The incidence of acute kidney injury (AKI) in the intensive care unit (ICU) population has been reported to be as high as greater than 50% in a recent study (1), often in the context of multiorgan failure and sepsis. AKI contributes significantly to the high mortality in the ICU population, where in-hospital mortality rates may exceed 50%. AKI is also strongly associated with a higher risk for the development of chronic kidney disease (2). The pathophysiology of AKI is multifactorial, involving hemodynamic changes, inflammation, and direct injury to tubule cells (2). The endothelium plays an important role in the pathophysiology of AKI. Injury to endothelial cells results in enhance leukocyte adherence, platelet aggregation, vasoconstriction, reduced blood flow to the nephron, and impairment of the permeability barrier of the glomerulus and the extensive network of peritubular capillaries. Loss of peritubular capillaries is a recognized consequence of AKI and chronic kidney disease. The glycocalyx is a negatively charged gel that coats the endothelium and creates a molecular sieve that prevents large molecules from passing through, and also likely protects the endothelial cells (3).
The endothelial glycocalyx was first visualized lining endothelial cells in the 1950s, after the invention of the transmission electron microscope (4). The Greek translation of glycocalyx is “sweet husk” (5). The major components of the endothelial glycocalyx are glycoproteins (cell surface receptors such as selectins and integrins), proteoglycans (membrane-bound proteins such as syndecans and glypicans), and glycosaminoglycans (GAGs; long, linear polysaccharides that carry a strong negative charge, including heparan sulfate, chondroitin sulfate, and hyaluronic acid or hyaluronan). Proteoglycans with covalently bound GAGs are prominent contributors to the endothelial glycocalyx (6). Heparan sulfate constitutes up to 90% of the GAGs on the surface of endothelial cells (7). Hyaluronic acid linked to the endothelial surface receptor, CD44, weaves through the endothelial glycocalyx (8). All these components, together with proteins and growth factors bound to the carbohydrates of GAGs, form a fence-like meshwork on the surface of endothelial cells and play an important role in vascular homeostasis and a barrier against protein filtration (9).
The endothelial glycocalyx is in dynamic balance between synthesis and degradation. In response to inflammatory mediators, both GAGs and proteoglycans can be shed from the endothelium. In a recent observational study by Ostrowski and colleagues (10), the authors found significantly elevated serum levels of syndecan-1 in patients with sepsis. Schmidt and colleagues (11), using intravital microscopy, found that endotoxemia in mice rapidly induced pulmonary microvascular glycocalyx degradation via tumor necrosis factor α–dependent mechanisms involving the activation of endothelial heparanase. Therefore, the extent of shedding of glycocalyx components might be considered a marker for endothelial glycocalyx stability. Because the presence of the glycocalyx on endothelial cells is not organ-specific, the shed glycocalyx components in blood are not specific to injury of a particular organ.
In this issue of the Journal, Schmidt and colleagues (pp. 439–449) used mass spectroscopy (12, 13) to analyze the urine GAG content in a small cohort of 30 patients with sepsis with varying levels of renal function. They validated their findings in a cohort of 70 patients with ARDS, all of whom had normal renal function at baseline. The authors demonstrated that the levels of urinary GAG fragmentation predict the development of AKI and mortality in critically ill patients. The measurement of urinary GAGs might be most reflective of glomerular endothelial glycocalyx disruption; however, released GAGs from endothelium in other organs may enter the circulation and be filtered by the damaged glomerulus. Glomerular endothelial glycocalyx was first confirmed by Rostgaard and colleagues (14), using scanning electron microscopy. They reported that the glycocalyx measures up to 300 nm and covers both the fenestral and the interfenestral domains of glomerular endothelial cells. Dane and colleagues (15) also demonstrated that the fenestrae in the glomerular endothelium are predominantly filled with hyaluronan. Earlier studies showed that GAG-degrading enzymes such as heparinase and chondroitinase could increase the permeability of the glomerular filter (16, 17). Furthermore, Gil and colleagues (18) demonstrated that heparanase is essential for the development of proteinuria in experimental diabetic nephropathy. Garsen and colleagues also demonstrated that heparanase deficiency ameliorated proteinuria, reduced glomerular damage, and reduced the proinflammatory cytokine milieu in the kidney during experimental glomerulonephritis (19). In another study, Lygizos and colleagues (20) observed septic induction of renal heparanase, using a mouse model of polymicrobial sepsis. Renal heparanase activation was associated with fragmentation of glomerular HS, contributing to the onset of septic renal dysfunction. Taking all these findings together, degradation of glomerular endothelial glycocalyx can be pathogenic, resulting in proteinuria.
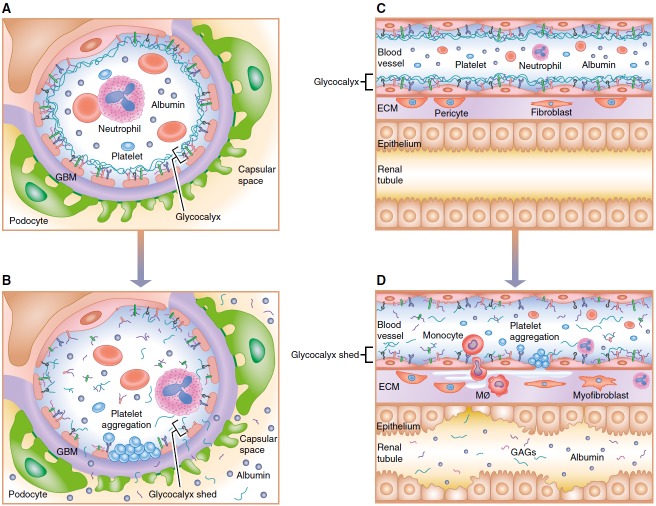
What is the relationship between the breakdown in glomerular glycocalyx and AKI? Is the AKI that is associated with urinary GAGs a direct result of glomerular endothelial cell glycocalyx breakdown, or are the urinary GAGs a marker of more generalized glycocalyx breakdown in the capillaries of the kidney and/or other organs? Schmidt and colleagues (12) argue for specificity of urine heparin sulfate to a renal origin, as previous data from their laboratory indicate an association between plasma heparan sulfate and infection during sepsis, and the current study shows urinary GAGs predict development of renal dysfunction, even when severity of illness is controlled for. Glomerular glycocalyx damage with subsequent increase in leakiness of the glomerular barrier would not alone be expected to reduce GFR, but a reduced GFR could result from secondary leukocyte– and/or platelet–endothelial interactions, which can cause capillary obstruction (Figures 1A and 1B). This could result in reduced postglomerular peritubular capillary perfusion and local ischemia to the tubules. It is important to recognize that the peritubular capillaries from one glomerulus provide blood flow to segments of a number tubules derived from a number of other glomeruli in addition to the glomerulus of origin (21). In addition, the changes in the glomerular capillary glycocalyx may also be present in the peritubular capillaries, and the latter may be more important in the tubular dysfunction that accompanies AKI. In the latter case, the urinary GAGs may be a surrogate for peritubular capillary changes, which then can lead to increased vascular obstruction, inflammation, interstitial edema, and reduced tubule oxygen supply particularly in the outer medulla of the kidney (2) (Figures 1C and 1D).
Figure 1.
Endothelial injury and glycocalyx disruption in acute kidney injury. (A) Normal and (B) injured glomerular endothelium. With injury, disruption of the glycocalyx can result in increased leukocyte– and platelet–endothelial interactions that can cause impaired capillary flow or obstruction. (C) Normal tubular epithelium and peritubular capillary endothelium separated by a small interstitial compartment. A glycocalyx coats the endothelium. (D) Disruption of the glycocalyx in peritubular capillaries may lead to increased leukocyte– and platelet–endothelial interactions, inflammation, interstitial edema, and reduced tubule oxygen supply, particularly in the outer medulla of the kidney. ECM = extracellular matrix; GAG = glycosaminoglycan; GBM = glomerular basement membrane; MØ = macrophage.
Another novel aspect of the study of Schmidt and colleagues (12) is the use of an inexpensive method to detect highly sulfated GAGs, including heparan sulfate and chondroitin sulfate, in the urine. As indicated above, the glycocalyx is a highly dynamic and fragile structure. GAGs and bound plasma proteins can be lost during fixation, dehydration, and staining of biopsy tissue (22). Although newly developed technologies of fixation and rapid freeze techniques have led to visualization of the glycocalyx, it would be useful to have a way to evaluate the glycocalyx without the need for obtaining tissue. Although mass spectrometry provides an accurate and sensitive method to analyze the GAG content and disaccharide composition of urine samples, the cost of mass spectrometry is high. Acknowledging this limitation, Schmidt and colleagues (12) compared the results of urinary GAGs from mass spectrometry with dimethylmethylene blue (DMMB) staining of the urinary GAGs, which would be much less expensive and more applicable at the point of care. Using the DMMB colorimetric assay to measure levels of sulfated GAGs, the association of urinary GAG concentration with subsequent onset/progression of kidney injury was preserved. On binding to polyanionic substrates such as GAGs, the characteristic blue of the cationic DMMB dye shifts to a violet hue, which can be assessed using a spectrophotometer. This assay can be affected, however, by duration of reaction, pH, salt content, and other polyanions such as DNA, RNA, or hyaluronic acid in the biological samples (23). Furthermore, the DMMB assay may overestimate the GAG level if the biological sample has less than 5 μg/ml of GAGs (23). Schmidt and colleagues (12) did consider and test potential interference of pH and DNA on the DMMB assay. DMMB levels were not normalized to urinary creatinine concentrations, whereas the authors present GAG levels both normalized and nonnormalized. As the authors pointed out, the generalization of the DMMB assay should be tested in a larger study.
Whether using mass spectroscopy or DMMB staining to measure urinary GAGs as biomarkers to predict AKI or mortality, the current study yields areas under the curve of the receiver operator curve that vary from 0.5 to 0.85, depending on what urinary GAGs are being used to predict or whether all GAGs are considered. One of the problems with evaluation of biomarkers for the prediction or identification of AKI is the use of changes in serum creatinine concentration as the gold standard for the diagnosis of AKI. Serum creatinine is well known to be flawed as a gold standard (24). Much more needs to be done to determine how useful urinary GAGs are as biomarkers, but this study is important in focusing more attention on the role of the glycocalyx in renal dysfunction in the critically ill patient.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9:12–20. doi: 10.2215/CJN.02730313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabelink TJ, de Zeeuw D. The glycocalyx--linking albuminuria with renal and cardiovascular disease. Nat Rev Nephrol. 2015;11:667–676. doi: 10.1038/nrneph.2015.162. [DOI] [PubMed] [Google Scholar]
- 4.Farquhar MG, Palade GE. Segregation of ferritin in glomerular protein absorption droplets. J Biophys Biochem Cytol. 1960;7:297–304. doi: 10.1083/jcb.7.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett HW, Hess FT. Rapid detection of excess glucose in coloring syrups used in tablet production. J Pharm Sci. 1963;52:607–608. doi: 10.1002/jps.2600520627. [DOI] [PubMed] [Google Scholar]
- 6.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oohira A, Wight TN, Bornstein P. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J Biol Chem. 1983;258:2014–2021. [PubMed] [Google Scholar]
- 8.Culty M, Miyake K, Kincade PW, Sikorski E, Butcher EC, Underhill C. The hyaluronate receptor is a member of the CD44 (H-CAM) family of cell surface glycoproteins. J Cell Biol. 1990;111:2765–2774. doi: 10.1083/jcb.111.6.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarbell JM, Cancel LM. The glycocalyx and its significance in human medicine. J Intern Med. doi: 10.1111/joim.12465. [online ahead of print] 8 Jan 2016; DOI: 10.1111/joim.12465. [DOI] [PubMed] [Google Scholar]
- 10.Ostrowski SR, Gaïni S, Pedersen C, Johansson PI. Sympathoadrenal activation and endothelial damage in patients with varying degrees of acute infectious disease: an observational study. J Crit Care. 2015;30:90–96. doi: 10.1016/j.jcrc.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt EP, Overdier KH, Sun X, Lin L, Liu X, Yang Y, Ammons LA, Hiller TD, Suflita MA, Yu Y, et al. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2016;194:439–449. doi: 10.1164/rccm.201511-2281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun X, Li L, Overdier KH, Ammons LA, Douglas IS, Burlew CC, Zhang F, Schmidt EP, Chi L, Linhardt RJ. Analysis of total human urinary glycosaminoglycan disaccharides by liquid chromatography-tandem mass spectrometry. Anal Chem. 2015;87:6220–6227. doi: 10.1021/acs.analchem.5b00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rostgaard J, Qvortrup K. Electron microscopic demonstrations of filamentous molecular sieve plugs in capillary fenestrae. Microvasc Res. 1997;53:1–13. doi: 10.1006/mvre.1996.1987. [DOI] [PubMed] [Google Scholar]
- 15.Dane MJ, van den Berg BM, Avramut MC, Faas FG, van der Vlag J, Rops AL, Ravelli RB, Koster BJ, van Zonneveld AJ, Vink H, et al. Glomerular endothelial surface layer acts as a barrier against albumin filtration. Am J Pathol. 2013;182:1532–1540. doi: 10.1016/j.ajpath.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 16.Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol. 2007;18:2885–2893. doi: 10.1681/ASN.2007010119. [DOI] [PubMed] [Google Scholar]
- 17.Jeansson M, Haraldsson B. Glomerular size and charge selectivity in the mouse after exposure to glucosaminoglycan-degrading enzymes. J Am Soc Nephrol. 2003;14:1756–1765. doi: 10.1097/01.asn.0000072742.02714.6e. [DOI] [PubMed] [Google Scholar]
- 18.Gil N, Goldberg R, Neuman T, Garsen M, Zcharia E, Rubinstein AM, van Kuppevelt T, Meirovitz A, Pisano C, Li JP, et al. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes. 2012;61:208–216. doi: 10.2337/db11-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garsen M, Benner M, Dijkman HB, van Kuppevelt TH, Li JP, Rabelink TJ, Vlodavsky I, Berden JH, Rops AL, Elkin M, et al. Heparanase is essential for the development of acute experimental glomerulonephritis. Am J Pathol. 2016;186:805–815. doi: 10.1016/j.ajpath.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Lygizos MI, Yang Y, Altmann CJ, Okamura K, Hernando AA, Perez MJ, Smith LP, Koyanagi DE, Gandjeva A, Bhargava R, et al. Heparanase mediates renal dysfunction during early sepsis in mice. Physiol Rep. 2013;1:e00153. doi: 10.1002/phy2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beeuwkes R, III, Bonventre JV. Tubular organization and vascular-tubular relations in the dog kidney. Am J Physiol. 1975;229:695–713. doi: 10.1152/ajplegacy.1975.229.3.695. [DOI] [PubMed] [Google Scholar]
- 22.Dane MJ, van den Berg BM, Lee DH, Boels MG, Tiemeier GL, Avramut MC, van Zonneveld AJ, van der Vlag J, Vink H, Rabelink TJ. A microscopic view on the renal endothelial glycocalyx. Am J Physiol Renal Physiol. 2015;308:F956–F966. doi: 10.1152/ajprenal.00532.2014. [DOI] [PubMed] [Google Scholar]
- 23.Zheng CH, Levenston ME. Fact versus artifact: avoiding erroneous estimates of sulfated glycosaminoglycan content using the dimethylmethylene blue colorimetric assay for tissue-engineered constructs. Eur Cell Mater. 2015;29:224–236. doi: 10.22203/ecm.v029a17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol. 2012;23:13–21. doi: 10.1681/ASN.2010111124. [DOI] [PMC free article] [PubMed] [Google Scholar]