Abstract
Graft infection following aortic aneurysms repair is an uncommon but devastating complication; its incidence ranges from <1% to 6% (mean 4%), with an associated perioperative and overall mortality of 12% and 17.5-20%, respectively. The most common causative organisms are Staphylococcus aureus and Escherichia coli; causative bacteria typically arise from the skin or gastrointestinal tract. The pathogenetic mechanisms of aortic graft infections are mainly breaks in sterile technique during its implantation, superinfection during bacteremia from a variety of sources, severe intraperitoneal or retroperitoneal inflammation, inoculation of bacteria during postoperative percutaneous interventions to manage various types of endoleaks, and external injury of the vascular graft. Mechanical forces in direct relation to the device were implicated in fistula formation in 35% of cases of graft infection. Partial rupture and graft migration leading to gradual erosion of the bowel wall and aortoenteric fistulas have been reported in 30.8% of cases.
Rarely, infection via continuous tissues may affect the spine, resulting in spondylitis. Even though graft explantation and surgical debridement is usually the preferred course of action, comorbidities and increased perioperative risk may preclude patients from surgery and endorse a conservative approach as the treatment of choice. In contrast, conservative treatment is the treatment of choice for spondylitis; surgery may be indicated in approximately 8.5% of patients with neural compression or excessive spinal infection. To enhance the literature, we searched the related literature for published studies on continuous spondylitis from infected endovascular grafts aiming to summarize the pathogenesis and diagnosis, and to discuss the treatment and outcome of the patients with these rare and complex infections.
Keywords: Endovascular aneurysm repair, Vascular graft, Aortic endograft, Continuous spondylitis.
Introduction
Conventional surgical repair of aortic aneurysms has been applied for many decades. However, despite the advances in operative techniques and perioperative management of patients with abdominal aortic aneurysms or occlusive aorto-iliac disease, there are many early and late complications related to aortic graft repair. One of the most devastating complication is infection of the aortic graft accounting for an incidence ranging from just under 1% to as high as 6%, with an overall incidence of approximately 4%, and an associated perioperative and overall mortality of 12% and 17.5-20%, respectively 1.
In recent years, a modern minimally invasive procedure, the endovascular aortic repair (EVAR) has been introduced for aortic aneurysm repairs 2-12. First described by Parodi in 1991, the latter technique consists of percutaneous insertion of a tubular graft within the aneurysmal sac, tethered with metallic stents with the use of special catheters and guidewires through arteriotomies 2. Hence, this minimally invasive procedure has emerged as an appealing treatment option, as compared to conventional open surgery, with comparatively better early outcomes and lower perioperative mortality and morbidity rates 3. However, there has been no reason to believe that endovascular aortic stent grafts would be immune from septic complications 1, 4. The rate of EVAR infections is unclear 6. Even though the reported incidence of EVAR infection is very low (mean, 0.4%; range, 0.2-0.7%) 5, 6, it represents a devastating complication with an estimated mortality ranging from 27.4% to 36.4% 6. Skin contamination during interventional procedures, which occur in up to 10-20%, and hematogenous graft seeding may occur 1-6.
In a few cases, infection may transmit to continuous tissues resulting in spondylitis 7-12. The incidence of spondylitis is even more rare and cannot be estimated because only case reports have been published in the related literature 7-12. To enhance the literature, we searched PubMed using as search terms [endograft] or [aortic graft] or [EVAR] and [spondylitis] aiming to summarize the pathogenesis and diagnosis, and to discuss the treatment and outcome of the patients with these rare and complex infections. Our literature search showed 67 studies (original and review articles) on endograft infections and spondylitis 1-57. These studies were read and evaluated for continuous tissues transmitted spondylitis from infected aortic grafts. Our review revealed 14 case reports 7-12, 32, 50-52, 54-57 on continuous spondylitis from infected aortic grafts (Table 1; Paper level: 4 [Reviews]; Data level: 1 [Neutral, n< 50]). Spondylitis transmitted from the infected aortic graft occurred within 9 days to 11 years after the vascular procedure (either endovascular or open vascular surgery); patients were treated with variable treatments (antibiotics and/or debridement); their follow-up was short for important conclusions.
Table 1.
Summary of published studies on spondylitis transmitted from an infected aortic graft.
| Study | Patients (Age/Sex) | Vascular graft | Time to and Site of spondylitis; Bacterial isolate | Treatment | Follow-up; Outcome |
|---|---|---|---|---|---|
| Lowe et al. [7] |
82/M | EVAR | 2 months; L4-L5; not reported | Long-term antibiotics (gentamicin, rifampicin, clindamycin) | 6 months; low back pain and neurological status did not improve |
| Mavrogenis et al. [8] | 64/M | EVAR | 7 years; L3; methicillin-resistant Staphylococcus epidermidis | Long-term antibiotics (moxifloxacin and linezolid) | 12 months; mild low back pain |
| Faccenna et al. [9] |
51/M | EVAR | 9 days; L2-L4; methicillin-susceptible Staphylococcus aureus | Antibiotics (ciprofloxacin, rifampicin), drainage, spinal brace | 4 years; low back pain, neurological impairment |
| de Koning et al. [10] | 74/M | EVAR | 7 months; L3-L4; Escherichia coli and multiple anaerobic bacteria | Surgical (endograft removal and debridement), antibiotics (clindamycin, piperacillin, tazobactam amoxicillin-clavulanate) | 7 months; mild right thigh pain |
| Blanch et al. [11] |
76/M | EVAR | 6 months; L3-L4; Streptococcus haemolyticus and Propionibacterium | Surgical (debridement, endograft preservation), antibiotics (sulfamethoxazole,trimetoprim, rifampicin, cefuroxime, linezolid) | 6 months; not reported |
| Laser et al. [12] |
73/M | EVAR | 6 months; lumbar spine and sacrum; Staphylococcus | Surgical (endograft removal, axillary bifemoral bypass), antibiotics | 3 months; alive |
| 54/M | EVAR | 45 months; not reported; negative | Surgical (endograft removal, repair with rifampin-soaked endograft), antibiotics | 7 months; alive | |
| d' Ettore et al. [32] |
61/M | EVAR | Not reported; T7-T9; coagulase-negative Staphylococcus | Antibiotics (vancomycin, rifampicin and ceftazidime, switched to levofloxacin, minocycline and teicoplanin) | Not reported; good clinical and radiological features |
| Bogaert et al. [50] |
72/M | Aortobiiliac graft | 11 years; L1-L2; Torulopsis glabrata | Bed rest, antibiotics (fluconazole, amphotericin B) | 11 weeks; deceased |
| Dreyfus et al. [51] |
71/M | Aortobiiliac graft | Not reported; L4-L5 spondylodiscitis; Staphylococcus aureus | Surgical (graft removal and extra-anatomic axillo-bifemoral arterial bypass), antibiotics (ciprofloxacin, oxacillin) | 3 months; asymptomatic |
| Piquet et al. [52] |
67/M | Aortobifemoral graft | 7 years; L2; Coxiella burnetii | Surgical (two-stage; removal of graft and bilateral aortofemoral bypass; L2 vertebroplasty), antibiotics (doxycycline, ofloxacin) | 3 years; asymptomatic |
| Brandt et al. [54] |
73/M | Aortobifemoral graft | 3 years; L2-L3; Aspergillus fumigatus | Surgical (graft removal and axillo-bifemoral arterial bypass), antibiotics (amphotericin B) | 6 months; ambulatory and relatively pain-free |
| Anderson et al. [55] | 73/M | Aortobifemoral graft | 3 years; L2-L3; Aspergillus species | Surgical (graft removal and axillo-bifemoral arterial bypass), antibiotics (amphotericin B) | 6 months; alive and well |
| Glotzbach et al. [56] | 71/M | Aortobifemoral graft | 2 years; L2-L3; Aspergillus terreus | Surgical (graft removal and aortic repair with a new aortobifemoral graft), antibiotics (amphotericin B) | 37 days; deceased |
| Solomon et al. [57] |
78/M | Aortic graft and EVAR | 7 years; L2; mixed enteric flora (Streptococcus viridans, Streptococcus anginosus, and Escherichia coli). | Antibiotics, drainage followed by debridement (excision of a portion of infected Dacron graft, aneurysm sac and abscess cavity; endograft was left in situ | 6 months; deceased from causes not related to aortic graft infection |
EVAR: endovascular aneurysm repair.
Pathogenesis
Causative organisms for endovascular infections are usually derived from skin flora or the gastrointestinal tract and may include Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Enterococci, or Streptococci. Staphylococcus aureus is reported to be the most common causative pathogen both in open aortic repairs and EVAR procedures 17, 18. Rare cases of aortic graft infections from Pasteurella multocida 13, 14, Propionibacterium acnes 15, Aspergillus fumigatus 16, Gemella haemolysans 49 and Torulopsis glabrata 50 have also been described. For all organisms, bacterial adherence to the vascular graft is the initial event in the process of graft infection 1. Adherence depends on the physical characteristics of the graft such as pore size and surface are, and on the chemical properties of the graft such as hydrophobicity 58.
The production of an extracellular glycocalyx (biofilm) by bacteria promotes adherence to biomaterials and provides protection against host defenses. Biofilm decreases antibiotic penetration, impairs phagocyte and antibody functions, and stimulates a chronic inflammatory process around an infected prosthesis. In this setting, bacteria are more difficult to culture from an infected graft 1, 58, 59. Proteases produced by gram-negative organisms contribute to the higher rates of vessel wall necrosis, anastomotic disruption, and pseudoaneurysm formation with infection from these bacteria 58, 59. In the canine model, it has been shown that in the presence of bacteremia, endografts become more resistant to infection after the first week due to a pseudointima formation over the endograft material, which propagates inwards from the edges of the graft 60. In humans, the ability of depth of stent graft incorporation has not been well documented. Since pseudointima formation in newly implanted vascular grafts starts from the edges of the graft and proceeds inwards, the first weeks after implantation are critical for possible infectious graft contamination by hematogenous seeding, as reported by Murphy et al. 61, that found most of the infections occurring within 3 months. However, it is probable that very long grafts may possibly never be fully incorporated, as indirectly proven by late detection of infection, up to 72 months in the series of Capoccia et al. 62.
Reported risk factors for aortic graft infections include cardiac, pulmonary and hepatic disease, diabetes mellitus, renal insufficiency, intestinal ischemia, immunosuppression especially that due to corticosteroid therapy or chemotherapy in cancer patients, coincident malignancy, postoperative wound infections, and urgent/emergency procedures 17, 19, 61. Possible routes for graft infection include perioperative contamination, hematogenous spread from a distant site, and mechanical erosion of the endograft or aneurysmal sac coils 1-51; reported sources of endograft infection include infection (groin, urinary or other) at index operation, contaminated index operation, and endoleak at index operation 33. Aortic graft infections may also be related with a non-evacuated thrombus, atherosclerotic plaques, persistent endoleak, presence of an aortoenteric fistula 8, 11, 20, 21, and suboptimal sterility conditions in the interventional radiology suites 18. Nonetheless, the majority of infections occur after bacterial contamination of the perigraft space at the time of the index graft implantation or during subsequent endovascular procedures, such as catheter manipulation inside the stent graft 8, 17. In a study 18, a 62.5% of stent graft infections were reported when the procedure was performed in interventional radiology suites, compared to 37.5% when the procedure was performed in conventional operating theaters; mechanical forces in direct relation to the device were implicated in fistula formation in 35% of infected endografts and in 30.8% of aortoenteric fistulas; partial rupture, excessive angulation at the level of the proximal neck, and graft migration led to gradual erosion of the bowel wall and secondarily to contamination of the stent graft 18.
Traditionally, prosthetic joint infections (PJIs) are classified as early (< 3 months after surgery), delayed (3-24 months after surgery), and late infections (> 2 years after surgery) 63. Early and delayed infections are mainly exogenously acquired in the perioperative period, whereas most late PJIs are hematogenously acquired. Acute hematogenous prosthetic joint infections of less than 3 weeks' duration and early post-interventional prosthetic joint infections (< 1 month after surgery) can generally be treated with implant retention. In contrast, in patients with chronic prosthetic joint infections, the biofilm on implant material can generally not be eliminated by antimicrobial agents; therefore, all foreign material has to be removed 63.
Early or late onset aortic graft infections are possible. It has been postulated that an infection may manifest within 4 months or >12 months after initial surgery 5, 33, 62-68. Smeds et al. 33 reported a mean interval of 18 months (range, 0.6-70 months) for the diagnosis of infection for thoracic stent grafts compared to a mean interval of 24 months (range, 0.2-158 months) for abdominal stent graft infections, without any difference between the two infected stent types. Capoccia et al. 62 reported a mean time from index endograft procedure to infection diagnosis of 20.5 ± 20.3 months (range, 1-72 months). Bacterial contamination during surgery commonly results in early infections, while in rare cases bacteremia or mechanical graft erosion into the surrounding tissues may result in late infections 5. Graft or arterial interface disruption may lead to hematoma formation 22. Bacteria may seed an adjacent hematoma, transforming it into an abscess 9. In the context of further disease progression, spondylitis secondary to aortic graft infection may occur either by bacteremia or by continuous tissues spread 7-12, 32, 49-51.
Reported risk factors for spondylitis include preexisting or synchronous non spinal infection including skin and soft tissue infection, infective endocarditis, catheter related infection, post-operative wound infection (non-spinal operation), pneumonia, arthritis, arteriovenous fistula infection, meningitis, genitourinary tract infection, intra-abdominal infection, previous bacteremia within 1 year, endovascular stent infection, and endophthalmitis 69-75. Risk factors associated with spondylitis in multivariate analysis include female gender (for gram negative infections), epidural block within 6 months, spinal prosthesis, previous bacteremia within 1 year, fever, sepsis, preexisting or synchronous genitourinary tract infection, and preexisting or synchronous intra-abdominal infection 75.
Spondylitis secondary to aortic graft infection probably should be considered a sign of low-virulent bacteria late infection. In this setting, infection extends into the adjacent tissue, and affects the spine and potentially the spinal cord or nerve roots 7-12. A specific time interval between the index vascular procedure and continuous spondylitis has not been reported to draw important conclusions.
Diagnostic Approach
Diagnosis of aortic graft infections is challenging. Clinical features may be ambiguous and nonspecific. A high index of suspicion is essential and diagnosis should be established based on clinical symptoms, imaging and laboratory findings. Infection may lead to graft/arterial interface disruption, hemorrhage, or sepsis 17. Infection can be low- or high-grade 5, 62. Low-grade infections (approximately 30%) are caused by low-virulent bacteria, and occur generally late after the index graft implantation; infection route is exogenous with only a few exceptions 63. Usually, these patients have been suffering from chronic, nonspecific symptoms and signs such as weakness, weight loss, and malaise 17, 33. In contrast, the type of symptoms is prominent at the local site of infection if virulent pathogens such as Staphylococcus aureus or Gram-negative bacteria are involved 63. High-grade infections are usually caused by high virulent bacteria; in this setting, symptoms appear acutely and include sepsis, fever, abdominal pain, lumbar pain, graft thrombosis, septic embolism, hematemesis, rectal blood loss, and hemorrhagic shock in the case of aorto-enteric fistula formation 6, 17, 18, 33, 41, 62. Although most of the infections originate from perioperative contamination, it has been reported that 50% of cases may present after the second year of follow-up 5, 17, 19. Constant, progressive back pain, malaise or weight loss is suggestive of infection spread to the spine 11, 23. Neurological impairment may also occur in some cases when the spinal cord or nerve roots are compressed 7.
White blood cell (WBC) count and C-reactive protein (CRP) should be included in the basic work-up. CRP has been suggested as a sensitive index for evaluating infection and treatment response in the follow-up period, also providing guidance for antibiotic therapy discontinuation 24.
CT is the imaging modality of choice when aortic graft infection is suspected 26. Signs such as air in the perigraft space, tissue infiltration, intrasac collection, fluid accumulation and soft-tissue attenuation, pseudoaneurysm or discontinuity of the vascular wall, or communication between the aortic wall and the bowel should be evaluated with caution, as they are indicative for infection 17. Nonetheless, in the early postoperative period, it is difficult to distinguish graft infection from the expected postoperative changes 8, 26. The presence of perigraft air is not pathognomonic of graft infection until 4-7 weeks after surgery, while the presence of perigraft fluid may be normal up to 4 months after surgery 8. In acute graft infections, sensitivity and specificity of CT are very high; however, accuracy decreases dramatically in chronic disease 27. In the presence of secondary spondylitis, diagnosis by means of CT may be difficult. Imaging findings may be nonspecific, as noninfectious vertebral destruction may also occur after endograft procedures or in the presence of a pseudoaneurysm 11.
When CT is inconclusive, positron emission tomography with 2-deoxy-2-fluoro-d-glucose (FDG-PET) may be considered. This promising imaging modality is advocated as superior to CT when aortic graft infection is suspected 28. Accordingly, the combination of FDG-PET with CT (PET-CT) is reported to increase sensitivity and specificity 17. FDG-PET may also provide evidence of spondylitis with high sensitivity, specificity and accuracy 29. Increased uptake not only at the location of the graft but also at the adjacent spinal region is suggestive of spinal spread of the disease. Indium111 labeled leukocyte scan may be helpful in evaluating vascular graft infection and assessing its extent in the perigraft region. It may also detect other sites of infection that need to be managed accordingly 30.
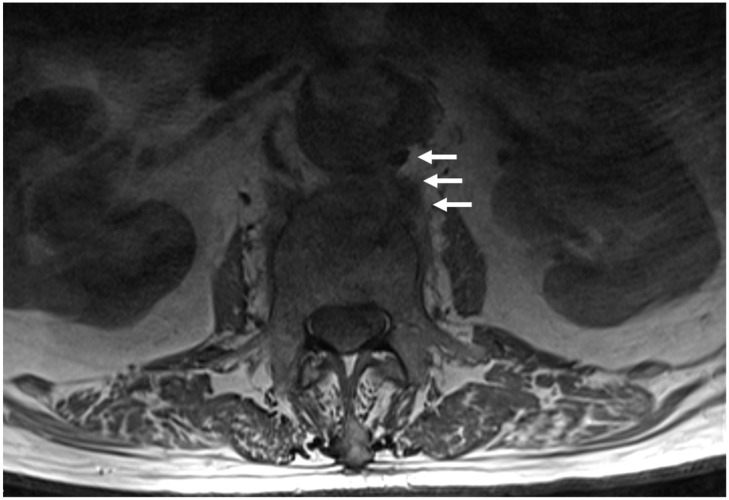
The role of magnetic resonance (MR) imaging in aortic graft infections is not clarified; however, it may be more accurate in cases of intra-abdominal graft infections, considering its superiority to discriminate tissue planes, particularly in the retroperitoneum 8, 26 (Figure 1). MR imaging may differentiate perigraft fluid and inflammatory changes of the adjacent tissues from acute and chronic hematoma 8, 26. Isolation of the causative bacteria or fungus from blood- or perigraft-drained fluid material is confirmative of the diagnosis of infection 17. Tissue sampling or drainage should be performed under CT guidance 25, 26. However, blood or tissue cultures are not always positive in patients with active aortic graft infection, even in cases of frank purulence 5, 12.
Figure 1.
A 93-year-old man presented with low back and right leg pain, malaise and low-grade fever of 4 month duration; he had an abdominal aortic aneurysm treated with vascular graft 18 years before. Axial T1-weighted MR image of the lumbar spine shows pathological signal intensity of the L3 vertebral body, and continuous abnormal soft tissue of similar signal intensity extending from the posterior aneurysmal sac to L3 vertebral body (arrows). The aortic lumen is irregular, with flame-shaped areas of pointing contrast suggesting inflammatory infiltration into the aortic wall. With the presumptive diagnosis of infection, he was treated by his local physicians with antibiotics (ciprofloxacin and rifampicin) and analgesics, in addition to lumbar spine immobilization with a brace. CT-guided biopsy was done at his admission; cultures were negative, probably because of antibiotics administration. Because of deteriorated general health status, a joint decision was obtained for conservative treatment with long term suppression with antibiotics. Four months later, the patient is afebrile with improved but constant low back pain.
A high clinical suspicion is important in patients with nonmechanical pain for early diagnosis of spondylitis. Serologic tests such as erythrocyte sedimentation rate and C-reactive protein are quite sensitive, but specificity is relatively low. Changes on radiographs appear at least 3 to 4 weeks after the onset of disease. Bone scan is a sensitive but not a specific test. CT provides structural details in the bone and intervertebral disc 76. MR imaging is the imaging modality of choice for the detection and evaluation of spondylitis, with superior imaging quality, 96% sensitivity, 92% specificity, and 94% accuracy 31, 76. Isolation of the causative bacteria from blood or bone tissue is confirmative of the diagnosis of infection. In many patients, percutaneous or open biopsy is required to make the definitive diagnosis of spondylitis and the responsible organism for the infection 76.
Surgical Treatment
The management of vascular graft infections should aim at eradication of the infectious source, without compromising perfusion to the corresponding end organ or extremity 1, 4-6, 13-22. However, the literature lacks information from large series on the management of patients with continuous spondylitis from infected aortic vascular grafts 7-12, 32. In the existing reports, the types of grafts, neurological symptoms of the patients, type of treatment and bacterial isolates varied, therefore, important conclusions cannot be drawn 7-12, 32, 33. By direct comparison of the respective results, it seems more likely for an infection recurrence or persistence after antibiotics administration and conservative treatment 7, 9. Therefore, since there are no specific guidelines for the treatment of aortic graft infections, probably, surgical treatment should be recommended for these patients 1, 11, 33-39. In this setting, the patients should be informed that the mortality and morbidity of this approach may be up to 20% 1, 39; reinfection of the aortic grafts may also occur, and usually proves fatal 1, 40.
Before the decision for surgical treatment, patient's comorbidities, abdomen hostility and overall clinical status should be evaluated 17. If the patient's general condition allows, conventional open surgery for complete removal of the infected graft is preferable 19. After graft explantation, necrotic tissue should be thoroughly debrided, and revascularization either via in situ or extra-anatomical aortic reconstruction should be performed 33. Aortic repair after explantation and subsequent coverage using omentum or fascia lata is often conducted 33. Biological vascular grafts (auto- or allografts) or a prosthesis with or without antimicrobial coating may be used 27. Extra-anatomical bypass (EAB) has theoretically a lower risk of infection than in situ repair, as it diverges from the site of infection; however, secondary infection has been reported in 10% of cases 34. There are also concerns about long term graft patency, lower flow rates and aortic stump blow-out after EAB. The risk of stump blow-out is avoided when in situ repair is performed, while the superiority of the latter in terms of mortality, amputation rate and patency is lately advocated 35.
Conservative treatment is the standard of care for spondylitis and is expected to be effective in most of cases 36. Surgical treatment is indicated for patients with spinal cord or cauda equina compression with progressive neurological deficits, and for those with spinal instability due to extensive bone destruction, significant deformity or when conservative treatment fails 37.
Conservative Treatment
To avoid submitting high-risk patients to a complex procedure with high morbidity and mortality, a conservative treatment approach with aggressive local wound care with preservation of most or all of the involved graft, percutaneous abscess drainage, and long-term antimicrobial suppression with systematic antimicrobial regimens and local antibiotic administration through the drains is often recommended in patients with aortic graft infection 1, 12, 30, 40, 41. However, conservative treatment is questionable for patients presenting with endograft infection after EVAR 18, 38, 39. Sharif et al. 38 reported an 100% mortality after conservative treatment, and Setacci et al. 39 reported a 38% mortality after conservative treatment, as opposed to 14.6% after graft explantation and EAB and 7.4% after explantation and in situ reconstruction. Other authors report no statistically significant differences in mortality rates between surgical and conservative treatment 41.
There have been few trials to study antimicrobial therapy as the main treatment of aortic graft infection. Most have been performed by surgeons, and, as such, antibiotic therapy was only mentioned as adjunct to surgical treatment. Therefore, there is no evidence on which to decide the optimal duration of antibiotic administration 1, 45. Oral antibiotics are not preferred as initial therapy for aortic graft infections. However, they are often used for long-term suppression, after intravenous agents have controlled sepsis and constitutional symptoms. These agents should be tailored based on cultures and susceptibilities, considering long-term side effects and potential development of microbial resistance 30. Accordingly, appropriate oral agents, with high bioavailability may allow a switch to per os administration 43.
There are no standardized guidelines regarding the optimal duration of antibiotic treatment against endograft infection; this may vary from 2 weeks to one year, although a minimum of 4-6 weeks of intravenous therapy, followed by up to 6 months of oral therapy is commonly recommended 42, 46. Most often, physicians rely on experts' opinion, individualized to each patient. There are a small number of cases reported in the literature, in which long-term suppressive antibiotic treatment was used when surgery was not possible or complete 47, 48, 64; in some occasions, lifelong antibiotics were administered 1, 47, 48. However, such therapy puts the patients at risk of adverse drug reactions and the acquisition of resistant organisms. Additionally, because of biofilm formation by bacteria and retention of the infected material, there is a possible risk for bacterial spreading or sepsis in patients with persistent percutaneous drainage and suppressive antibiotics administration. Successful antibiotic treatment with implant retention has been described in the setting of other prosthetic infections, including cochlear implants 65, spinal instrumentation 66, 67, and prosthetic joints. However, only in the last group has a randomized controlled trial confirmed the efficacy of this approach 67; in the other groups, as in aortic graft infections, details of this conservative approach come from case reports and small series only 7-12, 26, 32, 49-57. Therefore, in most cases, decision making is not clear and it is usually made by the input of the clinicians involved including vascular surgeons, microbiologists, infectious disease specialists and interventional radiologists, taking into consideration the individual patient's condition and health status. In contrast, the treatment of spondylitis includes antibiotic administration for at least 6 to 12 weeks, until CRP and ESR fall within normal ranges 44, and spinal immobilization. Bed rest may be recommended in the early stages, until resolution of acute symptoms, and ambulation with a brace may be recommended thereafter 42.
Conclusion
The rare incidence of aortic graft infection-related spondylitis and the nonspecific clinical manifestations may lead to confusing diagnosis. Physicians should be aware of this complication when treating patients with vascular grafts, especially in the case of persistent back pain, fever of unknown origin and abdominal pain. Management requires a multidisciplinary approach involving vascular surgeons, radiologists, infectious disease specialists and spine surgeons. Thus, the treatment of this clinical condition remains problematic. The patients' general health status and comorbidities may not allow an open vascular surgery, and spinal infection is generally treated successfully by conservative means. Therefore, often, long-term antibiotic therapy and close follow-up is the only treatment option for these patients.
References
- 1.Tsapralis D, Charalampopoulos A, Lazaris AM. Abdominal aortic graft infection; Grundmann R, ed; Diagnosis, Screening and Treatment of Abdominal, Thoracoabdominal and Thoracic Aortic Aneurysms; InTech 2011. [Google Scholar]
- 2.Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491–9. doi: 10.1007/BF02015271. [DOI] [PubMed] [Google Scholar]
- 3.Coselli JS, Spiliotopoulos K, Preventza O, de la Cruz KI, Amarasekara H, Green SY. Open aortic surgery after thoracic endovascular aortic repair. Gen Thorac Cardiovasc Surg. 2016;64:441–9. doi: 10.1007/s11748-016-0658-8. [DOI] [PubMed] [Google Scholar]
- 4.Chalmers N, Eadington DW, Gandanhamo D, Gillespie IN, Ruckley CV. Case report: infected false aneurysm at the site of an iliac stent. Br J Radiol. 1993;66:946–8. doi: 10.1259/0007-1285-66-790-946. [DOI] [PubMed] [Google Scholar]
- 5.Hobbs SD, Kumar S, Gilling-Smith GL. Epidemiology and diagnosis of endograft infection. J Cardiovasc Surg (Torino) 2010;51:5–14. [PubMed] [Google Scholar]
- 6.Fiorani P, Speziale F, Calisti A, Misuraca M, Zaccagnini D, Rizzo L. et al. Endovascular graft infection: preliminary results of an international enquiry. J Endovasc Ther. 2003;10:919–27. doi: 10.1177/152660280301000512. [DOI] [PubMed] [Google Scholar]
- 7.Lowe C, Chan A, Wilde N, Hardy S. Infected endovascular aneurysm repair graft complicated by vertebral osteomyelitis. J Vasc Surg. 2012;56:826–8. doi: 10.1016/j.jvs.2012.03.268. [DOI] [PubMed] [Google Scholar]
- 8.Mavrogenis AF, Triantafyllopoulos GK, Kokkinis K, Stefos A, Sipsas NV, Pneumaticos SG. Continuous L3 spondylitis caused by an infected endovascular aortic graft. Surg Infect (Larchmt) 2014;15:861–2. doi: 10.1089/sur.2013.219. [DOI] [PubMed] [Google Scholar]
- 9.Faccenna F, Alunno A, Castiglione A, Carnevalini M, Venosi S, Gossetti B. Large aortic pseudoaneurysm and subsequent spondylodiscitis as a complication of endovascular treatment of iliac arteries. Thorac Cardiovasc Surg. 2013;61:606–9. doi: 10.1055/s-0033-1333843. [DOI] [PubMed] [Google Scholar]
- 10.de Koning HD, van Sterkenburg SM, Pierie ME, Reijnen MM. Endovascular abdominal aortic aneurysm repair complicated by spondylodiscitis and iliaco-enteral fistula. J Vasc Surg. 2008;47:1330–2. doi: 10.1016/j.jvs.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 11.Blanch M, Berjon J, Vila R, Simeon JM, Romera A, Riera S, Cairols MA. The management of aortic stent-graft infection: endograft removal versus conservative treatment. Ann Vasc Surg. 2010;24:554. doi: 10.1016/j.avsg.2009.11.003. e1-5. [DOI] [PubMed] [Google Scholar]
- 12.Laser A, Baker N, Rectenwald J, Eliason JL, Criado-Pallares E, Upchurch GR Jr. Graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2011;54:58–63. doi: 10.1016/j.jvs.2010.11.111. [DOI] [PubMed] [Google Scholar]
- 13.Jayakrishnan TT, Keyashian B, Amene J, Malinowski M. Aortic Endograft Infection by Pasteurella multocida: A Rare Case. Vasc Endovascular Surg. 2016;50:435–7. doi: 10.1177/1538574416665987. [DOI] [PubMed] [Google Scholar]
- 14.Silberfein EJ, Lin PH, Bush RL, Zhou W, Lumsden AB. Aortic endograft infection due to Pasteurella multocida following a rabbit bite. J Vasc Surg. 2006;43:393–5. doi: 10.1016/j.jvs.2005.10.067. [DOI] [PubMed] [Google Scholar]
- 15.Etienne H, Touma J, Becquemin JP. Unusual Acute Onset of Abdominal Aortic Endograft Infection by Propionibacterium acnes after Coil Embolization for Type II Endoleak. Ann Vasc Surg; 2016. [DOI] [PubMed] [Google Scholar]
- 16.Ferrero E, Ferri M, Viazzo A, Trevisan A, Psacharopulo D, Ripepi M. et al. Fungal infection of aortic endograft because of Aspergillus fumigatus. Ann Vasc Surg. 2014;28:1795. doi: 10.1016/j.avsg.2014.04.012. e11-4. [DOI] [PubMed] [Google Scholar]
- 17.Setacci C, Chisci E, Setacci F, Ercolini L, de Donato G, Troisi N. et al. How To Diagnose and Manage Infected Endografts after Endovascular Aneurysm Repair. Aorta (Stamford) 2014;2:255–64. doi: 10.12945/j.aorta.2014.14-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ducasse E, Calisti A, Speziale F, Rizzo L, Misuraca M, Fiorani P. Aortoiliac stent graft infection: current problems and management. Ann Vasc Surg. 2004;18:521–6. doi: 10.1007/s10016-004-0075-9. [DOI] [PubMed] [Google Scholar]
- 19.Heyer KS, Modi P, Morasch MD, Matsumura JS, Kibbe MR, Pearce WH. et al. Secondary infections of thoracic and abdominal aortic endografts. J Vasc Interv Radiol. 2009;20:173–9. doi: 10.1016/j.jvir.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 20.Kieffer E, Sabatier J, Plissonnier D, Knosalla C. Prosthetic graft infection after descending thoracic/thoracoabdominal aortic aneurysmectomy: management with in situ arterial allografts. J Vasc Surg. 2001;33:671–8. doi: 10.1067/mva.2001.112314. [DOI] [PubMed] [Google Scholar]
- 21.Valentine RJ. Diagnosis and management of aortic graft infection. Semin Vasc Surg. 2001;14:292–301. doi: 10.1053/svas.2001.27874. [DOI] [PubMed] [Google Scholar]
- 22.McCready RA, Bryant MA, Divelbiss JL, Chess BA, Chitwood RW, Paget DS. Arterial infections in the new millenium: an old problem revisited. Ann Vasc Surg. 2006;20:590–5. doi: 10.1007/s10016-006-9107-y. [DOI] [PubMed] [Google Scholar]
- 23.Thiruganasambandamoorthy V, Turko E, Ansell D, Vaidyanathan A, Wells GA, Stiell IG. Risk factors for serious underlying pathology in adult emergency department nontraumatic low back pain patients. J Emerg Med. 2014;47:1–11. doi: 10.1016/j.jemermed.2013.08.140. [DOI] [PubMed] [Google Scholar]
- 24.Brouw LW, van Weerelt CT, van Guldener C, Geenen GP, van der Laan L. Non invasive treatment of peri-aortic inflammation after endovascular graft. Eur J Vasc Endovasc Surg. 2007;34:179–81. doi: 10.1016/j.ejvs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Duarte RM, Vaccaro AR. Spinal infection: state of the art and management algorithm. Eur Spine J. 2013;22:2787–99. doi: 10.1007/s00586-013-2850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.FitzGerald SF, Kelly C, Humphreys H. Diagnosis and treatment of prosthetic aortic graft infections: confusion and inconsistency in the absence of evidence or consensus. J Antimicrob Chemother. 2005;56:996–9. doi: 10.1093/jac/dki382. [DOI] [PubMed] [Google Scholar]
- 27.Legout L, D'Elia PV, Sarraz-Bournet B, Haulon S, Meybeck A, Senneville E. et al. Diagnosis and management of prosthetic vascular graft infections. Med Mal Infect. 2012;42:102–9. doi: 10.1016/j.medmal.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Fukuchi K, Ishida Y, Higashi M, Tsunekawa T, Ogino H, Minatoya K. et al. Detection of aortic graft infection by fluorodeoxyglucose positron emission tomography: comparison with computed tomographic findings. J Vasc Surg. 2005;42:919–25. doi: 10.1016/j.jvs.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 29.Love C, Palestro CJ. Nuclear medicine imaging of bone infections. Clin Radiol. 2016;71:632–46. doi: 10.1016/j.crad.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence PF. Conservative treatment of aortic graft infection. Semin Vasc Surg. 2011;24:199–204. doi: 10.1053/j.semvascsurg.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Modic MT, Feiglin DH, Piraino DW, Boumphrey F, Weinstein MA, Duchesneau PM. et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157:157–66. doi: 10.1148/radiology.157.1.3875878. [DOI] [PubMed] [Google Scholar]
- 32.D'Ettorre G, Ceccarelli G, Zaffiri L, Falcone M, Mastroianni CM, Venditti M. et al. Infectious aortitis and spondylodiscitis in patients with endovascular stents. Minerva Med. 2009;100:167–70. [PubMed] [Google Scholar]
- 33.Smeds MR, Duncan AA, Harlander-Locke MP, Lawrence PF, Lyden S, Fatima J. et al. Treatment and outcomes of aortic endograft infection. J Vasc Surg. 2016;63:332–40. doi: 10.1016/j.jvs.2015.08.113. [DOI] [PubMed] [Google Scholar]
- 34.Yeager RA, Taylor LM Jr, Moneta GL, Edwards JM, Nicoloff AD, McConnell DB. et al. Improved results with conventional management of infrarenal aortic infection. J Vasc Surg. 1999;30:76–83. doi: 10.1016/s0741-5214(99)70178-3. [DOI] [PubMed] [Google Scholar]
- 35.Berger P, Moll FL. Aortic graft infections: is there still a role for axillobifemoral reconstruction? Semin Vasc Surg. 2011;24:205–10. doi: 10.1053/j.semvascsurg.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Mavrogenis AF, Igoumenou V, Tsiavos K, Megaloikonomos P, Panagopoulos GN, Vottis C. et al. When and how to operate on spondylodiscitis: a report of 13 patients. Eur J Orthop Surg Traumatol. 2016;26:31–40. doi: 10.1007/s00590-015-1674-6. [DOI] [PubMed] [Google Scholar]
- 37.Guerado E, Cervan AM. Surgical treatment of spondylodiscitis. An update. Int Orthop. 2012;36:413–20. doi: 10.1007/s00264-011-1441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharif MA, Lee B, Lau LL, Ellis PK, Collins AJ, Blair PH. et al. Prosthetic stent graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2007;46:442–8. doi: 10.1016/j.jvs.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 39.Setacci C, De Donato G, Setacci F, Chisci E, Perulli A, Galzerano G. et al. Management of abdominal endograft infection. J Cardiovasc Surg (Torino) 2010;51:33–41. [PubMed] [Google Scholar]
- 40.Calligaro KD, Veith FJ, Yuan JG, Gargiulo NJ, Dougherty MJ. Intraabdominal aortic graft infection: Complete or partial graft preservation in patients at very high risk. J Vasc Surg. 2003;38(6):1199–205. doi: 10.1016/s0741-5214(03)01043-7. [DOI] [PubMed] [Google Scholar]
- 41.Cernohorsky P, Reijnen MM, Tielliu IF, van Sterkenburg SM, van den Dungen JJ, Zeebregts CJ. The relevance of aortic endograft prosthetic infection. J Vasc Surg. 2011;54:327–33. doi: 10.1016/j.jvs.2010.12.067. [DOI] [PubMed] [Google Scholar]
- 42.Zarghooni K, Rollinghoff M, Sobottke R, Eysel P. Treatment of spondylodiscitis. Int Orthop. 2012;36:405–11. doi: 10.1007/s00264-011-1425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. 2010;362:1022–9. doi: 10.1056/NEJMcp0910753. [DOI] [PubMed] [Google Scholar]
- 44.Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65(Suppl 3):iii11–24. doi: 10.1093/jac/dkq303. [DOI] [PubMed] [Google Scholar]
- 45.Perera GB, Fujitani RM, Kubaska SM. Aortic graft infection: update on management and treatment options. Vasc Endovascular Surg. 2006;40(1):1–10. doi: 10.1177/153857440604000101. [DOI] [PubMed] [Google Scholar]
- 46.Nevelsteen A, Lacroix H, Suy R. Autogenous reconstruction with the lower extremity deep veins: an alternative treatment of prosthetic infection after reconstructive surgery for aortoiliac disease. J Vasc Surg. 1995;22(2):129–34. doi: 10.1016/s0741-5214(95)70106-0. [DOI] [PubMed] [Google Scholar]
- 47.Roy D, Grove DI. Efficacy of long-term antibiotic suppressive therapy in proven or suspected infected abdominal aortic grafts. J Infect. 2000;40(2):184–7. doi: 10.1016/s0163-4453(00)80014-6. [DOI] [PubMed] [Google Scholar]
- 48.Baddour LM; Infectious Diseases Society of America's Emerging Infections Network. Long-term suppressive antimicrobial therapy for intravascular device-related infections. Am J Med Sci. 2001;322(4):209–12. doi: 10.1097/00000441-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Gatibelza ME, Laroye B, Lombard J, Mameli A, Thomas E. Management of a ruptured infected abdominal aortic aneurysm and a spondylodiscitis due to Gemella haemolysans. Ann Vasc Surg. 2009;23(4):536.e13–7. doi: 10.1016/j.avsg.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Bogaert J, Lateur L, Baert AL. Case report 762. Torulopsis glabrata spondylodiscitis as a late complication of an infected abdominal aortic graft. Skeletal Radiol. 1992;21(8):550–4. doi: 10.1007/BF00195242. [DOI] [PubMed] [Google Scholar]
- 51.Dreyfus J, Grange L, Sessa C, Juvin R. Pyogenic discitis revealing infrarenal aortic prosthetic graft infection impinging on the left ureter. Joint Bone Spine. 2003;70(2):140–2. doi: 10.1016/s1297-319x(03)00022-8. [DOI] [PubMed] [Google Scholar]
- 52.Piquet P, Raoult D, Tranier P, Mercier C. Coxiella burnetii infection of pseudoaneurysm of an aortic bypass graft with contiguous vertebral osteomyelitis. J Vasc Surg. 1994 Jan;19(1):165–8. doi: 10.1016/s0741-5214(94)70131-8. [DOI] [PubMed] [Google Scholar]
- 53.Aguado JM, Valle R, Arjona R, Ferreres JC, Gutierrez JA. Aortic bypass graft infection due to Aspergillus: report of a case and review. Clin Infect Dis. 1992 Apr;14(4):916–21. doi: 10.1093/clinids/14.4.916. [DOI] [PubMed] [Google Scholar]
- 54.Brandt SJ, Thompson RL, Wenzel RP. Mycotic pseudoaneurysm of an aortic bypass graft and contiguous vertebral osteomyelitis due to Aspergillus fumigatus. Am J Med. 1985 Aug;79(2):259–62. doi: 10.1016/0002-9343(85)90019-1. [DOI] [PubMed] [Google Scholar]
- 55.Anderson J, Kron IL. Treatment of Aspergillus infection of the proximal aortic prosthetic graft with associated vertebral osteomyelitis. J Vasc Surg. 1984 Jul;1(4):579–81. doi: 10.1067/mva.1984.avs0010579. [DOI] [PubMed] [Google Scholar]
- 56.Glotzbach RE. Aspergillus terreus infection of pseudoaneurysm of aortofemoral vascular graft with contiguous vertebral osteomyelitis. Am J Clin Pathol. 1982 Feb;77(2):224–7. doi: 10.1093/ajcp/77.2.224. [DOI] [PubMed] [Google Scholar]
- 57.Solomon B, Kim B, Rockman C, Veith FJ, Jacobowitz G. Aortic endograft infection with aortoduodenal fistula associated with adjacent vertebral body mycobacterial osteomyelitis (Pott's disease) Ann Vasc Surg. 2012;26(2):276.e1–4. doi: 10.1016/j.avsg.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 58.O'Brien T, Collin J. Prosthetic vascular graft infection. Br J Surg. 1992;79(12):1262–7. doi: 10.1002/bjs.1800791205. [DOI] [PubMed] [Google Scholar]
- 59.Kitamura T, Morota T, Motomura N, Ono M, Shibata K, Ueno K, Kotsuka Y, Takamoto S. Management of infected grafts and aneurysms of the aorta. Ann Vasc Surg. 2005;19(3):335–42. doi: 10.1007/s10016-005-0006-4. [DOI] [PubMed] [Google Scholar]
- 60.Kirksey L, Brener BJ, Hertz S, Parsonnet V. Prophylactic antibiotics prior to bacteremia decrease endovascular graft infection in dogs. Vasc Endovascular Surg. 2002;36(3):171–8. doi: 10.1177/153857440203600303. [DOI] [PubMed] [Google Scholar]
- 61.Murphy EH, Szeto WY, Herdrich BJ, Jackson BM, Wang GJ, Bavaria JE, Fairman RM, Woo EY. The management of endograft infections following endovascular thoracic and abdominal aneurysm repair. J Vasc Surg. 2013;58(5):1179–85. doi: 10.1016/j.jvs.2013.04.040. [DOI] [PubMed] [Google Scholar]
- 62.Capoccia L, Mestres G, Riambau V. Current technology for the treatment of infection following abdominal aortic aneurysm (AAA) fixation by endovascular repair (EVAR) J Cardiovasc Surg (Torino) 2014;55:381–9. [PubMed] [Google Scholar]
- 63.ZImmerli W. Bone and Joint Infections: From Microbiology to Diagnostics and Treatment. John Wiley & Sons, Inc; 2015. [Google Scholar]
- 64.Coselli JS, Koksoy C, LeMaire SA. Management of thoracic aortic graft infections. Ann Vasc Surg. 1999;67:1990–3. doi: 10.1016/s0003-4975(99)00355-0. [DOI] [PubMed] [Google Scholar]
- 65.Yu KCY, Hegarty JL, Gantz BJ. et al. Conservative management of infections in cochlear implant recipients. Otolaryngol Head Neck Surg. 2001;125:66–70. doi: 10.1067/mhn.2001.116444. [DOI] [PubMed] [Google Scholar]
- 66.Bose B. Delayed infection after instrumented spine surgery: case reports and review of the literature. Spine J. 2003;3:394–9. doi: 10.1016/s1529-9430(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 67.Jutte PC, Castelein RM. Complications of pedicle screws in lumbar and lumbosacral fusions in 105 consecutive primary operations. Eur Spine J. 2002;11:594–8. doi: 10.1007/s00586-002-0469-8. [DOI] [PubMed] [Google Scholar]
- 68.Zimmerli W, Widmer AF, Blatter M. et al. Role of rifampicin for treatment of orthopaedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279:1537–41. doi: 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]
- 69.Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976) 2000;25:1668–1679. doi: 10.1097/00007632-200007010-00010. [DOI] [PubMed] [Google Scholar]
- 70.McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34:1342–1350. doi: 10.1086/340102. [DOI] [PubMed] [Google Scholar]
- 71.Darouiche RO. Spinal epidural abscess. N Eng J Med. 2006;355:2012–2020. doi: 10.1056/NEJMra055111. [DOI] [PubMed] [Google Scholar]
- 72.Grammatico L, Baron S, Rusch E. et al. Epidemiology of vertebral osteomyelitis (VO) in France: analysis of hospital-discharge data 2002-2003. Epidemiol Infect. 2008;136:653–660. doi: 10.1017/S0950268807008850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39:10–17. doi: 10.1016/j.semarthrit.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 74.Park KH, Oh WS, Kim ES. et al. Factors associated with ciprofloxacin- and cefotaxime-resistant Escherichia coli in women with acute pyelonephritis in the emergency department. Int J Infect Dis. 2014;23:8–13. doi: 10.1016/j.ijid.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 75.Kang SJ, Jang HC, Jung SI. et al. Clinical characteristics and risk factors of pyogenic spondylitis caused by gram-negative bacteria. PLoS One. 2015;10(5):e0127126. doi: 10.1371/journal.pone.0127126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.An HS, Seldomridge JA. Spinal infections: diagnostic tests and imaging studies. Clin Orthop Relat Res. 2006;444:27–33. doi: 10.1097/01.blo.0000203452.36522.97. [DOI] [PubMed] [Google Scholar]