Summary
Approximately 6.5 million US men have both oral and penile human papillomavirus (HPV) infections. Genotype-concordant HPV infection between sites was associated with sexual behavior, which may partly explain the observed association between penile and oral HPV infections.
Keywords: NHANES, human papillomavirus, HPV, penile, oral, genotype, concordance.
Abstract
This study examined the concordance of penile and oral human papillomavirus (HPV) infections in the United States. A total of 1683 men aged 18–59 years who participated in the 2013–2014 National Health and Nutrition Examination Survey and had results of oral and penile HPV DNA testing were examined. The prevalence of any HPV genotype was 45.3% on the penis, 11.2% in the oral cavity, and 8.8% at both sites. The prevalence of HPV in the oral cavity was higher among those with than among those without penile HPV infection (19.3% vs 4.4%; prevalence ratio, 4.37 [95% confidence interval, 2.66–7.16]). The prevalence of ≥1 genotype-concordant HPV infection was 3.2% and was associated with sexual behavior, independent of demographic characteristics and smoking status. Sexual behavior may partly explain the observed association between penile and oral HPV infections.
Persistent infection with high-risk genotypes of human papillomavirus (HPV), particularly HPV-16, is causally associated with the development of genital cancers (cervical, penile, and anal) and oropharyngeal squamous cell carcinomas [1]. The incidence of oropharyngeal cancers has been increasing in industrialized countries, including in the United States, where the burden is disproportionately high among men [2]. In parallel, men in the United States have a higher prevalence of oral HPV infection as compared to women, which is suggested to be mediated by cumulative lifetime sexual exposure [3]. It was recently shown that almost half of US men also have penile HPV infection [4–6]. Estimates of detectable HPV prevalence vary by anatomic site [7], but there are limited population-based studies that have examined HPV infection at multiple anatomic sites among the same men [8]. In this study, we examined the prevalence of dual penile and oral HPV infections among a nationally representative sample of US men.
MATERIALS AND METHODS
Study Population
The National Health and Nutrition Examination Survey (NHANES) is a continuous cross-sectional survey conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) [9]. NHANES is designed to obtain a nationally representative sample of noninstitutionalized, civilian residents in the United States. The survey uses a complex, stratified, multistage probability sample design with unequal probabilities of selection. Respondents who provided informed consent participated in a comprehensive household-based interview and a physical health examination at a local mobile examination center. Data collection for NHANES was approved by the CDC Institutional Review Board.
The present study was conducted using data collected during the 2013–2014 cycle of NHANES. Overall, 68.1% of screened males were interviewed and physically examined. Of the 2042 men aged 18–59 years who were interviewed, 1975 (96.7%) received a physical health examination. Of those who were examined, 1868 (94.6%) had a valid oral HPV test result and 1757 (89.0%) had a valid penile HPV test result available for analysis. The present study was restricted to 1683 men who had valid oral and penile HPV test results.
Sociodemographic and Behavioral Data
Demographic information was self-reported. Household interviewers asked participants about tobacco use, using the computer-assisted personal interview system. Participants self-reported information on sexual history, using the audio computer-assisted self-interview system. Owing to limitations in sample size, we did not differentiate between male and female sex partners in this study.
HPV Detection and Genotyping
Details on collection, processing, storage, and HPV testing of oral rinse and penile swab specimens are extensively detailed on the NCHS website and/or in previous studies [5, 9, 10]. Different DNA extraction protocols and laboratories were used for oral and penile samples, but all DNA extracts were tested for the presence of 37 HPV genotypes, using the Linear Array HPV genotyping assay (Roche Diagnostics, Indianapolis, IN). Only specimens with detectable β-globin were considered valid.
Statistical Analysis
Prevalence estimates were weighted using the medical examination weight provided by the NCHS to adjust for the complex survey design and nonresponse to the medical examination. Taylor series linearization was used to approximate variance; estimates with a relative standard error (RSE) of >30% were considered unreliable and are indicated.
Log-binomial regression models were used to estimate prevalence ratios (PRs) and 95% confidence intervals (CIs). In the main analysis, we examined demographic and behavioral associations with having any concurrent penile and oral HPV infection (defined as detection of any genotype on the penis and in the oral cavity) and ≥1 genotype-concordant penile-oral HPV infection (defined as detection of the same genotype on the penis and in the oral cavity). All multivariable models included age, race/ethnicity, and smoking status as categorical variables, owing to a priori hypotheses [3, 4, 8]. In favor of parsimony and avoidance of collinearity, we built separate models for indicators related to participants’ sexual history. Since this study was limited to 1 NHANES cycle, there were only 15 degrees of freedom available to build multivariable models. Ptrend values were estimated by modeling ordinal categories as continuous variables. All P values for bivariable and multivariable models were calculated using a 2-sided design-adjusted Wald F test.
In a separate individual-level analysis, we assessed the association of penile and oral HPV infections by using methods described above. We also conducted an unweighted analysis in which the association of penile and oral HPV infections was examined at the genotype-level, such that each individual had 37 genotype-specific observations. For this exploratory analysis, we used modified Poisson regression with generalized estimating equations and robust variance estimation to test the effect of a genotype-specific penile HPV infection on detection of oral HPV infection of the same genotype.
Statistical analyses were conducted using Stata SE, version 14 (Stata, College Station, TX).
RESULTS
Participant Characteristics
This study represents 74.1 million US men between 18–59 years of age. Only 4.3% of respondents reported they never had any kind of sex (oral, vaginal, or anal). A small proportion of respondents reported ever having sex with another man (6.1%). There was a limited number of respondents who reported receiving at least 1 dose of an HPV vaccine (4.1%).
Prevalence of Penile and Oral HPV Infection
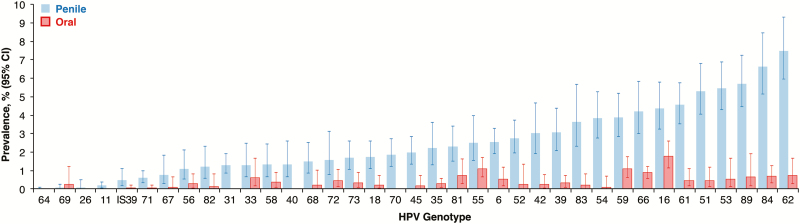
The prevalence of any detectable HPV infection was 45.3% (95% CI, 41.4%–49.4%) on the penis and 11.2% (95% CI, 8.8%–14.1%) in the oral cavity. Detection of multiple (≥2) HPV genotypes on the penis and in the oral cavity was 22.4% (95% CI, 19.9%–25.1%) and 2.0% (95% CI, 1.3%–3.1%), respectively. The diversity of detectable HPV genotypes was similar between sites; all 37 genotypes were detected at least once on the penis, while 31 genotypes were detected at least once in the oral cavity (Figure 1). The genotype most commonly detected in the oral cavity was HPV-16 (1.8% [95% CI, 1.2%–2.6%]), which was also among those most commonly detected on the penis (4.4% [95% CI, 3.3%–5.8%]; Figure 1). The frequency of detection of each genotype was higher on the penis than in the oral cavity, except for HPV-69 (RSE >30%; Figure 1).
Figure 1.
Genotype-specific prevalence of human papillomavirus (HPV) infection, by anatomic site, among men in the United States, National Health and Nutrition Examination Survey, 2013–2014. The bars on the left represent detectable penile HPV infection, and the bars on the right represent detectable oral HPV infection. All data shown are weighted, and error bars depict 95% confidence intervals (CIs). Estimates for many genotypes had a relative standard error of >30% but are provided to visualize the distribution of detectable genotypes between sites.
Association of Penile and Oral HPV Infection
Men with any detectable penile HPV infection were significantly more likely to have any detectable oral HPV infection (19.3% vs 4.4%; PR, 4.37 [95% CI, 2.66–7.16]). More specifically, compared with men without any detectable penile HPV infection (4.4%), the prevalence of any detectable oral HPV infection was 15.1% among men with 1 detectable penile HPV genotype (PR, 3.42 [95% CI, 1.76–6.64]) and 23.6% among men with ≥2 detectable penile HPV genotypes (PR, 5.34 [95% CI, 3.49–8.17]). In an unweighted genotype-level analysis (n = 62 271), detection of a specific genotype on the penis was significantly associated with the detection of the same genotype in the oral cavity (3.5% vs 0.3%; PR, 11.90 [95% CI, 8.86–16.00]).
Prevalence of Concurrent and Genotype-Concordant Penile and Oral HPV Infection
The prevalence of concurrent HPV infection of any genotype at both sites was 8.8% (95% CI, 6.6%–11.5%). Thus, approximately 6.5 million men in the United States between the ages 18–59 are concurrently infected with HPV in their oral and genital tract. Among men concurrently infected with any HPV genotype at both sites, 36.2% (95% CI, 25.6%–48.1%) were concordant for at least 1 genotype. However, the overall prevalence of ≥1 genotype-concordant HPV infections among all men in the study was only 3.2% (95% CI, 2.1%–4.7%). Genotypes with the highest concordance between sites were HPV-16 (0.2% [95% CI, .0%–.5%]), HPV-51 (0.2% [95% CI, .0%–.7%]), HPV-55 (0.3% [95% CI, .0%–1.2%]), HPV-59 (0.4% [95% CI, .2%–1.0%]), HPV-61 (0.2% [95% CI, .0%–.7%]), HPV-62 (0.3% [95% CI, .0%–.9%]), HPV-66 (0.2% [95% CI, .0%–.7%]), HPV-73 (0.2% [95% CI, .0%–.8%]), and HPV-89 (0.6% [95% CI, .2%–1.8%]); however, these estimates were unreliable (RSE >30%).
Risk Factors for Concurrent and Genotype-Concordant Penile and Oral HPV Infection
The prevalences of any detectable penile HPV infection and any detectable oral HPV infection stratified by demographic and behavioral characteristics are shown in Supplementary Table 1. In bivariable analyses, race/ethnicity and smoking status were associated with any concurrent HPV infection (Table 1). However, these effects were attenuated in the multivariable model that included the lifetime number of (any) sex partners (Table 1). Higher lifetime numbers of (any) sex partners, of oral sex partners, and of any recent sex partners were significantly associated with any concurrent HPV infection, independent of age, race/ethnicity, and smoking status (Table 1).
Table 1.
Correlates of Concurrent and Genotype-Concordant Human Papillomavirus (HPV) Infection Among Men in the United States, National Health and Nutrition Examination Survey, 2013–2014
| Characteristic | Men, No. | Concurrent Penile and Oral HPV Infection of Any Genotype | ≥1 Genotype-Concordant Penile and Oral HPV Infection | ||
|---|---|---|---|---|---|
| Prevalence, % (95% CI) |
Adjusted PR
(95% CI) |
Prevalence, % (95% CI) |
Adjusted PR
(95% CI) |
||
| Age, y a | |||||
| 18–24 | 351 | 4.4 (2.3–8.3) | Reference | 2.2 (1.3–3.6) | Reference |
| 25–29 | 187 | 7.8 (3.5–16.7)b | 1.31 (.52–3.29) | 4.0 (1.3–11.4)b | 1.79 (.52–6.16) |
| 30–39 | 402 | 8.7 (5.9–12.8) | 1.39 (.73–2.64) | 3.7 (1.8–7.2)b | 1.50 (.81–2.77) |
| 40–49 | 369 | 9.6 (6.0–14.9) | 1.48 (.75–2.91) | 2.5 (.1–6.0)b | 0.92 (.26–3.26) |
| 50–59 | 374 | 11.8 (6.8–19.4) | 1.68 (.62–4.59) | 3.7 (1.6–8.2)b | 1.38 (.41–4.70) |
| P (Ptrend) | .098 (.050) | .828 (.341) | .650 (.634) | .707 (.971) | |
| Race/ethnicity a | |||||
| White, non-Hispanic | 691 | 9.9 (6.8–14.2) | Reference | 3.7 (2.3–6.0) | Reference |
| Black, non-Hispanic | 348 | 12.5 (8.9–17.1) | 1.00 (.67–1.46) | 3.5 (2.1–5.7) | 0.70 (.32–1.54) |
| Asian, non-Hispanic | 189 | 3.0 (1.6–5.8) | 0.71 (.37–1.38) | 1.3 (.4–3.9)b | 0.59 (.17–2.05) |
| Hispanic | 386 | 4.7 (3.2–6.8) | 0.58 (.35–.96) | 1.4 (.5–4.1)b | 0.34 (.10–1.16) |
| P | .001 | .220 | .061 | .077 | |
| Current smoker a | |||||
| No | 1227 | 7.2 (5.1–10.3) | Reference | 2.4 (1.6–3.7) | Reference |
| Yes | 456 | 13.4 (9.0–19.5) | 1.30 (.75–2.25) | 5.6 (3.2–9.5) | 1.65 (.80–3.47) |
| P | .020 | .326 | .006 | .168 | |
| Lifetime no. of sex partners a (anyc) | |||||
| 0–5 | 686 | 1.7 (.7–3.7)b | Reference | 1.4 (.5–3.4)b | Reference |
| 6–10 | 324 | 8.0 (5.0–12.8) | 4.41 (1.83–10.60) | 2.4 (1.1–5.3)b | 1.55 (.53–4.53) |
| >10 | 564 | 16.5 (11.7–22.8) | 8.33 (3.09–22.48) | 5.7 (3.6–9.1) | 3.45 (1.01–11.75) |
| P (Ptrend) | <.001 (<.001) | .002 (.001) | .022 (.006) | .117 (.043) | |
| Lifetime no. of oral sex partners d | |||||
| 0–1 | 556 | 3.2 (1.4–7.3)b | Reference | 1.7 (.1–4.3)b | Reference |
| 2–5 | 601 | 5.1 (3.1–8.1) | 1.36 (.52–3.61) | 2.1 (.1–4.3)b | 0.99 (.34–2.91) |
| >5 | 414 | 18.2 (13.1–24.7) | 4.37 (1.65–11.58) | 6.2 (3.6–10.5) | 2.59 (.65–10.35) |
| P (Ptrend) | .001 (<.001) | .002 (.001) | .040 (.026) | .125 (.116) | |
| No. of recent sex partners e,d (anyc) | |||||
| None | 234 | 2.8 (.9–8.5)b | Reference | 0.4 (.0–2.2)b | Reference |
| 1 | 989 | 7.5 (4.9–11.5) | 2.27 (.69–7.51) | 3.1 (1.7–5.4) | 6.54 (.99–43.11) |
| ≥2 | 357 | 14.2 (10.7–18.7) | 4.61 (1.55–13.75) | 4.9 (2.9–8.4) | 9.91 (1.87–52.66) |
| P (Ptrend) | .013 (.002) | .007 (.001) | .017 (.004) | .028 (.020) | |
All prevalence estimates and 95% CIs were weighted. Log-binomial models were used to calculate PRs of any concurrent HPV infection (ie, detection of any genotype on the penis and in the oral cavity) and ≥1 genotype-concordant HPV infection (ie, detection of the same genotype on the penis and in the oral cavity). P and Ptrend values reflect findings of 2-sided design-adjusted Wald F tests from bivariable and multivariable regression models, but only adjusted PRs are shown. Ptrend values were estimated by modeling ordinal categories as a continuous variable. Participants in the “multiracial/other” race/ethnicity category were included in all estimates, but the prevalence among this subgroup was not shown because of a low sample size (n = 69).
Abbreviations: CI, confidence interval; PR, prevalence ratio.
aMultivariable model included age group, race/ethnicity, current smoking status, and lifetime number of partners for any type of sexual activity.
bThe relative standard error of the weighted prevalence estimate was >30%.
cAny type of sexual activity (oral, vaginal, or anal).
dMultivariable model included age group, race/ethnicity, current smoking status, and the sexual behavior characteristic of interest.
eThe number of sex partnerships in the past 12 months.
The prevalence of ≥1 genotype-concordant HPV infection was not associated with age (Table 1). There was no difference in the prevalence of ≥1 genotype-concordant HPV infection between non-Hispanic blacks and non-Hispanic whites (Table 1). However, compared with non-Hispanic whites (3.7%), non-Hispanic Asians (1.3%; PR, 0.35 [95% CI, .10–1.32]) and Hispanics (1.4%; PR, 0.37 [95% CI, .14–.98]) were less likely to have ≥1 genotype-concordant HPV infection. Current smokers were significantly more likely to have ≥1 genotype-concordant HPV infection than nonsmokers (5.6% vs 2.4%; PR, 2.33 [95% CI, 1.32–4.11]). These associations were no longer significant in the multivariable model that included the lifetime number of partners for any kind of sex (Table 1). Higher lifetime numbers of sex partners and recent partners for any kind of sex were significantly associated with having ≥1 genotype-concordant HPV infection, independent of age, race/ethnicity, and smoking status (Table 1); however, this was not statistically significant for the lifetime number of oral sex partners (Table 1).
DISCUSSION
This study described a high burden of penile HPV infection that was associated with oral HPV infection among men in the general US population. We estimate that 6.5 million US men aged 18–59 years have both penile and oral HPV infections. It has previously been shown that sexual behavior is associated with genital HPV infection and oral HPV infection individually [3–7]. In this study, we demonstrated that the cumulative and recent number of sex partnerships are associated with a higher prevalence of concurrent and genotype-concordant penile and oral HPV infections. High-risk sexual behaviors may partly explain the observed associations between penile and oral HPV infections.
The association between penile and oral HPV infections has been previously documented in a population-based study in China, which also found that men with multiple lifetime sex partners have a higher prevalence of concurrent HPV infection [8]. In addition, sexual behavior has been shown to be associated with genotype-concordant genital and oral HPV infections among women in the United States [11, 12]. By using multiple cycles of NHANES, Kedarisetty et al were able to incorporate multiple indicators of sexual history in the same multivariable model and found that recent oral sexual exposure was a more important risk factor of genotype-concordant vaginal and oral HPV infections than cumulative sexual exposure [12]. While it is plausible that autoinoculation may also play a role as a means of nonsexual HPV transmission [13], these cross-sectional data among men and women collectively support the genital-oral HPV transmission theory [12]. Nonetheless, longitudinal studies of stable couples with timely sampling at multiple anatomic sites are needed to elucidate HPV transmission dynamics [14].
The low overall prevalence of genotype-specific concordance (or high rate of discordance) between anatomic sites may be explained by a combination of factors: site variation in susceptibility to HPV infection, differential exposure characteristics, and differences in natural history at each mucosal site [15]. Accordingly, the lack of association between age and genotypic concordance when age is associated with both penile and oral HPV infections individually requires investigation [5, 10]. It has been previously suggested that the increase in detectable HPV infection with older age is due to potential loss in immune control and reactivation of latent HPV infection—which may be site specific; however, this hypothesis cannot be confirmed by using cross-sectional data, since we cannot distinguish HPV detection due to incident infection, reinfection, or reactivation. Although it is also difficult to highlight direct implications of our findings on cancer development, the high observed burden of HPV suggests the need for early preventive interventions.
This study has other limitations. To obtain more-precise and more-reliable prevalence estimates, especially at the genotype level, additional cycles of NHANES will be required. This will also help to address residual confounding in our analyses, since the number of covariates in our multivariable models were limited by study design. Finally, we likely underestimated the true prevalence and concordance of HPV infection, owing to cross-sectional sampling, sampling errors, and/or limitations in assay sensitivity.
Overall, this population-based study extends our understanding of penile and oral HPV epidemiology in men. Our descriptive findings suggest HPV infection at multiple sites is linked to sexual behavior, but further work is needed to investigate the association between penile and oral HPV infections. The low genotypic concordance despite high exposure at both sites and the age-specific prevalence of concordance observed in this study may guide future research regarding HPV infection and disease susceptibility and site-specific immune response to infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported in part by the National Institutes of Health (awards 1K23AI093152-01A1, 5R01AI120938-02, and 1R01AI128779-01 to A. A. R. T) and the Maryland Cigarette Restitution Fund (to A. F. R., via the Johns Hopkins Medical Institutions [fiscal year 2017]).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Schiffman M, Doorbar J, Wentzensen N, et al. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers 2016; 2:16086. [DOI] [PubMed] [Google Scholar]
- 2. Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell Carcinoma. J Clin Oncol 2015; 33:3235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chaturvedi AK, Graubard BI, Broutian T, et al. NHANES 2009–2012 Findings: association of sexual behaviors with higher prevalence of oral oncogenic human papillomavirus infections in U.S. men. Cancer Res 2015; 75:2468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han JJ, Beltran TH, Song JW, Klaric J, Choi YS. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013–2014. JAMA Oncol 2017; doi:10.1001/jamaoncol.2016.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gargano JW, Unger ER, Liu G, et al. Prevalence of genital human papillomavirus in males, United States, 2013–2014. J Infect Dis 2017; 215:1070–9. [DOI] [PubMed] [Google Scholar]
- 6. Gravitt PE. Human papillomavirus: the equal opportunity pathogen. J Infect Dis 2017; 215:1014–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis 2006; 194:1044–57. [DOI] [PubMed] [Google Scholar]
- 8. Liu F, Hang D, Deng Q, et al. Concurrence of oral and genital human papillomavirus infection in healthy men: a population-based cross-sectional study in rural China. Sci Rep 2015; 5:15637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey https://www.cdc.gov/nchs/nhanes/ Accessed 5 January 2017.
- 10. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 2012; 307:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steinau M, Hariri S, Gillison ML, et al. Prevalence of cervical and oral human papillomavirus infections among US women. J Infect Dis 2014; 209:1739–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kedarisetty S, Orosco RK, Hecht AS, Chang DC, Weissbrod PA, Coffey CS. Concordant oral and vaginal human papillomavirus infection in the United States. JAMA Otolaryngol Head Neck Surg 2016; 142:457–65. [DOI] [PubMed] [Google Scholar]
- 13. Pamnani SJ, Nyitray AG, Abrahamsen M, et al. Sequential acquisition of anal Human Papillomavirus (HPV) infection following genital infection among men who have sex with women: the HPV Infection in Men (HIM) study. J Infect Dis 2016; 214:1180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grabowski MK, Gravitt PE, Gray RH, et al. Trends and determinants of human papillomavirus concordance among HIV-positive and HIV-negative heterosexual couples in Rakai, Uganda. J Infect Dis 2017; 215:772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Veldhuijzen NJ, Snijders PJ, Reiss P, Meijer CJ, van de Wijgert JH. Factors affecting transmission of mucosal human papillomavirus. Lancet Infect Dis 2010; 10:862–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.