Abstract
Beneficial effects of cannabidiol (CBD) have been described for a wide range of psychiatric disorders, including anxiety, psychosis, and depression. The mechanisms responsible for these effects, however, are still poorly understood. Similar to clinical antidepressant or atypical antipsychotic drugs, recent findings clearly indicate that CBD, either acutely or repeatedly administered, induces plastic changes. For example, CBD attenuates the decrease in hippocampal neurogenesis and dendrite spines density induced by chronic stress and prevents microglia activation and the decrease in the number of parvalbumin-positive GABA neurons in a pharmacological model of schizophrenia. More recently, it was found that CBD modulates cell fate regulatory pathways such as autophagy and others critical pathways for neuronal survival in neurodegenerative experimental models, suggesting the potential benefit of CBD treatment for psychiatric/cognitive symptoms associated with neurodegeneration. These changes and their possible association with CBD beneficial effects in psychiatric disorders are reviewed here.
Keywords: cannabinoids, anxiety, depression, schizophrenia, neurogenesis, synaptic remodeling, autophagy
Introduction
Plasticity relates to the particular characteristic of a material that undergoes deformation under a load (Lubliner, 2005). In neuroscience, the term neuroplasticity applies to the capacity of the brain to adapt and change in response to experience (Fuchs and Flugge, 2014). William James was the first to propose this term in 1890, defending the idea that brain functions are not fixed during life (James, 1890). Several neuroscientists denied this concept for decades. Santiago Ramón y Cajal, however, used the term neuroplasticity to describe changes in the brain that were a consequence of, or related to, pathology. He also suggested that small protrusions in the dendrites of neurons stained with Golgi's method, which he later named as dendritic spines, are involved in synaptic connectivity and function (Stahnisch and Nitsch, 2002). Nowadays, the idea that brain continually changes along our lifetime is well accepted. Notably, the concept of neuroplasticity has expanded to include not only changes at a morphological level but also biochemical and pharmacological adaptations (intracellular pathways, receptors, synaptic proteins), alterations in neuronal networks (changes in connectivity, dendritic remodeling, and number and morphology of dendritic spines), as well as the generation of new neurons (i.e., adult neurogenesis) (Fuchs and Flugge, 2014). These neuroplastic modifications, moreover, can be either adaptive or maladaptive. Therefore, the mechanisms responsible for these changes may be a great window of opportunity for understanding the pathophysiology and treatment of mental illness (Kays et al., 2012).
Neuroplasticity and psychotropic drugs
Psychiatric disorders may result from significant neuroplastic changes that lead to new set points of brain functions (Pallanti, 2016). For instance, several neuropsychiatric conditions have been associated with stress-induced changes in dendritic remodeling and decreased adult hippocampal neurogenesis (Bessa et al., 2009; Campos et al., 2013b). Corroborating this proposal, decreased hippocampal volume and reduced proliferative activity of neurogenic niches have been described in mood disorders, posttraumatic stress disorder (PTSD) and schizophrenia (Reif et al., 2006; Dhikav and Anand, 2007; Lucassen et al., 2010).
The therapeutic effects of several psychotropic drugs usually need 2–6 weeks to be clinically recognized. It suggests that time-dependent structural reorganization of neuronal circuits and biochemical synaptic changes are required for the pharmacological action of these drugs (Konradi and Heckers, 2001; Fogaça et al., 2013).
Antidepressants are probably the most studied class of medication associated with plastic brain changes. For example, chronic, but not acute, treatment with antidepressants such as serotonin selective uptake inhibitors (SSRIs) and tricyclics increases the expression of Brain-derived Neurotrophic Factor (BDNF) in the hippocampus and prefrontal cortex (PFC) (Castren et al., 2007). Repeated antidepressant treatment also prevents stress-induced hippocampal dendritic atrophy (Bessa et al., 2009) and facilitates adult hippocampal neurogenesis in rodents (Malberg et al., 2000; Santarelli et al., 2003). In addition to standard antidepressant drugs, the rapid and sustained antidepressant effects induced by ketamine also seem to depend on neuroplastic events (Duman et al., 2016). Ketamine appears to act in the PFC and hippocampus modifying the number of dendritic spines and BDNF expression by facilitating mTOR (mechanistic Target of Rapamycin) intracellular pathway (Duman et al., 2016).
Neuroplastic changes have also been associated with the effects of antipsychotic drugs. Haloperidol modifies the number/shape of dendritic spines and synaptic strength and increases expression of synaptic proteins (Eastwood et al., 1997; Harris, 1999; Matus, 1999; Nakahara et al., 1999). Regarding adult hippocampal neurogenesis, the results are contradictory. Whereas Malberg et al. (2000) found no changes in haloperidol-treated adult rats, in gerbils haloperidol seems to facilitate neurogenesis (Dawirs et al., 1998). In the case of atypical antipsychotic drugs, clozapine induces proliferation in the subgranular zone of the rodent dentate gyrus 24-h after the treatment (Halim et al., 2004) and prevents the phencyclidine-induced decrease in hippocampal neurogenesis (Maeda et al., 2007).
Cannabidiol effects in psychiatric disorders
Cannabidiol (CBD) is one of the most abundant components among more than 100 compounds called cannabinoids present in the Cannabis sativa plant. CBD differs from it's the main psychoactive component, delta-9-tetrahydrocannabinol (THC), CBD does not cause psychotomimetic and anxiogenic effects or induce dependence after repeated use (for review, see Ligresti et al., 2016). In addition, it has a better safety profile compared to other cannabinoids, such as THC. For instance, high doses of CBD (up to 1,500 mg/day) are well tolerated in animals and humans.
Nowadays, CBD is one of the phytocannabinoid with the widest range of potential therapeutic actions (Izzo et al., 2009; Ligresti et al., 2016). There are a considerable number of clinical trials using CBD alone or in combination with other cannabinoids in progress (Campos et al., 2016). CBD has attracted considerable interest recently, as marihuana extracts enriched in CBD have been reported to exert a significant reduction in seizure number and severity in Dravet and Gaston-Leroux patients (Devinsky et al., 2014). Of note, the Food and Drug Administration and, the European Medicines Agency approved the use of CBD (Epidiolex, GW) for the treatment of this conditions. Additionaly, CBD exhibits a broad spectrum of other possible therapeutic actions, which include anxiolytic, antipsychotic, antidepressive, and neuroprotective effects over a large range of psychiatric and neurodegenerative disorders (Campos et al., 2016; Ligresti et al., 2016). Although most of these putative therapeutic properties were initially described in animal models, clinical studies have supported the beneficial effects of CBD in anxiety, schizophrenia, epilepsy, and multiple sclerosis (Bergamaschi et al., 2011a; Leweke et al., 2012; Ligresti et al., 2016; Table 1). Corroborating these findings, neuroimaging studies clearly demonstrated that CBD affects brain areas involved in the neurobiology of psychiatric disorders. Crippa et al. (2004) showed that a single dose of CBD, administered orally in healthy volunteers, alters the resting activity in limbic and paralimbic brain areas while decreasing subjective anxiety associated with the scanning procedure. CBD reduced the activity of the left amygdala-hippocampal complex, hypothalamus, and posterior cingulated cortex while increasing the activity of the left parahippocampal gyrus compared with placebo. In healthy volunteers treated with CBD and submitted to a presentation of fearful faces, a decreasing of the amygdala and anterior and posterior cingulate cortex activities and a disruption in the amygdala-anterior cingulated cortex connectivity have also been observed (Fusar-Poli et al., 2009, 2010). Furhter imaging studies also demonstrated that CBD changes activity in other brain areas involved in neuropsychiatric disorders such as the medial and left temporal and prefrontal cortex and insula (Borgwardt et al., 2008; Bhattacharyya et al., 2010; Table 1).
Table 1.
CBD effects in psychiatric disorders.
| Behavioral effect | Model | CBD dose/concentration range | Route of administration/schedule | Species/Strain | Mechanisms investigated | References |
|---|---|---|---|---|---|---|
| PRECLINICAL STUDIES | ||||||
| Antidepressant-like | Forced Swimming Test (FST) | 30 mg/kg | Acute, i.p. | Swiss mice | 5HT1A | Zanelati et al., 2010 |
| FST and Tail Suspension Test (TST) | 200 mg/kg | Acute, i.p. | Swiss Webster mice (FST) DBA/2 mice (TST) | Not determinated | El-Alfya et al., 2010 | |
| FST | 30 mg/kg | Acute and chronic, i.p. | Wistar rats | – | Réus et al., 2011 | |
| Antidepressant-/Anxiolytic-like | Chronic Unpredictable Stress | 30 mg/kg | Chronic, i.p. | GFAP-thymidine kinase (GFAP-TK) transgenic mice | CB1, increased neurogenesis and anandamide levels | Campos et al., 2013b |
| Novelty Suppressed Feeding | C57BL6 mice | |||||
| Elevated Plus Maze (EPM) | ||||||
| Antidepressant-like | FST and TST | 3 and 30 mg/kg | Acute and chronic, i.p. | Swiss mice | Increased neurogenesis | Schiavon et al., 2016 |
| Olfactory bulbectomy | 50 mg/kg | Acute and chronic, i.p. | C57BL6 mice | 5HT1A | Linge et al., 2016 | |
| FST | Intracerebral (mPFC), acute | Wistar rats | 5HT1A | Sartim et al., 2016 | ||
| FST | 30 mg/kg | Acute, i.p. | Swiss mice | Not determinated | Breuer et al., 2016 | |
| Saccharin consumption test | 30 mg/kg | Oral, acute | Wistar-Kyoto (WKY) rat | Not determinated | Shoval et al., 2016 | |
| Antipsychotic | Repeated administration of the NMDA receptor antagonist MK-801 | 15–60 mg/kg | 14 days, i.p. | C57BL6/J mice | Attenuated parvalbumin loss and glial activation in the mPFC, | Gomes et al., 2015a,b |
| Amphetamine sensitization model Prepulse inhibition (PPI) | 100 ng/0.5 μL | Intra-NAc shell/acute | Sprague Dawley rats | Attenuated PPI disruption and increased dopamine system activity via a mTOR/p70S6Kinase signaling pathway | Renard et al., 2016 | |
| Acute administration of the NMDA receptor antagonist MK-801 | 5 mg/kg | Acute, i.p. | Swiss mice | TRPV1 receptors | Long et al., 2006 | |
| Anxiolytic-like | EPM Vogel‘s conflict test | 30 nmol | Intra-periqueductal gray matter | Wistar rats | 5HT1A | Campos and Guimarães, 2008 |
| EPM | 60 nmol | Intra-periqueductal gray matter | Wistar rats | TRPV1 | Campos and Guimarães, 2009 | |
| Predator threat-induced long lasting behavioral alterations | 5 mg/kg | 7 days, i.p. | Wistar rats | 5HT1A | Campos et al., 2012a | |
| Elevated T-Maze | 5 mg/kg | 21 days, i.p. | Wistar rats | 5HT1A | Campos et al., 2013a | |
| Marble burying | 15–60 mg/kg | Acute, i.p. | Swiss mice | CB1 | Casarotto et al., 2010 | |
| Anxiogenic-like | Contextual Fear conditioning | 10 mg/kg | 14 days, i.p. | Lister-hooded rats | Decreased levels of the phosphorylated form of ERK1/2 in the PFC | ElBatsh et al., 2012 |
| CLINICAL STUDIES | ||||||
| Antipsychotic | Double blind controlled clinical trial | 600–800 mg | 28 days, oral | Schizophrenia patients | Reduces psychotic symptoms similar to amisulpride | Leweke et al., 2012 |
| Placebo-controlled clinical trial | Not informed | Oral | Schizophrenia patients | Reduces psychotic symptoms in patients that have previously failed to respond adequately to first line anti-psychotic medications | GW Pharmaceuticals, 2015 | |
| Anxiolytic | 400 mg | Acute, oral | ↓ Subjective anxiety and ↑ mental sedation. | Crippa et al., 2004 | ||
| ↓ Blood Flow in posterior cingulated cortex and Amygdala/Bed nucleus of stria terminalis and ↑ in left parahippocampal gyrus | ||||||
| 600 mg | Acute, oral | ↓ Blood-oxygen-level dependent contrast imaging (BOLD) of amydala signal and amygdala-anterior cingulated connectivity during fearful faces presentations | Fusar-Poli et al., 2009, 2010 | |||
| 600 mg | Acute, oral | ↓ Activation left temporal and insular cortex during motor inhibition task | Borgwardt et al., 2008 | |||
i.p., intraperitoneal; mPFC, medial prefrontal córtex; ↓, decreases; ↑, increases.
Mechanisms of CBD effects in neuropsychiatric disorders
The mechanism of action of CBD remains controversial and is yet unclear. Numerous signaling pathways have been proposed as candidates to mediate its neuroplastic effects (Izzo et al., 2009; Fernández-Ruiz et al., 2013). The possible targets of CBD action have been extensively examined recently (Ibeas Bih et al., 2015; Ligresti et al., 2016). The present review will focus on mechanisms more closely associated with the neuropsychiatric effects of CBD, particularly those related to its neuroplastic effects.
Although several CBD actions are not directly mediated by canonical metabotropic CB1/CB2 receptors, CBD can influence endocannabinoid (ECB) levels (Izzo et al., 2009). While there are contradictory results, it was suggested that CBD exerts an antagonist or negative modulatory action, but with a low affinity, at CB1 and CB2 receptors (Thomas et al., 2007; Laprairie et al., 2015; McPartland et al., 2015). At high concentrations, CBD can facilitate ECB-mediated actions in vitro, including CB1-neuromodulation by decreasing their hydrolysis mediated by Fatty acid amide hydrolase (FAAH) and Monoacylglycerol lipase (MAGL) or re-uptake (Bisogno et al., 2001; De Petrocellis et al., 2011). Another possible mechanism by which CBD decreases anandamide uptake/metabolism in humans, but not rodents, could be the binding to fatty acid binding proteins (FABPs), which is necessary for the transport of this ECB from the membrane to intracellular FAAH (Elmes et al., 2015). The interaction of plant-derived cannabinoids with the ECB metabolism enzymes explains partially why some of the anxiolytic effects of CBD are mediated by CB1 receptors (Casarotto et al., 2010; Campos et al., 2013a).
Like other cannabinoids, CBD produces bell-shaped dose-response curves and can act by different mechanisms accordingly to its concentration or the simultaneous presence of other cannabinoid-ligands (Campos et al., 2012b; Ligresti et al., 2016). CBD can regulate, directly or indirectly, the activity of peroxisome proliferator-activated receptor gamma (PPARγ), serotonin 5HT1A receptor, adenosine transporter, members of the TRPV family, and the metabotropic CB1 and CB2 receptors (Campos et al., 2012b; Ligresti et al., 2016).
5HT1A receptors and the effects of CBD
The acute anxiolytic and antidepressive actions of acute CBD are proposed to be mediated by serotonin 5HT1A receptors. The crosstalk between cannabinoids and serotoninergic signaling, however, is complex. In rats, CBD administration into the dorsal portions of periaqueductal gray matter (dPAG) produces anti-aversive effects in the elevated plus maze and flight-induced by local electric stimulation. These effects were prevented by WAY-100635, a 5HT1A antagonist (Campos and Guimarães, 2008; Soares et al., 2010). Other brain regions, such as the basal ganglia (Espejo-Porras et al., 2013), the bed nucleus of stria terminallis (Gomes et al., 2011), the prelimbic PFC (Fogaça et al., 2014) and the dorsal raphe nucleus (Rock et al., 2012; Katsidoni et al., 2013), also seem to mediate CBD effects via 5HT1A receptors. Similarly, a 5HT1A antagonist prevented the anxiolytic, antistress, and antidepressive-like effects of acute, subchronic (7 days) (Resstel et al., 2009; Zanelati et al., 2010; Campos et al., 2012a; Twardowschy et al., 2013), or chronic (14 days) systemic administration of CBD (Campos et al., 2013b).
The molecular mechanism by which CBD facilitates 5HT1A receptor activation remains unclear. Evidence suggests that it may involve allosteric modulation of this receptor, promoting 5HT1A agonist-related stimulation of [35S]GTPγS binding (Russo et al., 2005; Rock et al., 2012), increase in 5-HT release and/or reuptake inhibition (Linge et al., 2016) or the indirect formation of heterodimers consisting of 5HT1A and other receptors, such as CB1 (Mato et al., 2010).
TRPV1 and the effects of CBD
CBD and other non-psychotomimetic phytocannabinoids can also act, at least in some cases, via the transient receptor potential vanilloid (TRPV) ion channel receptor family. CBD and cannabidivarin (CBDV) activate and desensitize TRPV1 in vitro (Iannotti et al., 2014). TRPV1 receptors activation contributes to the bell-shaped dose-response curve of the anxiolytic action of CBD. The lack of effects observed with high doses of CBD was prevented when the animals were treated with a TRPV1 antagonist (Campos and Guimarães, 2009). TRPV1 also seem to participate in the antihyperalgesic effects of CBD (Costa et al., 2004) as well as in the CBD effects on the sensorimotor gating disruption induced by NMDA antagonists (Long et al., 2006).
Neuroplasticity and CBD effects in chronic stress
Several studies have addressed the effects of CBD administration in different models of stress. Among these models, chronic unpredictable stress (CUS) produces anxiety and depression-like behaviors and cognitive impairment, which are accompanied by reduced levels of neurotrophins (i.e., BDNF and others important for neuronal survival), impaired hippocampal neurogenesis and dendritic arborization, and neuroinflammatory response (microgliosis and astrogliosis; Farooq et al., 2012; Campos et al., 2013b). Chronic CBD administration counteracts the behavioral and neuroplastic consequences of CUS in adult mice by a complex interplay of different mechanisms (Campos et al., 2013b).
CBD and hippocampal adult neurogenesis
Adult neurogenesis is a complex process that involves division, survival (not all cells that divide will survive), migration, and differentiation of new cells (Kempermann, 2008; Suh et al., 2009; Deng et al., 2010). Although, neuronal proliferative capacity has been reported in different brain regions (Chaker et al., 2016), it is well accepted that two areas have an effective neurogenic potential under physiological conditions: the subventricular zone (SVZ), comprising the lateral walls of the lateral ventricle, and the subgranular zone (SGZ) of the dentate gyrus of the hippocampus (Kempermann et al., 2004). Both regions have a small population of neural stem/progenitor cells that originate neurons, astrocytes, and oligodendrocytes (Kempermann and Gage, 2000).
Hippocampal neurogenesis is necessary for at least some forms of learning and memory (Kempermann and Gage, 2000). In addition, decreased adult hippocampal neurogenesis has been associated with psychiatric disorders such as anxiety, schizophrenia, and mood disorders. Disturbed adult hippocampal neurogenesis may be one of the contributors to the loss of hippocampal volume reported in patients suffering from these disorders (Sheline et al., 1996). In animal models, exposure to chronic stress induces both depressive and anxiogenic-like behavior and an impairment of the dentate gyrus subgranular zone (SGZ) neurogenesis (Gould et al., 1997; Goul et al., 1998; Tanapat et al., 1998). Snyder et al. (2011) showed that neurogenesis-deficient mice presented a heightened stress-induced depressive-like behavior and impaired hypothalamus-pituitary-adrenal axis response to stress. Interestingly, classical antidepressants increase neurogenesis in a time span similar to the latency required for their therapeutic effects (Malberg et al., 2000; Manev et al., 2001). Additionally, when neurogenesis is blocked, some behavioral effects of fluoxetine and imipramine disappear (Santarelli et al., 2003; Airan et al., 2007; David et al., 2009).
Although several reports have investigated the potential use C. sativa derivatives in mood and anxiolytic disorders, the effects of cannabinoids on hippocampal neurogenesis was an unexplored issue until the mid-2000's. Jiang et al. (2005) observed that chronic treatment with the synthetic cannabinoid HU210 enhanced neurogenesis in rats. Wolf et al. (2010) investigated the effect of a 6 weeks treatment with a CBD-rich diet in mice and reported an increased number of cells positive for the thymidine analog bromodeoxyuridine (BrdU) in the hippocampus, reflecting increased neural progenitor cell proliferation. This facilitation of hippocampal neurogenesis seemed to depend on CB1 receptors since it was absent in animals lacking this cannabinoid receptor. Another report showed that repeated CBD administration prevented the reduction in neurogenesis in a murine model of Alzheimer's disease through a PPARγ-dependent mechanism (Esposito et al., 2011).
The first study to directly investigate if the behavioral effects of repeated CBD administration are mediated by its pro-neurogenic action was performed by Campos et al. (2013a). They showed that CBD reversed the anxiogenic effect and decreased neurogenesis in CUS-exposed wild-type mice. Interestingly, reforcing the pro-neurogenic effects of CBD, the anti-stress effect of CBD was not observed in transgenic GFAP/thymidine kinase mice where neurogenesis was abolished. CBD effects were also prevented by pharmacological antagonism of CB1 and CB2 receptors, suggesting that the anti-stress effects CBD depend on facilitation of hippocampal neurogenesis through a mechanism that involves increased ECB levels (Campos et al., 2013a). Interestingly, Demirakca et al. (2011) suggested that in chronic heavy Cannabis users, higher THC and lower CBD concentrations were associated with diminished hippocampal gray matter and low cognitive performance, while higher CBD concentrations in the consumed Cannabis samples prevented THC-induced neurotoxic effects. To explain these findings, the authors suggested that the CBD neuroprotective effects would occur through a mechanism that facilitates hippocampal neurogenesis (Demirakca et al., 2011). However, as will be discussed bellow, the role of neurogenesis in CBD effects is complex and may depend on the stress level (Schiavon et al., 2016).
CBD and synaptic remodeling
Synaptic plasticity could be a major target to treat mental disorders. Indeed, besides modulating neurogenesis, pre-clinical findings suggest that usual (SSRIs) and rapid-acting antidepressants, such as ketamine, may increase or restore the synaptic function impaired by chronic stress through a modulation of plastic changes. These effects may involve dendritic spines density, dendritic length and branches, neurotrophic factors (BNDF) and synaptic proteins such as synapsin, synaptophysin, post-synaptic density protein 95 (PSD95) and metabotropic glutamate receptors (mGluR), in the hippocampus and PFC (Li et al., 2010; Duman et al., 2016). These effects are mediated by several intracellular pathways that control neuronal plasticity, protection and survival, including protein kinase B (Akt), extracellular-signal regulated kinases (Erk1/2), glycogen synthase kinase 3β (GSK3β) and mammalian target of rapamycin (mTOR, Bockaert and Marin, 2015; Duman et al., 2016).
Evidence suggests that CBD can interfere with stress-induced synaptic remodeling. CBD normalized synaptophysin levels in rats submitted to a brain damage by iron overload (da Silva et al., 2014) and produced a neuritogenic effect in PC12 cells, increasing the expression of synaptophysin and synapsin I. These effects were inhibited by a TrkA antagonist (Santos et al., 2015). In addition, CBD can modulate intracellular pathways directly related to synaptic remodeling, such as Erk1/2 and Akt, in different types of cancer cell lines (McAllister et al., 2011; Solinas et al., 2013). Its precise effects in different brain regions, however, are still unclear. For example, repeated CBD administration (14 days) enhanced contextual conditioned fear responses and decreased phosphorylated forms of Erk1/2 levels in the PFC (ElBatsh et al., 2012). Since fear acquisition took place under treatment with CBD, learning/memory facilitation could also explain/contribute to this finding.
In chronically stressed mice, repeated CBD treatment also promoted dendritic remodeling and increased the expression of PSD95, Synapsin I/II, and p-GSK3β in the hippocampus of animals submitted to CUS (Fogaça, 2016).
CBD and antidepressant effects
The first experimental evidence indicating that CBD induces antidepressant-like effects is based on its ability to attenuate autonomic and behavioral responses induced by previously inescapable stress exposure in rats (Resstel et al., 2009). These CBD effects were blocked by pre-treatment with WAY100635, a 5HT1A antagonist. Following this study, our group investigated CBD effects in animals submitted to the forced swimming test (FST) (Zanelati et al., 2010) a widely used animal model predictive of antidepressant effects (Cryan et al., 2002). Systemic CBD treatment reduced immobility time in mice, as did the prototype tricyclic antidepressant imipramine (Zanelati et al., 2010). Similar results were shown in mice submitted to the FST or the tail suspension test (TST) (El-Alfya et al., 2010; Réus et al., 2011; Schiavon et al., 2016). More recently, the antidepressant-like effect of CBD was detected in the olfactory bulbectomy (Linge et al., 2016) and learned helplessness models (Pereira et al., 2016). CBD was also effective in rat strains that naturally express “depressive-like behaviors,” such as the Wistar-Kyoto (Shoval et al., 2016). Altogether, this data strengthen the possibility that CBD can induce antidepressant effects. However, the mechanisms involved in this effect have only recently started to be investigated. As discussed above, similar to the acute anxiolytic effects, the acute antidepressant effect of CBD seems to depend on facilitation of 5HT1A receptor-mediated neurotransmission (Zanelati et al., 2010).
Preclinical and clinical studies indicate that forebrain 5HT1A receptors are important targets of antidepressant drugs (Samuels et al., 2015; Kaufman et al., 2016). The mechanisms of this effect are still unclear, but these receptors interfere in neuroplastic events that have been associated with antidepressant action such as BDNF release and neurogenesis (Mahar et al., 2014; Serafini et al., 2014; Samuels et al., 2015; Zhang et al., 2016). However, acute or sub-chronic CBD treatment failed to change hippocampal BDNF levels (Zanelati et al., 2010; Campos et al., 2012a). Réus et al. (2011) reported similar results in three brain regions (PFC, hippocampus, and amygdala) after acute treatment with doses that induced antidepressant-like effects in rats submitted to the FST. Despite these negative results, BDNF involvement in the plastic changes induced by CBD cannot be ruled out. Methodological issues such as measurement of the whole BDNF content (not distinguishing stored and released pools), problems with the punching method, and the possibility that CBD increases BDNF release in specific subregions (Fanselow and Dong, 2010; O'Leary and Cryan, 2014; Suzuki et al., 2016) indicate that additional research on this topic is needed.
Another possibility to explain CBD-induced acute antidepressant effects would be the fast modulation of the neurochemical environment in limbic brain regions. In a recent work by Linge et al. (2016), a single CBD injection induced rapid antidepressant-like effect in the olfactory bulbectomy mouse model. This effect was associated with increased extracellular 5-HT and glutamate levels in the ventromedial prefrontal cortex (vmPFC). The 5HT1A-receptor antagonist WAY100635 prevented the behavioral and neurochemical effects of CBD. To explain these results, the authors suggested that CBD acute effects would be mediated by disinhibition of 5-HT and glutamatergic neurotransmission in the vmPFC through the modulation of 5HT1A receptor (Linge et al., 2016). In agreement with this proposal, bilateral microinjections of CBD into the vmPFC induced antidepressant-like effect in rats submitted to the FST. This effect was blocked by pre-treatment with WAY100635 or by the CB1 antagonist AM251 (Sartim et al., 2016). Since the antidepressant-like effect induced by the endogenous cannabinoid anandamide was also blocked by 5HT1A receptor antagonist, it was hypothesized that CBD effects on 5HT1A receptors would be due to indirect modulation of local 5-HT levels via CB1 activation. Therefore, this data support the hypothesis that acute CBD effects could involve rapid neurochemical changes, such as in the ECB and serotonergic systems, in the vmPFC. CBD effects in other brain regions remain to be examined.
Repeated administration of CBD can prevent the impairment in neurogenesis induced by CUS (Campos et al., 2013b). At a lower dose, CBD decreased depressive-like behaviors in non-stressed Swiss mice in the TST and increased the number of Ki67, BrdU, and doublecortin-positive cells, reflecting an enhanced SGZ neurogenesis (Schiavon et al., 2016). In this study, however, a higher CBD dose, despite still displaying an antidepressant-like effect, decreased the number of positive cell markers for the neurogenesis in the hippocampus (Schiavon et al., 2016). More studies, therefore, are needed to unveil an involvement of hippocampal neurogenesis in the antidepressive-like effects of CBD. Additionally, as discussed above, the participation of rapid plastic changes such as synaptic remodeling in acute CBD effects needs to be further investigated.
Epigenetics mechanisms could also be involved in the antidepressant effects of CBD. Psychiatric disorders are thought to originate from a complex interplay between genetic predisposition and environmental factors such as stress exposure. These factors may also modulate gene expression through interference with epigenetic mechanisms (Tsankova et al., 2007; Borrelli et al., 2008). They include covalent DNA modifications (e.g., DNA methylation), post-translational modifications of histone tails (e.g., methylation, acetylation, phosphorylation, and ubiquitination), as well as non-translational gene silencing mechanisms (e.g., micro-RNAs, ribonucleic acid; Krishnan and Nestler, 2008; Kim et al., 2009). Epigenetic changes have been related to the neurobiology of various neuropsychiatric disorders, including depression (Tsankova et al., 2007; Klengel et al., 2014), and antidepressant drugs can interfere with these changes. For example, antidepressants decrease the activity of DNA methyltransferases in vitro (Zimmermann et al., 2012) and alter gene transcription via epigenetic mechanisms in different brain structures associated with depression (Toffoli et al., 2014). Consistent with the idea that decreasing stress-induced DNA methylation could induce antidepressant effects, administration of DNMT inhibitors (Sales et al., 2011) as well as DNMT1 knockout (Morris et al., 2016) induced antidepressant-like effects in the FST.
A recent work by Pucci et al. (2013) demonstrated that CBD could also modulate epigenetic mechanisms by reducing global DNA methylation in human keratinocytes cells. Since stress appears to increase DNA methylation, this finding raised the possibility that the antidepressant-like effects of CBD involve epigenetic mechanism, such as DNA methylation.
CBD and antipsychotic effects
Preclinical and clinical studies indicate that CBD also has a potential therapeutic role in schizophrenia. CBD attenuates schizophrenia-related behavioral abnormalities (i.e., psychostimulant-induced hyperlocomotion, decreased sensorimotor gating, deficits in cognitive function, and decreased social interaction) in pharmacological, genetic, and neurodevelopmental animal models (Moreira and Guimarães, 2005; Gururajan et al., 2012; Long et al., 2012; Levin et al., 2014; Gomes et al., 2015a,b; Pedrazzi et al., 2015; Renard et al., 2016) with a profile similar to atypical antipsychotics (Zuardi et al., 1991, 1995; Guimarães et al., 2004). In humans, the antipsychotic properties of CBD were confirmed in a double-blind clinical trial where CBD reduced psychotic symptoms with a similar efficacy to the atypical antipsychotic amisulpride, but with significantly fewer side effects (Leweke et al., 2012). More recently, a placebo-controlled clinical trial with 88 schizophrenia patients who remained on their antipsychotic medication and were randomized to receive CBD or placebo as adjunct therapy also showed that it was consistently superior to placebo in alleviating psychotic symptoms (GW Pharmaceuticals, 2015). In this study, CBD also tended to improve negative and cognitive symptoms. Current antipsychotics show limited effectiveness in targeting these symptoms (Elvevag and Goldberg, 2000; Hanson et al., 2010).
The mechanisms by which CBD exert its antipsychotic effects are currently unknown. Leweke et al. showed that the alleviation of psychotic symptoms in patients treated with CBD was significantly associated with an increase in serum AEA levels (Leweke et al., 2012). In the same study, they confirmed that CBD inhibited FAAH activity in vitro (Leweke et al., 2012). Thus, by indirectly activating CB1 receptors via increased AEA levels, CBD could potentially modulate other neurotransmitters systems (Campos et al., 2012b). Additionally, a recent study using amphetamine sensitization model of schizophrenia suggest that its antipsychotic actions are dependent on the mTOR signaling pathway (Renard et al., 2016). However, other mechanisms such as anti-inflammatory and neuroprotective could also contribute to the beneficial effects of CBD in schizophrenia (Gomes et al., 2015b; Campos et al., 2016). The CBD antipsychotic effects may also involve parvalbumin-positive GABA neurons. These neurons are fast-spiking GABAergic interneurons, which synapse on the cell body or the axon initial segment of pyramidal neurons, promoting synchronization and temporal control of the information flow through the pyramidal neurons (Lewis et al., 2005). Parvalbumin-positive GABA neurons are selectively altered in schizophrenia patients (Lewis et al., 2005) and can account for abnormal circuit synchrony and cognitive deficits in this disorder (Gonzalez-Burgos and Lewis, 2012). Similar to patients, different rodent models of schizophrenia show deficits in the function of these cells (Penschuck et al., 2006; Gomes et al., 2015a; Canetta et al., 2016).
Recently, we observed that CBD attenuated the decreased number of parvalbumin-positive cells in the mPFC induced by repeated administration of the NMDA receptor antagonist MK-801 in mice (Gomes et al., 2015a). Parvalbumin loss induced by NMDA antagonists has been associated with increased oxidative stress (Behrens et al., 2007). The high-energy demands of parvalbumin interneurons make them particularly vulnerable to this process (Steullet et al., 2016). Impairment in antioxidant systems can lead to a diffusion of excessive reactive oxygen species outside the parvalbumin cell and induce glial activation (Morishita et al., 2015). However, glial changes could also be a consequence of high local levels of glutamate due to parvalbumin interneuron dysfunction, which leads to disinhibition of glutamate release from pyramidal neurons targeted by those interneurons (Nakazawa et al., 2012). Given that glial cells have a pivotal role in glutamate homeostasis (Bezzi et al., 1999; Shaked et al., 2005), changes in their activity may result from a compensatory mechanism in an attempt to normalize, for example, glutamate levels.
In our study, besides the changes in parvalbumin expression, the MK-801 treatment increased the expression of astrocytic and microglial cell markers in the mPFC (Gomes et al., 2015b), similar to what has been observed in post-mortem brain of schizophrenia patients (Radewicz et al., 2000; Catts et al., 2014). These effects were also attenuated by repeated CBD treatment (Gomes et al., 2015b) by mechanisms that are now under investigation. Suitable candidates are its antioxidant properties, activation of PPARγ or CB1 and/or CB2 receptors by the indirect increase in AEA levels (Campos et al., 2012b). These mechanisms can also be involved in the attenuation of increased glial reactivity by CBD in animal models related to other neuropathological conditions (Mecha et al., 2013; Perez et al., 2013; Schiavon et al., 2014).
CBD and neuroprotective mechanisms
Neuroprotection constitutes an important mechanism of neuropsychiatric drugs' action to preserve structure and function of neural cells, promoting a protection against oxidative stress, iron, excitotoxicity, protein aggregation, organelles damage, and inflammation (Kaur and Ling, 2008; Filipović et al., 2017). An imbalance in these processes are found in animal models of depression, anxiety, stroke and neurodegenerative diseases such as Alzheimer's, Huntington's, Parkinson's, and multiple sclerosis (Kaur and Ling, 2008; Filipović et al., 2017).
Antioxidants compounds act against oxidative stress, a condition characterized by an exacerbation of reactive oxygen/nitrogen species (ROS/RNS) production and, consequently, peroxidation of polyunsaturated fatty acids, DNA oxidation, and nitration/carbonylation of proteins, leading to cell damage or death (Pisoschi and Pop, 2015). One of the first studies relating CBD to neuroprotection showed that it acts as an antioxidant, preventing NMDA- and kainate receptor-mediated neurotoxicity and hydroperoxide-induced oxidative damage in rat cortical neuron culture, through a mechanism independent of cannabinoid receptors (Hampson et al., 1998). In these assays, CBD demonstrated a superior neuroprotective activity than other known antioxidants such as alpha-tocopherol and ascorbate (Hampson et al., 1998). Other studies have shown that neuroprotective effects of CBD are associated with its antioxidant properties (Table 2). CBD decreases the neuronal damage promoted by β-amyloid protein deposit (Iuvone et al., 2004; Esposito et al., 2006, 2011; Sagredo et al., 2007; Harvey et al., 2012; Janefjord et al., 2014; Scuderi et al., 2014) and attenuates the depletion of tyrosine hydroxylase, dopamine, and GABA levels by modulating the expression of the inducible isoform of nitric oxide synthase and reducing the production of ROS-generating NADPH oxidases (Esposito et al., 2006, 2007; Garcia-Arencibia et al., 2007; Sagredo et al., 2007, 2011; Pan et al., 2009). These effects seem to occur, in part, by activation of PPARγ receptors (Esposito et al., 2011; Scuderi et al., 2014) and ubiquitination of amyloid precursor protein (Scuderi et al., 2014). CBD protective responses were also accompanied by an increase in cell survival through inhibition of ROS/RNS production, a decrease in malondialdehyde and caspase-3 levels, inhibition of DNA fragmentation (Iuvone et al., 2004), and reduces de activity of NF-κB (Kozela et al., 2010). In addition, CBD exerts antioxidant activities against toxicity and/or oxidative stress produced by H2O2, tertbutyl hydroperoxide, and amphetamine (Valvassori et al., 2011; Harvey et al., 2012; Mecha et al., 2012). Moreover, CBD pre-treatment attenuates high-glucose-induced mitochondrial superoxide generation and NF-κB activation, along with the expression of the adhesion molecules ICAM-1 and VCAM-1 (Rajesh et al., 2007).
Table 2.
CBD and neuroprotective mechanisms.
| Main effect of CBD | Model | CBD Dose/concentration range | Route of administration | Species/Strain | Possible mechanism of action | References |
|---|---|---|---|---|---|---|
| Prevents NMDA receptor-induced excitoxicity | E17 cortical neurons culture | EC50 = 3.7 μM | In vitro | Wistar rat | Effect independent of cannabinoid receptors. | Hampson et al., 1998 |
| ↓Phosphorylated form of p38/MAP kinase, ↓Caspase 3 levels, and NFκ-b activation | β amyloid-induced neurotoxicity in PC12 cells | 10 μM | In vitro | PC12 cells | Antioxidant | Esposito et al., 2006 |
| Prevented gliosis, neuronal death and ↑ hippocampal neurogenesis | Genetic model of Alzheimer's Disease | 10 mg/kg | 15 days | C57BL6 mice | PPARγ | Esposito et al., 2011 |
| ↓Aβ cell viability and ↓LPS (conditioned media) induced microglia activation | β amyloid -induced neuronal toxicity in neuroblastoma cells. LPS-induced microglial-activation | 10 μM | In vitro | Neuroblastoma (SH-SY5Y) cells/Microglial (BV-2) cells | Not determinated | Janefjord et al., 2014 |
| Improved cell viability | Amyloid β -induced toxicity and tert-butyl hydroperoxide-induced oxidative stress | 0.01–10 μM | 15 min pre-incubation before Aβ or sAβ addition/ 24-h incubation for oxidative stress analysis | PC12 and Neuroblastoma (SH-SYS5) cells | Not determinated | Harvey et al., 2012 |
| ↓ Amyloid- β production | Amyloid β -induced neurotoxicity | 100 nM | 24 h | SHSY5Y (APP+) neurons | PPARγ | Scuderi et al., 2014 |
| Reversed 3-nitropropionic acid—induced ↓ GABA contents, ↓ substance P, ↓ neuronal-specific enolase and superoxide dismutase(SOD)-2 | (10 mg/kg) 3-nitropropionic acid-induced) striatal lesions | 5 mg/kg | 5 days, i.p. | Sprague-Dawley rats | Independent of CB1, TRPV1 and A2A receptors | Sagredo et al., 2007 |
| ↓ Levels of IL-1beta, GFAP and iNOS | Amyloid β -induced neurotoxicity | 10 mg/kg | i.p. | C57BL6 mice | Not determinated | Esposito et al., 2007 |
| Reduced dopamine depletion and ↑mRNA levels of SOD in the substantia nigra | 6-hydroxydopamine toxicity | 3 mg/kg | 14 days, i.p. | Sprague-Dawley rats | Antioxidant | Garcia-Arencibia et al., 2007 |
| ↓Cell death | H2O2-inducedoxidative stress in Oligodendrocyte progenitor cells | 1 μM | In vitro | Oligodendrocyte progenitor cells | Not determinated | Mecha et al., 2012 |
| ↓of carbonyl groups and prevents the decrease in BDNF expression | Amphetamine-induced oxidative stress | 60 mg/kg | 2 weeks, i.p. | Wistar rats | Not determinated | Valvassori et al., 2011 |
| ↓ NFκ-B, ↓ ICAM-1 and VACAM-1 | High glucose-induced mithocondrial superoxide generation | 4 μM | In vitro | Human coronary artery endothelial cells | Independent from CB1 and CB2 receptors | Rajesh et al., 2007 |
| Prevented Aβ-induced cognitive deficits, ↓ microglia activation, ↓ IL-6 mRNA expression Inhibited NO generation and ATP-induced intracellular Ca2+ levels | Rat primary cortical cultures, N13 and BV-2 microglial cells Morris water maze | 10–1,000 nM | In vitro 3 weeks: first week treated daily; second and third weeks treated 3 times/week, i.p. | Rat primary cortical cultures, N13 and BV-2 microglial cells C57BL6 mice | Some of the in vitro effects were mediated by A2A, CB1, and CB2 receptors | Martín-Moreno et al., 2011 |
| Blocked LPS-induced STAT1 activation | LPS-induced BV-2 activation | 10 μM | In vitro | BV-2 microglial cells | Not determinated | Kozela et al., 2010 |
| ↓Apoptosis; ↓Excitotoxicty and neuroinflamation | Newborn hypoxic-ischemic brain damage | 0.1–1,000 μM | Ex vivo | Brain slices from C57BL6 mice | CB2 and A2A receptors | Castillo et al., 2010 |
| Protects against the reduction in tyrosine hydroxylase activity | 6-hydroxydopamine-induced toxicity in the striatum and substantia nigra | 3 mg/kg | 14 days, i.p. | Sprague-Dawley rats | Not determinated | Lastres-Becker et al., 2005 |
| ↑ Viable neurons and ↓ excitoxicity, oxidative stress, and inflammation | Newborn hypoxic-ischemic brain damage (HI) | 1 mg/kg | 30 min after HI, i.p. | Newborn pigs | CB2 and 5HT1A receptors | Pazos et al., 2013 |
| Improve of cognition and motor activity. Restores BDNF levels | Encephalopathy (bile duct ligation) | 5 mg/kg | 28 days, i.p. | C57BL6 mice | 5HT1A | Magen et al., 2010 |
| Improvments od liver function, normalizes 5-HT levels, and improves brain pathology | Encephalopathy (thioacetamide) | 5 mg/kg | Single dose | C57BL6 mice | 5HT-dependent mechanism | Avraham et al., 2011 |
| Faciltates autophagic flux and decrease oxidative stress | Pilocarpine-Induced Seizure | 100 ng | Intracerebroventricular | Wistar rats | Induction of autophagy pathway | Hosseinzadeh et al., 2016 |
| Suppresses the transcription proinflammatory genes | MOG35-55-specific T cell in the presence of spleen-derived antigen presenting cells | 5 μM | In vitro | MOG35-55- and APCs isolated from spleens of C57BL6 | Not determinated | Kozela et al., 2016 |
| Attenuates TNF-α production and ↓ adenosine transport | murine microglia and RAW264.7 macrophages LPS-treated mice | 500 nM or 1 mg/kg | In vitro | Murine microglia | A2A adenosine receptor | Carrier et al., 2006 |
| In vivo (1 h before LPS injection, i.p.) | RAW264.7 macrophages C57BL6 mice | |||||
| Improves motor deficits in the chronic phase; ↓ microglial activation and Il-beta and TNF-α production | Viral model of multiple sclerosis | 5 mg/kg | 7 days, i.p | SJL/J mice | A2A adenosine receptor | Carrier et al., 2006 |
| Normalizes synaptophyisin and caspase 3 expression | Brain damage induced by iron overload during neonatal period | Not informed | 14 day, i.p. | Wistar rats | Not determinated | da Silva et al., 2014 |
| Prevented MPP-induced toxicity and induces neurite growth | MPP-induced toxicity in PC12 cells and SH-SY5Y | 1 μM | In vitro | PC12 and SH-SY5Y cells | TRKA | Santos et al., 2015 |
| Prevents cognitive and anxiogenic effects, ↓ TNF-α and IL-6 ↑ BDNF levels | Murine model of cerebral Malaria | 30 mg/kg | 10 days, i.p. | C57BL6 mice | Not determinated | Campos et al., 2015 |
i.p., intra peritoneal; ↓, decreases; ↑, increases.
In models based on lipopolysaccharide (LPS) media activation, CBD increased microglial viability and migration as well as inhibited nitric oxide generation and STAT1 activation induced by LPS (Kozela et al., 2010; Martín-Moreno et al., 2011; Janefjord et al., 2014). Some of these effects were mediated by adenosine A2A, CB1, and/or CB2 receptors. In addition, CBD demonstrated neuroprotection in forebrain slices from newborn mice that underwent hypoxic-ischemic brain damage, reducing glutamate, IL-6 levels, and the expression of TNFalpha, COX-2, and iNOS via activation of CB2 and adenosine A2A receptors (Castillo et al., 2010). In accordance with these in vitro results, CBD was also neuroprotective in vivo after acute or chronic treatment in animal models of neurodegenerative diseases (Lastres-Becker et al., 2005; Alzheimer's and Parkinson's, Esposito et al., 2007, 2011; Garcia-Arencibia et al., 2007; Sagredo et al., 2007), ischemia (Pazos et al., 2013) and encephalopathy (Magen et al., 2010; Avraham et al., 2011). In a model of β-amyloid hippocampal inoculation, CBD decreased IL-1beta, GFAP, and iNOS levels (Esposito et al., 2007). In models of Parkinson's disease, CBD reversed the reduction of tyrosine hydroxylase activity and the dopamine depletion in the substantia nigra and striatum after 6-hydroxydopamine microinjection (Lastres-Becker et al., 2005; Garcia-Arencibia et al., 2007) and upregulated superoxide dismutase (SOD) mRNA levels in the substantia nigra (Garcia-Arencibia et al., 2007). In this same direction, CBD also reversed 3-nitropropionic acid-induced reductions in GABA contents and mRNA levels for substance P, neuronal specific enolase and superoxide dismutase 2, effects that were independent of CB1, TRPV1, and A2A receptors (Sagredo et al., 2007).
Moreover, in hypoxic-ischemic animals, CBD prevented the decrease in the number of viable neurons and the increase in excitotoxicity, oxidative stress, and inflammation through mechanisms involving CB2 and 5HT1A receptors (Pazos et al., 2013). In the middle cerebral artery occlusion, a method used to evaluate ischemia-reperfusion injury, CBD suppressed the decrease in cerebral blood flow after reperfusion, inhibited myeloperoxidase (MPO) activity in neutrophils, and reduced the number of MPO immunopositive cells. In addition, in animal models of encephalopathy, CBD improved cognition, motor activity, and restored 5-HT and BDNF levels via 5HT1A receptor activation (Magen et al., 2010; Avraham et al., 2011).
Besides these proposed mechanisms, neuroprotection can also be promoted by an enhancement in the autophagic function. Autophagy, more specifically macroautophagy, is a lysosomal degradation pathway essential to recycle damaged organelles and promote cell survival, protecting the cell malfunction or death under stress conditions (Jia and Le, 2015). In fact, some SSRIs and mood stabilizers such as lithium increase autophagy in the brain (Heiseke et al., 2009; Gassen et al., 2014; Jia and Le, 2015). Although there are few studies relating CBD to autophagy in neuropsychiatric disorders, CBD can modulate this process (Koay et al., 2014; Yang et al., 2014; Hosseinzadeh et al., 2016). Specifically in the brain, CBD produced anticonvulsant effects concomitant to an activation of hippocampal autophagy pathway in the chronic phase of pilocarpine-induced seizure (Hosseinzadeh et al., 2016). In a genetic model of tauopathy, used to evaluate frontotemporal dementia, parkinsonism, and motor neuron disease, 1-month daily injections of Sativex®, a 1:1 mixture combination of CBD and THC, increased the ratio of reduced/oxidized glutathione and promoted autophagy in the brain (Casarejos et al., 2013). In this same study, Sativex® attenuated the abnormal behaviors and reduced free radicals produced during the metabolism of dopamine, iNOS levels and deposition of tau in the hippocampus and cortex (Casarejos et al., 2013). In this context, recent findings from our group suggest that chronic CBD treatment increase autophagy in animals submitted to CUS, as revealed by its impact in phosphorylated form of mTOR, Beclin-1 and LC3, signaling proteins involved in autophagy induction (Fogaça, 2016).
The CBD neuroprotective effects could also involve neuroinflammatory mechanisms. CBD administration counteracts the deleterious consequences of neuroinflammation reducing adaptive immune cell Th2 phenotype and microglial/macrophage innate immunity (Kozela et al., 2016).
CBD inhibits adenosine transporter activity in a microglial cell line, thus resulting in increased A2A receptor signaling, and attenuation of TNF-α cytokine production (Carrier et al., 2006). Likewise, adenosine 2A receptor signaling is involved in the protective effects of CBD in the Theiler virus model of multiple sclerosis preventing both leukocyte and microglial-mediated actions (Mecha et al., 2013). On the other hand, CBD protects oligodendrocyte progenitor cells in CB1, CB2, PPARγ, and TRPV1 independent manner (Mecha et al., 2012).
Conclusions
In addition to CBD-only actions, considerable interest is also derived from its ability to modulate the psychoactive effects of THC. CBD tempers the detrimental (cognitive impairment, psychosis) consequences of THC (Curran et al., 2016), while preserving its beneficial actions. For example, administration of a THC: CBD mixture 1:1 (the similar composition to Sativex) in the APPxPS1 exhibits a better therapeutic profile than each cannabis component alone (Aso et al., 2015). Similarly THC:CBD combination is neuroprotective actions in toxin-induced striatal neurodegeneration (Valdeolivas et al., 2012). In a recent double-blind crossover pilot clinical trial, Sativex administration in Huntington's disease patients was shown to be safe and well tolerated, while no significant effects or biomarker changes could be evidenced (López-Sendón et al., 2016). Similarly to CBD, other non-psychotomimetic phytocannabinoids exert significant neuroplasticity adaptations of therapeutic interest by this mechanism. Cannabigerol (CBG) a non-CB1/CB2 receptor acting cannabinoid shares with CBD its ability to activate PPARγ and has been evaluated in preclinical models of neurodegeneration (Valdeolivas et al., 2015). New molecules derived from the CBG structure have been tested in multiple sclerosis and Huntington's disease models (toxin- and mutant huntingtin-induced striatal neurodegeneration, Granja et al., 2012; Díaz-Alonso et al., 2016). Characterization of the neuroprotective actions of VCE3.2 compound indicates that it acts as a PPARγ modulator without the secondary actions of full PPARγ agonists, thus highlighting the translational implications offered by non-psychotomimetic cannabinoids.
The pre-clinical and clinical studies available so far indicate that CBD has a good safety record (Bergamaschi et al., 2011b). CBD or other non-psychotomimetic phytocannabinoids effects in brain development, however, have not yet been extensively investigated. This lack of studies is a significant point given that endogenous cannabinoid system regulates important steps of the nervous system development (Diaz-Alonso et al., 2012). The deleterious consequences in brain development and psychiatric implications in the adulthood induced by psychoactive THC exposure have been widely studied (Tortoriello et al., 2014; Alpár et al., 2015; de Salas-Quiroga et al., 2015). Recent evidence indicates that while CBD in the adult brain is safe and lacks undesired side effects, in the differentiating neurons it may increase their sensitivity to future oxidative insults (Schönhofen et al., 2015). Clearly, research on CBD safety during brain development is essential.
Even with this unanswered question, however, CBD ability to reduce inflammation-associated neurodegeneration and its antioxidant properties, lack of psychoactivity and a broad range of potentially beneficial effects indicates that this drug could be a useful new approach to treat several neuropsychiatric disorders. Actually, new CBD-derived molecules aimed at improving the efficacy and/or potency of natural phytocannabinoids have been recently developed (Haj et al., 2015; Breuer et al., 2016).
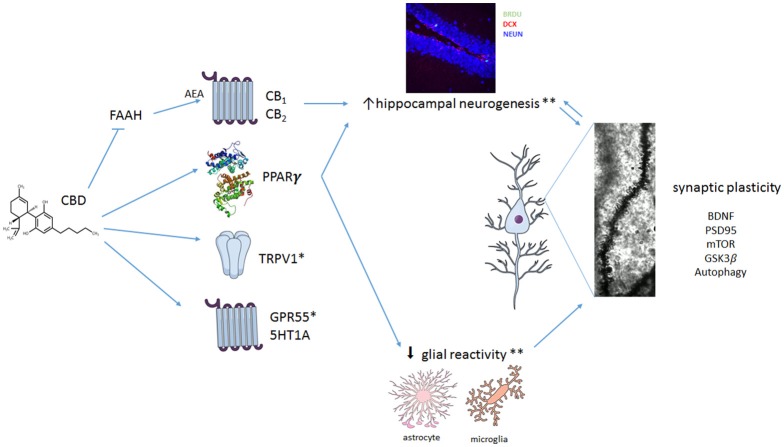
As discussed above, different mechanisms appear to be involved in CBD effects (Figure 1). The evidence available so far indicates that, in addition to its better described acute mechanisms (such as interaction with 5HT1A-mediated neurotransmission, TRPV1 receptors, and inhibition of anandamide metabolism), plastic changes take place over time and contribute to CBD-induced behavioral effects in response to chronic treatment. Even if the number of studies investigating these chronic effects is still sparse, it is clear that no single mechanism will explain the remarkable pharmacological profile of CBD. In this way, it joins a club of multi-target drugs that includes, for example, clozapine. These drugs challenge our familiar concept that acting in a single pharmacological target is always desirable (Imming et al., 2006) and agree with the observation made by Mencher and Wang (2005) that, sometimes, promiscuity could be a virtue.
Figure 1.
Multiple mechanisms proposed to explain CBD effect in neuropsychiatric disorders. CBD seems to interact with numerous different targets. It can act as a positive modulator of 5HT1A-mediated neurotransmission or as an agonist at TRPV1 and PPARγ receptors. In addition, CBD can facilitate anandamide (AEA)-mediated neurotransmission (by inhibiting FAAH) and induce antioxidant actions. CBD also promotes a complex set of changes in crucial intra-cellular pathways such as mTOR, autophagy and GSK3β, resulting in neuroprotection, decreased proinflammatory responses and facilitation of neuroplastic events. Taken together, these mechanisms would lead to an overall beneficial effect of CBD in neuropsychiatric disorders. *Little is known about the contribution of TRPV1 and 5HT1A for the effects of CBD on glial reactivity and on adult hippocampal neurogenesis. **CBD effects in reducing glial cell reactictivity and preventing stress-induced or amyloid-β-induced decreased adult hippocampal neurogenesis seem to depend on activation of CB1/CB2 (indirectly) and PPARγ. Because CB1 and CB2 are expressed in both neural precursor cells (NPCs) and glial cells, CBD effects on adult hippocampal neurogenesis could be a result of its actions on NPCs and/or attenuation of glial reactivity. BDNF, Brain derived neurotrophic factor; PSD95, postsynaptic density protein 95; mTOR, mechanistic target of rapamycin; GSK3β, Glycogen synthase kinase 3-beta.
Author contributions
AC, MF, FS, SJ, AJS, FVG, ABS, NR, and IG participated in the writing process of the first draft of the manuscript. AC design the Figure 1. FSG, AC, and IG revised the final version of the manuscript.
Conflict of interest statement
FG is co-inventor of the patent “Fluorinated CBD compounds, compositions and uses thereof.” Pub. No.: WO/2014/108899. International Application No.: PCT/IL2014/050023. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank CNPq, Capes, and Fapesp for their financial support. Research in IG lab is supported by Instituto de Salud Carlos III (PI15-00310) and Ministerio de Economía y Competitividad (RTC-2015-3364-1) and Regional Madrid Government S2010/BMD-2336 cofinanced by Fondo Europeo de Desarrollo Regional (FEDER) “Una manera de hacer Europa.” Illustrations were designed in Mind the Graph tool (www.mindthegraph.com).
References
- Airan R. D., Meltzer L. A., Roy M., Gong Y., Chen H., Deisseroth K. (2007). High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 317, 819–823. 10.1126/science.1144400 [DOI] [PubMed] [Google Scholar]
- Alpár A., Di Marzo V., Harkany T. (2015). At the tip of an iceberg: prenatal marijuana and its possible relation to neuropsychiatric outcome in the offspring. Biol. Psychiatry 79, 33–45. 10.1016/j.biopsych.2015.09.009 [DOI] [PubMed] [Google Scholar]
- Aso E., Sánchez-Pla A., Vegas-Lozano E., Maldonado R., Ferrer I. (2015). Cannabis-based medicine reduces multiple pathological processes in AβPP/PS1 mice. J. Alzheimer's Dis. 43, 977–991. 10.3233/JAD-141014 [DOI] [PubMed] [Google Scholar]
- Avraham Y., Grigoriadis N., Poutahidis T., Vorobiev L., Magen I., Ilan Y., et al. (2011). Cannabidiol improves brain and liver function in a fulminant hepatic failure-induced model of hepatic encephalopathy in mice. Br. J. Pharmacol. 162, 1650–1658. 10.1111/j.1476-5381.2010.01179.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens M. M., Ali S. S., Dao D. N., Lucero J., Shekhtman G., Quick K. L., et al. (2007). Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 318, 1645–1647. 10.1126/science.1148045 [DOI] [PubMed] [Google Scholar]
- Bergamaschi M. M., Queiroz R. H., Chagas M. H., de Oliveira D. C., De Martinis B. S., Kapczinski F., et al. (2011a). Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology 36, 1219–1226. 10.1038/npp.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi M. M., Queiroz R. H., Zuardi A. W., Crippa J. A. (2011b). Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr. Drug Saf. 6, 237–249. 10.2174/157488611798280924 [DOI] [PubMed] [Google Scholar]
- Bessa J. M., Ferreira D., Melo I., Marques F., Cerqueira J. J., Palha J. A., et al. (2009). The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol. Psychiatry 14, 764–773, 739. 10.1038/mp.2008.119 [DOI] [PubMed] [Google Scholar]
- Bezzi P., Vesce S., Panzarasa P., Volterra A. (1999). Astrocytes as active participants of glutamatergic function and regulators of its homeostasis. Adv. Exp. Med. Biol. 468, 69–80. 10.1007/978-1-4615-4685-6_6 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S., Morrison P. D., Fusar-Poli P., Martin-Santos R., Borgwardt S., Winton-Brown T., et al. (2010). Opposite effects of Δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 35, 764–774. 10.1038/npp.2009.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T., Hanus L., De Petrocellis L., Tchilibon S., Ponde D. E., Brandi I., et al. (2001). Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 134, 845–852. 10.1038/sj.bjp.0704327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J., Marin P. (2015). mTOR in brain physiology and pathologies. Physiol. Ver. 95, 1157–1187. 10.1152/physrev.00038.2014 [DOI] [PubMed] [Google Scholar]
- Borgwardt S. J., Allen P., Bhattacharyya S., Fusar-Poli P., Crippa J. A., Seal M. L., et al. (2008). Neural basis of Δ-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol. Psychiatry 64, 966–973. 10.1016/j.biopsych.2008.05.011 [DOI] [PubMed] [Google Scholar]
- Borrelli E., Nestler E. J., Allis C. D., Sassone-Corsi P. (2008). Decoding the epigenetic language of neuronal plasticity. Neuron 60, 961–974. 10.1016/j.neuron.2008.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer A., Haj C. G., Fogaça M. V., Gomes F. V., Silva N. R., Pedrazzi J. F., et al. (2016). Fluorinated cannabidiol derivatives: enhancement of activity in mice models predictive of anxiolytic, antidepressant and antipsychotic effects. PLoS ONE 11:e0158779. 10.1371/journal.pone.0158779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos A. C., Brant F., Miranda A. S., Machado F. S., Teixeira A. L. (2015). Cannabidiol increases survival and promotes rescue of cognitive function in a murine model of cerebral malaria. Neuroscience 19, 166–180. 10.1016/j.neuroscience.2014.12.051 [DOI] [PubMed] [Google Scholar]
- Campos A. C., de Paula Soares V., Carvalho M. C., Ferreira F. R., Vicente M. A., Brandao M. L., et al. (2013a). Involvement of serotonin-mediated neurotransmission in the dorsal periaqueductal gray matter on cannabidiol chronic effects in panic-like responses in rats. Psychopharmacology (Berl) 226, 13–24. 10.1007/s00213-012-2878-7 [DOI] [PubMed] [Google Scholar]
- Campos A. C., Ferreira F. R., Guimarães F. S. (2012a). Cannabidiol blocks long-lasting behavioral consequences of predator threat stress: possible involvement of 5HT1A receptors. J. Psychiatr. Res. 46, 1501–1510. 10.1016/j.jpsychires.2012.08.012 [DOI] [PubMed] [Google Scholar]
- Campos A. C., Fogaca M. V., Sonego A. B., Guimarães F. S. (2016). Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 112, 119–127. 10.1016/j.phrs.2016.01.033 [DOI] [PubMed] [Google Scholar]
- Campos A. C., Guimarães F. S. (2008). Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl) 199, 223–230. 10.1007/s00213-008-1168-x [DOI] [PubMed] [Google Scholar]
- Campos A. C., Guimarães F. S. (2009). Evidence for a potential role for TRPV1 receptors in the dorsolateral periaqueductal gray in the attenuation of the anxiolytic effects of cannabinoids. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 1517–1521. 10.1016/j.pnpbp.2009.08.017 [DOI] [PubMed] [Google Scholar]
- Campos A. C., Moreira F. A., Gomes F. V., Del Bel E. A., Guimarães F. S. (2012b). Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 367, 3364–3378. 10.1098/rstb.2011.0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos A. C., Ortega Z., Palazuelos J., Fogaça M. V., Aguiar D. C., Díaz-Alonso J., et al. (2013b). The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid system. Int. J. Neuropsychopharmacol. 16, 1407–1419. 10.1017/S1461145712001502 [DOI] [PubMed] [Google Scholar]
- Canetta S., Bolkan S., Padilla-Coreano N., Song L. J., Sahn R., Harrison N. L., et al. (2016). Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Mol. Psychiatry 21, 956–968. 10.1038/mp.2015.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier E. J., Auchampach J. A., Hillard C. J. (2006). Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. U.S.A. 103, 7895–7900. 10.1073/pnas.0511232103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarejos M. J., Perucho J., Gomez A., Mu-oz M. P., Fernandez-Estevez M., Sagredo O., et al. (2013). Natural cannabinoids improve dopamine neurotransmission and tau and amyloid pathology in a mouse model of tauopathy. J. Alzheimers Dis. 35, 525–539. 10.3233/JAD-130050 [DOI] [PubMed] [Google Scholar]
- Casarotto P. C., Gomes F. V., Resstel L. B. M., Guimarães F. S. (2010). Cannabidiol inhibitory effect on marble-burying behaviour: involvement of CB1 receptors. Behav. Pharmacol. 21, 353–358. 10.1097/FBP.0b013e32833b33c5 [DOI] [PubMed] [Google Scholar]
- Castillo A., Tolón M. R., Fernández-Ruiz J., Romero J., Martinez-Orgado J. (2010). The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol. Dis. 37, 434–440. 10.1016/j.nbd.2009.10.023 [DOI] [PubMed] [Google Scholar]
- Castren E., Voikar V., Rantamaki T. (2007). Role of neurotrophic factors in depression. Curr. Opin. Pharmacol. 7, 18–21. 10.1016/j.coph.2006.08.009 [DOI] [PubMed] [Google Scholar]
- Catts V. S., Wong J., Fillman S. G., Fung S. J., Shannon Weickert C. (2014). Increased expression of astrocyte markers in schizophrenia: association with neuroinflammation. Aust. N.Z. J. Psychiatry 48, 722–734. 10.1177/0004867414531078 [DOI] [PubMed] [Google Scholar]
- Chaker Z., Codega P., Doetsch F. (2016). A mosaic world: puzzles revealed by adult neural stem cell heterogeneity. Wiley Interdiscip. Rev. Dev. Biol. 5, 640–658. 10.1002/wdev.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B., Giagnoni G., Franke C., Trovato A. E., Colleoni M. (2004). Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br. J. Pharmacol. 143, 247–250. 10.1038/sj.bjp.0705920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa J. A., Zuardi A. W., Garrido G. E., Wichert-Ana L., Guarnieri R., Ferrari L., et al. (2004). Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology 29, 417–426. 10.1038/sj.npp.1300340 [DOI] [PubMed] [Google Scholar]
- Cryan J. F., Markou A., Lucki I. (2002). Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol. Sci. 23, 238–245. 10.1016/S0165-6147(02)02017-5 [DOI] [PubMed] [Google Scholar]
- Curran H. V., Freeman T. P., Mokrysz C., Lewis D. A., Morgan C. J., Parsons L. H. (2016). Keep off the grass? Cannabis, cognition and addiction. Nat. Rev. Neurosci. 17, 293–306. 10.1038/nrn.2016.28 [DOI] [PubMed] [Google Scholar]
- da Silva V. K., de Freitas B. S., da Silva Dornelles A., Nery L. R., Falavigna L., Ferreira R. D., et al. (2014). Cannabidiol normalizes caspase 3, synaptophysin, and mitochondrial fission protein DNM1L expression levels in rats with brain iron overload: implications for neuroprotection. Mol. Neurobiol. 49, 222–233. 10.1007/s12035-013-8514-7 [DOI] [PubMed] [Google Scholar]
- David D. J., Samuels B. A., Rainer Q., Wang J.-W., Marsteller D., Mendez I., et al. (2009). Behavioral effects of fluoxetine in an animal model of anxiety/depression are mediated by both neurogenesis-dependent and independent mechanisms. Neuron 62, 479–493. 10.1016/j.neuron.2009.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawirs R. R., Hildebrandt K., Teuchert-Noodt G. (1998). Adult treatment with haloperidol increases dentate granule cell proliferation in the gerbil hippocampus. J. Neural. Transm. (Vienna) 105, 317–327. 10.1007/s007020050061 [DOI] [PubMed] [Google Scholar]
- Demirakca T., Sartorius A., Ende G., Meyer N., Welzel H., Skopp G., et al. (2011). Diminished gray matter in the hippocampus of cannabis users: possible protective effects of cannabidiol. Drug Alcohol Depend. 114, 242–245. 10.1016/j.drugalcdep.2010.09.020 [DOI] [PubMed] [Google Scholar]
- Deng W., Aimone J. B., Gage F. H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Ver. Neurosci. 11, 339–350. 10.1038/nrn2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L., Ligresti A., Moriello A. S., Allarà M., Bisogno T., Petrosino S., et al. (2011). Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 163, 1479–1494. 10.1111/j.1476-5381.2010.01166.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Salas-Quiroga A., Díaz-Alonso J., García-Rincón D., Remmers F., Veja D., Gómez-Cañas M., et al. (2015). Prenatal exposure to cannabinoids evokes long-lasting functional alterations by targeting CB 1 receptors on developing cortical neurons. Proc. Natl. Acad. Sci. U.S.A. 112, 13693–13698. 10.1073/pnas.1514962112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O., Cilio M. R., Cross H., Fernandez-Ruiz J., French J., Hill C., et al. (2014). Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 55, 791–802. 10.1111/epi.12631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhikav V., Anand K. S. (2007). Is hippocampal atrophy a future drug target? Med. Hypotheses 68, 1300–1306. 10.1016/j.mehy.2006.09.040 [DOI] [PubMed] [Google Scholar]
- Diaz-Alonso J., Guzman M., Galve-Roperh I., Díaz-Alonso J., Guzmán M., Galve-Roperh I. (2012). Endocannabinoids via CB1 receptors act as neurogenic niche cues during cortical development. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 367, 3229–3241. 10.1098/rstb.2011.0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Alonso J., Paraíso-Luna J., Navarrete C., del Río C., Cantarero I., Palomares B., et al. (2016). VCE-003.2, a novel cannabigerol derivative, enhances neuronal progenitor cell survival and alleviates symptomatology in murine models of Huntington's disease. Sci. Rep. 6:29789. 10.1038/srep29789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R. S., Aghajanian G. K., Sanacora G., Krystal J. H. (2016). Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 22, 238–249. 10.1038/nm.4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood S. L., Heffernan J., Harrison P. J. (1997). Chronic haloperidol treatment differentially affects the expression of synaptic and neuronal plasticity-associated genes. Mol. Psychiatry 2, 322–329. 10.1038/sj.mp.4000238 [DOI] [PubMed] [Google Scholar]
- El-Alfya A. T., Iveya K., Robinsona K., Ahmedb S., Radwanb M., Sladeb D., et al. (2010). Antidepressant-like effect of Δ9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol. Biochem. Behav. 95, 434–442. 10.1016/j.pbb.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElBatsh M. M., Assareh N., Marsden C. A., Kendall D. A. (2012). Anxiogenic-like effects of chronic cannabidiol administration in rats. Psychopharmacology (Berl) 221, 239–247. 10.1007/s00213-011-2566-z [DOI] [PubMed] [Google Scholar]
- Elmes M. W., Kaczocha M., Berger W. T., Leung K., Ralph B. P., Wang L., et al. (2015). Fatty acid-binding proteins (FABPs) are intracellular carriers for Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J. Biol. Chem. 290, 8711–8721. 10.1074/jbc.M114.618447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvevag B., Goldberg T. E. (2000). Cognitive impairment in schizophrenia is the core of the disorder. Crit. Rev. Neurobiol. 14, 1–21. 10.1615/CritRevNeurobiol.v14.i1.10 [DOI] [PubMed] [Google Scholar]
- Espejo-Porras F., Fernandez-Ruiz J., Pertwee R. G., Mechoulam R., Garcia C. (2013). Motor effects of the non-psychotropic phytocannabinoid cannabidiol that are mediated by 5-HT1A receptors. Neuropharmacology 75, 155–163. 10.1016/j.neuropharm.2013.07.024 [DOI] [PubMed] [Google Scholar]
- Esposito G., De Filippis D., Maiuri M. C., De Stefano D., Carnuccio R., Iuvone T. (2006). Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in beta-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-kappaB involvement. Neurosci. Lett. 399, 91–95. 10.1016/j.neulet.2006.01.047 [DOI] [PubMed] [Google Scholar]
- Esposito G., Scuderi C., Savani L., Steardo D., Jr., De Filippis P., Cottone T., et al. (2007). Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br. J. Pharmacol. 151, 1272–1279. 10.1038/sj.bjp.0707337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G., Scuderi C., Valenza M., Togna G. I., Latina V., de Filippis D., et al. (2011). Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS ONE 6:e28668 10.1371/journal.pone.0028668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow M. S., Dong H. W. (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19. 10.1016/j.neuron.2009.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq R. K., Isingrini E., Tanti A., Le Guisquet A. M., Arlicot N., Minier F., et al. (2012). Is unpredictable chronic mild stress (UCMS) a reliable model to study depression-induced neuroinflammation? Behav. Brain. Res. 231, 130–137. 10.1016/j.bbr.2012.03.020 [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J., Sagredo O., Pazos M. R., García C., Pertwee R. G., Mechoulam R., et al. (2013). Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 75, 323–333. 10.1111/j.1365-2125.2012.04341.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipović D., Todorović N., Bernardi R. E., Gass P. (2017). Oxidative and nitrosative stress pathways in the brain of socially isolated adult male rats demonstrating depressive- and anxiety-like symptoms. Brain Struct. Funct. 222, 1–20. 10.1007/s00429-016-1218-9 [DOI] [PubMed] [Google Scholar]
- Fogaça M. V. (2016). Mechanisms Involved in the Behavioral and Pro-neurogenic Effects of Repeated Administration of Cannabidiol in Mice Submitted to the Chronic Unpredictable Stress. Ph.D. Thesis, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo. [Google Scholar]
- Fogaça M. V., Galve-Roperh I., Guimarães F. S., Campos A. C. (2013). Cannabinoids, Neurogenesis and Antidepressant Drugs: is there a Link? Curr. Neuropharmacol. 11, 263–275. 10.2174/1570159X11311030003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogaça M. V., Reis F. M., Campos A. C., Guimarães F. S. (2014). Effects of intra-prelimbic prefrontal cortex injection of cannabidiol on anxiety-like behavior: involvement of 5HT1A receptors and previous stressful experience. Eur. Neuropsychopharmacol. 24, 410–419. 10.1016/j.euroneuro.2013.10.012 [DOI] [PubMed] [Google Scholar]
- Fuchs E., Flugge G. (2014). Adult neuroplasticity: more than 40 years of research. Neural. Plast. 2014:541870 10.1155/2014/541870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Allen P., Bhattacharyya S., Crippa J. A., Mechelli A., Borgwardt S., et al. (2010). Modulation of effective connectivity during emotional processing by Δ9-tetrahydrocannabinol and cannabidiol. Int. J. Neuropsychopharmacol. 13, 421–432. 10.1017/S1461145709990617 [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Crippa J. A., Bhattacharyya S., Borgwardt S. J., Allen P., Martin-Santos R., et al. (2009). Distinct effects of Δ9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch. Gen. Psychiatry 66, 95–105. 10.1001/archgenpsychiatry.2008.519 [DOI] [PubMed] [Google Scholar]
- Garcia-Arencibia M., Gonzalez S., de Lago E., Ramos J. A., Mechoulam R., Fernandez-Ruiz J. (2007). Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson's disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 1134, 162–170. 10.1016/j.brainres.2006.11.063 [DOI] [PubMed] [Google Scholar]
- Gassen N. C., Hartmann J., Zschocke J., Stepan J., Hafner K., Zellner A., et al. (2014). Association of FK P51 with priming of autophagy pathways and mediation of antidepressant treatment response: evidence in cells, mice, and humans. PLoS Med. 11:e1001755. 10.1371/journal.pmed.1001755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes F. V., Issy A. C., Ferreira F. R., Viveros M. P., Del Bel E. A., Guimarães F. S. (2015a). Cannabidiol attenuates sensorimotor gating disruption and molecular changes induced by chronic antagonism of NMDA receptors in mice. Int. J. Neuropsychopharmacol. 18:pyu041. 10.1093/ijnp/pyu041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes F. V., Llorente R., Del Bel E. A., Viveros M. P., Lopez-Gallardo M., Guimarães F. S. (2015b). Decreased glial reactivity could be involved in the antipsychotic-like effect of cannabidiol. Schizophr. Res. 164, 155–163. 10.1016/j.schres.2015.01.015 [DOI] [PubMed] [Google Scholar]
- Gomes F. V., Resstel L. B., Guimarães F. S. (2011). The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology (Berl) 213, 465–473. 10.1007/s00213-010-2036-z [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G., Lewis D. A. (2012). NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr. Bull. 38, 950–957. 10.1093/schbul/sbs010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goul E., Tanapat P., McEwen B. S., Flügge G., Fuchs E. (1998). Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc. Natl. Acad. Sci. U.S.A. 95, 3168–3171. 10.1073/pnas.95.6.3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E., McEwen B. S., Tanapat P., Galea L. A. M., Fuchs E. (1997). Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 17, 2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granja A. G., Carrillo-Salinas F., Pagani A., Gómez-Cañas M., Negri R., Navarrete C., et al. (2012). A cannabigerol quinone alleviates neuroinflammation in a chronic model of multiple sclerosis. J. Neuroimmune Pharmacol. 7, 1002–1016. 10.1007/s11481-012-9399-3 [DOI] [PubMed] [Google Scholar]
- Guimarães V. M., Zuardi A. W., Del Bel E. A., Guimarães F. S. (2004). Cannabidiol increases Fos expression in the nucleus accumbens but not in the dorsal striatum. Life Sci. 75, 633–638. 10.1016/j.lfs.2004.01.015 [DOI] [PubMed] [Google Scholar]
- Gururajan A., Taylor D. A., Malone D. T. (2012). Cannabidiol and clozapine reverse MK-801-induced deficits in social interaction and hyperactivity in Sprague-Dawley rats. J. Psychopharmacol. 26, 1317–1332. 10.1177/0269881112441865 [DOI] [PubMed] [Google Scholar]
- GW Pharmaceuticals (2015). GW Pharmaceuticals Announces Positive Proof of Concept Data in Schizophrenia. Available online at: https://www.gwpharm.com/about-us/news/gw-pharmaceuticals-announces-positive-proof-concept-data-schizophrenia
- Haj C. G., Sumariwalla P. F., Hanuš L., Kogan N. M., Yektin Z., Mechoulam R., et al. (2015). HU-444, a novel, potent anti-inflammatory, nonpsychotropic cannabinoid. J. Pharmacol. Exp. Ther. 355, 66–75. 10.1124/jpet.115.226100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim N. D., Weickert C. S., McClintock B. W., Weinberger D. R., Lipska B. K. (2004). Effects of chronic haloperidol and clozapine treatment on neurogenesis in the adult rat hippocampus. Neuropsychopharmacology 29, 1063–1069. 10.1038/sj.npp.1300422 [DOI] [PubMed] [Google Scholar]
- Hampson A. J., Grimaldi M., Axelrod J., Wink D. (1998). Cannabidiol and Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. U.S.A. 95, 8268–8273. 10.1073/pnas.95.14.8268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson E., Healey K., Wolf D., Kohler C. (2010). Assessment of pharmacotherapy for negative symptoms of schizophrenia. Curr. Psychiatry Rep. 12, 563–571. 10.1007/s11920-010-0148-0 [DOI] [PubMed] [Google Scholar]
- Harris K. M. (1999). Structure, development, and plasticity of dendritic spines. Curr Opin. Neurobiol. 9, 343–348. 10.1016/S0959-4388(99)80050-6 [DOI] [PubMed] [Google Scholar]
- Harvey B. S., Ohlsson K. S., Mååg J. L., Musgrave I. F., Smid S. D. (2012). Contrasting protective effects of cannabinoids against oxidative stress and amyloid-β evoked neurotoxicity in vitro. Neurotoxicology 33, 138–146. 10.1016/j.neuro.2011.12.015 [DOI] [PubMed] [Google Scholar]
- Heiseke A., Aguib Y., Riemer C., Baier M., Schätzl H. M. (2009). Lithium induces clearance of protease resistant prion protein in prion-infected cells by induction of autophagy. J. Neurochem. 109, 25–34. 10.1111/j.1471-4159.2009.05906.x [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh M., Nikseresht S., Khodagholi F., Naderi N., Maghsoudi N. (2016). Cannabidiol post-treatment alleviates rat epileptic-related behaviors and activates hippocampal cell autophagy pathway along with antioxidant defense in chronic phase of pilocarpine-induced seizure. J. Mol. Neurosci. 58, 432–440. 10.1007/s12031-015-0703-6 [DOI] [PubMed] [Google Scholar]
- Iannotti F. A., Hill C. L., Leo A., Alhusaini A., Soubrane C., Mazzarella E., et al. (2014). Nonpsychotropic Plant Cannabinoids, Cannabidivarin (CBDV) and Cannabidiol (CBD), Activate and Desensitize Transient Receptor Potential Vanilloid 1 (TRPV1) Channels in vitro: Potential for the Treatment of Neuronal Hyperexcitability. ACS Chem. Neurosci. 5, 1131–1141. 10.1021/cn5000524 [DOI] [PubMed] [Google Scholar]
- Ibeas Bih C., Chen T., Nunn A. V., Bazelot M., Dallas M., Whalley B. J. (2015). Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 12, 699–730. 10.1007/s13311-015-0377-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imming P., Sinning C., Meyer A. (2006). Drugs, their targets and the nature and number of drug targets. Nat. Rev. Drug Discov. 5, 821–834. 10.1038/nrd2132 [DOI] [PubMed] [Google Scholar]
- Iuvone T., Esposito G., Esposito R., Santamaria R., Di Rosa M., Izzo A. A. (2004). Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J. Neurochem. 89, 134–141. 10.1111/j.1471-4159.2003.02327.x [DOI] [PubMed] [Google Scholar]
- Izzo A., Borrelli F., Capasso R., Di Marzo V., Mechoulam R. (2009). Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends. Pharmacol. Sci. 30, 515–527. 10.1016/j.tips.2009.07.006 [DOI] [PubMed] [Google Scholar]
- James W. (1890). The Principles of Psychology, Chapter IV-Habits. New York, NY: Henry Holt and Company. [Google Scholar]
- Janefjord E., Mååg J. L., Harvey B. S., Smid S. D. (2014). Cannabinoid effects on β amyloid fibril and aggregate formation, neuronal and microglial-activated neurotoxicity in vitro. Cell Mol. Neurobiol. 34, 31–42. 10.1007/s10571-013-9984-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J., Le W. (2015). Molecular network of neuronal autophagy in the pathophysiology and treatment of depression. Neurosci. Bull. 31, 427–434. 10.1007/s12264-015-1548-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Zhang Y., Xiao L., Van Cleemput J., Ji S. P., Bai G., et al. (2005). Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J. Clin. Invest. 115, 3104–3116. 10.1172/JCI25509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsidoni V., Anagnostou I., Panagis G. (2013). Cannabidiol inhibits the reward-facilitating effect of morphine: involvement of 5-HT1A receptors in the dorsal raphe nucleus. Addict. Biol. 18, 286–296. 10.1111/j.1369-1600.2012.00483.x [DOI] [PubMed] [Google Scholar]
- Kaufman J., DeLorenzo C., Choudhury S., Parsey R. V. (2016). The 5-HT1A receptor in Major Depressive Disorder. Eur. Neuropsychopharmacol. 26, 397–410. 10.1016/j.euroneuro.2015.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C., Ling E. A. (2008). Antioxidants and neuroprotection in the adult and developing central nervous system. Curr. Med. Chem. 15, 3068–3080. 10.2174/092986708786848640 [DOI] [PubMed] [Google Scholar]
- Kays J. L., Hurley R. A., Taber K. H. (2012). The dynamic brain: neuroplasticity and mental health. J. Neuropsychiatry Clin. Neurosci. 24, 118–124. 10.1176/appi.neuropsych.12050109 [DOI] [PubMed] [Google Scholar]
- Kempermann G. (2008). The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends. Neurosci. 31, 163–169. 10.1016/j.tins.2008.01.002 [DOI] [PubMed] [Google Scholar]
- Kempermann G., Gage F. H. (2000). Neurogenesis in the adult hippocampus. Novartis Found Symp. 231, 220–235; discussion 235–241, 302–226. 10.1002/0470870834.ch14 [DOI] [PubMed] [Google Scholar]
- Kempermann G., Wiskott L., Gage F. H. (2004). Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 14, 186–191. 10.1016/j.conb.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Kim J. K., Samaranayake M., Pradhan S. (2009). Epigenetic mechanisms in mammals. Cell. Mol. Life Sci. 66, 596–612. 10.1007/s00018-008-8432-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T., Pape J., Binder E. B., Mehta D. (2014). The role of DNA methylation in stress-related psychiatric disorders. Neuropharmacology 80, 115–132. 10.1016/j.neuropharm.2014.01.013 [DOI] [PubMed] [Google Scholar]
- Koay L. C., Rigby R. J., Wright K. L. (2014). Cannabinoid-induced autophagy regulates suppressor of cytokine signaling-3 in intestinal epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G140–G148. 10.1152/ajpgi.00317.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C., Heckers S. (2001). Antipsychotic drugs and neuroplasticity: insights into the treatment and neurobiology of schizophrenia. Biol. Psychiatry 50, 729–742. 10.1016/S0006-3223(01)01267-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozela E., Juknat A., Gao F., Kaushansky N., Coppola G., Vogel Z. (2016). Pathways and gene networks mediating the regulatory effects of cannabidiol, a nonpsychoactive cannabinoid, in autoimmune T cells. J. Neuroinflammation 13:136. 10.1186/s12974-016-0603-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozela E., Pietr M., Juknat A., Rimmerman N., Levy R., Vogel Z. (2010). Cannabinoids Delta(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-beta/STAT proinflammatory pathways in BV-2 microglial cells. J. Biol. Chem. 285, 1616–1626. 10.1074/jbc.M109.069294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Nestler E. J. (2008). The molecular neurobiology of depression. Nature 455, 894–902. 10.1038/nature07455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie R. B., Bagher A. M., Kelly M. E., Denovan-Wright E. M. (2015). Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 172, 4790–4805. 10.1111/bph.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastres-Becker I., Molina-Holgado F., Ramos J. A., Mechoulam R., Fernández-Ruiz J. (2005). Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson's disease. Neurobiol. Dis. 19, 96–107. 10.1016/j.nbd.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Levin R., Peres F. F., Almeida V., Calzavara M. B., Zuardi A. W., Hallak J. E., et al. (2014). Effects of cannabinoid drugs on the deficit of prepulse inhibition of startle in an animal model of schizophrenia: the SHR strain. Front. Pharmacol. 5:10. 10.3389/fphar.2014.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke F. M., Piomelli D., Pahlisch F., Muhl D., Gerth C. W., Hoyer C., et al. (2012). Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2:e94. 10.1038/tp.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. A., Hashimoto T., Volk D. W. (2005). Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 6, 312–324. 10.1038/nrn1648 [DOI] [PubMed] [Google Scholar]
- Li N., Lee B., Liu R. J., Banasr M., Dwyer J. M., Iwata M., et al. (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329, 959–964. 10.1126/science.1190287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligresti A., De Petrocellis L., Di Marzo V. (2016). From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol. Rev. 96, 1593–1659. 10.1152/physrev.00002.2016 [DOI] [PubMed] [Google Scholar]
- Linge R., Jiménez-Sánchez L., Campa L., Pilar-Cuéllar F., Vidal R., Pazos A., et al. (2016). Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: role of 5-HT1A receptors. Neuropharmacology 103, 16–26. 10.1016/j.neuropharm.2015.12.017 [DOI] [PubMed] [Google Scholar]
- Long L. E., Chesworth R., Huang X. F., Wong A., Spiro A., McGregor I. S., et al. (2012). Distinct neurobehavioural effects of cannabidiol in transmembrane domain neuregulin 1 mutant mice. PLoS ONE 7:e34129. 10.1371/journal.pone.0034129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L. E., Malone D. T., Taylor D. A. (2006). Cannabidiol reverses MK-801-induced disruption of prepulse inhibition in mice. Neuropsychopharmacology 31, 795–803. 10.1038/sj.npp.1300838 [DOI] [PubMed] [Google Scholar]
- López-Sendón J. L., Caldentey J. G., Cubillo P. T., Romero C. R., Ribas G. G., Arias M. A. A., et al. (2016). A double-blind, randomized, cross-over, placebo-controlled, pilot trial with Sativex in Huntington's disease. J. Neurol. 263, 1390–1400. 10.1007/s00415-016-8145-9 [DOI] [PubMed] [Google Scholar]
- Lubliner J. (2005). Plasticity Theory. New York, NY: Macmillan Publishing Company. [Google Scholar]
- Lucassen P. J., Meerlo P., Naylor A. S., van Dam A. M., Dayer A. G., Fuchs E., et al. (2010). Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: implications for depression and antidepressant action. Eur. Neuropsychopharmacol. 20, 1–17. 10.1016/j.euroneuro.2009.08.003 [DOI] [PubMed] [Google Scholar]
- Maeda K., Sugino H., Hirose T., Kitagawa H., Nagai T., Mizoguchi H., et al. (2007). Clozapine prevents a decrease in neurogenesis in mice repeatedly treated with phencyclidine. J. Pharmacol. Sci. 103, 299–308. 10.1254/jphs.FP0061424 [DOI] [PubMed] [Google Scholar]
- Magen I., Avraham Y., Ackerman Z., Vorobiev L., Mechoulam R., Berry E. M. (2010). Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br. J. Pharmacol. 159, 950–957. 10.1111/j.1476-5381.2009.00589.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar I., Bambico F. R., Mechawar N., Nobrega J. N. (2014). Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 38, 173–192. 10.1016/j.neubiorev.2013.11.009 [DOI] [PubMed] [Google Scholar]
- Malberg J. E., Eisch A. J., Nestler E. J., Duman R. S. (2000). Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 20, 9104–9110. Available online at: http://www.jneurosci.org/content/jneuro/20/24/9104.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H., Uz T., Smalheiser N. R., Manev R. (2001). Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro. Eur. J. Pharmacol. 411, 67–70. 10.1016/S0014-2999(00)00904-3 [DOI] [PubMed] [Google Scholar]
- Martín-Moreno A. M., Reigada D., Ramírez B. G., Mechoulam R., Innamorato N., Cuadrado A., et al. (2011). Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer's disease. Mol. Pharmacol 79, 964–973. 10.1124/mol.111.071290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato S., Vidal R., Castro E., Diaz A., Pazos A., Valdizan E. M. (2010). Long-term fluoxetine treatment modulates cannabinoid type 1 receptor-mediated inhibition of adenylyl cyclase in the rat prefrontal cortex through 5-hydroxytryptamine 1A receptor-dependent mechanisms. Mol. Pharmacol. 77, 424–434. 10.1124/mol.109.060079 [DOI] [PubMed] [Google Scholar]
- Matus A. (1999). Postsynaptic actin and neuronal plasticity. Curr. Opin. Neurobiol. 9, 561–565. 10.1016/S0959-4388(99)00018-5 [DOI] [PubMed] [Google Scholar]
- McAllister S. D., Murase R., Christian R. T., Lau D., Zielinski A. J., Allison J., et al. (2011). Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res. Treat. 129, 37–47. 10.1007/s10549-010-1177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland J. M., Duncan M., Di Marzo V., Pertwee R. G. (2015). Are cannabidiol and Δ(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 172, 737–753. 10.1111/bph.12944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecha M., Feliu A., Inigo P. M., Mestre L., Carrillo-Salinas F. J., Guaza C., et al. (2013). Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol. Dis. 59, 141–150. 10.1016/j.nbd.2013.06.016 [DOI] [PubMed] [Google Scholar]
- Mecha M., Torrao A. S., Mestre L., Carrillo-Salinas F. J., Mechoulam R., Guaza C. (2012). Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis. 3:e331. 10.1038/cddis.2012.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencher S. K., Wang L. G. (2005). Promiscuous drugs compared to selective drugs (promiscuity can be a virtue). BMC Clin. Pharmacol. 5:3. 10.1186/1472-6904-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira F. A., Guimarães F. S. (2005). Cannabidiol inhibits the hyperlocomotion induced by psychotomimetic drugs in mice. Eur. J. Pharmacol. 512, 199–205. 10.1016/j.ejphar.2005.02.040 [DOI] [PubMed] [Google Scholar]
- Morishita H., Cabungcal J. H., Chen Y., Do K. Q., Hensch T. K. (2015). Prolonged period of cortical plasticity upon redox dysregulation in fast-spiking interneurons. Biol. Psychiatry 78, 396–402. 10.1016/j.biopsych.2014.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M. J., Na E. S., Autry A. E., Monteggia L. M. (2016). Impact of DNMT1 and DNMT3a forebrain knockout on depressive- and anxiety like behavior in mice. Neurobiol. Learn. Mem. 135, 139–145. 10.1016/j.nlm.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara T., Kuroki T., Hondo H., Tsutsumi T., Fukuda K., Yao H., et al. (1999). Effects of atypical antipsychotic drugs vs. haloperidol on expression of heat shock protein in the discrete brain regions of phencyclidine-treated rats. Brain Res. Mol. Brain Res. 73, 193–197. 10.1016/S0169-328X(99)00248-X [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Zsiros V., Jiang Z., Nakao K., Kolata S., Zhang S., et al. (2012). GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 62, 1574–1583. 10.1016/j.neuropharm.2011.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary O. F., Cryan J. F. (2014). A ventral view on antidepressant action: roles for adult hippocampal neurogenesis along the dorsoventral axis. Trends. Pharmacol. Sci. 35, 675–687. 10.1016/j.tips.2014.09.011 [DOI] [PubMed] [Google Scholar]
- Pallanti S. (2016). ICD and DSM: neuroplasticity and staging are still missing. CNS Spectr. 21, 276–278. 10.1017/S1092852916000146 [DOI] [PubMed] [Google Scholar]
- Pan H., Mukhopadhyay P., Rajesh M., Patel V., Mukhopadhyay B., Gao B., et al. (2009). Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J. Pharmacol. Exp. Ther. 328, 708–714. 10.1124/jpet.108.147181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos M. R., Mohammed N., Lafuente H., Santos M., Martínez-Pinilla E., Moreno E., et al. (2013). Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: role of 5HT(1A) and CB2 receptors. Neuropharmacology, 71, 282–291. 10.1016/j.neuropharm.2013.03.027 [DOI] [PubMed] [Google Scholar]
- Pedrazzi J. F., Issy A. C., Gomes F. V., Guimarães F. S., Del-Bel E. A. (2015). Cannabidiol effects in the prepulse inhibition disruption induced by amphetamine. Psychopharmacology (Berl) 232, 3057–3065. 10.1007/s00213-015-3945-7 [DOI] [PubMed] [Google Scholar]
- Penschuck S., Flagstad P., Didriksen M., Leist M., Michael-Titus A. T. (2006). Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. Eur. J. Neurosci. 23, 279–284. 10.1111/j.1460-9568.2005.04536.x [DOI] [PubMed] [Google Scholar]
- Pereira V. S., Sales A. J., Joca S., Wegener G. (2016). Antidepressant-like effects of Cannabidiol on a genetic animal model of depression. Acta. Neuropsychiatr. 28 (Suppl. 2), 1–40. 10.1017/neu.2016.18 [DOI] [Google Scholar]
- Perez M., Benitez S. U., Cartarozzi L. P., Del Bel E., Guimarães F. S., Oliveira A. L. (2013). Neurorotection and reduction of glial reaction by cannabidiol treatment after sciatic nerve transection in neonatal rats. Eur. J. Neurosci. 38, 3424–3434. 10.1111/ejn.12341 [DOI] [PubMed] [Google Scholar]
- Pisoschi A. M., Pop A. (2015). The role of antioxidants in the chemistry of oxidative stress: a review. Eur. J. Med. Chem. 97, 55–74. 10.1016/j.ejmech.2015.04.040 [DOI] [PubMed] [Google Scholar]
- Pucci M., Rapino C., Di Francesco A., Dainese E., D'Addario C., Maccarrone M. (2013). Epigenetic control pf skin differentiation genes by phytocannabinoids. Brit. J. Pharmacol. 170, 581–591. 10.1111/bph.12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radewicz K., Garey L. J., Gentleman S. M., Reynolds R. (2000). Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J. Neuropathol. Exp. Neurol. 59, 137–150. 10.1093/jnen/59.2.137 [DOI] [PubMed] [Google Scholar]
- Rajesh M., Mukhopadhyay P., Bátkai S., Haskó G., Liaudet L., Drel V. R., et al. (2007). Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am. J. Physiol. Heart Circ. Physiol. 293, H610–H619. 10.1152/ajpheart.00236.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A., Fritzen S., Finger M., Strobel A., Lauer M., Schmitt A., et al. (2006). Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol. Psychiatry 11, 514–522. 10.1038/sj.mp.4001791 [DOI] [PubMed] [Google Scholar]
- Renard J., Loureiro M., Rosen L. G., Zunder J., de Oliveira C., Schmid S., et al. (2016). Cannabidiol counteracts amphetamine-induced neuronal and behavioral sensitization of the mesolimbic dopamine pathway through a novel mTOR/p70S6 Kinase Signaling Pathway. J. Neurosci. 36, 5160–5169. 10.1523/JNEUROSCI.3387-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel L. B. M., Tavares R. F., Lisboa S. F. S., Joca S. R. L., Corrêa F. M. A., Guimarães F. S. (2009). 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Brit. J. Pharmacol. 156, 181–188. 10.1111/j.1476-5381.2008.00046.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réus G. Z., Stringari R. B., Ribeiro K. F., Luft T., Abelaira H. M., Fries G. R., et al. (2011). Administration of cannabidiol and imipramine induces antidepressant-like effects in the forced swimming test and increases brain-derived neurotrophic factor levels in the rat amygdala. Acta. Neuropsychiatr. 23, 241–248. 10.1111/j.1601-5215.2011.00579.x [DOI] [PubMed] [Google Scholar]
- Rock E. M., Bolognini D., Limebeer C. L., Cascio M. G., Anavi-Goffer S., Fletcher P. J., et al. (2012). Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 165, 2620–2634. 10.1111/j.1476-5381.2011.01621.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E. B., Burnett A., Hall B., Parker K. K. (2005). Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 30, 1037–1043. 10.1007/s11064-005-6978-1 [DOI] [PubMed] [Google Scholar]
- Sagredo O., Pazos M. R., Satta V., Ramos J. A., Pertwee R. G., Fernández-Ruiz J. (2011). Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington's disease. J. Neurosci. Res. 89, 1509–1518. 10.1002/jnr.22682 [DOI] [PubMed] [Google Scholar]
- Sagredo O., Ramos J. A., Decio A., Mechoulam R., Fernández-Ruiz J. (2007). Cannabidiol reduced the striatal atrophy caused 3-nitropropionic acid in vivo by mechanisms independent of the activation of cannabinoid, vanilloid TRPV1 and adenosine A2A receptors. Eur. J. Neurosci. 26, 843–851. 10.1111/j.1460-9568.2007.05717.x [DOI] [PubMed] [Google Scholar]
- Sales A. J., Biojone C., Terceti M. S., Guimarães F. S., Gomes M. V., Joca S. R. (2011). Antidepressant-like effect induced by systemic and intra-hippocampal administration of DNA methylation inhibitors. Br. J. Pharmacol. 164, 1711–1721. 10.1111/j.1476-5381.2011.01489.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels B. A., Anackerm C., Hu A., Levinstein M. R., Pickenhagen A., Tsetsenis T., et al. (2015). 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat. Neurosci. 18, 1606–1616. 10.1038/nn.4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., et al. (2003). Requirement of hipocampal neurogenesis for the behavioral effects of antidepressants. Science, 301, 805–809. 10.1126/science.1083328 [DOI] [PubMed] [Google Scholar]
- Santos N. A., Martins N. M., Sisti F. M., Fernandes L. S., Ferreira R. S., Queiroz R. H., et al. (2015). The neuroprotection of cannabidiol against MPPâ-induced toxicity in PC12 cells involves trkA receptors, upregulation of axonal and synaptic proteins, neuritogenesis, and might be relevant to Parkinson's disease. Toxicol. In vitro 30, 231–240. 10.1016/j.tiv.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Sartim A. G., Guimarães F. S., Joca S. R. (2016). Antidepressant-like effect of cannabidiol injection into the ventral medial prefrontal cortex-Possible involvement of 5-HT1A and CB1 receptors. Behav. Brain Res. 303, 218–227. 10.1016/j.bbr.2016.01.033 [DOI] [PubMed] [Google Scholar]
- Schiavon A. P., Bonato J. M., Milani H., Guimarães F. S., Weffort de Oliveira R. M. (2016). Influence of single and repeated cannabidiol administration on emotional behavior and markers of cell proliferation and neurogenesis in non-stressed mice. Prog. Neuro Psychopharmacol. Biol. Psychiatry 64, 27–34. 10.1016/j.pnpbp.2015.06.017 [DOI] [PubMed] [Google Scholar]
- Schiavon A. P., Soares L. M., Bonato J., Milani H., Guimarães F. S., Weffort de Oliveira R. M. (2014). Protective effects of cannabidiol against hippocampal cell death and cognitive impairment induced by bilateral common carotid artery occlusion in mice. Neurotox. Res. 26, 307–316. 10.1007/s12640-014-9457-0 [DOI] [PubMed] [Google Scholar]
- Schönhofen P., de Medeiros L. M., Bristot I. J., Lopes F. M., De Bastiani M. A., Kapczinski F., et al. (2015). Cannabidiol exposure during neuronal differentiation sensitizes cells against redox-active neurotoxins. Mol. Neurobiol. 52, 26–37. 10.1007/s12035-014-8843-1 [DOI] [PubMed] [Google Scholar]
- Scuderi C., Steardo L., Esposito G. (2014). Cannabidiol promotes amyloid precursor protein ubiquitination and reduction of beta amyloid expression in SHSY5YAPP+ cells through PPARγ involvement. Phytother. Res. 28, 1007–1013. 10.1002/ptr.5095 [DOI] [PubMed] [Google Scholar]
- Serafini G., Hayley S., Pompili M., Dwivedi Y., Brahmachari G., Girardi P., et al. (2014). Hippocampal neurogenesis, neurotrophic factors and depression: possible therapeutic targets? CNS Neurol. Disord. Drug Targets 13, 1708–1721. 10.2174/1871527313666141130223723 [DOI] [PubMed] [Google Scholar]
- Shaked I., Tchoresh D., Gersner R., Meiri G., Mordechai S., Xiao X., et al. (2005). Protective autoimmunity: interferon-gamma enables microglia to remove glutamate without evoking inflammatory mediators. J. Neurochem. 92, 997–1009. 10.1111/j.1471-4159.2004.02954.x [DOI] [PubMed] [Google Scholar]
- Sheline Y. I., Wang P. W., Gado M. H., Csernansky J. G., Vannier M. W. (1996). Hippocampal atrophy in recurrent major depression. Proc. Natl. Acad. Sci. U. S. A. 93, 3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoval G., Shbiro L., Hershkovitz L., Hazut N., Zalsman G., Mechoulam R., et al. (2016). Prohedonic effect of cannabidiol in a rat model of depression. Neuropsychobiology 73, 123–129. 10.1159/000443890 [DOI] [PubMed] [Google Scholar]
- Snyder J. S., Soumier A., Brewer M., Pickel J., Cameron H. A. (2011). Adult hipocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461. 10.1038/nature10287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares Vde. P., Campos A. C., Bortoli V. C., Zangrossi H., Jr., Guimarães F. S., et al. (2010). Intra-dorsal periaqueductal gray administration of cannabidiol blocks panic-like response by activating 5-HT1A receptors. Behav. Brain Res. 213, 225–229. 10.1016/j.bbr.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Solinas M., Massi P., Cinquina V., Valenti M., Bolognini D., Gariboldi M., et al. (2013). Cannabidiol, a non-psychoactive cannabinoid compound, inhibits proliferation and invasion in U87-MG and T98G glioma cells through a multitarget effect. PLoS ONE 8:e76918. 10.1371/journal.pone.0076918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahnisch F. W., Nitsch R. (2002). Santiago Ramon y Cajal's concept of neuronal plasticity: the ambiguity lives on. Trends Neurosci. 25, 589–591. 10.1016/S0166-2236(02)02251-8 [DOI] [PubMed] [Google Scholar]
- Steullet P., Cabungcal J. H., Monin A., Dwir D., O'Donnell P., Cuenod M., et al. (2016). Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology? Schizophr. Res. 176, 41–51. 10.1016/j.schres.2014.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H., Deng W., Gage F. H. (2009). Signaling in adult neurogenesis. Annu. Rev. Cell Dev. Biol. 25, 253–275. 10.1146/annurev.cellbio.042308.113256 [DOI] [PubMed] [Google Scholar]
- Suzuki S., Saitoh A., Ohashi M., Yamada M., Oka J., Yamada M. (2016). The infralimbic and prelimbic medial prefrontal cortices have differential functions in the expression of anxiety-like behaviors in mice. Behav. Brain Res. 304, 120–124. 10.1016/j.bbr.2016.01.044 [DOI] [PubMed] [Google Scholar]
- Tanapat P., Galea L. A., Gould E. (1998). Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int. J. Dev. Neurosci. 16, 235–239. 10.1016/S0736-5748(98)00029-X [DOI] [PubMed] [Google Scholar]
- Thomas A., Baillie G. L., Phillips A. M., Razdan R. K., Ross R. A., Pertwee R. G. (2007). Cannabidiol displays unexpectedly high potency as an antagonist of CB 1 and CB 2 receptor agonists in vitro. Br. J. Pharmacol. 150, 613–623. 10.1038/sj.bjp.0707133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffoli L. V., Rodrigues G. M., Jr., Oliveira J. F., Silva A. S., Moreira E. G., Pelosi G. G., et al. (2014). Maternal exposure to fluoxetine during gestation and lactation affects the DNA methylation programming of rat's offspring: modulation by folic acid supplementation. Behav. Brain Res. 265, 142–147. 10.1016/j.bbr.2014.02.031 [DOI] [PubMed] [Google Scholar]
- Tortoriello G., Morris C. V., Alpar A., Fuzik J., Shirran S. L., Calvigioni D., et al. (2014). Miswiring the brain: Δ9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J. 33, 668–685. 10.1002/embj.201386035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N., Renthal W., Kumar A., Nestler E. J. (2007). Epigenetic regulation in psychiatric disorders. Nat. Ver. Neurosci. 8, 355–367. 10.1038/nrn2132 [DOI] [PubMed] [Google Scholar]
- Twardowschy A., Castiblanco-Urbina M. A., Uribe-Mariño A., Biagioni A. F., Salgado-Rohner C. J., Crippa J. A., et al. (2013). The role of 5-HT1A receptors in the anti-aversive effects of cannabidiol on panic attack-like behaviors evoked in the presence of the wild snake Epicrates cenchria crassus (Reptilia, Boidae). J. Psychopharmacol. 27, 1149–1159. 10.1177/0269881113493363 [DOI] [PubMed] [Google Scholar]
- Valdeolivas S., Navarrete C., Cantarero I., Bellido M. L., Muñoz E., Sagredo O. (2015). Neuroprotective properties of cannabigerol in Huntington's disease: studies in R6/2 mice and 3-nitropropionate-lesioned mice. Neurotherapeutics 12, 185–199. 10.1007/s13311-014-0304-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdeolivas S., Satta V., Pertwee R. G., Fernandez-Ruiz J., Sagredo O. (2012). Sativex-like combination of phytocannabinoids is neuroprotective in malonate-lesioned rats, an inflammatory model of Huntington's disease: role of CB1 and CB2 receptors. ACS Chem. Neurosci. 3, 400–406. 10.1021/cn200114w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvassori S. S., Elias G., de Souza B., Petronilho F., Dal-Pizzol F., Kapczinski F., et al. (2011). Effects of cannabidiol on amphetamine-induced oxidative stress generation in an animal model of mania. J. Psychopharmacol. 25, 274–280. 10.1177/0269881109106925 [DOI] [PubMed] [Google Scholar]
- Wolf S. A., Bick-Sander A., Fabel K., Leal-Galicia P., Tauber S., Ramirez-Rodriguez G., et al. (2010). Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun. Signal. 8:12 10.1186/1478-811X-8-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Rozenfeld R., Wu D., Devi L. A., Zhang Z., Cederbaum A. (2014). Cannabidiol protects liver from binge alcohol-induced steatosis by mechanisms including inhibition of oxidative stress and increase in autophagy. Free Radic. Biol. Med. 68, 260–267. 10.1016/j.freeradbiomed.2013.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanelati T. V., Biojone C., Moreira F. A., Guimarães F. S., Joca S. R. (2010). Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br. J. Pharmacol. 159, 122–128. 10.1111/j.1476-5381.2009.00521.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Cai C. Y., Wu H. Y., Zhu L. J., Luo C. X., Zhu D. Y. (2016). CREB-mediated synaptogenesis and neurogenesis is crucial for the role of 5-HT1a receptors in modulating anxiety behaviors. Sci. Rep. 6:29551. 10.1038/srep29551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann N., Zschocke J., Perisic T., Yu S., Holsboer F., Rein T. (2012). Antidepressants inhibit DNA methyltransferase 1 through reducing G9a levels. Biochem. J. 448, 93–102. 10.1042/BJ20120674 [DOI] [PubMed] [Google Scholar]
- Zuardi A. W., Morais S. L., Guimarães F. S., Mechoulam R. (1995). Antipsychotic effect of cannabidiol. J. Clin. Psychiatry 56, 485–486. [PubMed] [Google Scholar]
- Zuardi A. W., Rodrigues J. A., Cunha J. M. (1991). Effects of cannabidiol in animal models predictive of antipsychotic activity. Psychopharmacology (Berl) 104, 260–264. 10.1007/BF02244189 [DOI] [PubMed] [Google Scholar]