Abstract
Several phytoceuticals and extracts of medicinal plants are reported to mitigate deleterious effects of ionizing radiation. The potential of hydro-alcoholic extract of Clerodendron infortunatum (CIE) for providing protection to mice exposed to gamma radiation was investigated. Oral administration of CIE bestowed a survival advantage to mice exposed to lethal doses of gamma radiation. Radiation-induced depletion of the total blood count and bone marrow cellularity were prevented by treatment with CIE. Damage to the cellular DNA (as was evident from the comet assay and the micronucleus index) was also found to be decreased upon CIE administration. Radiation-induced damages to intestinal crypt cells was also reduced by CIE. Studies on gene expression in intestinal cells revealed that there was a marked increase in the Bax/Bcl-2 ratio in mice exposed to whole-body 4 Gy gamma radiation, and that administration of CIE resulted in significant lowering of this ratio, suggestive of reduction of radiation-induced apoptosis. Also, in the intestinal tissue of irradiated animals, following CIE treatment, levels of expression of the DNA repair gene Atm were found to be elevated, and there was reduction in the expression of the inflammatory Cox-2 gene. Thus, our results suggest a beneficial use of Clerodendron infortunatum for mitigating radiation toxicity.
Keywords: Clerodendron infortunatum, radiomodifiers, radioprotectors, comet assay, Bax/Bcl-2 ratio, Cox-2, Atm
INTRODUCTION
With the widespread application of nuclear energy and radioisotopes in various human activities (such as in industry, healthcare, agriculture, food processing, power production and defence), there is an increasing risk of radiation exposures to life-forms. Thus, protecting humans from the harmful effects of ionizing radiation is a major challenge. The reactive species of oxygen (ROS) and nitrogen (RNS) formed in biological systems upon exposure to ionizing radiation deplete the antioxidants and damage the vital cellular DNA and membranes, resulting in cell death, altered cell division, depletion of stem cells, organ system dysfunction and, at high doses, death of the organism. Depending on the dose of the exposure, ionizing radiation damages the hematopoietic system, gastrointestinal system, central nervous system and reproductive system. Antioxidants can reduce the damage produced by both low and high doses of radiation [1, 2]. The use of an appropriate antioxidant type, dose and dose schedule is very important in reducing radiation damage, because most of the adverse effects of ionizing radiation are due to ROS formed in the cellular milieu from the radiolysis of water, which generate ROS-like hydrogen peroxide (H2O2), molecular hydrogen (H2) and a number of highly active free radicals, such as superoxide hydrogen radical (H•), hydroxyl radical (OH•), hydroperoxyl radical (HO2•) and superoxide anion radical (O2−•) [3]. Along with the production of ROS, ionizing radiation causes direct DNA damage, resulting in double- or single-strand breaks. Cells suffering such insults can undergo mortality (through apoptosis, etc.) and be removed from the body, or can mutate and turn malignant [4]. Several compounds, dietary ingredients, plant extracts and formulations having antioxidant activity can help in preventing radiation-induced oxidative stress, thereby acting as radioprotectors [5]. We have investigated the antioxidant and radioprotecting properties of the plant Clerodendron infortunatum, which belongs to the family Verbenaceae and is widely used in Indian indigenous medicine for its various therapeutic properties [6, 7]. Different parts of the plant have been used in tribal and folk medicine in India for colic, scorpion sting, snake bite, tumour, certain skin diseases, and for various conditions such as bronchitis, asthma, fever, diseases of the blood and inflammation [8]. The roots of the plant have laxative, diuretic, analgesic, anti-inflammatory, anti-tumour and antibacterial activities. In the present paper, we present data on the radioprotective efficacy of extract of Clerodendron infortunatum (CIE) in mice against whole-body gamma radiation exposure.
MATERIALS AND METHODS
Chemicals
All the chemicals and reagents used in this study were of analytical grade and purchased from Sigma Chemicals; the molecular reagents were purchased from Origin Diagnostics and Research.
Animals
Male Swiss albino mice of 8–10 weeks old, weighing 22–25 g, were obtained from the Small Animal Breeding Section (SABS), Kerala Agricultural University, Mannuthy, Thrissur, Kerala. They were kept under standard conditions of temperature and humidity in the Centre's Animal House Facility. The animals were provided with standard mouse chow (Sai Durga Feeds and Foods, Bangalore, India) and water ad libitum. All animal experiments were carried out with the prior approval of the Institutional Animal Ethics Committee (IAEC) and were conducted strictly adhering to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) constituted by the Animal Welfare Division of the Government of India.
Preparation of hydro-alcoholic extract of Clerodendron infortunatum
Roots of Clerodendron infortunatum were dried and finely powdered. The powder was weighed and subjected to soxhlet extraction with 50% ethyl alcohol. The extract was evaporated in a rotary evaporator at 50°C under vacuum. Finally, the extract was subjected for lyophilization to yield a solid with 12% yield. This was labelled as CIE and stored at 4°C.
High-pressure liquid chromatography analysis of CIE
A solution of CIE (10 mg/ml) was filtered through a 0.2 µm filter, and 20 µl of the filtrate was injected into an Agilent Model No. 1260 high-pressure liquid chromatography (HPLC) System, equipped with a Pixel Array Detector (PAD) detector and a SunFire C18, 5 µm column. The HPLC profile of the standard compound quercetin was obtained by injecting 20 µl of 1 mg/ml solution. The solvents used for gradient elution were acetonitrile and water. The detection wavelength was 280 nm. As quercetin is one of the components in the extract, its percentage in CIE was calculated using the peak areas.
Free radical scavenging activity of CIE
The free radical scavenging activity of CIE was determined by the method of Aquino et al. [9], with minor modifications, using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) stable free radical.
Gamma irradiation and treatments
CIE was dissolved in distilled water. The animals were administered per os with various quantities of CIE 1 h prior to gamma irradiation. The animals were divided into 10 groups of 10 animals each and were exposed to whole-body 60Co gamma radiation in a blood irradiator (BRIT, DAE, Mumbai, India) at a dose rate of 1.95 Gy/min. Out of the 10 groups, the first 5 were taken for biochemical and molecular studies, in which Group II to Group V received 4 Gy whole-body gamma radiation. Group I served as the unirradiated control. The remaining five groups (VI–X) were taken for survival studies. Group VII to Group X each received 8 Gy whole-body gamma radiation. Group VI served as the unirradiated control for survival studies. The details of the CIE administration and the irradiation of each group are provided below. Various quantities of CIE were administered by oral gavage to animals 1 h prior to gamma irradiation. There were 10 groups of 10 animals.
Group I. Distilled water (0.1 ml/animal) + sham irradiation (0 Gy)
Group II. Distilled water (0.1 ml/animal) + 4 Gy gamma irradiation
Group III. CIE (100 mg/kg) + 4 Gy gamma irradiation
Group IV. CIE (200 mg/kg) + 4 Gy gamma irradiation
Group V. CIE (300 mg/kg) + 4 Gy gamma irradiation
Group VI. Distilled water (0.1 ml/animal) + sham irradiation (0 Gy)
Group VII. Distilled water (0.1 ml/animal) + 8 Gy gamma irradiation
Group VIII. CIE (100 mg/kg) + 8 Gy gamma irradiation
Group IX. CIE (200 mg/kg) + 8 Gy gamma irradiation
Group X. CIE (300 mg/kg) + 8 Gy gamma irradiation
Following irradiation, the animals of Groups I–V were sacrificed at a range of time intervals, and the blood and various tissues were extracted for various studies. Three hours after irradiation, from each of the groups, three animals were sacrificed; bone marrow cells, peripheral blood leukocytes and spleenocytes of these animals were collected for alkaline comet assay. At 24 h after irradiation, five animals from each group were sacrificed and the various tissues were extracted. The bone marrow cellularity and the total WBC count were monitored. Also, antioxidant parameters such as glutathione (GSH), glutathione peroxidase (GPx) and superoxide dismutase (SOD), along with levels of peroxidation of membrane lipids, were analyzed in a number of tissues such as liver, heart, kidney intestine and brain. After 48 h of radiation exposure, blood was collected from two mice from each group by tail vein puncture to perform the micronucleus assay. Animals of Groups VI–X were checked on a daily basis to record the mortality in each group. The survival in each group was represented in terms of percentage.
Determination of bone marrow cellularity and white blood cell count
After 24 h of radiation exposure, the animals were sacrificed by cervical dislocation, and blood was collected by heart puncture into heparinized tubes for the determination of various haematological parameters using an automated haematological analyser unit. The bone marrow was collected from femurs into phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS), and the bone marrow cellularity was determined using a haemocytometer.
Effect of CIE on a number of antioxidant parameters of various tissues of animals exposed to gamma radiation
After 24 h of radiation exposure, the animals were sacrificed by cervical dislocation and the liver, brain, kidney, intestine and heart were excised and washed with ice-cold PBS. Homogenates [10% (w/v)] of these tissues were prepared in PBS. The levels of reduced GSH, lipid peroxidation, GPx, SOD and protein were estimated in the homogenates. The level of GSH was assayed by the method of [10], based on the reaction with Ellman's reagent [5,5′-dithiobis-(2-nitrobenzoic acid) or DTNB]. GPx activity was measured based on the method of Hafeman et al. [11], based on the degradation of H2O2. Activity of SOD was measured by the nitroblue tetrazolium (NBT) reduction method of McCord and Fridovich [12]. Protein levels in the tissue were measured by following the method of Lowry et al. [13]. Levels of peroxidation in membrane lipids were determined using the method of Buege and Aust [14].
Histopathological studies
Intestinal tissue was fixed in 10% formalin solution and dehydrated in ethanol, cleared in xylene and embedded in paraffin wax. Sections of 5 μ thickness were made using a microtome, and stained with haematoxylin–eosin and observed under microscope [15]. Photographs of each of the slides were taken at ×40 magnification.
Alkaline single-cell gel electrophoresis (comet assay)
Alkaline single-cell gel electrophoresis was performed using the method given by Singh [16], with minor modifications [17]. Microscope slides were coated with normal melting point agarose (1% in PBS) and kept at 4°C till the agarose was solidified. To each of these slides, 200 µl of 0.8% low-melting-point agarose containing 50 µl of treated cells was added. After solidification of the low-melting-point agarose, the slides were immersed in pre-chilled Lysing Solution [containing 2.5 M NaCl, 100 mM ethylenediaminetetraacetic acid (EDTA), 10 mM Tris-HCl, pH-10, 1% dimethyl sulfoxide (DMSO) and 1% TritonX] and kept for 1 h at 4°C for lysis of the cells. After lysis, the slides were drained properly and placed in a horizontal electrophoretic apparatus filled with freshly prepared electrophoresis buffer (containing 300 mM NaOH, 1 mM EDTA, 0.2% DMSO, pH ≥13). The slides were equilibrated in buffer for 20 min, and electrophoresis was carried out for 30 min at 20 V. After electrophoresis, the slides were washed gently with 0.4 mM Tris-HCl buffer at pH 7.4 to remove alkali. The slides were again washed with distilled water and kept at 37°C for 2 h to dry the gel. The slides were again washed with distilled water stained with 100 µl propidium iodide (100 µg/ml). The comets were visualized under a binocular microscope and the images captured were analyzed using the software ‘CASP’ to find out the extent of DNA damage (measured in terms of Olive Tail Moment) [18]. The parameter Olive Tail Moment (OTM) is the product of the distance between the centre of gravity of the head and the centre of gravity of the tail, and the percentage DNA in the tail. The results are presented as mean ± standard deviation.
Micronucleus assay
The micronucleus assay with mouse peripheral blood reticulocytes (as reported by Hayashi et al. [19] using acridine orange (AO)-coated slides) was carried out to evaluate the chromosomal damage. From each of the mice in each treatment group, 5 µl of peripheral blood was collected from the tail vein without any anticoagulant at the 48th hour of irradiation and placed on a AO-coated slide then covered immediately with a coverglass; the slides were allowed to stand for a few hours or overnight in a refrigerator to allow the cells to settle and to maximize staining. The slides were observed under a blue excitation (488 nm) and a yellow-to-orange barrier filter (515 nm), and 2000 reticulocytes of peripheral blood (identified by their reticulum structure with red fluorescence) were observed in order to determine the percentage of micronucleated (round in shape with a strong yellow-green fluorescence) reticulocytes were scored.
Effect of CIE on the expression profile of various genes in mice exposed to 4 Gy gamma radiation
Mice exposed to 4 Gy gamma radiation were administered with various doses of CIE. Genes involved in apoptosis, the inflammatory response and DNA damage repair were evaluated in the intestinal tissue of mice after 24 h of whole-body irradiation.
Briefly, the isolated RNA was subjected for cDNA synthesis by using reverse transcriptase. The expression of various genes controlling pathways for apoptosis, inflammation and survival was studied. The synthesized cDNA was subjected to polymerase chain reaction (PCR) on an Applied Biosystems Geneamp Thermal Cycler 2720 to find out the levels of expression of Bax, Bcl-2, Atm and Cox-2. Gapdh was used as the housekeeping control gene. The forward and reverse primers of various genes are given in Table 1.
Table 1.
Primer sequences of the genes studied
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Bax | 5′- AAGCTGAGCGAGTGTCTCCGGCG -3′ | 5′-GCCACAAAGATGGTCACTGTCTGCC-3′ |
| Bcl-2 | 5′-CTCGTCGCTACCGTCGTGACTTCG-3′ | 5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ |
| Atm | 5′-CGTAGGCTGGGAAGTGATAA-3′ | 5′-ACACATATGGGATGCGTTCT-3′ |
| Cox-2 | 5′-TCAAAAGAAGTGCTGGAAAAGGTT-3′ | 5′-TCTACCTGAGTGTCTTTGACTGTG-3′ |
| Gapdh | 5′-AAGGGCTCATGACCACAGTC-3′ | 5′-TGTGAGGGAGATGCTCAGTG-3′ |
The cycling conditions of Bcl-2 and Bax were the same: 94°C for 1 min (denaturation), 64°C for 1 min (annealing) and for 1 min (extension). For the housekeeping gene Gapdh, the denaturation temperature was at 95°C (10 min), annealing at 56°C (30 s) and extension at 72°C (59 s). Around 35 cycles of denaturation, annealing and extension were carried out. The initial heat activation of primers occurs at 95°C for all of the three genes; 5 min is programmed for Bcl-2 and Bax, whereas 10 min is programmed for Gapdh. Post extension, however, occurs at 72°C for each of the three genes.
The final amplicons were run in a 2% agarose gel electrophoresis and the gel was visualized under a gel documentation system.
Statistical analysis
All the results except for survival are presented as mean ± S.D. of the studied groups. Statistical analyses of the results were performed using analysis of variance (ANOVA) with the Tukey–Kramer multiple comparisons test. Statistical analysis of the survival data was performed by ‘Z’ test.
RESULTS
Free radical scavenging activity of Clerodendron infortunatum extract
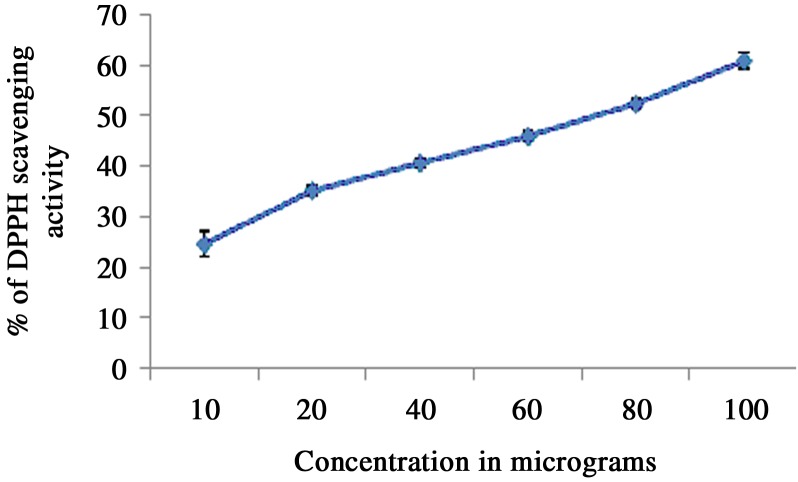
The hydro-alcoholic CIE reduced the DPPH radical in a concentration-dependent manner, as was evident from the data presented in Fig. 1. The stable free radical DPPH, with characteristic absorption at 515 nm, was reduced by the extract, resulting in a decrease in the absorption that was directly proportional to the electron-scavenging activity of CIE.
Fig. 1.
The DPPH free radical scavenging activity of CIE at various concentrations was determined from the reduction of DPPH when incubated with various concentrations (10–100 µg/ml) of CIE.
High-pressure liquid chromatography analysis of Clerodendron infortunatum extract
Quercetin has been reported to be one of the components of the CIE [20]. The HPLC chromatogram of hydro-alcoholic CIE and quercetin at 280 nm are presented in Fig. 2a and b, respectively. The presence of several compounds can be inferred from the several peaks seen in the HPLC profile of CIE (Fig. 2a).
Fig. 2.
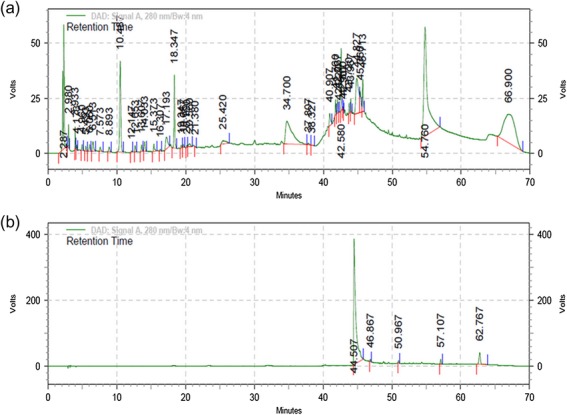
(a) The HPLC chromatogram of hydro-alcoholic CIE. (b) The HPLC chromatogram of the reference standard quercetin.
The HPLC chromatogram of reference compound quercetin showed a peak at 44.507 Volt.Minutes (V.S) with an area of 16424464, while hydro-alcoholic CIE showed a corresponding peak at 44.827 Volt.Minutes (V.S) and an area of 975343. From these results, the percentage of quercetin present in the CIE extract was calculated to be 6.52%. Quercetin is a strong antioxidant molecule and is present in various dietary sources and plant extracts [21]. It has been reported to be effective in alleviating various free radical–induced physiological stresses [22, 23]. Gamma radiation manifests deleterious effects by producing free radicals inside the cells, causing a variety of damages. The presence of free radical–scavenging quercetin in the extract could offer protection from damages induced by gamma radiation.
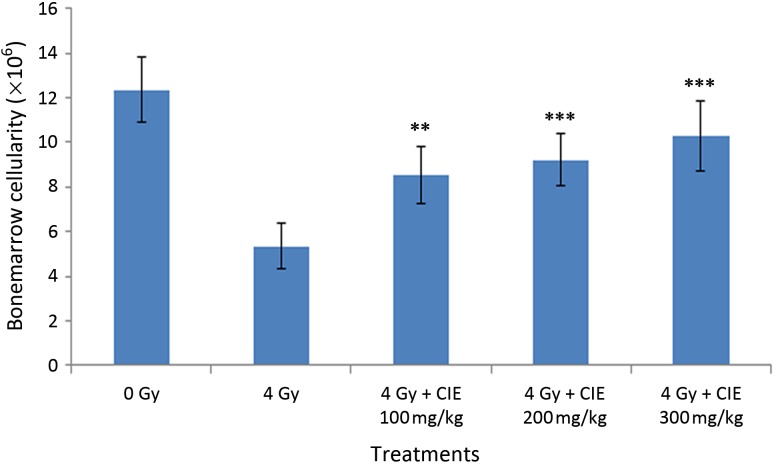
Effect of CIE on radiation-induced changes in bone marrow cellularity
Whole-body exposure to ionizing radiation causes severe alterations in bone marrow cellularity, and a drop in the total white blood cell (WBC) count. The number of bone marrow cells decreased drastically in animals exposed to 4 Gy whole-body radiation, as is evident from the data presented in Fig. 3. Administration of CIE could prevent the depletion of bone marrow cells and elevate the level of cellularity in a dose-dependent manner (Fig. 3).
Fig. 3.
Effect of oral administration of Clerodendron infortunatum (100–300 mg/kg) on the bone marrow cellularity of mice at 24th hour of 4 Gy whole-body gamma irradiation. Note: ***P < 0.001 and **P < 0.01 when compared with the respective control.
Effect of CIE on haematological parameters of irradiated animals
Exposure to gamma radiation lowers the total WBC count of the animals, as can be realized from the data presented in Fig. 4. Whole-body exposure to 4 Gy irradiation significantly lowered the total leukocyte count, while administration of CIE 1 h prior to irradiation was able to protect the animals from a radiation-induced decrease in total WBC count.
Fig. 4.
Effect of CIE (100–300 mg/kg) on total white blood cell (WBC) count after exposure to 4 Gy gamma radiation. Note: ***P < 0.001, *P < 0.05 and ns indicates ‘Not significant’ when compared with the respective control.
Antioxidant parameters in mice following CIE treatment and radiation exposure
Data on the evaluation of antioxidant status—the levels of GPx, GSH and SOD—in various tissues (such as heart, liver, kidney, intestine and brain) of the various groups of mice treated with different concentrations of CIE (100–300 mg/kg) 1 h prior to 4 Gy whole-body gamma irradiation are presented in Tables 2–4. The levels of GPx, GSH and SOD were decreased significantly following irradiation. Administration of CIE prevented this radiation-induced decrease of antioxidant levels in a concentration-dependent manner in CIE-treated animals.
Table 3.
Changes in reduced glutathione levels (nmol/mg protein) in various tissues of 4 Gy whole-body–irradiated mice administered CIE (100–300 mg/kg)
| Heart | Liver | Kidney | Intestine | Brain | |
|---|---|---|---|---|---|
| Normal (0 Gy) | 4.01 ± 0.36 | 22.91 ± 2.00 | 8.11 ± 1.00 | 7.07 ± 0.81 | 1.28 ± 0.04 |
| Control (4 Gy) | 1.33 ± .30 | 13.51 ± 1.50 | 4.81 ± 0.53 | 4.16 ± 0.63 | 0.49 ± 0.11 |
| 100 mg/kg (4 Gy+CIE) | 1.77 ± 0.23ns | 15.33 ± 1.17ns | 5.63 ± 0.47ns | 5.25 ± 0.59* | 0.83 ± 0.07*** |
| 200 mg/kg (4 GY+CIE) | 2.13 ± 0.25* | 17.86 ± 1.54*** | 6.21 ± 0.49** | 5.47 ± 0.34* | 1.14 ± 0.1*** |
| 300 mg/kg (4 GY+CIE) | 2.94 ± 0.62*** | 19.19 ± 1.16*** | 7.08 ± 0.42*** | 6.02 ± 0.30*** | 1.15 ± 0.10*** |
Note: ‘***’ corresponds to P < 0.001; ‘**’ corresponds to P < 0.01; ‘*’ corresponds to P < 0.05; and ns, ‘Not significant’ when compared with the respective control.
Table 2.
Changes in glutathione peroxidase levels (units/mg protein) in various tissues of 4 Gy whole-body–irradiated mice administered CIE (100–300 mg/kg)
| Heart | Liver | Kidney | Intestine | Brain | |
|---|---|---|---|---|---|
| Normal (0 Gy) | 2.07 ± 0.34 | 2.91 ± 0.38 | 3.97 ± 0.43 | 2.91 ± 0.37 | 0.50 ± 0.03 |
| Control (4 Gy) | 0.77 ± 0.15 | 1.49 ± 0.20 | 1.14 ± 0.14 | 1.56 ± .14 | 0.29 ± 0.04 |
| 100 mg/kg (4 Gy+CIE) | 1.16 ± 0.28ns | 1.60 ± 0.26ns | 1.75 ± 0.23* | 1.78 ± 0.15ns | 0.39 ± 0.02** |
| 200 mg/kg (4 Gy+CIE) | 1.75 ± 0.35** | 1.92 ± 0.23* | 2.39 ± 0.28*** | 2.31 ± 0.25*** | 0.43 ± 0.05*** |
| 300 mg/kg (4 Gy+CIE) | 2.40 ± 0.45*** | 2.28 ± 0.19*** | 3.07 ± 0.39*** | 2.42 ± 0.19*** | 0.47 ± 0.03*** |
Note: ‘***’ corresponds to P < 0.001; ‘**’ corresponds to P < 0.01; ‘*’ corresponds to P < 0.05; and ns, ‘Not significant’ when compared with the respective control.
Table 4.
Changes in superoxide dismutase levels (units/mg protein) in various tissues of 4 Gy whole-body–irradiated mice administered CIE (100–300 mg/kg)
| Heart | Liver | Kidney | Intestine | Brain | |
|---|---|---|---|---|---|
| Normal (0 Gy) | 3.88 ± 0.48 | 6.21 ± 0.52 | 2.14 ± 0.25 | 7.21 ± 0.50 | 1.26 ± 0.21 |
| Control (4 Gy) | 1.33 ± 0.20 | 3.47 ± 0.45 | 0.66 ± 0.12 | 2.90 ± 0.54 | 0.37 ± 0.06 |
| 100 mg/kg (4 Gy+CIE) | 1.72 ± 0.20ns | 4.00 ± 0.36ns | 1.16 ± 0.29ns | 4.82 ± 0.67ns | 0.77 ± 0.07*** |
| 200 mg/kg (4 GY+CIE) | 2.87 ± 0.47*** | 5.05 ± 0.42*** | 1.58 ± 0.21*** | 5.18 ± 0.46*** | 0.94 ± 0.15*** |
| 300 mg/kg (4 GY+CIE) | 3.63 ± 0.68*** | 5.34 ± 0.42*** | 1.88 ± 0.18*** | 5.92 ± 0.34*** | 1.21 ± 0.08*** |
Note: ‘***’ corresponds to P < 0.001; ‘**’ corresponds to P < 0.01; ‘*’ corresponds P < 0.05; and ns, ‘Not significant’ when compared with the respective control.
Effect of CIE on peroxidation of lipids in tissues of mice exposed to whole-body gamma radiation
Whole-body–irradiated mice showed elevated malondialdehyde (MDA) levels, reflecting peroxidation of membrane lipids in liver, kidney, heart, intestine and brain, while CIE administration significantly brought down the levels of MDA to near-normal levels, suggesting a decrease in radiation-induced membrane damage, as is evident from the results presented in Table 5.
Table 5.
Changes in lipid peroxidation levels (units/mg protein) in various tissues of 4 Gy whole-body–irradiated mice administered CIE (100–300 mg/kg)
| Heart | Liver | Kidney | Intestine | Brain | |
|---|---|---|---|---|---|
| Normal (0 Gy) | 1.69 ± 0.15 | 1.29 ± 0.24 | 5.15 ± 0.54 | 3.12 ± 0.43 | 4.45 ± 0.83 |
| Control (4 Gy) | 4.71 ± 0.37 | 2.73 ± 0.32 | 13.21 ± 1.01 | 8.42 ± 1.25 | 15.14 ± 2.34 |
| 100 mg/kg (4 Gy+CIE) | 4.01 ± 0.42* | 2.24 ± 0.20* | 11.11 ± 0.92* | 5.92 ± 0.47*** | 10.71 ± 2.39** |
| 200 mg/kg (4 GY+CIE) | 3.24 ± 0.31*** | 2.04 ± 0.28*** | 8.95 ± 1.43*** | 4.96 ± 0.44*** | 8.73 ± 0.83*** |
| 300 mg/kg (4 GY+CIE) | 2.20 ± 0.20*** | 1.75 ± 0.22*** | 7.42 ± 0.75*** | 4.18 ± 0.47*** | 6.14 ± 1.17*** |
Note: ‘***’ corresponds to P < 0.001; ‘**’ corresponds to P < 0.01; ‘*’ corresponds to P < 0.05; and ns, ‘Not significant’ when compared with the respective control.
Effect of CIE on the tissue ultrastructure of the gastrointestinal tract of whole-body–irradiated mice
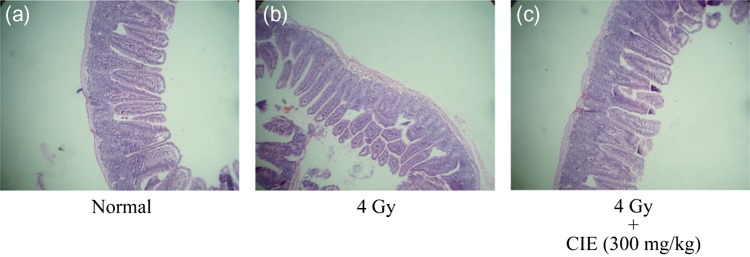
Whole-body exposure of animals to gamma radiation (4 Gy) resulted in alteration in tissue ultrastructure, particularly in the small intestine as is characteristic of gastrointestinal syndrome. Histopathological studies of the gastrointestinal system revealed that 24 h after 4 Gy gamma radiation, the irradiated mice exhibited gastrointestinal damages as blunting of the villi, as can be seen in Fig. 5b. In the animals administered with CIE, normal architecture of the intestine tissue was discernible (Fig. 5c). These histopathological observations supported the previous results that CIE administration could prevent ionizing radiation–induced damages [24, 25].
Fig. 5.
Effect of CIE on gastrointestinal injury in mice after whole-body gamma irradiation. Histopathology of intestinal sections from mice 24 h after irradiations and CIE treatments. (a) control; 0 Gy, no CIE treatment; (b) 4 Gy, no CIE treatment; (c) 4 Gy, + CIE (300 mg/kg) treatment.
Effect of CIE on gamma radiation–induced strand breaks
Whole-body exposure of mice to 4 Gy gamma radiation caused damage to cellular DNA of various tissues, such as blood leukocyte bone marrow and spleen. The comet parameter Olive Tail Moment was found to be increased in the irradiated groups. Oral administration of CIE to mice was able to prevent the formation of DNA strand breaks, as evident from a decreased comet parameter. The data are presented in Fig. 6a–c. Figure 6d is a representative image of bone marrow cells of mice that were irradiated with 4 Gy gamma radiation, with or without administration of CIE (300 mg/kg).
Fig. 6.
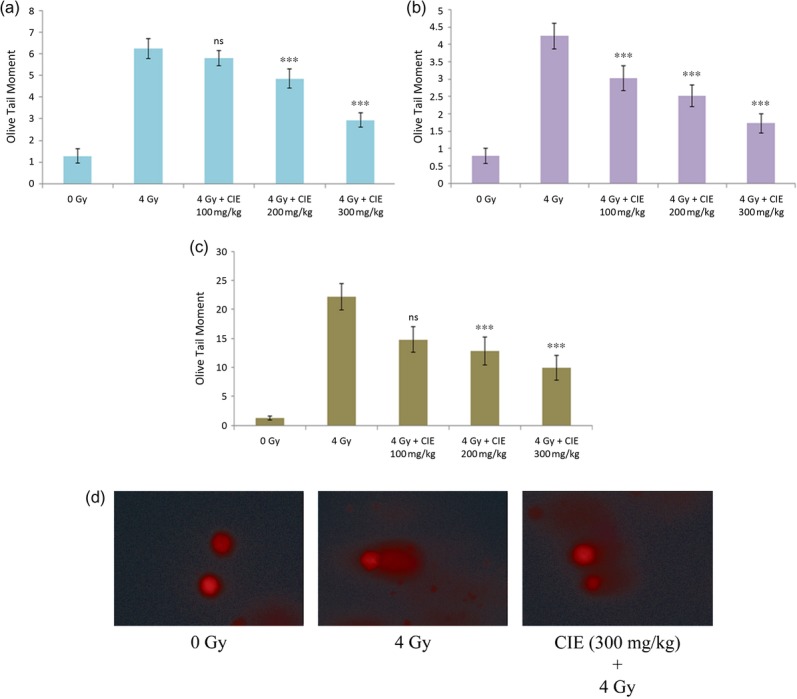
(a) Effect of CIE (100–300 mg/kg) on prevention of 4 Gy gamma-radiation–induced strand breaks in cellular DNA in various tissues of mice, expressed as Olive Tail Moment: (a) bone marrow cells, (b) peripheral blood leukocytes, (c) spleenocytes. Note: ***P < 0.001 and ns indicates ‘Not significant’ when compared with the respective control. (d) Representative image of bone marrow cells of mice that received CIE (300 mg/kg) 1 h prior to irradiation with 4 Gy gamma radiation.
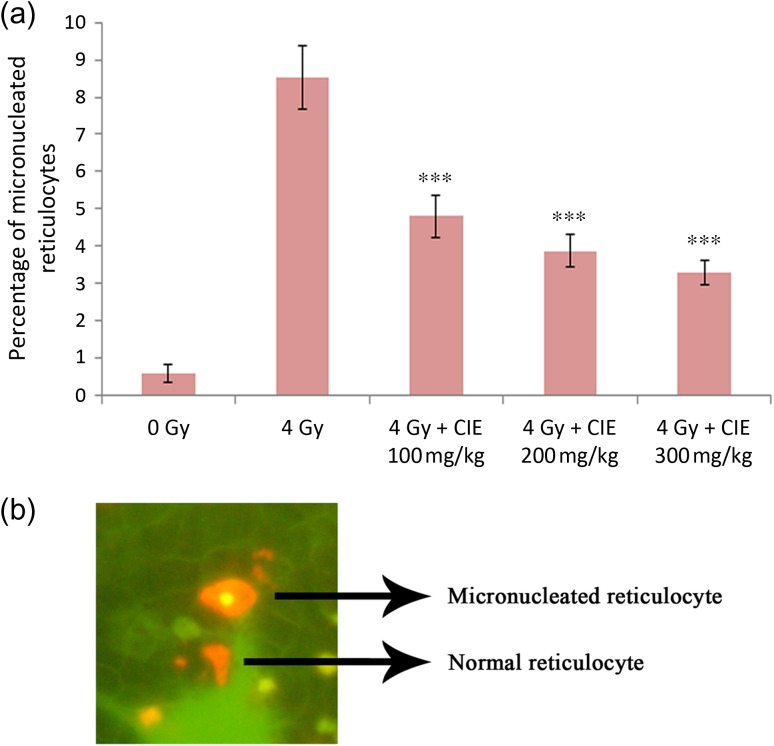
Effect of CIE on induction of micronuclei in mouse reticulocytes
The number of circulating micronucleated reticulocytes were found to be significantly increased in animals exposed to 4 Gy whole-body gamma irradiation after 48 h. Oral administration of CIE 1 h before radiation exposure significantly prevented this increase in the number of circulating micronucleated reticulocytes (Fig. 7a). A representative image of a micronucleated reticulocyte and a normal reticulocyte is shown in Fig. 7b.
Fig. 7.
(a) Effect of administration of CIE (100–300 mg/kg) on the induction of micronuclei in mouse reticulocytes at the 48th hour of 4 Gy whole-body gamma irradiation. Note: ***P < 0.001 when compared with the respective control. (b) Representative image of mice peripheral blood stained with acridine orange. The red cell with an orange spot in it is the micronucleated reticulocyte.
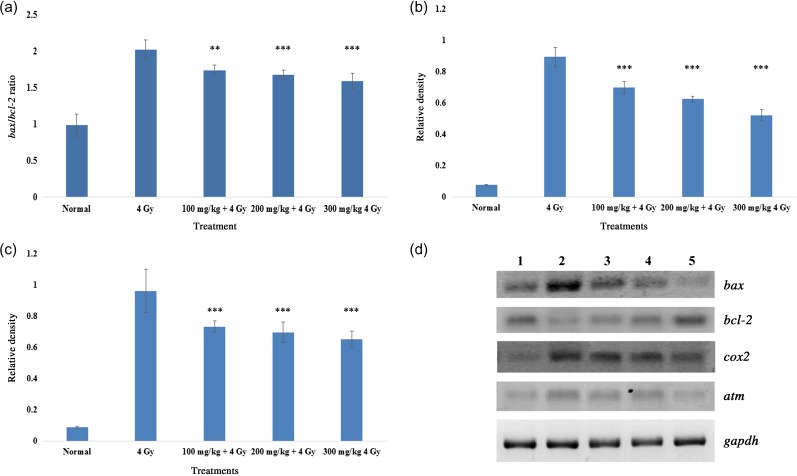
Effect of CIE on gene expression in the intestinal cells of mice exposed to 4 Gy whole-body gamma radiation
Genes involved in apoptosis, the inflammatory response and DNA damage repair were evaluated in the intestinal tissue of whole-body–irradiated mice.
The Bcl-2/Bax ratio is a classical marker for predicting the probability of a cell entering the apoptotic pathway. This ratio increased in animals exposed to whole-body irradiation, while administration of CIE could prevent the increase of the ratio, as can be seen in Fig. 8a.
Fig. 8.
(a) The Bax/Bcl-2 ratio of the intestinal tissue of mice exposed to whole body 4 Gy gamma radiation with or without oral administration of various doses of CIE (100–300 mg/kg). The ratios were calculated from densitometric scans of the PCR amplicons of the respective genes. Note: ‘***’ corresponds to P < 0.001, and ‘**’ corresponds to P < 0.01 when compared with the respective control. (b) Levels of Cox-2 expression in the intestinal tissue of mice exposed to 4 Gy whole-body gamma irradiation with or without oral administration of various doses of CIE (100–300 mg/kg). The densities relative to Gapdh expression were calculated from densitometric scans of the PCR amplicons of Cox-2 and Gapdh. Note: ‘***’ corresponds to P < 0.001 when compared with the respective control. (c) Levels of Atm expression in intestinal tissue of mice exposed to 4 Gy whole-body gamma irradiation with or without oral administration of CIE (100–300 mg/kg). The densities relative to Gapdh expression were calculated from densitometric scans of the PCR amplicons of Atm and Gapdh. Note: ‘***’ corresponds to P < 0.001 when compared with the respective control. (d) Electrophoretic separation of RT–PCR on mice intestine samples. Lane 1: unirradiated mice. Lane 2: 4 Gy irradiated animals. Lane 3: CIE (100 mg/kg) + 4 Gy. Lane 4: CIE (200 mg/kg) + 4 Gy. Lane 5: CIE (300 mg/kg) + 4 Gy.
Exposure of animals to 4 Gy whole-body gamma radiation upregulated Cox-2 gene expression in intestinal tissue, and administration of CIE prevented the increased expression of Cox-2, as is evident from the data presented in Fig. 8b.
Elevated Atm expression in intestinal cells of whole-body–irradiated mice indicated the activation of a DNA repair mechanism. Expression of Atm in the irradiated CIE-treated group was found to be lower than in the irradiated control group (Fig. 8c). The low expression of Atm in this case could be ascribed to a reduced number of radiation-induced strand breaks in this group, which corroborates the results of the comet assay (where CIE administration was found to reduce radiation-induced strand breaks).
A representative gel image of each gene is given in Fig. 8d.
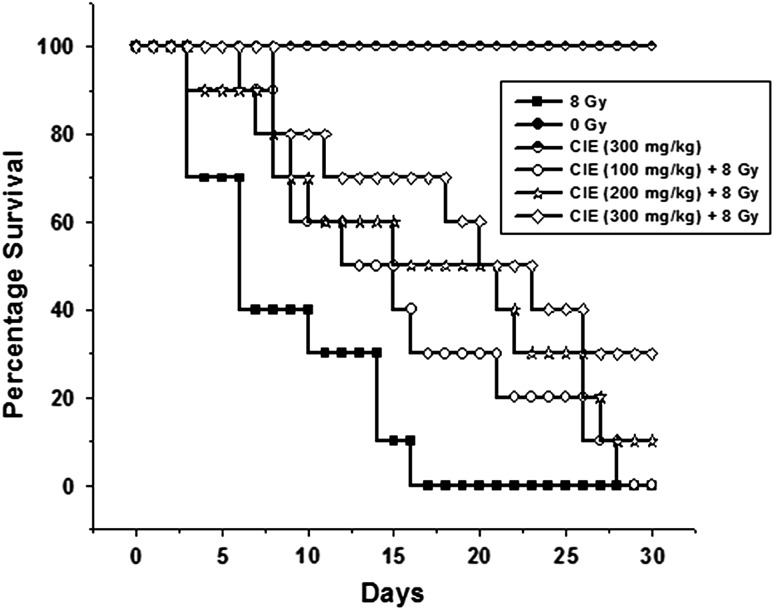
Effect of CIE on gamma radiation–induced alterations in survival period
Exposure to lethal doses of ionizing radiation results in mortality due to irreparable damage to various organ systems. As presented in Fig. 9, on the 17th day of irradiation 100% mortality was observed in the group that received 8 Gy whole-body gamma radiation. Pre-oral administration of CIE at various concentrations provided survival advantage to the animals. The group that received CIE (300 mg/kg) showed a 30% survival at the end of 30 days. The P-value obtained by comparison of the group that received 300 mg/kg CIE with the irradiated control group was 0.0601. The result was statistically significant (P < 0.10).
Fig. 9.
The effect of various concentrations of CIE on 8 Gy whole-body gamma irradiation–induced mortality in mice (n = 10).
DISCUSSION
Ionizing radiation exerts its deleterious effects through direct action on the vital cellular molecules and also by indirect action through aqueous free radicals generated by radiolysis of water. Free radicals generate a cascade pathway through which DNA, protein and carbohydrate get oxidized. Higher doses of ionizing radiation exposure cause depletion to antioxidant enzymes to an unrecoverable status [26]. Radiation-induced direct and indirect DNA damage will lead to single- or double-strand breaks that affect the fidelity of DNA replication and cause genomic instability and cell mortality [27]. Extracts of several plants have been reported to give protection to mammalian systems against the deleterious effects of ionizing radiation [28–31]. These extracts contain a number of phytoceutical compounds, and many of these individually and collectively act as radioprotectors [32–34]. Plants are known to be a reliable source of antioxidants, and many studies have shown the potential of plant-derived antioxidant compounds to act as radioprotectors due to their ability to mitigate the oxidative stress resulting from free radicals induced by ionizing radiation [35].
The present study explored the mechanism of radioprotection in mice administered with CIE through antioxidant assays, the alkaline comet assay and the micronucleus assay. Radiation-induced oxidative injury is attributed to the depletion of free radical scavenging enzymes such as SOD, GPx, catalase and reduced GSH. Enhancement of the antioxidants-mediated endogenous cellular defence mechanism has prime importance in radioprotection. The administration of CIE to irradiated mice elevated their cellular antioxidant status to a safe level. Elevation of peroxidation of membrane lipids by ionizing radiation was prevented by oral administration of CIE prior to irradiation. The damage inflicted on the intestinal architecture by ionizing radiation was also reduced by CIE administration; this may have been due to the efficacy of CIE in scavenging free radicals.
The alkaline comet assay is an elegant and effective technique for monitoring the extent of DNA damage. Whole-body–irradiated mice showed DNA damage that was reflected in an increase in the comet parameter Olive Tail Moment. Administration of CIE before irradiation prevented DNA damage, as observed from a decrease in the comet parameter in a concentration-dependent manner, indicative of its radioprotecting ability.
A decrease in the comet parameter indicates a decrease in strand breaks. This decrease can be due to either legitimate repair of damaged DNA or improper joining of strands. Improper strand joining in precursors of blood cells will be reflected as micronuclei in the peripheral reticulocytes, since these small fragments fail to integrate properly into the nucleus. The percentage of micronucleated reticulocytes of whole-body–irradiated mice after 48 h indicates the extent of improper DNA repair; CIE-administered mice had fewer micronucleus, revealing the augmentation of proper DNA repair.
The small intestine is a very radiosensitive tissue [36], so the effect of CIE on the expression of various genes was studied in this tissue. Ionizing radiation can trigger the activation of various pathways such as apoptosis, DNA damage repair and inflammation. Damaged cells are eliminated through apoptosis; DNA damage is repaired through Atm; atr activation and damage to various tissues induces the activation of inflammatory pathways.
Apoptosis is activated when ionizing radiation inflicts unrepairable damage on cells [37]. The elevated pro-apoptotic gene expression of Bax and decreased expression of anti-apoptotic Bcl-2 leads to the pathway of radiation-induced programmed cell death in the intestine. An increased Bax/Bcl-2 ratio is a classical marker for apoptosis [28]. The CIE-administered irradiated mice showed enhanced anti-apoptotic gene expression and a simultaneous decrease in pro-apoptotic gene expression, resulting in enhancement of cell survival after irradiation.
CIE was able to maintain the tissue antioxidant levels at subcritical levels. This would have facilitated scavenging of the ROS produced inside the cells during the radiation exposure. Maintaining an optimal level of cellular antioxidants could reduce radiation-induced DNA damages [38, 39]. Lower levels of DNA damage will reduce the recruiting of Atm to damaged sites [40, 41]. This was observed in our results: groups that received CIE prior to radiation exposure showed lower levels of Atm expression than the control group.
The inflammatory response is one of the major pathways activated during radiation exposure [42, 43]. Cox2 is one of the mediators of this pathway. Animals that received CIE prior to exposure to radiation showed a decrease in Cox-2 expression, suggesting a decrease in the inflammatory response. This result also suggests the ability of CIE to inhibit initiation of the necrotic pathway by protecting cells from radiation-induced damages.
Exposure to lethal doses of ionizing radiation results in mortality due to the failure of all the organ systems. The lethal effect of ionizing radiation was found to be reduced by the administration of CIE in a concentration-dependent manner. This can be attributed to the ability of this extract to provide protection against ionizing radiation–induced deleterious effects in various tissues. Thus, the present work provides compelling evidence suggesting the use of CIE as a radioprotector in cases of planned exposure to ionizing radiation.
ACKNOWLEDGEMENTS
The authors thank Dr Jayakrishnan, Dr Ramachandran and Dr Vivek [LLRRL (DAE), Quilon, Kerala] for their help and support in irradiation of the animals.
CONFLICT OF INTEREST
The authors state that there are no conflicts of interest.
REFERENCES
- 1. Nair CK, Parida DK, Nomura T. Radioprotectors in radiotherapy. J Radiat Res 2001;42:21–37. [DOI] [PubMed] [Google Scholar]
- 2. Weiss JF, Landauer MR. Radioprotection by antioxidants. Ann NY Acad Sci 2000;899:44–60. [PubMed] [Google Scholar]
- 3. Luc R, Vergely C. Forgotten radicals in biology. Int J Biomed Sci 2008;4:255–9. [PMC free article] [PubMed] [Google Scholar]
- 4. Halliwell B. Free Radicals and Other Reactive Species in Disease in eLS. John Wiley & Sons, Ltd, 2001. [Google Scholar]
- 5. Paul P, Unnikrishnan MK, Nagappa AN. Phytochemicals as radioprotective agents: a review. Indian J Nat Prod Resour 2011;2:137–50. [Google Scholar]
- 6. Bhattacharjee D, Das A, Das SK, et al. Clerodendrum infortunatum Linn.: a review. J Adv Pharm Healthc Res 2011;1:82–5. [Google Scholar]
- 7. Sen A, Kar P, Goyal A, et al. Antioxidant and pharmaceutical potential of Clerodendrum L.: an overview.Int J Green Pharm 2014;8:210–6. [Google Scholar]
- 8. Shrivastava N, Patel T. Clerodendrum and heathcare: an overview. Med Aromat Plant Sci Biotechnol 2007;1:142–50. [Google Scholar]
- 9. Aquino R, Morelli S, Lauro MR, et al. Phenolic constituents and antioxidant activity of an extract of Anthurium versicolor leaves. J Nat Prod 2001;64:1019–23. [DOI] [PubMed] [Google Scholar]
- 10. Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 1979;582:67–78. [DOI] [PubMed] [Google Scholar]
- 11. Hafeman DG, Sunde RA, Hoekstra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr 1974;104:580–7. [DOI] [PubMed] [Google Scholar]
- 12. McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 1969;244:6049–55. [PubMed] [Google Scholar]
- 13. Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–75. [PubMed] [Google Scholar]
- 14. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol 1978;52:302–10. [DOI] [PubMed] [Google Scholar]
- 15. Galeano M, Altavilla D, Cucinotta D, et al. Recombinant human erythropoietin stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetes 2004;53:2509–17. [DOI] [PubMed] [Google Scholar]
- 16. Singh NP. Microgels for estimation of DNA strand breaks, DNA protein crosslinks and apoptosis. Mutat Res 2000;455:111–27. [DOI] [PubMed] [Google Scholar]
- 17. Chandrasekharan DK, Kagiya TV, Nair CKK. Radiation protection by 6-palmitoyl ascorbic acid-2-glucoside: studies on DNA damage in vitro, ex vivo, in vivo and oxidative stress in vivo. J Radiat Res 2009;50:203–12. [DOI] [PubMed] [Google Scholar]
- 18. Końca K, Lankoff A, Banasik A, et al. A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res 2003;534:15–20. [DOI] [PubMed] [Google Scholar]
- 19. Hayashi M, Morita T, Kodama Y, et al. The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange–coated slides. Mutat Res 1990;245:245–9. [DOI] [PubMed] [Google Scholar]
- 20. Gupta S, Gupta R. Detection and quantification of quercetin in roots, leaves and flowers of Clerodendrum infortunatum L. Asian Pac J Trop Dis 2012;2:S940–3. [Google Scholar]
- 21. Hollman PC, van Trijp JM, Buysman MNC, et al. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett 1997;418:152–6. [DOI] [PubMed] [Google Scholar]
- 22. Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 2008;585:325–37. [DOI] [PubMed] [Google Scholar]
- 23. Mahesh T, Menon VP. Quercetin allievates oxidative stress in streptozotocin-induced diabetic rats. Phytother Res 2004;18:123–7. [DOI] [PubMed] [Google Scholar]
- 24. Deniz M, Atasoy BM, Dane F, et al. Radiation-induced oxidative injury of the ileum and colon is alleviated by glucagon-like peptide-1 and -2. J Radiat Res Appl Sci 2015;8:234–42. [Google Scholar]
- 25. Ha D, Bing SJ, Cho J, et al. Phloroglucinol Protects Small Intestines of Mice from Ionizing Radiation by Regulating Apoptosis-Related Molecules: A Comparative Immunohistochemical Study. J Histochem Cytochem 2013;61:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Samarth RM, Panwar M, Kumar M, et al. Evaluation of antioxidant and radical-scavenging activities of certain radioprotective plant extracts. Food Chem 2008;106:868–73. [Google Scholar]
- 27. Uma Devi P. Radioprotective, anticarcinogenic and antioxidant properties of the Indian holy basil, Ocimum sanctum (Tulasi). Indian J Exp Biol 2001;39:185–90. [PubMed] [Google Scholar]
- 28. Yi X, Yin X-M, Dong Z. Inhibition of Bid-induced apoptosis by Bcl-2. tBid insertion, Bax translocation, and Bax/Bak oligomerization suppressed. J Biol Chem 2003;278:16992–9. [DOI] [PubMed] [Google Scholar]
- 29. Samarth RM, Kumar A. Radioprotection of Swiss albino mice by plant extract Mentha piperita (Linn.). J Radiat Res 2003;44:101–9. [DOI] [PubMed] [Google Scholar]
- 30. Jagetia GC, Venkatesh P, Baliga MS. Evaluation of the radioprotective effect of bael leaf (Aegle marmelos) extract in mice. Int J Radiat Biol 2004;80:281–90. [DOI] [PubMed] [Google Scholar]
- 31. Plamadeala C, Aparaschivei A, Bara I, et al. Comparative cytogenetic analysis of radioprotector effect of two vegetal extracts. J Adv Res Phys 2013;4:1–4. [Google Scholar]
- 32. Abdel-Hamid NM. Phytoceuticals for radioprotection with special reference to Egyptian flora In: Arora R.(ed.). Herbal Radiomodulators: Applications in Medicine, Homeland Defence and Space. Wallingford: CABI, 2008, 83–6. [Google Scholar]
- 33. Choudhary D, Chandra D, Kale RK. Modulation of radioresponse of glyoxalase system by curcumin. J Ethnopharmacol 1998;64:1–7. [DOI] [PubMed] [Google Scholar]
- 34. Shimoi K, Masuda S, Shen B, et al. Radioprotective effects of antioxidative plant flavonoids in mice. Mutat Res 1996;350:153–61. [DOI] [PubMed] [Google Scholar]
- 35. Arora R, Gupta D, Chawla R, et al. Radioprotection by plant products: present status and future prospects. Phytother Res 2005;19:1–22. [DOI] [PubMed] [Google Scholar]
- 36. Suciu D. Cellular death by apoptosis in some radiosensitive and radioresistant mammalian tissues. J Theor Biol 1983;105:391–401. [DOI] [PubMed] [Google Scholar]
- 37. Richardson RB. Ionizing radiation and aging: rejuvenating an old idea. Aging 2009;1:887–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fang Y-Z, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition 2002;18:872–9. [DOI] [PubMed] [Google Scholar]
- 39. Sun J, Chen Y, Li M, et al. Role of antioxidant enzymes on ionizing radiation resistance. Free Radic Biol Med 1998;24:586–93. [DOI] [PubMed] [Google Scholar]
- 40. Derheimer FA, Kastan MB. Multiple roles of ATM in monitoring and maintaining DNA integrity. FEBS Lett 2010;584:3675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Panier S, Boulton SJ. Double-strand break repair: 53BP1 comes into focus. Nat Rev Mol Cell Biol 2013;15:7–18. [DOI] [PubMed] [Google Scholar]
- 42. Sonis ST, O'Donnell KE, Popat R, et al. The relationship between mucosal cyclooxygenase-2 (COX-2) expression and experimental radiation-induced mucositis. Oral Oncol 2004;40:170–6. [DOI] [PubMed] [Google Scholar]
- 43. Stewart FA, Heeneman S, Te Poele J, et al. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE−/− mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol 2006;168:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]