Abstract
Herpes simplex virus type 1 (HSV-1) causes disease in humans and animals. Infection usually occurs via the neural route and possibly occurs via the hematogenous route. The latter, however, is the main route by which immunosuppressed individuals and neonates are infected. Gender-dependent differences in the incidence and severity of some viral infections have been reported. To detect differences between the sexes with respect to HSV-1 colonization and disease, the characteristics of both acute and latent infections in hematogenously infected male and female mice were compared. In acute infection, the female mice had a poorer outcome: HSV-1 colonization was more effective, especially in the gonads and brain. In the encephalon, the midbrain had the highest viral load. In latent infection, brain viral loads were not significantly different with respect to sex. Significant differences were seen, however, in the blood and trigeminal ganglia: HSV-1 seroprevalence was observed in females, with no virus detected in males. In brain dissections, only the cerebral cortex of the females had viral loads statistically higher than those observed in the males. The spread of the virus to several organs of interest during acute infection was examined immunohistochemically. Female mice showed greater viral immunostaining, especially in the adrenal cortex, gonads, and midbrain. In male mice, HSV-1 was detected predominantly in the adrenal cortex. It was also found that apolipoprotein E promotes virus colonization of the ovaries, the APOE gene dose being directly related to viral invasiveness.
It is well known that males and females differ in their susceptibilities to a variety of infections and diseases (1, 10, 18). Despite early reports of differences between the sexes with respect to herpes simplex virus type 1 (HSV-1) antibody titers in mice (22), in the severity of paralysis in mice after HSV-1 inoculation (17, 39), and in the outcome of HSV-1 infection by corneal scarification (14), neither sex-dependent differences in infections with this pathogen nor associated diseases have been well studied. In addition, the literature contains little information on sex-dependent differences with respect to the outcome of hematogenous infection with HSV-1.
HSV-1 infection is among the most common of human viral infections and classically causes oral-labial herpes (cold sores or fever blisters). Serological studies from the 1940s indicated that HSV-1 infection is almost universal in adulthood (8), the virus causing persistent and latent infection. Reactivation from latency in humans is triggered by a number of stimuli, including menstruation. These data suggest that certain hormones could be involved (16, 23, 38).
HSV-1 may reach the central nervous system (CNS) and peripheral organs via either the neural or the hematogenous route. There is extensive evidence for the spread of HSV-1 within the peripheral nerves and the CNS; latent infection of the trigeminal ganglia is nearly always present in humans (16, 35). However, few studies have examined the characteristics of the hematogenous route of infection. Although the main route for HSV-1 infection in humans is certainly neural, hematogenous infection could be important in some contexts. In fact, inoculation into the bloodstream has been used consistently in animal models for the study of HSV-1 pathogenesis (17, 24, 30, 37). It is accepted that intraperitoneal (i.p.) inoculation mimics hematogenous infection (21), the adrenal glands playing a crucial role in CNS infection after viremia (5, 17). After i.p. infection of mice, HSV-1 spreads by means of viremia to many of the visceral organs, the ovaries and the adrenal glands becoming the major replication sites (29). High levels of HSV-1 have been detected in the adrenal glands after inoculation by different routes and with different viral strains (5, 17, 26, 30). In these studies, the virus was detected mainly in the adrenal cortex, the immunoreaction generally being restricted to the zona fasciculata and the zona reticularis (17, 26). In the ovaries, the virus has been detected in the follicles and the stroma (37).
Recent findings suggested that apolipoprotein E (ApoE) may influence the response to HSV-1 infection. We reported that ApoE, particularly the ApoE4 variant, is involved in HSV-1 colonization of the brain from the blood (4, 5). ApoE is a constituent of very-low-density lipoprotein and a subclass of the high-density lipoproteins involved in cholesterol transport between cells (25). The binding of HSV-1 to the different subclasses of serum lipoproteins has been described, as has the interaction of purified HSV-1 glycoprotein B with ApoE (19). It is therefore clear that ApoE is involved in HSV-1 infection via the bloodstream.
This report shows that gender influences hematogenous HSV-1 infection in the CNS and that infection of the gonads is ApoE dependent. This report also extends knowledge of how HSV-1 infects the brain in a gender-specific manner, both in the early phase of infection and in latency. This is the first in vivo study to relate gender dependence in hematogenous HSV-1 infection to ApoE, and its findings could be important in the context of the potential neurological and sexually transmitted diseases associated with HSV-1 infection.
MATERIALS AND METHODS
Animals and viruses.
Experiments were performed in accordance with the guidelines of the European Community Animals (Scientific Procedures) Act (1986). All animals underwent a period of quarantine. Strict precautions were taken to prevent contamination during inoculation and dissection.
A total of 192 C57BL/6J mice were monitored to determine the characteristics of hematogenous HSV-1 infection: 105 wild-type females, 21 wild-type males, 47 APOE knockout females, and 19 APOE hemizygote females. All mice were marked, examined, and analyzed individually. Five or more mice (depending on availability) were used at each time point in each experimental group. APOE knockout mice (C57BL/6J-ApoEtm1Unc) generated by using the protocol described by Piedrahita et al. (28) were obtained from Jackson Laboratory (Bar Harbor, Maine). The hemizygote females were produced from matings between wild-type females and knockout males. Male and female mice were housed in the same suite under specific-pathogen-free conditions and were used at 14 weeks of age.
Vero cells were grown in Dulbecco's modified Eagle medium supplemented with 5% fetal calf serum and gentamicin. HSV-1 (strain KOS; kindly supplied by L. Carrasco) was propagated and titrated by plaque assays of confluent monolayers of these cells (11) for use in all experiments.
Inoculation and dissection.
Mice were inoculated i.p. with HSV-1 as previously described (5) and monitored daily for symptoms. Mock-infected animals (inoculated with saline solution) were used as controls (used in parallel in all experiments). To evaluate the role of ApoE in the hematogenous route of infection, APOE knockout, hemizygote, and wild-type mice were infected with HSV-1 (106 PFU). These animals were sacrificed with CO2-enriched air at different intervals (between 18 h and 37 days postinfection [dpi]), depending on the experimental design. Their organs (blood, adrenal glands, spinal cord, brain, gonads, trigeminal ganglia, pancreas, spleen, heart, liver, and bone marrow) were removed and frozen at −70°C. The brain was defined as the sum of the midbrain, ventricles, cerebral cortex, and cerebellum. In acute-infection assays, infected animals (106 PFU) were culled at day 5.7. This time point was selected because it coincided with the highest viral load in the brain (5). In latent-infection assays, several groups of animals infected with 106 PFU were sacrificed at 37 dpi. Since the ovaries are derived from the same embryonic tissue as the adrenal cortex and also produce steroid hormones, these organs were carefully examined for evidence of HSV-1 infection. To study the influence of ApoE on infection of the ovaries, wild-type and knockout mice were culled at 0.8, 2, 3, 4, 5.7, 7, and 10 days after inoculation, while hemizygote mice were killed at 4 days (when differences in viral loads between wild-type and knockout mice were large).
Virus detection.
HSV-1 DNA was extracted from homogenized samples by conventional methods (NucleoSpin, catalog no. K3053-2; Clontech) and quantified for several organs by real-time quantitative PCR as previously described (6). An appropriate range of virus concentrations was used for the optimization of the standard curve, and viral loads were expressed as PFU. PCR calibration was performed by using the β-actin housekeeping gene (results were expressed as nanograms of β-actin). Cross-contamination of samples and false-positive PCR results were carefully avoided by frequent changes of gloves, the exclusive use of pipettes, and the strict spatial separation of the three main PCR stages. Real-time PCR was performed by using a LightCycler rapid thermal cycler (Roche Diagnostics Ltd., Lewes, United Kingdom), 1 μM concentrations of primers, and 2 mM MgCl2. β-Actin primers (5′-AAC CCT AAG GCC AAC CGT GAA AAG ATG ACC-3′ and 5′-CCA GGG AGG AAG AGG ATG CGG C-3′) were used as a positive control for the reaction (379-bp PCR product). Specific primers for a sequence in the viral DNA polymerase gene (5′-GGT GAA CGT CTT TTC GCA CT-3′ and 5′-GTG TTG TGC CGC GGT CTC AC-3′; 120-bp amplicon) and the thymidine kinase gene (5′-ATA CCG ACG ATC TGC GAC CT-3′ and 5′-TTA TTG CCG TCA TAG CGC GG-3′; 110-bp product) were used. The reaction conditions were 95°C for 10 min; 50 cycles at 95°C for 30 s; 55°C for 30 s (for β-actin), 60°C for 5 s (for thymidine kinase), or 60°C for 30 s (for polymerase); and 72°C for 40 s. Each experiment was performed in triplicate. Melting curve analysis, agarose gel electrophoresis, and restriction analysis confirmed the specificities of the amplification products.
Detection of HSV-1 in blood fractions.
To determine whether HSV-1 in the bloodstream is carried predominantly in the plasma fraction or in the cellular fraction, whole blood from female mice infected with 106 PFU and sacrificed at 5.7 dpi was separated by centrifugation at 1,870 × g for 25 min at 4°C. DNA was extracted from both fractions, and HSV-1 was detected as described above. Viral loads were expressed as PFU equivalents of virus normalized in terms of nanograms of β-actin. Viral loads were also normalized by counting the number of cells in the blood fraction. Cell numbers were determined (four replicates) by using a Neubauer chamber hemocytometer. Cell viability was assessed by trypan blue exclusion as previously described (34).
Histological procedures and immunodetection of viral antigens.
To assess the importance of the various target organs in murine HSV-1 infection, the correlation between viral load and immunohistochemical detection was studied. VP16, a tegument protein which acts as a transcriptional activator of immediate-early gene products (32), was used as the target antigen. In other histological examinations, murine adrenal glands, gonads, and brain were examined for evidence of HSV-1 infection and to localize viral antigens.
For the analysis of acute infection, groups of five mice, both male and female (infected i.p. with 106 PFU of HSV-1 and sacrificed at 5.7 dpi), were examined. These animals were intracardially perfused with 10 ml of phosphate-buffered saline followed by 10 ml of Bouin's solution. The organs were removed, fixed in Bouin's solution, dehydrated, and embedded in paraffin wax. Sections (7 μm thick) were processed by using the avidin-biotin-phosphatase complex method. To retrieve antigens, sections were deparaffinized, hydrated, and incubated with 0.1 M citrate buffer (pH 6) for 1 min in a conventional pressure cooker (27). After the sections were rinsed in Tris-buffered saline (TBS), they were incubated with 3% normal donkey serum (NDS) in TBS containing 0.05% Triton X-100 for 30 min to prevent nonspecific binding of the primary antibody. The sections then were incubated overnight at 4°C with rabbit polyclonal anti-VP16 (BD Biosciences, Palo Alto, Calif.) primary antibody diluted 1:100 in TBS containing 3% NDS and 0.05% Triton X-100. The sections then were washed in TBS and incubated for 1 h with anti-rabbit biotinylated immunoglobulin (Amersham Biosciences, Little Chalfont, United Kingdom) diluted 1:500 in TBS containing 0.3% NDS and 0.005% Triton X-100. After the sections were washed in TBS, they were incubated with avidin-biotin-phosphatase complex (Dako, Barcelona, Spain) for 45 min and developed with an AP-red substrate kit (Zymed Laboratories Inc., San Francisco, Calif.). The sections then were counterstained with hematoxylin to emphasize the absence of nonspecific immunostaining and were mounted in Acuatex (Merck, Bärmstabt, Germany).
For negative controls, contiguous sections of each sample were incubated with the primary or secondary antibody omitted. HSV-1-infected mice and mock-infected organs were included in every experiment to monitor the specificity of viral localization. At least three sections per organ were examined to ensure that sufficient observations were made.
Statistical analysis.
Differences between the groups with respect to viral loads were examined by using Fisher's exact test. The χ2 test was used for comparisons of genders. Significance was set at a P value of <0.01.
RESULTS
Clinical scores.
After inoculation, only a small number of mice (27 out of 171; 15.8%) showed any clinical symptoms of disease (5). Briefly, these mice were bilaterally affected, starting with a slight weakness and loss of movement, followed by signs of ataxia, complete paralysis of the hindquarters with loss of postural control, and eventually death. All mice remained asymptomatic until day 4; none of the mock-infected animals showed any clinical abnormalities. The difference between the genders in terms of signs of disease was statistically significant at 5.7 dpi in 25 mice that had been injected with 106 PFU; the incidence was much higher in females (44.4% of the mice in a 25-mouse sample) than in males (14.3% of the mice in the same sample) (P < 0.01). On the contrary, no significant difference was seen in the number of sick mice with respect to APOE gene dose (P > 0.1). After day 10, asymptomatic mice remained without clinical disease and survived until the end of the experiment.
Effect of viral dose.
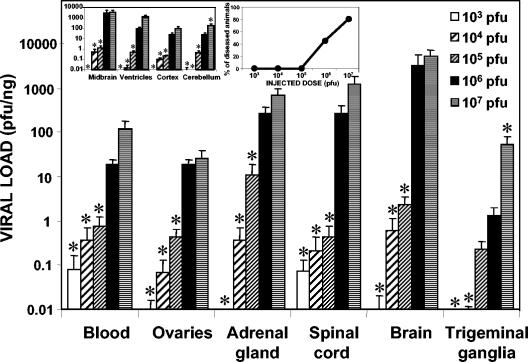
To evaluate the influence of the injected viral dose on colonization, experiments with doses of between 103 and 107 PFU were performed with female mice (sacrificed at 5.7 dpi). Figure 1 shows the viral loads detected in several organs in relation to the administered doses. The right inset in Fig. 1 shows the percentage of animals with signs of disease and consequently the percentage that died with respect to the injected dose. HSV-1 is evidently highly virulent, with a 50% lethal dose close to 106 PFU. The viral load was always directly dependent on the viral dose, although similar patterns of infection were seen for all doses analyzed. Independent of the dose, the organs with the highest viral loads were the adrenal glands and the CNS (spinal cord and brain), confirming the anatomical and functional connections between the adrenal glands and the brain in HSV-1 migration. Viral loads in the four brain regions analyzed (left inset in Fig. 1) were also dose dependent. The midbrain had the highest viral load for every dose tested. In the remaining organs analyzed (pancreas, spleen, heart, liver, and bone marrow), no virus was detected.
FIG. 1.
Quantification of viral loads with respect to the viral doses injected into several organs (the viral load in the brain is the sum of those in the midbrain, ventricles, cortex, and cerebellum). A total of 33 female mice that were 14 weeks old were inoculated i.p. with HSV-1 suspensions ranging from 103 to 107 PFU, sacrificed, and dissected at 5.7 dpi, when an acute infection was established. The bar graph represents the viral load detected in each organ, expressed on a logarithmic scale. Values are means and standard errors of the means (SEMs) for the quantity of viral DNA, expressed as PFU equivalents, and normalized with respect to the quantity of mouse genomes, expressed as nanograms of amplified β-actin housekeeping gene. Fisher's exact test was used to compare the viral loads achieved with the different doses (always compared to a dose of 106 PFU) (an asterisk indicates a P value of <0.01). (Left inset) Dissected areas of the brain analyzed in this study. Values are means and SEMs on a logarithmic scale. (Right inset) Clinical disease scores related to the various injected doses, expressed as percentages of animals with disease.
Detection of viral loads in blood fractions.
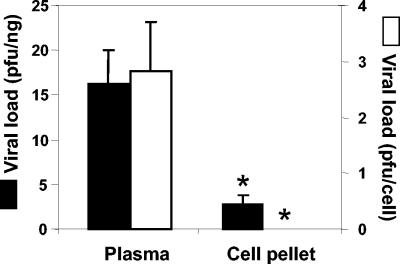
Significant differences were seen in HSV-1 levels in the plasma and cellular fractions obtained by centrifugation (P < 0.01) with either viral load normalization protocol (Fig. 2). When normalized with respect to the number of mouse genomes and expressed as PFU per nanograms, viruses in the plasma fraction made up nearly 85% of the total (mean and standard error of the mean, 16.2 ± 3.8 PFU/ng in the plasma fraction compared to 2.8 ± 1.0 PFU/ng in the cellular fraction). The analysis of viral loads normalized with respect to the number of cells showed a similar trend, with plasma fraction HSV-1 levels of 2.8 ± 0.9 PFU/cell and cellular fraction levels of 0.0 ± 0.0 PFU/cell.
FIG. 2.
Viral loads in plasma and cellular fractions of whole blood. A total of 11 female mice that were 14 weeks old were inoculated i.p. with 106 PFU of HSV-1 and bled at 5.7 dpi, when an acute infection was established. Blood was fractionated by centrifugation at 1,870 × g for 25 min at 4°C to leave a cell pellet and the plasma supernatant. DNA from both fractions was extracted, and HSV-1 was detected; viral loads were expressed as PFU equivalents of virus normalized with respect to the number of mouse genomes (expressed in nanograms of amplified β-actin housekeeping gene) (black bars) or with respect to the number of cells counted in each fraction (white bars). The plasma supernatant showed significantly higher viral loads than the cell pellet, when normalized with respect to both the number of mouse genomes (16.2 ± 3.8 compared to 2.8 ± 1.0 PFU/ng in the cellular fraction) and the number of cells (2.8 ± 0.9 compared to 0.0 ± 0.0 PFU/cell in the cellular fraction). Values are means and standard errors of the means. Fisher's exact test was used to compare the viral loads in the two fractions (an asterisk indicates a P value of <0.01).
Influence of gender on HSV-1 infection.
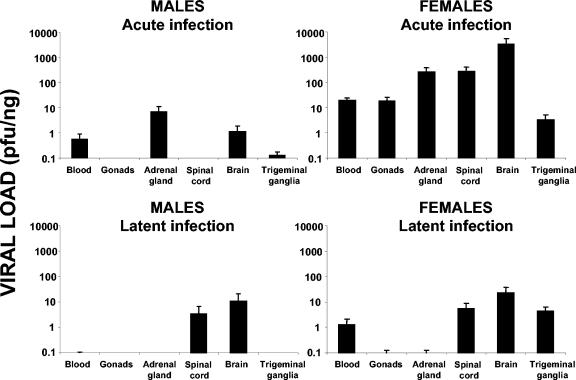
In acute infection, the viral loads in all organs tested in females were significantly higher than those in males. These differences decreased, however, in latent infection (Fig. 3). In acute infection, HSV-1 was detected only in the blood, adrenal glands, and nervous system of male mice. Dramatic differences were seen in gonadal viral loads; while HSV-1 was undetectable in the testes, the ovaries were confirmed as an important target of infection. Viral levels in the trigeminal ganglia were consistently higher in females than in males (P < 0.001). Significant differences were also detected between the sexes with respect to viremia and adrenal gland viral loads (P < 0.001) (Fig. 3). The CNS also showed important sex-dependent differences with respect to viral levels, with spinal cord and brain female/male ratios of over 3,000 (P < 0.001). In latent infection, however, viral loads in the CNS were not significantly different with respect to gender, but the whole blood (P < 0.01) and trigeminal ganglia showed very significant differences (P < 0.001). In fact, in male mice, viral levels were undetectable (Fig. 3). The organ with the highest viral load was consistently the brain, in both males and females, indicating a preferential tropism of HSV-1 for the CNS.
FIG. 3.
Quantification of viral loads in several organs by gender in acute and latent infections. Totals of 28 female and 16 male mice that were 14 weeks old were inoculated i.p. with a suspension of 106 PFU of HSV-1, sacrificed, and dissected at 5.7 dpi (acute infection) or at 37 dpi (latent infection). The bar graphs represent the viral load detected in each organ, expressed on a logarithmic scale. Values are means and standard errors of the means for the quantity of viral DNA, expressed as PFU equivalents, and normalized with respect to the quantity of mouse genomes, expressed as nanograms of amplified β-actin housekeeping gene.
Gender-dependent brain colonization.
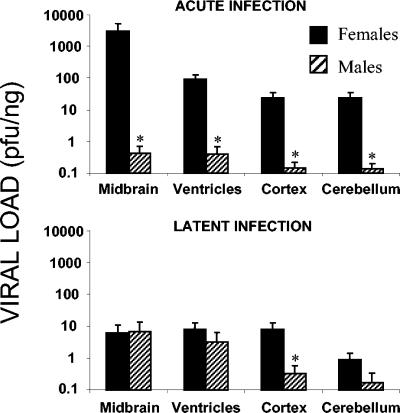
In acute infection, viruses were more easily detected in female brains than in male brains (P < 0.001). All of the encephalic subregions of female mice showed considerably higher viral loads than those of male mice. The midbrain was the area of greatest infectivity, followed by the ventricles, cortex, and cerebellum (Fig. 4). The viral loads in male mice, although significantly lower (P < 0.01), followed the same order. In latent infection, however, gender-dependent differences in whole-brain viral loads were not significantly different (P > 0.5). With respect to the different brain regions, only the cerebral cortex in female mice showed viral loads statistically higher than those in male mice (P < 0.01) (Fig. 4). Surprisingly, the viral loads detected in the brains of male mice were significantly higher (P < 0.001) in latent infection than in acute infection, suggesting an enrichment of virus in the brain over time.
FIG. 4.
Quantification of viral loads in dissected areas of the brain (midbrain, ventricles, cortex, and cerebellum) by gender in acute and latent infections. Totals of 28 female and 16 male mice that were 14 weeks old were inoculated i.p. with a suspension of 106 PFU of HSV-1, sacrificed, and dissected at 5.7 dpi (acute infection) or at 37 dpi (latent infection). The bar graphs represent the viral load detected in each brain region, expressed on a logarithmic scale. Values are means and standard errors of the means for the quantity of viral DNA, expressed as PFU equivalents, and normalized with respect to the quantity of mouse genomes, expressed as nanograms of amplified β-actin housekeeping gene. Fisher's exact test was used to compare the values for the two genders (an asterisk indicates a P value of <0.01).
Gender-dependent viral immunodetection.
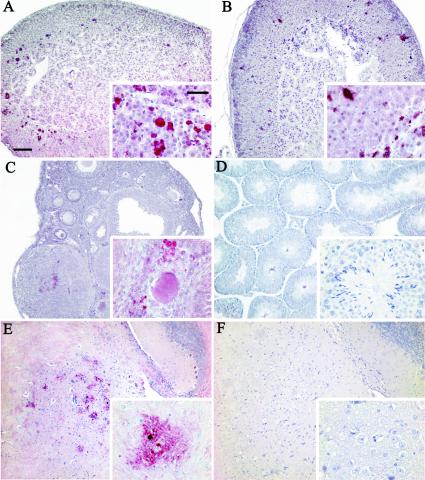
HSV-1 was detected immunohistochemically with an antibody against viral tegument protein VP16 (Fig. 5) and was found mainly in the adrenal glands, ovaries, and midbrain of females and only in the adrenal glands of males. In the adrenal glands, a more intense immunoreaction and more foci of infection were detected in females (Fig. 5A) than in males (Fig. 5B). HSV-1 staining was seen mainly in the zona fasciculata of the adrenal cortex. In the ovaries, VP16 was consistently detected and showed intense signals of infection in the stromal, thecal, and granulosa cells of follicles at different stages of development (Fig. 5C). In contrast, the testes of infected mice were completely negative (Fig. 5D). In the CNS, viruses were immunodetected mainly in the midbrain of female mice, specifically in the ganglion cells of the mesencephalic trigeminal nucleus (Fig. 5E). Male mice showed no detectable signals (Fig. 5F).
FIG. 5.
Immunohistochemical detection of HSV-1 in several organs in acute infection. A total of 10 mice that were 14 weeks old were inoculated i.p. with a suspension of 106 PFU of HSV-1, sacrificed, and dissected at 5.7 dpi. The organs were embedded in paraffin wax and serially sectioned. Immunodetection was performed with anti-tegument viral protein VP16 and a red chromogenic substrate as detailed in Material and Methods. (A) Immunohistochemical analysis of an adrenal gland from a female mouse, showing many foci of infection preferentially located in the zona fasciculata and zona reticularis and with involvement of the corticomedullary junction. (B) Immunohistochemical analysis of an adrenal gland from a male mouse, with a pattern of expression of HSV-1 similar to that of the female mouse but with fewer foci of infection. (C) Immunodetection of HSV-1 in ovaries, showing VP16 staining in the stroma and in some follicles at different stages of development. None of the oocytes observed showed HSV-1 infection. (D) Immunohistochemical analysis of the testis, showing no reaction to HSV-1. (E) Sagittal section of female brain showing HSV-1 staining at some foci of infection in the midbrain. (Inset) Intense detection of the virus in neurons of the mesencephalic trigeminal nucleus. (F) Negative staining of the midbrain from a male mouse. Insets show higher magnifications of a region of each organ. Bars: A, 100 μm; inset, 20 μm.
Effect of ApoE on ovary colonization.
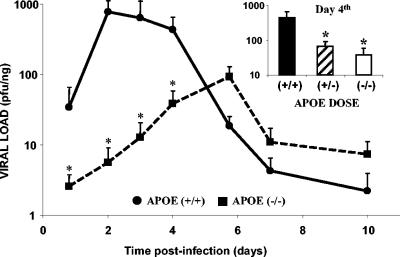
Fig. 6 shows the time course of HSV-1 colonization in ovaries of wild-type and APOE knockout mice; the kinetics of infection were very different in these two kinds of animals. Wild-type mice were more susceptible to HSV-1 infection than APOE knockout mice, with a peak of infection between days 2 and 4. Viral levels then decreased dramatically, and at 5.7 dpi, they were close to those in the APOE knockout mice. The virus arrived later in the ovaries and in smaller quantities in the APOE knockout mice than in the control mice. The peak of detection for the wild-type mice was 1 log unit higher than that for the knockout mice (P < 0.001). Hemizygote mice showed significant differences (P < 0.001) in viral loads with respect to both wild-type and knockout mice at day 4 (inset in Fig. 6).
FIG. 6.
Time course of HSV-1 infection in the ovaries in relation to APOE gene dose. Totals of 48 wild-type, 47 APOE knockout, and 19 APOE hemizygote female mice that were 14 weeks old were inoculated i.p. with a suspension of 106 PFU of HSV-1, sacrificed, and dissected at intervals ranging from 18 h to 10 dpi. Solid lines represent the wild-type group; broken lines represent the APOE knockout group. Values are expressed on a logarithmic scale as means and standard errors of the means for the quantity of viral DNA, expressed as PFU equivalents, and normalized with respect to the quantity of mouse genomes, expressed as nanograms of amplified β-actin housekeeping gene. Fisher's exact test was used to compare the values for the different APOE gene dose groups (an asterisk indicates a P value of <0.001). (Inset) Viral loads in ovaries of wild-type, APOE knockout, and APOE hemizygote mice at a selected time point (day 4).
DISCUSSION
We previously showed that, after i.p. inoculation of mice, HSV-1 is immediately detected in the blood, the virus replicating in the adrenal glands and eventually reaching the spinal cord and brain. In the encephalon, HSV-1 is first detected in the midbrain, then in the brain ventricles, and finally in the cortex and cerebellum (5). The midbrain is important in the access of HSV-1 to the brain from the bloodstream due to the anatomical and functional connections between the adrenal glands and the hypothalamic suprachiasmatic nucleus (3). Another alternative mechanism of viral infection might involve transneuronal migration after infection of the peripheral nerves. Replication might occur in synaptically linked populations of neurons from which the virus could be transported through a multisynaptic pathway. Although neuronal infection is the product of the transsynaptic migration of the virus rather than the lytic release of virions into the extracellular space (36), the precise route of viral spread in the nervous system is still to be established.
To confirm the participation of the bloodstream after i.p. inoculation, the viral loads in the two main centrifugation fractions of whole blood (the plasma and the cell pellet) were determined. The results showed that plasma is the compartment mainly involved in the transport of the virus, confirming the in vivo (5) and in vitro (19) interactions between ApoE and HSV-1.
One of the most interesting findings in the present study was the seroprevalence in the females. Recently, HSV-1 seropositivity was associated with the female sex (9, 31). This finding agrees with studies showingthat HSV-1-specific antibodies are generally more abundant in female mice than in male mice—a difference that disappears upon castration (22). Some authors have also reported HSV-1-infected males to have less severe clinical disease (17, 39). Yirrell et al. (39) showed that male mice were less susceptible to paralysis than female mice and that castration prior to infection increased the probability of disease in males to that in females; treatment with testosterone reversed this change. On the contrary, Han et al. (14) showed that after infection with HSV-1 by corneal scarification, mortality and reactivation were more common in males. These discrepancies might be attributable to the different inoculation routes used in these experiments. Moreover, mouse strains may vary in susceptibility to infection with herpesviruses. It has been shown that different strains of mice vary in their resistance to HSV-1 infection: strain C57BL/6 is very resistant compared to BALB/c, CBA, AKR, and A/J following i.p. inoculation of HSV-1 (24). The incidence of disease reported here with C57BL/6 mice shows that female sex is a risk factor for HSV-1 infection via the bloodstream. Our data, together with those of a number of clinical studies (9, 31), indicate gender-dependent differences in the pathobiology of HSV-1 infection.
Another interesting result was the clear confirmation of the ovaries as targets for HSV-1 infection; acute infection of these organs plays an important role in viral spread. Lesions were previously detected in follicles and in the stroma (37), and in the present study, HSV-1 was immunodetected in both sites during acute infection. On the contrary, the testes were always HSV-1 negative, independent of infection status. There are a number of connections between the adrenal glands and the gonads at different levels. Necrotic lesions in the adrenal glands have been detected following disseminated HSV-1 infection in children (2, 15), and in mice, the adrenal glands are the organs most severely infected after intranasal, intravenous, or i.p. inoculation with HSV-1 (17, 26, 30). Ovaries are derived from the embryonic mesoderm, as is the adrenal cortex; they are therefore similar targets for the virus. Finally, there are multisynaptic neuronal connections among the adrenal glands, the gonads, and certain brain structures (13): structures belonging to the CNS and nerves coming from the adrenal glands and the ovaries all connect with the spinal cord, suggesting that two synergistic or alternative routes exist for infecting the brain. At the peak of infection, HSV-1 loads in the ovaries and adrenal glands were similar to those previously reported (5), indicating that HSV-1 replication might be positively influenced by the presence of immunosuppressive steroid hormones. The cells of the adrenal glands are known to synthesize corticosteroids and sex steroids. Estrogens might enhance susceptibility to infection, whereas testosterone results in the suppression of HSV-1 (29). It might be assumed that, following hematogenous infection, replication in the adrenal glands and ovaries of mice is directed by hormone-dependent mechanisms. Our observation that HSV-1 colonizes the adrenal glands of male mice but not their testes supports the idea of the dependence of HSV-1 replication on certain hormones. HSV-1 immunodetection was observed mainly in the adrenal glands, ovaries, and midbrain of female mice but only in the adrenal glands of male mice.
Latent infection of the trigeminal ganglia in both humans and mice is virtually ubiquitous (7, 16, 35). Periodically, the virus is reactivated from latency and colonizes other regions, generally in the CNS. The results of the present study show that the virus is present in blood only when the trigeminal ganglia are infected. These data suggest that the latter play an important role as a viral reservoir in latently infected mice, supplying significant quantities of virus to the blood. This scenario, however, appears to be gender dependent, since HSV-1 was detected only in the blood and trigeminal ganglia of latently infected female mice. Analysis of the different brain regions led to findings possibly related to the pathobiology of a number of neurological disorders. It has been demonstrated that the combination of the ApoE4 isoform and the presence of HSV-1 in the brain confers an increased risk of Alzheimer's disease (AD) (20). Our results show that in latent infection, the cerebral cortex of females has a significantly higher viral load; this finding is of special interest, since female sex is a risk factor for AD (12) and the regions involved in AD pathology (temporal lobe, frontal lobe, and hippocampus) are all grouped in the cerebral cortex sample defined by our dissection protocol. In previous work, viral colonization was shown to be dependent on ApoE4 (4), and ApoE4 is recognized as one of the most important risk factors for AD (33).
The present results also show that ApoE is also important in the colonization of the ovaries. In APOE-deficient mice, viral load was significantly lower and viral arrival was delayed in these organs. Moreover, these results were dependent on the gene dose, since APOE hemizygote mice had an ovarian viral load intermediate between those of wild-type and knockout mice. As far as we know, this is the first report of ApoE promoting HSV-1 colonization of the ovaries. Although more research is needed to define the precise mechanisms involved, gender-dependent differences in viral binding to ApoE-containing lipoproteins may exist. This scenario may lead to more efficient transport to the ovaries or favor viral spread within the gonads.
The data reported here show that gender is an important factor in the invasiveness of HSV-1 in the nervous system and gonads. Moreover, infection of the ovaries is dependent on ApoE levels. These findings imply that the bloodstream plays a much more pivotal role in HSV-1 infection in female mice than in male mice. Detailed comparisons of HSV-1 infection with respect to human gender are required to determine whether the same differences exist.
Acknowledgments
We thank the Asociación de Familiares de Enfermos de Alzheimer (AFAL), the Fundación Areces, the Obra Social Caja Madrid, the Universidad Autónoma de Madrid, and the Comunidad de Madrid for supporting our research.
We thank F. Mayor for continuous encouragement and help and L. Carrasco for providing HSV-1 strain KOS.
REFERENCES
- 1.Barna, M., T. Komatsu, Z. Bi, and C. S. Reiss. 1996. Sex differences in susceptibility to viral infection of the central nervous system. J. Neuroimmunol. 67:31-39. [DOI] [PubMed] [Google Scholar]
- 2.Brain, R. T., R. C. Pugh, and J. A. Dudgeon. 1957. Adrenal necrosis in generalized herpes simplex. Arch. Dis. Child. 32:120-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buijs, R. M., J. Wortel, J. J. Van Heerikhuize, M. G. Feenstra, G. J. Ter Horst, H. J. Romijn, and A. Kalsbeek. 1999. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur. J. Neurosci. 11:1535-1544. [DOI] [PubMed] [Google Scholar]
- 4.Burgos, J. S., C. Ramirez, I. Sastre, M. J. Bullido, and F. Valdivieso. 2003. ApoE4 is more efficient than E3 in brain access by herpes simplex virus type 1. Neuroreport 14:1825-1827. [DOI] [PubMed] [Google Scholar]
- 5.Burgos, J. S., C. Ramirez, I. Sastre, M. J. Bullido, and F. Valdivieso. 2002. Involvement of apolipoprotein E in the hematogenous route of herpes simplex virus type 1 to the central nervous system. J. Virol. 76:12394-12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgos, J. S., C. Ramirez, R. Tenorio, I. Sastre, and M. J. Bullido. 2002. Influence of reagents formulation on real-time PCR parameters. Mol. Cell. Probes 16:257-260. [DOI] [PubMed] [Google Scholar]
- 7.Cabrera, C. V., C. Wohlenberg, H. Openshaw, M. Rey-Mendez, A. Puga, and A. L. Notkins. 1980. Herpes simplex virus DNA sequences in the CNS of latently infected mice. Nature 288:288-290. [DOI] [PubMed] [Google Scholar]
- 8.Corey, L., and A. Wald. 1999. Genital herpes, p. 285-312. In K. K. Holmes et al. (ed.), Sexually transmitted diseases. New York: McGraw-Hill Book Co., New York, N.Y.
- 9.Cowan, F. M., R. S. French, P. Mayaud, R. Gopal, N. J. Robinson, S. A. de Oliveira, T. Faillace, A. Uuskula, M. Nygard-Kibur, S. Ramalingam, G. Sridharan, R. El Aouad, K. Alami, M. Rbai, N. P. Sunil-Chandra, and D. W. Brown. 2003. Seroepidemiological study of herpes simplex virus types 1 and 2 in Brazil, Estonia, India, Morocco, and Sri Lanka. Sex. Transm. Infect. 79:286-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curiel, R. E., M. H. Miller, R. Ishikawa, D. C. Thomas, and N. J. Bigley. 1993. Does the gender difference in interferon production seen in picornavirus-infected spleen cell cultures from ICR Swiss mice have any in vivo significance? J. Interferon Res. 13:387-395. [DOI] [PubMed] [Google Scholar]
- 11.Enjuanes, L., A. L. Carrascosa, M. A. Moreno, and E. Vinuela. 1976. Titration of African swine fever (ASF) virus. J. Gen. Virol. 32:471-477. [DOI] [PubMed] [Google Scholar]
- 12.Evans, D., M. Ganguli, T. Harris, C. Kawas, and E. B. Larson. 1999. Women and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 13:187-189. [DOI] [PubMed] [Google Scholar]
- 13.Gerendai, I., and B. Halasz. 2000. Central nervous system structures connected with the endocrine glands. findings obtained with the viral transneuronal tracing technique. Exp. Clin. Endocrinol. Diabetes 108:389-395. [DOI] [PubMed] [Google Scholar]
- 14.Han, X., P. Lundberg, B. Tanamachi, H. Openshaw, J. Longmate, and E. Cantin. 2001. Gender influences herpes simplex virus type 1 infection in normal and gamma interferon-mutant mice. J. Virol. 75:3048-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haynes, R. E., P. H. Azimi, and H. G. Cramblett. 1968. Fatal herpesvirus hominis (herpes simplex virus) infections in children. Clinical, pathologic, and virologic characteristics. JAMA 206:312-319. [PubMed] [Google Scholar]
- 16.Hill, T. J. 1985. Herpes simplex virus latency, p. 175-240. In B. Roizman (ed.), The herpesvirus, 3rd ed. Plenum Press, New York, N.Y.
- 17.Hill, T. J., D. L. Yirrell, and W. A. Blyth. 1986. Infection of the adrenal gland as a route to the central nervous system after viraemia with herpes simplex virus in the mouse. J. Gen. Virol. 67:309-320. [DOI] [PubMed] [Google Scholar]
- 18.Huber, S. A., J. Kupperman, and M. K. Newell. 1999. Hormonal regulation of CD4+ T-cell responses in coxsackievirus B3-induced myocarditis in mice. J. Virol. 73:4689-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huemer, H. P., H. J. Menzel, D. Potratz, B. Brake, D. Falke, G. Utermann, and M. P. Dierich. 1988. Herpes simplex virus binds to human serum lipoprotein. Intervirology 29:68-76. [DOI] [PubMed] [Google Scholar]
- 20.Itzhaki, R. F., W. R. Lin, D. Shang, G. K. Wilcock, B. Faragher, and G. A. Jamieson. 1997. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet 349:241-244. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, R. T. 1964. The pathogenesis of herpes virus encephalitis. I. Virus pathways to the nervous system of suckling mice demonstrated by fluorescent antibody staining. J. Exp Med. 119:343-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knoblich, A., J. Gortz, V. Harle-Grupp, and D. Falke. 1983. Kinetics and genetics of herpes simplex virus-induced antibody formation in mice. Infect. Immun. 39:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurane, I., Y. Tsuchiya, T. Sekizawa, and K. Kumagai. 1984. Inhibition by indomethacin of in vitro reactivation of latent herpes simplex virus type 1 in murine trigeminal ganglia. J. Gen. Virol. 65:1665-1674. [DOI] [PubMed] [Google Scholar]
- 24.Lopez, C. 1975. Genetics of natural resistance to herpesvirus infections in mice. Nature 258:152-153. [DOI] [PubMed] [Google Scholar]
- 25.Mahley, R. W. 1988. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240:622-630. [DOI] [PubMed] [Google Scholar]
- 26.Nachtigal, M., and J. B. Caulfield. 1984. Early and late pathologic changes in the adrenal glands of mice after infection with herpes simplex virus type 1. Am. J. Pathol. 115:175-185. [PMC free article] [PubMed] [Google Scholar]
- 27.Norton, A. J., S. Jordan, and P. Yeomans. 1994. Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J. Pathol. 173:371-379. [DOI] [PubMed] [Google Scholar]
- 28.Piedrahita, J. A., S. H. Zhang, J. R. Hagaman, P. M. Oliver, and N. Maeda. 1992. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc. Natl. Acad. Sci. USA 89:4471-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Podlech, J., K. Weise, and D. Falke. 1990. Colonization of adrenal glands and ovaries of mice by HSV-2 variants. I. Virological studies. Arch. Virol. 110:165-177. [DOI] [PubMed] [Google Scholar]
- 30.Potratz, D., B. Brake, H. P. Dienes, T. F. Schulz, M. Hosp, M. P. Dierich, and D. Falke. 1986. Herpes simplex virus type 1 and 2 in the adrenal glands: replication and histopathology. Arch. Virol. 90:207-222. [DOI] [PubMed] [Google Scholar]
- 31.Roest, R. W., W. I. van der Meijden, G. van Dijk, J. Groen, P. G. Mulder, G. M. Verjans, and A. D. Osterhaus. 2001. Prevalence and association between herpes simplex virus type 1- and 2-specific antibodies in attendees at a sexually transmitted disease clinic. Int. J. Epidemiol. 30:580-588. [DOI] [PubMed] [Google Scholar]
- 32.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 33.Saunders, A. M., and A. D. Roses. 1993. Apolipoprotein E4 allele frequency, ischemic cerebrovascular disease, and Alzheimer's disease. Stroke 24:1416-1417. [DOI] [PubMed] [Google Scholar]
- 34.Spiecker, M., H. Darius, K. Kaboth, F. Hubner, and J. K. Liao. 1998. Differential regulation of endothelial cell adhesion molecule expression by nitric oxide donors and antioxidants. J. Leukoc. Biol. 63:732-739. [PubMed] [Google Scholar]
- 35.Steiner, I., and P. G. Kennedy. 1995. Herpes simplex virus latent infection in the nervous system. J. Neurovirol. 1:19-29. [DOI] [PubMed] [Google Scholar]
- 36.Strick, P. L., and J. P. Card. 1992. In J. P. Bolam (ed.), Experimental neuroanatomy. A practical approach, 4th ed., p. 81-101. Oxford University Press, Oxford, United Kingdom.
- 37.Wiegand, H., H. P. Dienes, P. Schirmacher, J. Podlech, K. Bohl, M. Bohle, D. Neumann-Haefelin, and D. Falke. 1991. Colonization of adrenal glands and ovaries of mice by variants of HSV 1 and 2. II. Histopathological, immunohistochemical and in situ hybridization studies. Arch. Virol. 117:237-249. [DOI] [PubMed] [Google Scholar]
- 38.Wildy, P., H. J. Field, and N. N. Nash. 1982. Classical herpes latency revisted. Symp. Soc. Gen. Microbiol. 33:148-149. [Google Scholar]
- 39.Yirrell, D. L., W. A. Blyth, and T. J. Hill. 1987. The influence of androgens on paralysis in mice following intravenous inoculation of herpes simplex virus. J. Gen. Virol. 68:2461-2464. [DOI] [PubMed] [Google Scholar]