Abstract
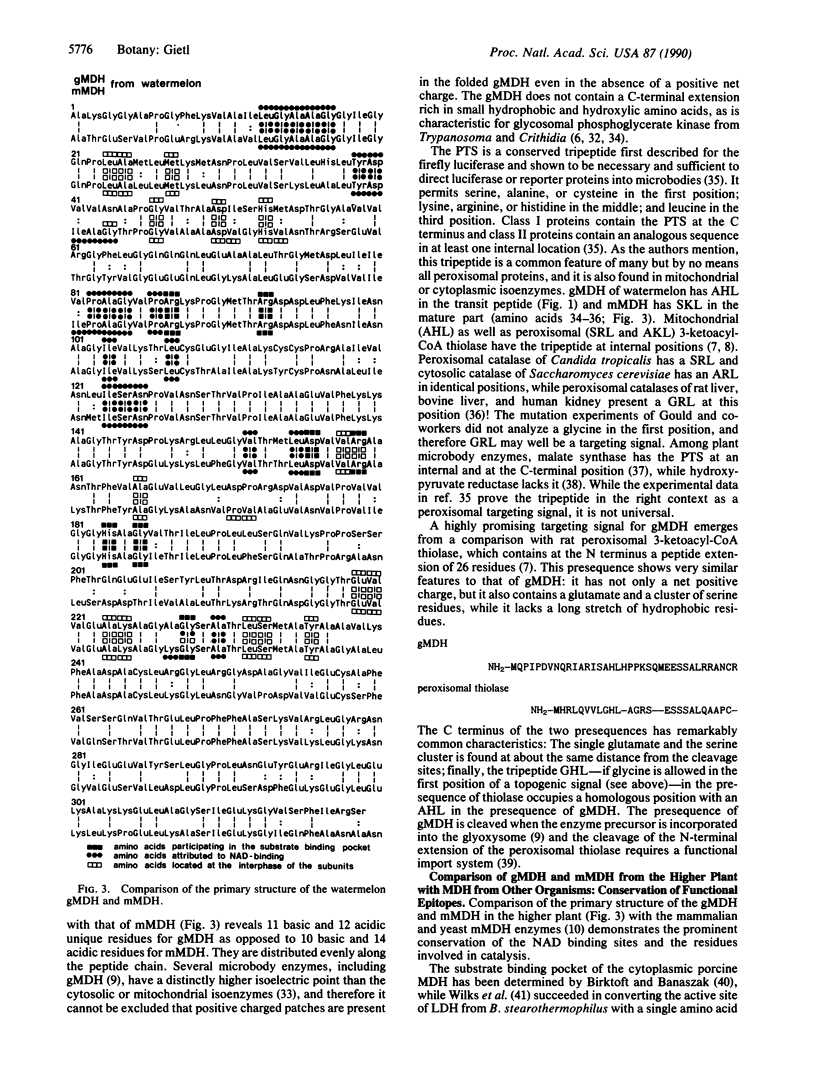
The isolation and sequence of a cDNA clone encoding the complete glyoxysomal malate dehydrogenase [gMDH; (S)-malate:NAD+ oxidoreductase, EC 1.1.1.37] of watermelon cotyledons are presented. Partial cDNA clones were synthesized in a three part strategy, taking advantage of the polymerase chain reaction technology with oligonucleotides based on directly determined amino acid sequences. Subsequently, the complete clone for gMDH was synthesized with a sense primer corresponding to the nucleotide sequence of the N-terminal end of pre-gMDH and an antisense primer corresponding to the adenylylation site found in the mRNA. The amino acids for substrate and cofactor binding identified by x-ray crystallography for pig heart cytoplasmic malate dehydrogenase are conserved in the 319-amino-acid-long mature plant enzyme. The pre-gMDH contains an N-terminal transit peptide of 37 residues. It has a net positive charge, lacks a long stretch of hydrophobic residues, and contains besides acidic amino acids a cluster of serine residues. This N-terminal extension is cleaved off upon association with or import into glyoxysomes. It contains a putative AHL topogenic signal for microbody import and has no sequence similarity to the 27-residue-long presequence of the watermelon mitochondrial malate dehydrogenase precursor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa H., Takiguchi M., Amaya Y., Nagata S., Hayashi H., Mori M. cDNA-derived amino acid sequence of rat mitochondrial 3-oxoacyl-CoA thiolase with no transient presequence: structural relationship with peroxisomal isozyme. EMBO J. 1987 May;6(5):1361–1366. doi: 10.1002/j.1460-2075.1987.tb02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birktoft J. J., Banaszak L. J. The presence of a histidine-aspartic acid pair in the active site of 2-hydroxyacid dehydrogenases. X-ray refinement of cytoplasmic malate dehydrogenase. J Biol Chem. 1983 Jan 10;258(1):472–482. doi: 10.2210/pdb2mdh/pdb. [DOI] [PubMed] [Google Scholar]
- Borst P. Peroxisome biogenesis revisited. Biochim Biophys Acta. 1989 Jun 1;1008(1):1–13. doi: 10.1016/0167-4781(89)90163-2. [DOI] [PubMed] [Google Scholar]
- Comai L., Baden C. S., Harada J. J. Deduced sequence of a malate synthase polypeptide encoded by a subclass of the gene family. J Biol Chem. 1989 Feb 15;264(5):2778–2782. [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., McCammon M. T., Vassarotti A. Targeting proteins into mitochondria. Microbiol Rev. 1986 Jun;50(2):166–178. doi: 10.1128/mr.50.2.166-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietl C., Hock B. Organelle-bound malate dehydrogenase isoenzymes are synthesized as higher molecular weight precursors. Plant Physiol. 1982 Aug;70(2):483–487. doi: 10.1104/pp.70.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Hosken N., Wilkinson J., Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989 May;108(5):1657–1664. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenler J. M., Sloan J. S., Schwartz B. W., Becker W. M. Isolation, characterization and sequence analysis of a full-length cDNA clone encoding NADH-dependent hydroxypyruvate reductase from cucumber. Plant Mol Biol. 1989 Aug;13(2):139–150. doi: 10.1007/BF00016133. [DOI] [PubMed] [Google Scholar]
- Haeuptle M. T., Flint N., Gough N. M., Dobberstein B. A tripartite structure of the signals that determine protein insertion into the endoplasmic reticulum membrane. J Cell Biol. 1989 Apr;108(4):1227–1236. doi: 10.1083/jcb.108.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F. U., Pfanner N., Nicholson D. W., Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989 Jan 18;988(1):1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hijikata M., Ishii N., Kagamiyama H., Osumi T., Hashimoto T. Structural analysis of cDNA for rat peroxisomal 3-ketoacyl-CoA thiolase. J Biol Chem. 1987 Jun 15;262(17):8151–8158. [PubMed] [Google Scholar]
- Keller G. A., Gould S., Deluca M., Subramani S. Firefly luciferase is targeted to peroxisomes in mammalian cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3264–3268. doi: 10.1073/pnas.84.10.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. A., de la Cruz V. F., Ferreira P. C., Morel C., Simpson L. Evolution of parasitism: kinetoplastid protozoan history reconstructed from mitochondrial rRNA gene sequences. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4779–4783. doi: 10.1073/pnas.85.13.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Misset O., Bos O. J., Opperdoes F. R. Glycolytic enzymes of Trypanosoma brucei. Simultaneous purification, intraglycosomal concentrations and physical properties. Eur J Biochem. 1986 Jun 2;157(2):441–453. doi: 10.1111/j.1432-1033.1986.tb09687.x. [DOI] [PubMed] [Google Scholar]
- Okada H., Ueda M., Sugaya T., Atomi H., Mozaffar S., Hishida T., Teranishi Y., Okazaki K., Takechi T., Kamiryo T. Catalase gene of the yeast Candida tropicalis. Sequence analysis and comparison with peroxisomal and cytosolic catalases from other sources. Eur J Biochem. 1987 Dec 30;170(1-2):105–110. doi: 10.1111/j.1432-1033.1987.tb13673.x. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R., Baudhuin P., Coppens I., De Roe C., Edwards S. W., Weijers P. J., Misset O. Purification, morphometric analysis, and characterization of the glycosomes (microbodies) of the protozoan hemoflagellate Trypanosoma brucei. J Cell Biol. 1984 Apr;98(4):1178–1184. doi: 10.1083/jcb.98.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H., Weir E. M., Leaver C. J., Titus D. E., Becker W. M. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: 3. IN VITRO TRANSLATION AND CHARACTERIZATION OF FOUR GLYOXYSOMAL ENZYMES. Plant Physiol. 1980 Jan;65(1):40–46. doi: 10.1104/pp.65.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. The transport of proteins into chloroplasts. Annu Rev Biochem. 1986;55:879–912. doi: 10.1146/annurev.bi.55.070186.004311. [DOI] [PubMed] [Google Scholar]
- Schram A. W., Strijland A., Hashimoto T., Wanders R. J., Schutgens R. B., van den Bosch H., Tager J. M. Biosynthesis and maturation of peroxisomal beta-oxidation enzymes in fibroblasts in relation to the Zellweger syndrome and infantile Refsum disease. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6156–6158. doi: 10.1073/pnas.83.16.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinkels B. W., Evers R., Borst P. The topogenic signal of the glycosomal (microbody) phosphoglycerate kinase of Crithidia fasciculata resides in a carboxy-terminal extension. EMBO J. 1988 Apr;7(4):1159–1165. doi: 10.1002/j.1460-2075.1988.tb02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Walk R. A., Hock B. Cell-free synthesis of glyoxysomal malate dehydrogenase. Biochem Biophys Res Commun. 1978 Mar 30;81(2):636–643. doi: 10.1016/0006-291x(78)91583-8. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Swinkels B., Michels P. A., Osinga K., Misset O., Van Beeumen J., Gibson W. C., Postma J. P., Borst P., Opperdoes F. R. Common elements on the surface of glycolytic enzymes from Trypanosoma brucei may serve as topogenic signals for import into glycosomes. EMBO J. 1987 Jan;6(1):215–221. doi: 10.1002/j.1460-2075.1987.tb04741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks H. M., Hart K. W., Feeney R., Dunn C. R., Muirhead H., Chia W. N., Barstow D. A., Atkinson T., Clarke A. R., Holbrook J. J. A specific, highly active malate dehydrogenase by redesign of a lactate dehydrogenase framework. Science. 1988 Dec 16;242(4885):1541–1544. doi: 10.1126/science.3201242. [DOI] [PubMed] [Google Scholar]
- von Figura K., Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]


