ABSTRACT
Clinical and laboratory parameters including blood and cerebrospinal fluid (CSF) neopterin were investigated in human-T-lymphotropic-virus-type-I associated-myelopathy/tropical-spastic-paraparesis-HAM/TSP and in HTLV-I carriers. HAM/TSP (n = 11, 2 males/9 females, median age = 48 years), recently diagnosed HTLV-I carriers (n = 21, 15 females/6 males, median age = 44 years), healthy individuals (n = 20, 10 males/10 females, median age = 34.6 years) from the Brazilian Amazon (Manaus, Amazonas State) were investigated. Neopterin was measured (IBL ELISA Neopterin, Germany) in serum samples of all the participants, in CSF of 9 HAM/TSP patients as well as in 6 carriers. In HAM/TSP patients, CSF cell counts, protein and glucose were measured, the Osame’s motor-disability-score/OMDS was determined, and brain/spinal cord magnetic-resonance-imaging (MRI) was performed. HAM/TSP patients had normal CSF glucose, leukocyte counts; and normal protein levels predominated. Brain-MRI showed white-matter lesions in 7 out of 11 HAM/TSP patients. OMDS varied from 2-8: 9 were able to walk, 2 were wheel-chair-users. The median serum neopterin concentration in HAM/TSP patients was 6.6 nmol/ L; min. 2.8- max. 12.5 nmol/ L); was lower in carriers (4.3 nmol/L; min. 2.7- max. 7.2 nmol/ L) as well as in healthy participants (4.7 nmol/ L; min. 2.7- max. 8.0 nmol/ L) (p < 0.05). CSF neopterin concentrations in HAM/TSP patients were higher than in serum samples, and higher compared to carriers (p < 0.05). Carriers had similar serum-CSF neopterin concentrations compared to healthy participants. Variable clinical and laboratory profiles were seen in HAM/TSP patients, however our results support the neopterin measurement as a potential biomarker of disease activity.
KEYWORDS: HAM/TSP patients, HTLV-I carriers, Neopterin, Cerebrospinal fluid
INTRODUCTION
Worldwide, around 5-10 million people may be infected with the human T cell lymphotropic virus type I (HTLV-I) with high prevalences in southern Japan, parts of West Africa, the Caribbean Islands, Melanesia and South America 1 , 2 . Brazil is considered one of the largest endemic areas of HTLV-I and associated diseases, and the city of Salvador, capital of the State of Bahia presents the highest prevalence (1.76% in the overall population) 3 . Nevertheless, HTLV-I worldwide prevalence is probably underestimated since it remains uninvestigated in many large and highly populated regions 4 . In endemic areas, mother to child transmission, which is mainly linked to extended breastfeeding is a main route of HTLV-I transmission, but sexual intercourse, blood transfusion and contaminated needle sharing are other forms of transmission 4 . Most HTLV-I infected individuals remain lifelong asymptomatic carriers, while 2-5% may develop adult T-cell leukemia/ATL and 0.25-3.8% may develop HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) 4 .
HAM/TSP, the most common neurological manifestation of HTLV-1 is a slowly progressive inflammatory demyelinating disease of the central nervous system (CNS) with gradual spastic paraparesis, neurogenic bladder disturbances, and other sensory signs 5 . High proviral loads are associated with faster disease progression, increased proliferation and migration of HTLV-I infected lymphocytes to the CNS 6 . HAM/TSP is an immune-mediated disease of the CNS, but its precise immunopathogenic mechanism is not completely understood. CD4+ T cells represent the main virus reservoirs and infiltrates of virus infected CD4+/CD8+ T cells in the spinal cord and increased production of inflammatory cytokines such as IFN𝛾, TNFα and IL1β have been described 6 , 7 , 8 . Alterations in magnetic resonance imaging (MRI) of the brain and spinal cord in HAM/TSP are probably a result of the virus-induced inflammatory process 9 , 10 . Several laboratory alterations in systemic and cerebrospinal fluid (CSF) have been described in HAM/TSP patients 11 , 12 , 13 .
In neuro-inflammatory diseases, neopterin concentration in biological specimens has been proposed as a marker of disease activity indicating cellular-mediated immunity (CMI) activation. Neopterin, a pteridine derived from guanosine triphosphate is produced by activated monocytes-macrophages and dendritic cells following IFN𝛾 production by TH1 cells 14 , 15 . Neopterin has been associated with HAM/TSP diagnosis and prognosis indicating the degree of intrathecal CMI activation 16 , 17 .
This study describes clinical and laboratory parameters which include serum and CSF neopterin measurements in HAM/TSP patients and in asymptomatic anti HTLV-I positive carriers from the Brazilian Amazon.
MATERIAL AND METHODS
HAM/TSP Patients and HTLV-I Carriers
Fifty-two participants from the city of Manaus (Amazonas State, North Brazil) were investigated: 11 HAM/TSP patients, 21 HTLV-I carriers and 20 healthy individuals. HAM/TSP patients (> 18 years old, positive anti HTLV-I in serum and CSF, confirmed by western blot or PCR) were recruited at the Neurology Ambulatory, Getúlio Vargas University Hospital, Federal University of Amazonas. The following exclusion criteria were considered: neoplastic or vascular diseases of the spinal cord, neurodegenerative or demyelinating diseases, myelopathy associated with vitamin B12 deficiency, infectious myelopathies, auto-immune diseases, positive serology to CMV, HBV, HCV, HIV, HTLV-II, syphilis or Chagas disease; history of malaria or pulmonary tuberculosis; previous treatment with corticosteroids or other immune modulating drug. The clinical diagnosis of HAM/TSP was conducted by an expert neurologist (Takatani M) according to criteria proposed by the World Health Organization 18 . Patients were classified according to the Osame’s motor disability score/OMDS grading incapacity level from 0 (normal gait and running), to 10 (completely bedridden)19. MRI of the brain and spinal column was performed for the differential diagnosis with other brain or spinal cord pathologies as multiple sclerosis. Gender, age, duration of disease and symptoms were also recorded.
Recently diagnosed, asymptomatic anti HTLV-I positive carriers, confirmed by western-blot or PCR were recruited among blood donors of a main public blood bank (Hematology and Hemotherapy Foundation from Amazonas State /FHEMOAM), Manaus, Amazonas State. HTLV-I carriers (both genders, > 18 years old, without myelopathy-related symptoms) were included. Exclusion criteria were: chronic liver, kidney or heart disease, pneumopathy, dermatological diseases, any sign of infectious disease such as fever, auto-immune diseases, positive serology for HBV, HCV, HIV, HTLV-II, syphilis, Chagas disease and pregnancy.
For comparisons of serum neopterin levels, 20 healthy individuals (10 males, 10 females; median age = 34.6 years, min.23- max. 44) were recruited among blood donors at FHEMOAM. They were all seronegative for HIV, HCV, HBV, Chagas disease, syphilis and HTLV-I/II.
Clinical Specimens
HAM/TSP patients had venous blood and CSF collected at Getúlio Vargas University Hospital; HTLV-I carriers had biological samples collected at FHEMOAM. CSF (8mL) was collected by lumbar puncture performed by a single expert professional (Takatani M); only samples devoid of red blood cells were used. For HAM/TSP patients and HTLV-I carriers who consented, paired blood and CSF samples were collected within a two-hours period. Biological samples were aliquoted and stored at -80oC until tested. For ethical reasons, only blood samples were collected from healthy participants.
HTLV-I Diagnosis and Neopterin Measurements
HTLV-I status of all the participants was determined by ELISA (MUREX HTLV I+ II GE80/81, MUREX Diagnosticos, Brasil) and confirmed by western blot (HTLV BLOT 2.4, Genelabs Diagnostics, Singapore) or PCR as previously described 20 .
Competitive ELISA was used to evaluate neopterin levels in serum and CSF (IBL neopterin ELISA, Hamburg, Germany), the normal serum neopterin concentration being < 10 nmol/ L. As previously reported, the normal neopterin CSF value was considered 4.2 nmol/ L. 21 CSF was also used for routine laboratory tests (cell count, total protein, glucose levels). Comorbidities and other exclusion conditions were also evaluated through the following laboratory parameters: blood cell count, erythrocyte sedimentation rate/ESR, lipidogram, glucose, urea, creatinine, AST, ALT, ALP, prothrombin and bilirubin levels.
Statistical Analysis
Exploratory data analyses including mean, medians, box-plots, interquartile ranges, frequencies were calculated by using the t student test, Pearson’s correlation. Kruskal Wallis and Man Whitney tests were used for both HAM/TSP and asymptomatic carriers data; when applicable the Graph Pad Prism 6 was used; p values < 0.05 were considered significant.
RESULTS
Clinical and Demographic Profiles of HAM/TSP Patients and HTLV-I Carriers
Among HAM/TSP patients (Table 1), females predominated; the median age was 48 years (27-66 years), the duration of symptoms ranged from 1-16 years and the Osame’s motor disability score/OMDS varied from 2 to 8. Most HAM/TSP patients (9/11, 81,9%) were able to walk (OMDS ≤ 6) while 2 (18,1%) were wheel chair-users. The lowest disability scores were seen in patients with the shortest duration of disease (patients # 7 and 10: 1 and 3 years of disease, respectively). All the HAM/TSP patients reported difficulty to walk, around half (54.5%) had low-back pain and paresthesia in the lower limbs was the most common sensory complaint (45.4%). The two male HAM/TSP patients did not report erectile or bladder dysfunction while 8 out of 9 female patients reported bladder dysfunction, with urinary frequency alterations, urgency or incontinence. Among 21 HTLV-I carriers (15 females, 6 males), the median age was 44 years (20-54 years).
Table 1. Main demographic, clinical and laboratory features of HAM/TSP patients.
| Case # | Gender | Age (years) | OMDS | Disease (years) | Neopterin Serum nmol/L | Neopterin CSF nmol/L | Protein CSF mg/dL | Glucose CSF mg/dL | Cell count CSF cell/mm3 |
| 1 | F | 56 | 4 | 10 | 6.5 | 7.8 | 32 | 50 | 4 |
| 2 | F | 52 | 4 | 5 | 6.2 | 16.5 | 40 | 11 | 3 |
| 3 | F | 66 | 8 | 16 | 35 | 166 | 44 | 47 | 4 |
| 4 | F | 62 | 4 | 6 | 2.8 | 7.8 | 41 | 41 | 21 |
| 5 | F | 55 | 5 | 8 | 6.8 | NA | NA | NA | NA |
| 6 | F | 44 | 5 | 15 | 4.8 | 12 | 45.7 | 50 | 1 |
| 7 | M | 48 | 2 | 1 | 12 | 20 | 73 | 58 | 3 |
| 8 | M | 27 | 7 | 4 | 10 | 17 | 47 | 58 | 18 |
| 9 | F | 40 | 4 | 7 | 4 | 17 | 42 | 53 | 13 |
| 10 | F | 40 | 2 | 3 | 12.5 | 18 | 18 | 50 | 7 |
| 11 | F | 44 | 6 | 14 | 12.5 | 26 | 16 | 26 | 1 |
M: male, F: Female; OMDS: Osame’s motor disability score; CSF: cerebrospinal fluid; Reference values for: serum neopterin concentration<=10nmol/L, CSF neopterin concentration<= 4.2nmol/L, CSF protein concentration=15-40mg/dl; CSF glucose levels=2/3 of serum glucose levels or 50-70mg/dl, CSF cell count<=4 cell/mm3; NA= data not available because patient did not consent CSF collection.
Leukocyte, Protein and Glucose levels in CSF of HAM/TSP patients and HTLV-I Carriers
Three HAM/TSP patients had CSF protein levels above the normal range (patients # 6, 7 and 8, Table 1) and patients # 3, 4 and 9 presented CSF protein concentration slightly higher than the normal range. The highest CSF protein concentration was seen in the most recently diagnosed patient (# 7: 1 year of disease). All the HAM/TSP patients had normal levels CSF glucose, four patients had CSF leukocyte counts above the normal range (# 4, 8, 9, 10), and lymphocytes represented the predominating cell population. In HTLV-I carriers (Table 2), only case # 2 had normal CSF protein level, all the carriers had normal CSF glucose levels and leukocyte cell counts were above the normal limit in 2 out of 6 carriers.
Table 2. Main demographic and laboratory features of HTLV-I carriers* .
| Control # | Gender | Age (years) | Neopterin serum nmol/L | Neopterin CSF nmol/L | Protein CSF mg/dL | Glucose CSF mg/dL | Cell count CSF cel/mm3 |
| 1 | M | 38 | 4.3 | 2.2 | 56 | 68 | 2 |
| 2 | F | 46 | 3.6 | 2.7 | 39 | 77 | 0 |
| 3 | F | 47 | 4.5 | 3.2 | 75 | 61 | 8 |
| 4 | F | 35 | 4.8 | 5.2 | 98 | 57 | 14 |
| 5 | M | 54 | 6.6 | 3.0 | 88 | 78 | 1 |
| 6 | F | 36 | 2.8 | 4.5 | 52 | 67 | 3 |
* HTLV-I carriers who consented to have CSF collected; M: male, F: Female; Age (years); CSF: cerebrospinal fluid; Reference values for: serum neopterin concentration ≤ 10 nmol/L, CSF neopterin concentration ≤ 4.2 nmol/L, CSF protein concentration = 15-40 mg/dL; CSF glucose levels = 2/3 of serum glucose levels or 50-70 mg/dL, CSF cell count ≤ 4 cell/mm3.
Neurologic Characterization of HAM/TSP Patients
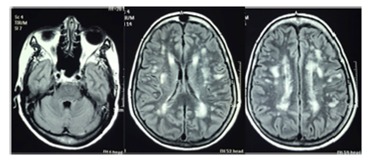
Brain MRI of 7 out of 11 HAM/TSP patients (# 1, 2, 3, 4, 5, 9, 10; Table 1) presented unspecific lesions in the white matter (WM) described as gliosis. Patient #1 showed multiple white matter confluent lesions extended from periventricular to subcortical locations, suggestive of a demyelinating disease (Figure 1) without lesions in the posterior fossa or in the thoracic spine. This 56 years old female patient, with 10 years of disease, had a motor incapacity score = 4 (needs support while using stairs but could walk without assistance). Only one patient (# 3) showed spinal cord MRI alteration while no sign of spinal cord swelling was seen in the other patients. Cerebral atrophy was observed in one HAM/TSP patient (# 5, Table 1) without association with dementia. No correlation between the symptoms and the number of WM lesions was found, and no evidence of cerebellar dysfunction was detected. Cranial nerve functions were normal in all the patients.
Figure 1. Brain magnetic resonance imaging (MRI, axial flair) showing hyper intense multiple lesions in the periventricular and subcortical white matter in a 56-year-old female HAM/TSP patient with 10 years of disease symptoms and motor disability grade 4.
Neopterin Levels in Peripheral Blood and CSF in HAM/TSP Patients and HTLV-I Carriers
Serum neopterin levels in healthy individuals (n = 20, median = 4.7 nmol/ L; min. 2.7- max. 8.0 nmol/L) and asymptomatic HTLV-I carriers were similar (n = 21; median = 4.3 nmol /L; min. 2.7- max. 7.2 nmol/ L) (Figure 2A; p > 0.05). However serum neopterin concentrations in HAM/TSP patients (n = 11; median = 6.8; min. 2.8-max. 35 nmol/ L, Table 1, Fig. 2A) were higher compared to the HTLV-I carriers (p = 0.0033) and the healthy participants (p < 0.0001).
Figure 2. (A). Neopterin concentrations in serum of healthy individuals, in serum and cerebrospinal fluid (CSF) of HTLV-I asymptomatic carriers and of HAM/TSP patients. Boxes encompass 25th and 75th percentiles of the distribution; black lines within boxes refer to the median values; (B). Neopterin concentrations in paired serum and CSF samples of HAM/TSP patients; (C). Neopterin concentrations in paired serum and CSF samples of HTLV-I carriers.

In HAM/TSP patients, the median serum concentration of neopterin was 6.8 nmol/ L (2.8-35 nmol/ L) while the median CSF neopterin was 17 nmol/ L (7.8-166 nmol/ L), Table 1, Figures 2A, 2B). In HTLV-I carriers (n = 6) normal serum and CSF neopterin levels were detected (serum: median = 4.4 nmol/ L; min. 2.7- max. 7.2 nmol/ L; CSF median = 3.1 nmol/L; min. 2.2 - max. 5.2 nmol/ L).
CSF neopterin concentrations in HAM/TSP patients (n = 10) were higher when compared to HTLV-I carriers (p= 0.0016, Fig. 2A). In HAM/TSP, paired serum-CSF analysis of neopterin showed consistently higher levels in CFS (p = 0.024, Figures 2A, 2B). In contrast, in HTLV-I carriers (n = 6), the median neopterin concentration in serum and in CSF was similar (p > 0.05) (Figures 2A, 2C). However, in HTVL-I carriers a heterogeneous pattern was seen: in 4 out of 6 carriers neopterin levels in serum samples were higher compared to CSF, whereas in two other carriers, neopterin levels were higher in CSF compared to the serum samples.
In HAM/TSP patients, a moderate correlation was found between CSF neopterin and motor incapacity level/OMDS (r = 0.62, p = 0.043). In HAM/TSP patients we have also analyzed if the difference of neopterin levels between the two compartments (CSF minus serum) would correlate with the data available. These analyses showed a moderate correlation between the difference of neopterin in CSF minus serum and OMDS (r = 0.62, p = 0.05). No correlation was found between CSF neopterin and duration of disease, CSF cell count, CSF glucose, CSF protein concentration (p> 0.05). No correlation was found between serum neopterin levels and duration of disease or motor incapacity level (p > 0.05). In asymptomatic HTLV-I carriers no correlation was observed between CSF neopterin concentrations and CSF cell counts, protein or glucose concentrations.
DISCUSSION
Brazil represents one of the largest endemic areas for HTLV-I, however there are scarce data from many areas in the country including the Amazonas State. In this context, our study provides detailed laboratory and clinical features of HAM/TSP patients from the Brazilian Amazon. As previously reported, in our study young adult females predominated in both symptomatic HAM/TSP patients and asymptomatic HTLV-I carriers 22 . Females are reported to have higher incidences of HAM/TSP with faster disease progression especially when symptoms precede menopause, suggesting a role for sex hormones 23 . A Brazilian nationwide survey (1994-1995) has shown that the ratio of infected females increased from South to North 24 . It is also stated that, regardless of different socio-economic and cultural environments, in highly endemic regions, HTLV-I seroprevalence increases gradually with age, especially in women. This increment may be associated with the overtime accumulation of risky sexual exposure or of other transmission risk factors 25 , 26 , 27 . A study showed that among HTLV-I positive blood donor carriers from Iran, males predominated, probably reflecting the lower tradition of female blood donors in this country, whereas among HAM/TSP patients, females predominated 28 .
We acknowledge limitations in our study including the small sample size and the lack of HTLV-I proviral loads; however, our data on serum and CSF neopterin measurements confirms its role as a marker of central nervous system disease with inflammatory activity 29 . In our study, HAM/TSP patients had higher serum neopterin concentrations compared to HTLV-I carriers and healthy participants. Moreover, in HAM/TSP patients, paired serum-CSF neopterin analysis showed higher values in CSF, which were also higher compared to CSF neopterin in asymptomatic carriers. In contrast, among HTLV-I carriers, serum and CSF neopterin concentrations were similar. HAM/TSP patients compared to HTLV-I carriers had higher neopterin concentrations both in serum and in CSF, indicating that a less invasive sample as the peripheral blood may also give valuable information about the level of CNS/spinal cord inflammation and CMI activation. Our results corroborate previous data indicating that neopterin represents another potential marker of disease activity in HAM/TSP patients with potential applications to assess the disease progression and to monitor the treatment efficacy 17 , 30 .
In fact, in our study group, the oldest HAM/TSP patient (66 years old) had the longest duration of symptoms (16 years), and the highest motor disability score (OMDS = 8). This patient had the highest neopterin concentrations in both serum (35 nmol/L) and CSF (166 nmol/ L). This patient had a slowly progressive myelopathy, which evolved rapidly to superior member incapacity in the last 6 months prior to the study enrollment. Serial brain and spinal cord MRI did not reveal any alteration that could be possibly related to this rapid clinical deterioration. During the disease course, no immunosuppressive or corticosteroid therapy was prescribed. There is no cure for HAM/TSP but different treatments can alleviate symptoms, so that biomarkers to indicate the risk and the rate of disease progression may have a potential clinical relevance and application 31 .
Among routine laboratory tests, moderate pleocytosis and raised protein content represent the most frequent CSF findings in HAM/TSP, especially during recent clinical manifestations, and these parameters tend to gradually decline 32 . Mild CSF mononuclear pleocytosis and discrete increase of CSF protein have been reported 11 . In our HAM/TSP patients, mild pleocytosis, mainly of mononuclear cells was reported, as previously described 18 . A study in 20 HAM/TSP Brazilian patients described mild pleocytosis in 30% 13 . In our HAM/TSP patients the highest CSF protein level was detected in the patient with the most recent diagnosis (# 7, 1 year, Table 1) indicating the possible association of CSF inflammatory markers with recent disease 33 . Interestingly, in our study, in 5 out of 6 asymptomatic carriers who had CSF collected, protein levels were abnormal, probably reflecting a more recent infection. In our HAM/TSP patients and asymptomatic carriers, there was lack of correlation between CSF neopterin concentrations and CSF cell counts, protein or glucose concentrations, similarly to data previously described 34 . However, one study showed correlation between both CSF neopterin and pleocytosis/ glucose levels in 41 HAM/TSP patients 35 . In our small group of HAM/TSP, time since diagnosis ranged widely and it is possible that differences in viral loads, which were not available, may have influenced these correlations.
A slowly progressive disease course is considered the hallmark of HAM/TSP 33 , 36 . However, the rate at which symptoms progress varies widely including patients that deteriorate rapidly while others remain clinically stable over the years. Therefore, biomarkers able to predict the level and speed of disease progression are important to identify patients at higher risk and can provide clinicians with a more precise prognosis and more appropriate treatment. A retrospective study has compared the prognostic value of several potential biomarkers for HAM/TSP in peripheral blood and CSF of deteriorating and stable HAM/TSP patients 37 . This study has identified CSF levels of CXCL10, CXCL9 and neopterin as promising candidate prognostic biomarkers for HAM/TSP progression, with better correlations than HTLV-I proviral loads in peripheral blood mononuclear cells. These results corroborate the importance of neopterin as a biomarker for HAM/TSP as shown in our study. Moreover, these results suggest that assessing multiple prognostic biomarkers in both, blood and CSF, may better discriminate patients with higher risk of disease progression and in need of a more effective treatment.
Most HAM/TSP patients from our study had slowly progressive symptoms, except for one patient (#8, Table 1, 27 years old male, OMDS=7, 4 years of symptoms) that progressed rapidly. Disease progression in HAM/TSP can reflect an active inflammatory process in the spinal cord. In fact, this patient had elevated neopterin levels in serum and CSF indicating inflammation and reactivation or maintenance of the inflammatory process which has been reported in HAM/TSP patients with long disease duration 35 . Mononuclear inflammatory infiltrates were reported in the white and gray matter of the spinal cord in a 74 years old, female HAM/TSP patient with 29 years of disease 36 . However, two autopsy cases of HAM/TSP patients reporting 15 and 21 years of symptoms showed very few inflammatory cells in the spinal cord and brain 38 . Another autopsy study of a HAM/TSP patient with 28 years of disease showed degenerative lesions in the spinal cord without inflammation 39 . Corticosteroid and immunosuppressive treatment for HAM/TSP patients reduce the intensity of the inflammatory reaction and consequently the tissue damage, however prolonging the reparative process 40 . In our observational study, no HAM/TSP patient was under immunosuppressive or corticosteroid treatment as these interventions are known to influence the inflammatory marker levels. Disease progression in HAM/TSP is considered multifactorial and the potential host and viral factors that influence the natural course of disease are not completely understood 41 and are beyond the scope of our study.
Brain and spinal cord MRI is highly sensitive to detect and control CNS abnormalities and complications and is important for the diagnosis and treatment follow up in HAM/TSP 42 , 31 . Unspecific periventricular and subcortical white matter (WM) lesions on brain MRI are frequent in HAM/TSP patients 41 . It has been suggested that WM lesions may represent small vessel vasculitis and since they predominate in middle aged/older patients, they could be due to degenerative microangiopathy 43 , 44 . Among our HAM/TSP patients, most showed small lesions in the subcortical and periventricular WM, however these lesions were not associated with symptoms, as reported 45 , 46 . MRI findings of 28 Brazilian HAM/TSP patients, mostly with severe neurological incapacity, showed hyper intense lesions in brain WM, indirect atrophy signs and occasionally spinal cord lesions 44 . Among our HAM/TSP patients, no sign of spinal cord swelling was reported, whereas only one patient showed spinal cord atrophy, however variable levels of spinal cord abnormalities have been reported 46 , 47 . For logistic and operational reasons, our study did not include MRI to investigate possible WM abnormalities in the spinal cord and brain of HTLV-I carriers and in negative controls.
In conclusion, our study shows a wide range of clinical and laboratory profiles among HAM/TSP patients, suggesting the influence of host and viral factors. Consistent with the literature, no association was found between cerebral WM lesions and disease duration or degree of motor disability. Our laboratory data reinforce the importance of neopterin measurement as a biomarker associated with CNS disease activity and as a potential biomarker of CNS disease progression. Biomarkers of CNS activity can be helpful in the clinical management of high-risk HAM/TSP patients helping to anticipate interventions to minimize spinal cord damage and to monitor treatment efficacy.
ACKNOWLEGEMENTS
The authors are thankful to the participants’ cooperation, and to the support of FHEMOAM. MM Stefani is a recipient of a fellowship from CNPq/ Brazil (PQ grant # 308381/2015-7).
Footnotes
ETHICAL CONSIDERATIONS This study was approved by the institutional review board at FHEMOAM (protocol # 0010). All the participants signed an informed consent form before the collection of biological specimens.
REFERENCES
- 1.Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388–388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.González-Alcaide G, Ramos JM, Huamaní C, Mendoza Cd, Soriano V. Human T-lymphotropic virus 1 (HTLV-1) and human T-lymphotropic virus 2 (HTLV-2) geographical research trends and collaboration networks (1989-2012) Rev Inst Med Trop Sao Paulo. 2016;58:11–11. doi: 10.1590/S1678-9946201658011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galvão-Castro B, Loures L, Rodriques LG, Sereno A, Ferreira OC, Júnior, Franco LG. Distribution of human T-lymphotropic virus type I among blood donors a nationwide Brazilian study. Transfusion. 1997;37:242–243. doi: 10.1046/j.1537-2995.1997.37297203532.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamano Y, Sato T. Clinical pathophysiology of human T-lymphotropic virus-type 1-associated myelopathy/tropical spastic paraparesis. Front Microbiol. 2012;3:389–389. doi: 10.3389/fmicb.2012.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araujo AQ, Silva MT. The HTLV-1 neurological complex. Lancet Neurol. 2006;5:1068–1076. doi: 10.1016/S1474-4422(06)70628-7. [DOI] [PubMed] [Google Scholar]
- 6.Saito M, Bangham CR. Immunopathogenesis of human T-cell leukemia virus type-1-associated myelopathy/tropical spastic paraparesis recent perspectives. Leuk Res Treatment. 2012;2012:259045–259045. doi: 10.1155/2012/259045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Umehara F, Izumo S, Nakagawa M, Ronquillo AT, Takahashi K, Matsumuro K. Immunocytochemical analysis of the cellular infiltrate in the spinal cord lesions in HTLV-I-associated myelopathy. J Neuropathol Exp Neurol. 1993;52:424–430. doi: 10.1097/00005072-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Matsuoka E, Takenouchi N, Hashimoto K, Kashio N, Moritoyo T, Higuchi I. Perivascular T cells are infected with HTLV-I in the spinal cord lesions with HTLV-I associated myelopathy/tropical spastic paraparesis double staining of immunohistochemistry and polymerase chain reaction in situ hybridization. Acta Neuropathol. 1998;96:340–346. doi: 10.1007/s004010050903. [DOI] [PubMed] [Google Scholar]
- 9.Umehara F, Nose H, Saito M, Fukuda M, Ogino M, Toyota T. Abnormalities of spinal magnetic resonance images implicate clinical variability in human T-cell lymphotropic virus type I-associated myelopathy. J Neurovirol. 2007;13:260–267. doi: 10.1080/13550280701258431. [DOI] [PubMed] [Google Scholar]
- 10.Godoy AJ, Kira J, Hasuo K, Goto I. Characterization of cerebral white matter lesions of HTLV-I-associated myelopathy/tropical spastic paraparesis in comparison with multiple sclerosis and collagen-vasculitis a semiquantitative MRI study. J Neurol Sci. 1995;133:102–111. doi: 10.1016/0022-510x(95)00161-t. [DOI] [PubMed] [Google Scholar]
- 11.Milagres AC, Jorge ML, Marchiori PE, Segurado AA. Human T cell lymphotropic virus type 1-associated myelopathy in Sao Paulo, Brazil Epidemiological and clinical features of a university hospital cohort. Neuroepidemiology. 2002;21:153–158. doi: 10.1159/000054813. [DOI] [PubMed] [Google Scholar]
- 12.Bhagavati S, Ehrlich G, Kula RW, Kwok S, Sninsky J, Udani V. Detection of human T-cell lymphoma/leukemia virus type I DNA and antigen in spinal fluid and blood of patients with chronic progressive myelopathy. N Engl J Med. 1988;318:1141–1147. doi: 10.1056/NEJM198805053181801. [DOI] [PubMed] [Google Scholar]
- 13.Puccioni-Sohler M, Kitze B, Felgenhauer K. HTLV-I associated myelopathy in patients from Brazil and Iran neurological manifestations and cerebrospinal fluid findings. Arq Neuropsiquiatr. 1995;53:213–217. doi: 10.1590/s0004-282x1995000200005. [DOI] [PubMed] [Google Scholar]
- 14.Berdowska A, Zwirska-Korczala K. Neopterin measurement in clinical diagnosis. J Clin Pharm Ther. 2001;26:319–329. doi: 10.1046/j.1365-2710.2001.00358.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann G, Wirleitner B, Fuchs D. Potential role of immune system activation-associated production of neopterin derivatives in humans. Inflamm Res. 2003;52:313–321. doi: 10.1007/s00011-003-1181-9. [DOI] [PubMed] [Google Scholar]
- 16.Ali A, Rudge P, Dalgleish AG. Neopterin concentrations in serum and cerebrospinal fluid in HTLV-I infected individuals. J Neurol. 1992;239:270–272. doi: 10.1007/BF00810351. [DOI] [PubMed] [Google Scholar]
- 17.Nomoto M, Utatsu Y, Soejima Y, Osame M. Neopterin in cerebrospinal fluid a useful marker for diagnosis of. HTLV-I associated myelopathy/tropical spastic paraparesis.Neurology. 1991;41:457–457. doi: 10.1212/wnl.41.3.457. [DOI] [PubMed] [Google Scholar]
- 18.Osame M. Blattner W. Human retrovirology: HTLV; Review of WHO Kagoshima Meeting and Diagnostic Guidelines for HAM/TSP; New York: Raven; 1990. [Google Scholar]
- 19.Nakagawa M, Izumo S, Ijishi S, Kubota H, Arimura K, Kawabata M. HTLV-I associated myelophathy analysis of 213 patients based on clinical features and laboratory findings. J Neurovirol. 1995;1:50–61. doi: 10.3109/13550289509111010. [DOI] [PubMed] [Google Scholar]
- 20.Tuke PW, Luton P, Garson JA. Differential diagnosis of HTLV-I and HTLV-II infections by restriction enzyme analysis of nested PCR products. J Virol Methods. 1992;40:163–173. doi: 10.1016/0166-0934(92)90065-l. [DOI] [PubMed] [Google Scholar]
- 21.Hagberg L, Dotevall L, Norkrans G, Larsson M, Wachter H, Fuchs D. Cerebrospinal fluid neopterin concentrations in central nervous system infection. J Infect Dis. 1993;168:1285–1288. doi: 10.1093/infdis/168.5.1285. [DOI] [PubMed] [Google Scholar]
- 22.Gonçalves DU, Proietti FA, Ribas JG, Araújo MG, Pinheiro SR, Guedes AC. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Rev. 2010;23:577–589. doi: 10.1128/CMR.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lima MA, Bica RB, Araújo AQ. Gender influence on the progression of HTLV-I associated myelopathy/tropical spastic paraparesis. J Neurol Neurosurg Psychiatry. 2005;76:294–296. doi: 10.1136/jnnp.2004.035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araújo AQ, Andrade AS, Filho, Castro-Costa CM, Menna-Barreto M, Almeida SM. HTLV-I-associated myelopathy/tropical spastic paraparesis in Brazil a nationwide survey. HAM/TSP Brazilian Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:536–541. doi: 10.1097/00042560-199812150-00014. [DOI] [PubMed] [Google Scholar]
- 25.Blattner WA, Nomura A, Clark JW, Ho GY, Nakao Y, Gallo R. Modes of transmission and evidence for viral latency from studies of human T-cell lymphotrophic virus type I in Japanese migrant populations in Hawaii. Proc Natl Acad Sci U S A. 1986;83:4895–4898. doi: 10.1073/pnas.83.13.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller N. The epidemiology of HTLV-I infection. Cancer Causes Control. 1991;2:37–52. doi: 10.1007/BF00052359. [DOI] [PubMed] [Google Scholar]
- 27.Murphy EL, Figueroa JP, Gibbs WN, Holding-Cobham M, Cranston B, Malley K. Human T-lymphotropic virus type I (HTLV- I) seroprevalence in Jamaica I. Demographic determinants. Am J Epidemiol. 1991;133:1114–1124. doi: 10.1093/oxfordjournals.aje.a115824. [DOI] [PubMed] [Google Scholar]
- 28.Shoeibi A, Rafatpanah H, Azarpazhooh A, Mokhber N, Hedayati-Moghaddam MR, Amiri A. Clinical features of HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in northeast Iran. Acta Neurol Belg. 2013;113:427–433. doi: 10.1007/s13760-013-0194-6. [DOI] [PubMed] [Google Scholar]
- 29.Dale RC, Brilot F, Fagan E, Earl J. Cerebrospinal fluid neopterin in paediatric neurology a marker of active central nervous system inflammation. Dev Med Child Neurol. 2009;51:317–323. doi: 10.1111/j.1469-8749.2008.03225.x. [DOI] [PubMed] [Google Scholar]
- 30.Nagai M, Tsujii T, Iwaki H, Nishikawa N, Nomoto M. Cerebrospinal fluid neopterin, but not osteopontin, is a valuable biomarker for the treatment response in patients with HTLV-I-associated myelopathy. Intern Med. 2013;52:2203–2208. doi: 10.2169/internalmedicine.52.0869. [DOI] [PubMed] [Google Scholar]
- 31.Araújo AQ. Update on neurological manifestations of HTLV-1 infection. Curr Infect Dis Rep. 2015;17:459–459. doi: 10.1007/s11908-014-0459-0. [DOI] [PubMed] [Google Scholar]
- 32.Moreno-Carvalho OA, Nascimento-Carvalho CM, Galvão-Castro B. HTLV-I associated tropical spastic paraparesis Cerebral spinal fluid evolutive aspects in 128 cases. Arq Neuropsiquiatr. 1995;53:604–607. doi: 10.1590/s0004-282x1995000400009. [DOI] [PubMed] [Google Scholar]
- 33.Araujo AQ, Alfonso CR, Schor D, Leite AC, de Andrada-Serpa MJ. Clinical and demographic features of HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) in Rio de Janeiro, Brazil. Acta Neurol Scand. 1993;88:59–62. doi: 10.1111/j.1600-0404.1993.tb04188.x. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida Y, Une F, Utatsu Y, Nomoto M, Furukawa Y, Maruyama Y. Adenosine and neopterin levels in cerebrospinal fluid of patients with neurological disorders. Intern Med. 1999;38:133–139. doi: 10.2169/internalmedicine.38.133. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa M, Nakahara K, Maruyama Y, Kawabata M, Higuchi I, Kubota H. Therapeutic trial in 200 patients with HTLV-I associated myelopathy/tropical spastic paraparesis. J Neurovirol. 1996;2:345–355. doi: 10.3109/13550289609146899. [DOI] [PubMed] [Google Scholar]
- 36.Iwasaki Y, Sawada K, Aiba I, Mukai E, Yoshida M, Hashizume Y. Widespread active inflammatory lesions in a case of HTLV-I-associated myelopathy lasting 29 years. Acta Neuropathol. 2004;108:546–551. doi: 10.1007/s00401-004-0924-1. [DOI] [PubMed] [Google Scholar]
- 37.Sato T, Coler-Reilly A, Utsunomiya A, Araya N, Yagishita N, Ando H. CSF CXCL10, CXCL9, and neopterin as candidate prognostic biomarkers for HTLV-1-associated myelopathy/tropical spastic paraparesis. PLoS Negl Trop Dis. 2013;7:e2479. doi: 10.1371/journal.pntd.0002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aye MM, Matsuoka E, Moritoyo T, Umehara F, Suehara M, Hokezu Y. Histopathological analysis of four autopsy cases of HTLV-I-associated myelopathy/tropical spastic paraparesis inflammatory changes occur simultaneously in the entire central nervous system. Acta Neuropathol. 2000;100:245–252. doi: 10.1007/s004019900170. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki S, Komori T, Maruyama S, Takeishi M, Iwasaki Y. An autopsy case of human T lymphotropic virus type I associated myelopathy (HAM) with a duration of 28 years. Acta Neuropathol. 1990;81:219–222. doi: 10.1007/BF00334512. [DOI] [PubMed] [Google Scholar]
- 40.Iwasaki Y. Pathology of chronic myelopathy associated with HTLV-I infection (HAM/TSP) J Neurol Sci. 1990;96:103–123. doi: 10.1016/0022-510x(90)90060-z. [DOI] [PubMed] [Google Scholar]
- 41.Cooper SA, van der Loeff MS, Taylor GP. The neurology of HTLV-1 infection. Pract Neurol. 2009;9:16–26. doi: 10.1136/jnnp.2008.167155. [DOI] [PubMed] [Google Scholar]
- 42.Howard AK, Li DK, Oger J. MRI contributes to the differentiation between MS and HTLV-I associated myelopathy in British Columbian coastal natives. Can J Neurol Sci. 2003;30:41–48. doi: 10.1017/s0317167100002420. [DOI] [PubMed] [Google Scholar]
- 43.Kira J, Fujihara K, Itoyama Y, Goto I, Hasuo K. Leukoencephalopathy in HTLV-I-associated myelopathy/tropical spastic paraparesis MRI analysis and a two year follow-up study after corticosteroid therapy. J Neurol Sci. 1991;106:41–49. doi: 10.1016/0022-510x(91)90192-a. [DOI] [PubMed] [Google Scholar]
- 44.Puccioni-Sohler M, Gasparetto E, Cabral-Castro MJ, Slatter C, Vidal CM, Cortes RD. HAM/TSP association between white matter lesions on magnetic resonance imaging, clinical and cerebrospinal fluid findings. Arq Neuropsiquiatr. 2012;70:246–251. doi: 10.1590/s0004-282x2012000400004. [DOI] [PubMed] [Google Scholar]
- 45.Morgan DJ, Caskey MF, Abbehusen C, Oliveira J, Filho, Araujo C, Porto AF. Brain magnetic resonance imaging white matter lesions are frequent in HTLV-I carriers and do not discriminate from HAM/TSP. AIDS Res Hum Retroviruses. 2007;23:1499–1504. doi: 10.1089/aid.2007.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bagnato F, Butman JA, Mora CA, Gupta S, Yamano Y, Tasciyan TA. Conventional magnetic resonance imaging features in patients with tropical spastic paraparesis. J Neurovirol. 2005;11:525–534. doi: 10.1080/13550280500385039. [DOI] [PubMed] [Google Scholar]
- 47.Cervilla J, Cartier L, García L. Resonancia magnética de médula espinal y cerebro en el correlato clínico de la paraparesia espástica progresiva que se asocia al virus humano linfotrópico tipo-I (HTLV-I) Rev Med Chil. 2006;134:1010–1018. doi: 10.4067/s0034-98872006000800010. [DOI] [PubMed] [Google Scholar]


