Abstract
It is not yet clear to what extent depletion of intracellular GTP pools contributes to the antiviral activity of ribavirin. Therefore, the antiviral activities of (i) ribavirin, (ii) its 5-ethynyl analogue, 5-ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide (EICAR), and (iii) mycophenolic acid (MPA) (a compound that inhibits only cellular IMP dehydrogenase activity) were studied on the replication of flaviviruses and paramyxoviruses. In addition, the effects of these three compounds on intracellular GTP pools were assessed. A linear correlation was observed over a broad concentration range between the antiviral activities of ribavirin, EICAR, and MPA and the effects of these compounds on GTP pool depletion. When the 50% effective concentrations (EC50s) for the antiviral activities of ribavirin, EICAR, and MPA were plotted against the respective EC50 values for GTP pool depletion, a linear correlation was calculated. These data provide compelling evidence that the predominant mechanism of action of ribavirin in vitro against flavi- and paramyxoviruses is based on inhibition of cellular IMP dehydrogenase activity.
Ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide) (see Fig. 1) has broad-spectrum antiviral activity against a wide range of (RNA) viruses. Ribavirin is being used with various degrees of success for the treatment of respiratory syncytial virus (6, 23) and Lassa fever virus (11) infections. Ribavirin, in combination with pegylated interferon, has become the standard therapy for the treatment of infections with hepatitis C virus (18).
FIG. 1.
Structural formulas of ribavirin, EICAR, and mycophenolic acid (MPA).
Various mechanisms of action have been suggested to be responsible for the antiviral activity of ribavirin (12). These mechanisms include the following: (i) depletion of intracellular GTP pools (by inhibition of the cellular IMP dehydrogenase [IMPDH] by the 5′-monophosphate metabolite of ribavirin), (ii) inhibition of viral polymerase activity by the 5′-triphosphate metabolite of ribavirin, (iii) inhibition of viral capping (by inhibition of [viral or cellular] guanylyltransferase activity by ribavirin 5′-triphosphate), and (iv) induction of error catastrophe as a result of accumulation of mutations (some of them lethal) in the viral genome. Although none of these mechanisms may be mutually exclusive, one (or more) mechanism(s) may be predominantly responsible for the antiviral activity of ribavirin.
The aim of this study was to assess to what extent inhibition of intracellular GTP pools contributes to the antiviral activity of ribavirin. For model viruses, we employed a plus-strand single-stranded RNA (ssRNA) virus (i.e., the yellow fever virus 17D vaccine strain [YFV 17D]) and a minus-strand ssRNA virus (i.e., the human parainfluenza virus 3 [hPIV3]; ATCC VR-93). 5-Ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide (EICAR) and mycophenolic acid (MPA) were selected for comparison with ribavirin because of the following. (i) EICAR (Fig. 1), which is the 5-ethynyl imidazole analogue of ribavirin, has the same spectrum of antiviral activity as ribavirin but is 20- to 60-fold more potent (9, 14). Furthermore, the 5′-monophosphate metabolite of EICAR, like ribavirin 5′-monophosphate, has proven to be an inhibitor of IMPDH and is at least 10-fold more potent than ribavirin in inhibiting IMPDH and thus reducing intracellular GTP pools (2, 3). (ii) MPA [6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-4-hexenoic acid] (Fig. 1) is clinically used by way of its prodrug mycophenolate mofetil (CellCept) as a second-line immunosuppressive agent to avert acute graft rejection in organ transplant patients (for a review, see reference 4). MPA is also endowed with potent antiviral activity against flaviviruses (10, 14, 16) and paramyxoviruses (our unpublished observations) among other viruses. MPA is a highly potent uncompetitive inhibitor of IMPDH and unlike ribavirin and EICAR, does not need to be metabolically activated (21).
First, the dose-response effects of ribavirin, EICAR, and MPA on the replication of YFV 17D and hPIV3 were determined. One-day-old confluent Vero cell monolayers, grown in 96-well microtiter plates, were infected with either YFV 17D (Stamaril; Aventis Pasteur MSD, Brussels, Belgium) or hPIV3 at a multiplicity of infection (MOI) of ∼0.1 in the absence or presence of serial dilutions of the respective compounds. Cultures were incubated at 37°C for 5 days, when infected, untreated cultures exhibited an obvious cytopathic effect (CPE). For each condition, the supernatant from two to four wells was pooled, and then total RNA was extracted (QIAamp viral RNA mini kit; QIAGEN, Leusden, The Netherlands). Viral RNA was quantified using one-step reverse transcription-quantitative PCR (RT-qPCR). For YFV 17D, TaqMan one-step RT-PCR master mix reagent kit, a forward primer (TGG CAT ATT CCA GTC AAC CTT CT), reverse primer (GAA GCC CAA GAT GGA ATC AAC T), and 6-carboxyfluorescine (FAM)-6-carboxytetramethylrhodamine (TAMRA) probe (TTC CAC ACA ATG TGG CAT GTC ACA AGA G) were used. For hPIV3, SYBR green RT-PCR reagent kit, a forward primer (GGA GGT GCA CGT CTG GTC TT), and a reverse primer (ACA GTC GTT GGC ATG GCT AAT A; Applied Biosystems, Lennik, Belgium) were used.
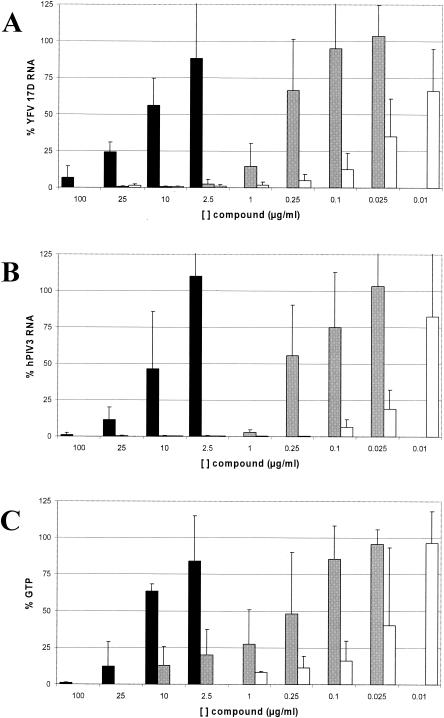
Each of the compounds (ribavirin, EICAR, and MPA) caused a concentration-dependent inhibition of synthesis of both YFV 17D and hPIV3 RNA (Fig. 2A and B). MPA proved to be the most potent compound, and ribavirin was the least potent compound (for YFV, the EC50 for inhibition of RNA synthesis [EC50 RNA] of ribavirin, 12.3 ± 5.6 μg/ml; EC50 RNA of EICAR, 0.35 ± 0.23 μg/ml; EC50 RNA of MPA, 0.020 ± 0.013 μg/ml; for hPIV3, EC50 RNA of ribavirin, 9.4 ± 6.1 μg/ml; EC50 RNA of EICAR, 0.27 ± 0.22 μg/ml; EC50 RNA of MPA, 0.015 ± 0.007 μg/ml).
FIG. 2.
(A) Antiviral activities of ribavirin (black), EICAR (grey), and MPA (white) against YFV 17D in Vero cells as assessed by RT-qPCR and presented as a percentage of the value for infected, untreated controls. The concentrations of the three compounds are shown on the x axis. Data are means ± standard deviations (error bars) obtained from five independent experiments. (B) Antiviral activities of ribavirin (black), EICAR (grey), and MPA (white) against hPIV3 in Vero cells as assessed by RT-qPCR and presented as a percentage of the value for infected, untreated controls. Data are mean values ± standard deviations (error bars) obtained from six independent experiments. (C) Inhibition of intracellular GTP pools in Vero cells by ribavirin (black), EICAR (grey), and MPA (white) and presented as a percentage of the value for untreated controls. Data are mean values ± standard deviations (error bars) obtained from three independent experiments.
A parallel set of samples was titrated six times for infectious virus content on 1-day-old confluent Vero cell monolayers grown in 96-well microtiter plates. For each sample, the 50% cell culture infectious dose (CCID50) was determined by the method of Reed and Muench (18a). Similar to the experiment described above, a dose-response effect was observed on the production of infectious YFV 17D and hPIV3 (data not shown) (for YFV, the EC50 for inhibition of infectious virus production [EC50 CCID50] of ribavirin, 48.5 ± 41.3 μg/ml; EC50 CCID50 of EICAR, 0.79 ± 0.47 μg/ml; EC50 CCID50 of MPA, 0.079 ± 0.053 μg/ml; for hPIV3, EC50 CCID50 of ribavirin, 17.2 ± 6.9 μg/ml; EC50 CCID50 of EICAR, 0.7 ± 0.9 μg/ml; EC50 CCID50 of MPA, 0.064 ± 0.08 μg/ml). Again, and as was the case for the RT-qPCR data, MPA was about 10-fold more potent than EICAR, which in turn was about 50-fold more potent than ribavirin.
Subsequently, the effect of each compound on GTP pool depletion was determined. Serial dilutions of compound, identical to those that were used in the antiviral assays, were added to 1-day-old confluent Vero cell monolayers grown in 75-cm2 culture flasks. Cells were collected 24 h later, and nucleotides were extracted from the cells and quantified by anion-exchange high-performance liquid chromatography (HPLC) as described previously (17). The data were corrected for the number of cells per culture.
Each of the compounds caused a concentration-dependent depletion of intracellular GTP pools (Fig. 2C). MPA proved to be the most potent in reducing intracellular GTP pools, EICAR had intermediate activity, and ribavirin was the least active (EC50 for depletion of the intracellular GTP pool [EC50 GTP] of ribavirin, 12.8 ± 6.0 μg/ml; EC50 GTP of EICAR,0.48 ± 0.33 μg/ml; EC50 GTP of MPA, 0.023 ± 0.021 μg/ml). Each compound, at the highest, nontoxic concentration tested, almost completely reduced intracellular GTP levels (ribavirin by 100% at 100 μg/μl, EICAR by 87% at 10 μg/ml, and MPA by 92% at 1 μg/ml). Similar effects of ribavirin and MPA on GTP pool depletion were also observed in 4-day-old cultures; the EC50 GTP values at day 4 were 24 and 0.06 μg/ml for ribavirin and MPA, respectively.
To determine whether ribavirin is preferentially phosphorylated in infected cells, as was suggested by others using cultures infected with the foot-and-mouth disease virus (1), we determined the levels of intracellular metabolites of ribavirin in YFV 17D-infected Vero cells at day 3 postinfection (when cultures exhibited 60% CPE). Cultures were pulse-labeled with [3H]ribavirin (Moravek, Brea, Calif.) for 6 h, and then methanol extracts were analyzed by HPLC on a Partisil SAX column. Following correction for the cell number, the levels of the phosphorylated metabolites of ribavirin, namely, the 5′-monophosphate, 5′-diphosphate, and 5′-triphosphate forms of ribavirin were 99, 100, and 101%, respectively, compared to those of parallel uninfected cultures. The levels of intracellular GTP in YFV 17D-infected Vero cells at day 3 postinfection were 1.5 ± 0.1-fold higher than in uninfected cells, and at day 4, GTP pools were 2.2 ± 0.05-fold higher than in uninfected cells.
For each compound, for both viruses and over the entire concentration range studied, data on inhibition of viral RNA replication were plotted against the data on GTP depletion (data not shown). The following linear correlations were calculated for YFV 17D (R2 = 0.966 for ribavirin, R2 = 0.954 for EICAR, and R2 = 0.974 for MPA) as well as for hPIV3 (R2 = 0.885 for ribavirin, R2 = 0.943 for EICAR, and R2 = 0.982 for MPA). This indicates a strong relationship between the antiviral activity of each of these three compounds and their effect on GTP depletion. Similar linear correlations were calculated when the data on reduction of viral infectivity were plotted against the data on GTP depletion (data not shown) (for YFV 17D, R2 = 0.868 for ribavirin, R2 = 0.797 for EICAR, and R2 = 0.982 for MPA; for hPIV3, R2 = 0.846 for ribavirin; R2 = 0.859 for EICAR, and R2 = 0.983 for MPA).
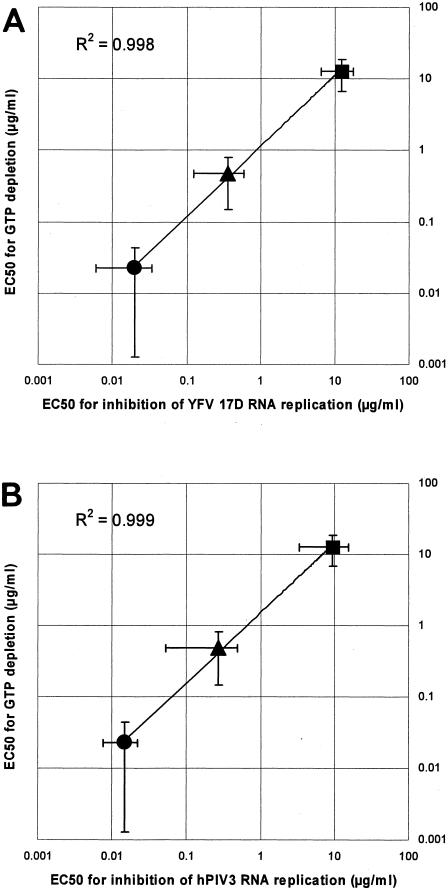
Finally, when the EC50 RNA values for antiviral activity of each of the three compounds were plotted against the respective EC50 GTP values for GTP depletion, a linear correlation was noted. The calculated curve had an intercept of nearly 1 on both axes and a slope of nearly 1 (Fig. 3, R2 = 0.998 for YFV in panel A and R2 = 0.999 for hPIV3 in panel B). Plotting the EC50 CCID50 values against the respective EC50 GTP values yielded similar R2 values (data not shown) (R2 = 0.982 for YFV; R2 = 0.997 for hPIV3). To further corroborate these findings, EC50s for inhibition of viral replication (YFV 17D; CPE assay) were generated using different MOIs and were then plotted against the EC50s for GTP depletion. At an MOI of 1, R2 was 0.95; at an MOI of 0.1, R2 was 0.93, and at a MOI of 0.01 R2 was 0.92.
FIG. 3.
(A) Correlation between the EC50 RNA values for antiviral activity against YFV 17D and EC50 GTP values for GTP depletion in Vero cells for MPA (•), EICAR (▴), and ribavirin (▪). (B) Correlation between the EC50 RNA values for antiviral activity against hPIV3 and EC50 GTP values for GTP depletion in Vero cells for MPA (•), EICAR (▴), and ribavirin (▪).
We then determined whether a similar correlation also holds for other flaviviruses and paramyxoviruses. Respiratory syncytial virus (RSV) (strain Long) was selected as another member of the paramyxoviruses. The replication of RSV in HeLa cells and the effects of the compounds on GTP pools were studied. The EC50 for inhibition of RSV-induced CPE in HeLa cells by ribavirin was 3.74 ± 0.87 μg/ml (n = 10), which is almost identical to the EC50 for GTP pool depletion in this cell line by ribavirin (3.80 ± 0.14 [n = 2]). The EC50 for inhibition of RSV-induced CPE in HeLa cells by MPA was 0.095 ± 0.022 μg/ml (n = 5), which is almost identical to the EC50 for GTP pool depletion in these cells (0.14 ± 0.09 μg/ml [n = 2]). Thus, both for hPIV3 in Vero cells and for RSV in HeLa cells, there is a strong correlation between GTP depletion and antiviral activity.
We reported earlier on the antiviral activities of ribavirin, EICAR, and MPA on the replication of four different flaviviruses (dengue virus [type 2], YFV 17D, Modoc virus, and Montana myotis leukoencephalitis virus) in Vero cells (5, 15). Using this set of data (obtained in CPE reduction assays), the following R2 values were calculated between the EC50s for antiviral activity and the EC50s for GTP pool depletion in Vero cells for the three compounds: R2 = 0.991 for dengue virus, R2 = 0.994 for YFV 17D, R2 = 0.999 for Modoc virus, and R2 = 0.987 for Montana myotis leukoencephalitis virus.
To further corroborate that depletion of intracellular GTP pools is the predominant mechanism of antiviral activity of ribavirin (and EICAR and MPA) against YFV 17D and hPIV3, the effect of exogenously added guanosine on the antiviral activity of the compounds was studied (Table 1). Guanosine at a concentration of 25 μg/ml efficiently reversed the antiviral activities of ribavirin, EICAR, and MPA against YFV 17D and hPIV3. A dose-dependent effect was observed when lower concentrations were used.
TABLE 1.
Effect of exogeneously added guanosine on the anti-YFV 17D and anti-hPIV3 activity of ribavirin, EICAR, and MPA in Vero cellsa
| Amt (μg/ml) of guanosine added | EC50 for antiviral activity
|
|||||
|---|---|---|---|---|---|---|
| YFV 17Db
|
hPIV3c
|
|||||
| Ribavirin | EICAR | MPA | Ribavirin | EICAR | MPA | |
| 0 (control) | 40 ± 9.0 | 1.05 ± 0.07 | 0.06 ± 0.03 | 27 ± 4.6 | 1.90 ± 0.22 | 0.055 ± 0.005 |
| 25 | >100 | ≥21 | >2.5d | >100 | >100 | >100 |
| 10 | >100 | 10 ± 5.6 | 0.15 ± 0.06 | >100 | >100 | 2.5 |
| 2.5 | >100 | 1.5 ± 0.1 | 0.06 ± 0.06 | 60 | 3.97 | 0.25 |
| 1.0 | ≥86 | 1.2 ± 0.1 | 0.057 ± 0.008 | 50 | 1.99 | 0.059 |
Guanosine at the concentrations used had no effect on virus-induced CPE formation.
Data against YFV 17D are from two independent experiments (and up to eight separate determinations).
Data against hPIV3 are from one or two independent experiments (and up to three separate determinations in the latter case).
The highest concentration used in one experiment was 2.5 μg/ml. In another experiment, the EC50 was >100 μg/ml.
It can be concluded that the predominant mechanism of antiviral activity of ribavirin and EICAR against flaviviruses and paramyxoviruses in vitro is mediated by depletion of intracellular GTP pools because of the following. (i) A linear correlation was calculated between inhibition of replication (as determined by RT-qPCR, titration for infectious virus content, and CPE reduction assays) of a plus-strand ssRNA virus as well as a minus-strand ssRNA virus on the one hand and GTP pool depletion on the other hand. (ii) A linear correlation was detected between the EC50s for antiviral activity and the corresponding EC50s for GTP depletion. (iii) Exogenously added guanosine efficiently reversed the antiviral activity of the compounds. (iv) The sole mechanism of action of MPA is inhibition of IMPDH activity. A shortage of GTP for viral RNA synthesis is thus predominantly responsible for the antiviral activity of ribavirin.
Although GTP depletion has long been proposed to be one of the mechanisms by which ribavirin exerts its antiviral activity (24), it is still not clear to what extent this mechanism of action contributes to the antiviral activity of ribavirin. Recently, it has been proposed that ribavirin-enhanced mutagenesis, leading to an error catastrophe of the virus population (8), would be the major mechanism for the in vitro antiviral activity of ribavirin (1, 7, 13, 19, 20, 25). In contrast to the present study and a study on the anti-vaccinia virus activity of ribavirin and MPA (22), none of these studies provided any substantial information on the dose-response effect of ribavirin on intracellular GTP pools in the antiviral concentration range of this molecule.
We have now provided compelling evidence that like MPA, ribavirin and EICAR cause depletion of the intracellular GTP pools and that this depletion tightly correlates with the antiviral activity of these compounds (against flavi- and paramyxoviruses) in vitro. Thus, the predominant mechanism of antiviral activity of ribavirin against these viruses in vitro is based on inhibition of IMPDH activity.
Acknowledgments
This study has been carried out with financial support from the Commission of the European Communities, specific program “Quality of Life and Management of Living Resources,” QLK2-CT2001-01225, “Towards the design of new potent antiviral drugs: structure function analysis of Paramyxoviridae RNA polymerase.” The study does not reflect the Commission’s views and in no way anticipates the Commission’s future policy in this area. P. Leyssen is a fellow of the “Onderzoeksfonds van de Katholieke Universiteit Leuven.”
We thank M.-H. Stuyck and Ria Van Berwaer for excellent technical assistance and D. Brabants and I. Aerts for editorial help.
REFERENCES
- 1.Airaksinen, A., N. Pariente, L. Menendez-Arias, and E. Domingo. 2003. Curing of foot-and-mouth disease virus from persistently infected cells by ribavirin involves enhanced mutagenesis. Virology 311:339-349. [DOI] [PubMed] [Google Scholar]
- 2.Balzarini, J., A. Karlsson, L. Wang, C. Bohman, K. Horska, I. Votruba, A. Fridland, A. Van Aerschot, P. Herdewijn, and E. De Clercq. 1993. Eicar (5-ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide). A novel potent inhibitor of inosinate dehydrogenase activity and guanylate biosynthesis. J. Biol. Chem. 268:24591-24598. [PubMed] [Google Scholar]
- 3.Balzarini, J., L. Stet, A. Matsuda, L. Wiebe, E. Knauss, and E. De Clercq. 1998. Metabolism of EICAR (5-ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide), a potent inhibitor of inosinate dehydrogenase. Adv. Exp. Med. Biol. 431:723-728. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter, P. A., and J. E. Sanders. 2003. Steroid-refractory graft-vs.-host disease: past, present and future. Pediatr. Transplant. 7(Suppl. 3):19-31. [DOI] [PubMed] [Google Scholar]
- 5.Charlier, N., P. Leyssen, J. Paeshuyse, C. Drosten, H. Schmitz, A. Van Lommel, E. De Clercq, and J. Neyts. 2002. Infection of SCID mice with Montana Myotis leukoencephalitis virus as a model for flavivirus encephalitis. J. Gen. Virol. 83:1887-1896. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, A. C., N. C. Banasiak, and P. J. Allen. 2003. Management and prevention strategies for respiratory syncytial virus (RSV) bronchiolitis in infants and young children: a review of evidence-based practice interventions. Pediatr. Nurs. 29:452-456. [PubMed] [Google Scholar]
- 7.Crotty, S., D. Maag, J. J. Arnold, W. Zhong, J. Y. Lau, Z. Hong, R. Andino, and C. E. Cameron. 2000. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat. Med. 6:1375-1379. [DOI] [PubMed] [Google Scholar]
- 8.Crotty, S., C. Cameron, and R. Andino. 2002. Ribavirin's antiviral mechanism of action: lethal mutagenesis? J. Mol. Med. 80:86-95. [DOI] [PubMed] [Google Scholar]
- 9.De Clercq, E., M. Cools, J. Balzarini, R. Snoeck, G. Andrei, M. Hosoya, S. Shigeta, T. Ueda, N. Minakawa, and A. Matsuda. 1991. Antiviral activities of 5-ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide and related compounds. Antimicrob. Agents Chemother. 35:679-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond, M. S., M. Zachariah, and E. Harris. 2002. Mycophenolic acid inhibits dengue virus infection by preventing replication of viral RNA. Virology 304:211-221. [DOI] [PubMed] [Google Scholar]
- 11.Haas, W. H., T. Breuer, G. Pfaff, H. Schmitz, P. Kohler, M. Asper, P. Emmerich, C. Drosten, U. Golnitz, K. Fleischer, and S. Gunther. 2003. Imported Lassa fever in Germany: surveillance and management of contact persons. Clin. Infect. Dis. 36:1254-1258. [DOI] [PubMed] [Google Scholar]
- 12.Hong, Z., and C. E. Cameron. 2002. Pleiotropic mechanisms of ribavirin antiviral activities. Prog. Drug Res. 59:41-69. [DOI] [PubMed] [Google Scholar]
- 13.Lanford, R. E., D. Chavez, B. Guerra, J. Y. Lau, Z. Hong, K. M. Brasky, and B. Beames. 2001. Ribavirin induces error-prone replication of GB virus B in primary tamarin hepatocytes. J. Virol. 75:8074-8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leyssen, P., E. De Clercq, and J. Neyts. 2000. Perspectives for the treatment of infections with Flaviviridae. Clin. Microbiol. Rev. 13:67-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leyssen, P., A. Van Lommel, C. Drosten, H. Schmitz, E. De Clercq, and J. Neyts. 2001. A novel model for the study of the therapy of flavivirus infections using the Modoc virus. Virology 279:27-37. [DOI] [PubMed] [Google Scholar]
- 16.Neyts, J., A. Meerbach, P. McKenna, and E. De Clercq. 1996. Use of the yellow fever virus vaccine strain 17D for the study of strategies for the treatment of yellow fever virus infections. Antivir. Res. 30:125-132. [DOI] [PubMed] [Google Scholar]
- 17.Neyts, J., G. Andrei, and E. De Clercq. 1998. The novel immunosuppressive agent mycophenolate mofetil markedly potentiates the antiherpesvirus activities of acyclovir, ganciclovir, and penciclovir in vitro and in vivo. Antimicrob. Agents Chemother. 42:216-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawlotsky, J. M. 2003. Mechanisms of antiviral treatment efficacy and failure in chronic hepatitis C. Antivir. Res. 59:1-11. [DOI] [PubMed] [Google Scholar]
- 18a.Reed, L.J., and H. Muench. 1938. A simple method of estimating 50 per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 19.Ruiz-Jarabo, C. M., C. Ly, E. Domingo, and J. C. de la Torre. 2003. Lethal mutagenesis of the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV). Virology 308:37-47. [DOI] [PubMed] [Google Scholar]
- 20.Severson, W. E., C. S. Schmaljohn, A. Javadian, and C. B. Jonsson. 2003. Ribavirin causes error catastrophe during Hantaan virus replication. J. Virol. 77:481-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sintchak, M. D., M. A. Fleming, O. Futer, S. A. Raybuck, S. P. Chambers, P. R. Caron, M. A. Murcko, and K. P. Wilson. 1996. Structure and mechanism of inosine monophosphate dehydrogenase in complex with the immunosuppressant mycophenolic acid. Cell 85:921-930. [DOI] [PubMed] [Google Scholar]
- 22.Smee, D. F., M. Bray, and J. W. Huggins. 2001. Antiviral activity and mode of action studies of ribavirin and mycophenolic acid against orthopoxviruses in vitro. Antivir. Chem. Chemother. 12:327-335. [DOI] [PubMed] [Google Scholar]
- 23.Snell, N. J. 2001. Ribavirin—current status of a broad spectrum antiviral agent. Exp. Opin. Pharmacother. 2:1317-1324. [DOI] [PubMed] [Google Scholar]
- 24.Wray, S. K., B. E. Gilbert, M. W. Noall, and V. Knight. 1985. Mode of action of ribavirin: effect of nucleotide pool alterations on influenza virus ribonucleoprotein synthesis. Antivir. Res. 5:29-37. [DOI] [PubMed] [Google Scholar]
- 25.Zhou, S., R. Liu, B. M. Baroudy, B. A. Malcolm, and G. R. Reyes. 2003. The effect of ribavirin and IMPDH inhibitors on hepatitis C virus subgenomic replicon RNA. Virology 310:333-342. [DOI] [PubMed] [Google Scholar]