006;1:43–51. [DOI] [PubMed] [Google Scholar]
- 22. Massaccesi M, Ferro M, Cilla S, et al. Accelerated intensity-modulated radiotherapy plus temozolomide in patients with glioblastoma: a phase I dose-escalation study (ISIDE-BT-1). Int J Clin Oncol 2013;18:784–91. [DOI] [PubMed] [Google Scholar]
- 23. Chen C, Damek D, Gaspar LE, et al. Phase I trial of hypofractionated intensity-modulated radiotherapy with temozolomide chemotherapy for patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys 2011;81:1066–74. [DOI] [PubMed] [Google Scholar]
- 24. Jastaniyah N, Murtha A, Pervez N, et al. Phase I study of hypofractionated intensity modulated radiation therapy with concurrent and adjuvant temozolomide in patients with glioblastoma multiforme. Radiat Oncol 2013;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]