ABSTRACT
Latex from Carica papaya is rich in bioactive compounds, especially papain, which may help to control parasitic diseases. This study evaluated the efficacy of latex from C. papaya and purified papain against Strongyloides venezuelensis. The Egg Hatching Test (EHT) and the Larval Motility Test (LMT) using fresh and frozen latex (250mg/mL), lyophilized latex (34mg/mL), and purified papain (2.8 mg/mL) were performed. Albendazole (0.025 mg/mL) and ivermectin (316 ppm) were used as positive controls. EHT and LMT were carried out through the incubation of each solution with S. venezuelensis eggs or larvae (± 100 specimens), and results were analyzed after 48h (EHT) or 24, 48, and 72h (LMT). EHT showed that latex preparations at higher concentrations (1:10 to 1:100) resulted in partial or complete destruction of eggs and larvae inside the eggs. The result from the 1:1,000 dilution was similar to the positive control. LMT showed effectiveness in all the tested dilutions compared to negative controls. Purified papain showed a dose-dependent response in the EHT. Purified papain (2.8 mg/ mL) showed similar results to lyophilized latex at 1:1,000 in the EHT. Latex and purified papain from C. papaya were effective against S. venezuelensis eggs and larvae in vitro, suggesting their potential use as an alternative treatment for strongyloidiasis.
KEYWORDS: Anthelmintic, Carica papaya, Latex, Papain, Strongyloidiasis
INTRODUCTION
Strongyloidiasis is a global emerging infectious disease with highest prevalence in Southern, Eastern, and Central Europe, the Caribbean Islands, Southeast Asia, Latin America, and sub-Saharan Africa 1 . This parasitic infection is caused by Strongyloides species and can be life threatening, particularly in immunossupressed hosts due to chronicity or hyper infection and dissemination of the parasite to other parts of the body 2 , 3 .
Frequently used drug therapies such as thiabendazole have not been effective in cases of disseminated Strongyloides sp. infection 4 . Recent reports have also indicated that these parasites have developed some level of resistance to most drug therapies 5 , 6 , and that many current therapies have high toxicity, making treatment difficult to maintain 6 , 7 .
Latex from Carica papaya is effective in controlling helminthic infections in animals 8 , 9 , 10 . Papain, another compound from this plant has anthelmintic activity through cuticle damage leading to high internal hydrostatic pressure and consequently, rupture of the parasite body 10 , 11 , 12 .
New studies searching for biologically and functionally active plant compounds are essential for the development and synthesis of new drugs with less toxicity and less prone to drug resistance. S. venezuelensis can be maintained in laboratory animals to produce a large number of eggs and larvae for experiments such as the ones set up to investigate S. stercoralis 13 .The aim of this study was to analyze the in vitro activity of latex and purified papain from C. papaya against Strongyloides venezuelensis eggs and larvae.
MATERIALS AND METHODS
Strongyloides venezuelensis eggs and larvae
Strongyloides venezuelensis strain was maintained through serial passages of infective larvae (L3) in Wistar rats (Rattusnovergicus) by subcutaneous inoculation. Feces from infected animals were used for egg collection and charcoal culture (72 h at 28 ºC) to obtain infective (L3) larvae 14 .
Collection and preparation of latex and purified papain
Latex from C. papaya was collected from the green papaya fruit according to the procedure described by Monti et al. 15 with some modifications. Three to four incisions of 2 to 3 mm were made in the green fruit using a stainless steel blade. The latex was collected after 1 to 2 min. in a Falcon tube, and was used fresh, frozen (after storage at -20oC) or lyophilized. The quantity of purified papain used was calculated according to Azarkanet et al. 16 . Fresh and frozen latex were diluted in phosphate buffered saline (PBS, pH 7.2) to acquire initial concentrations of 250 mg/mL, lyophilized latex and purified papain were diluted to 34 mg/mL and 2.8 mg/mL, respectively. Then 1:10 to 1:100,000 dilutions of all preparations were performed.
Egg Hatching Test (EHT) and Larval Motility Test (LMT)
The EHT was performed according to Coles et al. 17 with some modifications. Feces from S. venezuelensis-infected rats were previously homogenized in water and filtered using a 50 µm sieve. One hundred microliters of this solution containing 100 eggs were added to 100 µL of each preparation, then incubated at 28 ºC for 48 h, after which egg development was analyzed. Egg hatching and larvae from hatching were quantified using an optical microscope. Albendazole solution (0.025 mg/ mL) was used as the positive control 18 .
The LMT was performed according to Cordeiro et al. 19 with some modifications. One hundred microliters of a solution containing 100 infective larvae (L3) were added to 100 µL of each preparation to be tested in a 24-well plate. The solution was incubated at 28 ºC, and larval motility was analyzed and quantified after 24, 48 and 72h using an optical microscope. Ivermectin solution (316 ppm) was used as the positive control 20 .
Water and PBS were used as negative controls in both tests. Microscope images were obtained using the Leica Microsystems DM750 model camera.
Data analysis
The Inhibition of Eggs Hatching (IEH) was carried out according to Wood et al. 21 and to the Guidelines of the World Association for the Advancement of Veterinary Parasitology (WAAVP). Results were calculated using the following formula:
IEH (%) = [(number of eggs) / (number of eggs + number of larvae)] x 100.
The Inhibition of Larval Motility (ILM) was carried out according to Al-Rofaai et al. 22 by using the following formula:
ILM (%) = [(%) motilityin the treatment group - (%) motilityin negative controls)/ (100 - (%) motilityin the negative control)] x 100.
Data were analyzed using the GraphPad Prism 5.0 software by calculating the half-maximal inhibitory concentration (IC50). The Fisher’s exact test was used to compare the experimental groups, positive and negative controls. One-way analysis of variance (ANOVA) for parametric data and the Kruskal-Wallis tests for non-parametric data were used to compare the groups. The Mann-Whitney test was used to compare data from purified papain and lyophilized latex with the same dilutions. It was considered statistically significant when p < 0.05.
RESULTS
Morphological changes in the development of S. venezuelensis eggs after treatment with latex and purified papain from C. papaya
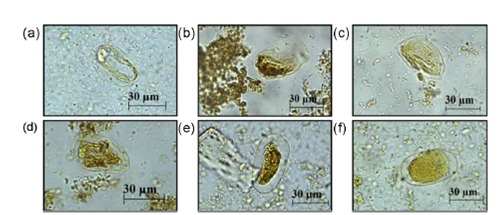
High concentrations of latex preparations such as pure and 1:10 to 1:100 dilutions were not analyzed because they caused total or partial destruction of eggs represented by scarce or condensed material inside the egg, and damaged or fragmented larvae. Treatment with latex preparations and purified papain resulted in a small number of embryonated eggs compared to the negative controls (Figure 1).
Figure 1. Morphological changes in Strongyloides venezuelensis eggs after treatment with lyophilized latex from (a) Carica papaya at 1:1,000 and (b and c) purified papain at 2.8 mg/mL. Positive control: (d) Albendazole (0.025 mg/mL). Negative controls: (e) PBS and (f) water.
Inhibition of Egg Hatching (IEH) after treatment with latex and purified papain from C. papaya
Latex preparations from C. papaya had variable results in the EHT (Table 1). The 1:1,000 dilutions from fresh (p< 0.05), frozen (p < 0.01) and lyophilized (p < 0.001) latex has efficiently inhibited eggs hatching, a similar effect observed in the positive control (Albendazole, 0.025 mg/mL).
Table 1. Effects of different dilutions of fresh, frozen and lyophilized latex on Innibition of Egg Hatching (IEH) of S. venezuelensis 48h post-treatment.
| Tests | Number of eggs | Number of larvae | Inhibition of Eggs Hatching (%) |
| Fresh latex (250mg/mL) | |||
| 1:1000 | 90 | 13 | 87.38 ± 5.07A |
| 1:10000 | 82 | 17 | 82.83 ± 1.01AC |
| 1:100000 | 79 | 31 | 71.82 ± 4.24BC |
| Frozen latex (250mg/mL) | |||
| 1:1000 | 86 | 11 | 88.66 ± 0.51A |
| 1:10000 | 94 | 14 | 87.04 ± 4.62A |
| 1:100000 | 78 | 19 | 80.41 ± 4.80AB |
| Lyophilized latex (34mg/mL) | |||
| 1:1000 | 90 | 9 | 90.91 ± 1.69A |
| 1:10000 | 80 | 14 | 85.11 ± 2.15A |
| 1:100000 | 80 | 13 | 86.02 ± 2.04A |
| Water | 73 | 30 | 70.87 ± 4.55B |
| PBS | 64 | 25 | 71.91 ± 1.29B |
| Albendazole (0.025mg/mL) | 88 | 9 | 90.72 ± 1.29A |
Different letters in the lines indicate statistically significant difference (P<0.05); PBS: phosphate buffered saline
There were no statistical differences among groups with different latex storage methods at the same concentrations.
Purified papain presented a dose-dependent response in the IEH (Figure 2). At 2.8 mg/ mL it was able to inhibit 91.18% (± 1.24) of eggs hatching. Purified papain was also effective by using 1:10 dilution compared to negative controls (p < 0.05). It was observed that the inhibition gradually decreased to 67.71% ± 2.05 (28 ηg/mL purified papain). Purified papain IC50 was 12.34 µg/mL.
Figure 2. Dose-dependent response of purified papain from Carica papaya on Inhibition of Egg Hatching (IEH) of Strongyloides venezuelensis. Results are presented as mean values ± standard error.
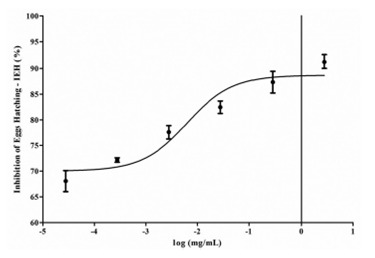
It was observed that, at the same dilutions, the activity of lyophilized latex and purified papain was different. Purified papain at 2.8 mg/mL showed similar efficacy to the lyophilized latex at 1:1,000 dilutions.
Inhibition of Larval Motility (ILM) after treatment with latex and purified papain from C. papaya
There were no statistical differences among groups by using the three latex preparation methods or dilutions at 24, 48 and 72 h post-treatment. It was shown that high concentrations of latex preparations inhibited larval motility at 24 h post-treatment. Lyophilized latex showed to be effective in the ILM compared to negative controls (Table 2).
Table 2. Effects of different dilutions of lyophilized latex on Inhibition of Larvas Motility (ILM) of S. venezuelensis 24h post-treatment.
| Tests | Immobility (%) | Efficacy (%) |
| Lyophilized latex (34mg/mL) | ||
| 1:10 | 76.74 ± 1.47AD | 73.98 |
| 1:100 | 75.26 ± 1.33A | 72.31 |
| 1:1000 | 67.71 ± 2.22A | 63.86 |
| 1:10000 | 60.00 ± 2.97AD | 55.24 |
| 1:100000 | 74.07 ± 1.48A | 70.99 |
| Water | 10.64 ± 3.58B | 0.00 |
| PBS | 14.55 ± 0.57B | 4.37 |
| Ivermectin (316ppm) | 94.17 ± 0.46C | 93.48 |
Different letters in the lines indicate statistically significant difference (p < 0.05); ppm: parts per million.
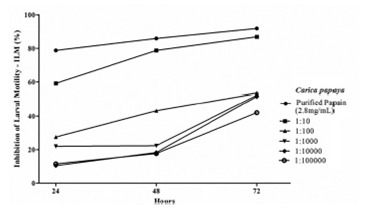
As IEH result, the purified papain presented a dose-dependent response in the ILM, with stronger reduction in motility at higher concentrations (Figure 3). There were no statistical differences among groups at 24, 48, and 72 h post-treatment and no differences between purified papain and lyophilized latex. The IC50 for purified papain at 24 h was 0.6839 µg/mL.
Figure 3. Dose-dependent response of different dilutions of purified papain from Carica papaya on Inhibition of Larval Motility (ILM) in Strongyloides venezuelensis. Results are presented as mean values ± standard error.
DISCUSSION
Strongyloidiasis is a serious public health problem with worldwide distribution, and is particularly frequent in developing countries 23 , 24 . Helminthes control has been limited mainly by the high toxicity of drug therapies and also by the development of parasite resistance to anthelmintics 25 .
The present study demonstrates for the first time that latex and purified papain from C. papaya had potential activity against S. venezuelensis eggs and larvae. These results are consistent with previous studies suggesting anthelmintic potential of C. papaya latex against other nematodes. Other studies report a significant reduction in egg production and gastrointestinal nematode burden in various animals, including rodents 26 , 27 , pigs 28 , birds 29 , sheep 12 , and humans 30 .
Pure latex was prepared and stored using different procedures to test whether methods such as lyophilization and freezing may interfere with efficacy. None of the processes has affected the activity of latex compounds, as no statistical differences were observed among fresh, frozen or lyophilized latex. High concentrations of latex showed some toxicity, revealed by the total or partial destruction of parasitic forms.
Fresh, frozen and lyophilized latex at 1:1,000 dilutions were very effective in inhibiting egg hatching and larval motility (i.e., activity was similar to the commercial anthelmintic). Other concentrations have also shown inhibitory effects compared to negative controls, and have demonstrated moderate effectiveness in accordance with the standards recommended by the WAAVP31. The WAAVP describes an anthelmintic as highly effective when it presents an inhibitory effect greater than 90% regarding the parasite activity, and moderately effective at 80-90% inhibition.
Latex from C. papaya contains cysteine proteinases, such as papain and chymopapain which have demonstrated anthelmintic potential 8 , 9 , 10 , 12 , 28 , 32 . In the present study EHT results differed when the same concentrations of purified papain and lyophilized latex were tested, suggesting that the effect of latex is not only due to the presence of papain. It can be infered that there are other compounds in the pure latex that promote parasite destruction and the observed synergistic effects.
An anthelmintic treatment is considered effective in the EHT if it inhibits egg development and hatching of larvae, or if it results in a high number of non-embryonated eggs 33 . According to Camurça-Vasconcelos et al. 34 , eggs embryonation is typically complete within 48 h after elimination in feces, and at that time, the development and eclosion of eggs may be observed under a microscope. In the current study, all latex treatments and purified papain have inhibited the eggs development 24 h post-treatment, and resulted in a high number of non-embryonated eggs.
The mechanism by which cysteine proteinases from plants such as C. papaya decrease the motility in larval nematodes is via impairment and degradation of the parasite cuticle due to the papain catalytic activity on disulfide bonds 26 , 27 . The reduction in larval motility observed at 24 h is consistent with the observations of Stepek et al. 26 , who reported papain-associated cuticle digestion in Meleidoginesp. and decreased numbers of larvae after treatment. C. papaya latex has also been found to cause paralysis in Pheritimaposthuma in vitro 32 , and is effective in the control of Haemonchus contortus 10 and Trichuris muris 9 , both in vivo.
The IC50 discrepancy regarding the treatment of eggs and larvae indicates the difference between the larval cuticle and the eggs shell. In addition, emphasizes the effectiveness of another compound, that is not papain, against eggs. Papain acts by releasing the internal structures of larvaes, leading to the death of parasites 26 . In this sense, papain inhibits the action of the larvae, preventing reinfection cycles in disseminated strongyloidiasis and could possibly interfere with parthenogenetic females by preventing eggs posture.
This study has demonstrated for the first time that C. papaya latex and purified papain were effective against S. venezuelensis eggs and larvae. Our results suggest that these bioactive compounds may be used, in the future, as alternative therapies for the control of gastrointestinal nematodes such as Strongyloides stercoralis. Further studies should be conducted to evaluate the toxicity and in vivo, as well as side effects, prior to clinical trials for the treatment of strongyloidiasis.
ACKNOWLEDGEMENTS
We are grateful to Prof. Dr. Ricardo de Mattos Santa-Rita from the Laboratório de Microscopia, Universidade Federal de Goiás-Jataí, for his technical skills in gathering microscope images.
Footnotes
FINANCIAL SUPPORT This research was supported by the Brazilian Research Council (Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq), Brazil, D.M, grant number 1227624.
REFERENCES
- 1.Puthiyakunnon S, Boddu S, Li Y, Zhou X, Wang C, Li J. Strongyloidiasis an insight into its global prevalence and management. PLoS Negl Trop Dis. 2014;8:e3018. doi: 10.1371/journal.pntd.0003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zueter AM, Mohamed Z, Abdullah AD, Mohamad N, Arifin N, Othman N. Detection of Strongyloides stercoralis infection among cancer patients in a major hospital in Kelantan, Malaysia. Singapore Med J. 2014;55:367–371. doi: 10.11622/smedj.2014088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skukla S, Chauhan R, Wadhwa S, Sehgal S, Singh S. Strongyloides stercoralis hyperinfection causing eosinophilic ascites. Diagn Cytopathol. 2015;43:731–733. doi: 10.1002/dc.23281. [DOI] [PubMed] [Google Scholar]
- 4.Grove DI. Human strongyloidiasis. Adv Parasitol. 1996;38:251–309. doi: 10.1016/s0065-308x(08)60036-6. [DOI] [PubMed] [Google Scholar]
- 5.Kundu S, Roy S, Lyndem LM. Cassia alata L potential role as anthelmintic agent against Hymenolepis diminuta. Parasitol Res. 2012;111:1187–1192. doi: 10.1007/s00436-012-2950-6. [DOI] [PubMed] [Google Scholar]
- 6.Rupa S, Jayanta B. Comparative studies on anthelmintic potential of Cucurbita maxima (Pumpkin) seeds and Carica papaya (Papaya) seeds. Int J Res Ayurveda Pharm. 2013;4:530–532. [Google Scholar]
- 7.Martín del Barco OH., Manzanares PA, Izquierdo RL. Parasitosis intestinal. FMC Form Med Contin Aten Prim. 2009;16:14–24. [Google Scholar]
- 8.Satrija F, Nansen P, Bjorn H, Murtini S, He S. Effect of papaya against Ascarissuum in naturally infected pigs. J Helminthol. 1994;68:343–346. doi: 10.1017/s0022149x00001619. [DOI] [PubMed] [Google Scholar]
- 9.Stepek G, Lowe AE, Buttle DJ, Duce IR, Behnke JM. In vitro and in vivo anthelmintic efficacy of plant cysteine proteinases against the rodent gastrointestinal nematode, Trichuris muris. Parasitology. 2006;132:681–689. doi: 10.1017/S003118200500973X. [DOI] [PubMed] [Google Scholar]
- 10.Buttle DJ, Behnke JM, Bartley Y, Elsheikha HM, Bartley DJ, Garnett MC. Oral dosing with papaya latex is an effective anthelmintic treatment for sheep infected with Haemonchus contortus. Parasit Vectors. 2011;4:36–36. doi: 10.1186/1756-3305-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okeniyi JA, Ogunlesi TA, Oyelami OA, Adeyemi LA. Effectiveness of dried Carica papaya seeds against human intestinal parasitosis a pilot study. J Med Food. 2007;10:194–196. doi: 10.1089/jmf.2005.065. [DOI] [PubMed] [Google Scholar]
- 12.Behnke JM, Buttle DJ, Stepek G, Lowe A, Duce IR. Developing novel anthelmintics from plant cysteine proteinases. Parasit Vectors. 2008;1:29–29. doi: 10.1186/1756-3305-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues RM, Silva NM, Gonçalves AL, Cardoso CR, Alves R, Gonçalves FA. Major histocompatibility complex (MHC) class II but not MHC class I molecules are required for efficient control of Strongyloides venezuelensis infection in mice. Immunology. 2009;128:e432–e441. doi: 10.1111/j.1365-2567.2008.02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rugai E, Mattos T, Brisola AP. Nova técnica para isolar larvas de nematoides das fezes modificações do método de Baermann. Rev Inst Adolfo Lutz. 1954;14:5–8. [PubMed] [Google Scholar]
- 15.Monti R, Basilio CA, Trevisan HC, Contiero J. Purification of papain from fresh latex of Carica papaya. Braz Arch Biol Technol. 2000;43:501–507. [Google Scholar]
- 16.Azarkan M, El Moussaoui A, van Wuytswinkel D, Dehon G, Looze Y. Fractionation and purification of the enzymes stored in the latex of Carica papaya. J Chromatogr B Analyt Technol Biomed Live Sci. 2003;790:229–238. doi: 10.1016/s1570-0232(03)00084-9. [DOI] [PubMed] [Google Scholar]
- 17.Coles GC, Bauer C, Borgsteede FH, Geerts S, Klei TR, Taylor MA. World Association for the Advancement of Veterinary Parasitology (W A.A.V.P.) methods for detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 1992;44:35–44. doi: 10.1016/0304-4017(92)90141-u. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho CO, Chagas AC, Cotinguiba F, Furtan M, Brito LG, Chaves FC. The anthelmintic effect of plant extract on Haemonchus contortus and Strongyloides venezuelensis. Vet Parasitol. 2012;183:260–268. doi: 10.1016/j.vetpar.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 19.Cordeiro LN, Athayde AC, Vilela VL, Costa JG, Silva WA, Araujo MM. Efeito in vitro do extrato etanólico das folhas do melão-de-São-Caetano (Momordica charantia L ) sobre ovos e larvas de nematoides gastrintestinais de caprinos. Rev Bras Plantas Med. 2010;12:421–426. [Google Scholar]
- 20.Rebollo CD, Taira N, Ura S, Williams JC. Larvicidal effects of several chemicals on Strongyloides infective larvae. Vet Parasitol. 2003;118:165–168. doi: 10.1016/j.vetpar.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Wood IB, Amaral NK, Bairden K, Duncan JL, Kassai T, Malone JB., Jr World Association for the Advancement of Veterinary Parasitology (W A.A.V.P.) second edition of guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine, ovine, caprine) Vet Parasitol. 1995;58:181–213. doi: 10.1016/0304-4017(95)00806-2. [DOI] [PubMed] [Google Scholar]
- 22.Al-Rofaai A, Rahman WA, Abdulghani M. Sensitivity of two in vitro assays for evaluating plant activity against the infective stage of Haemonchus contortus strains. Parasitol Res. 2013;112:893–898. doi: 10.1007/s00436-012-3113-5. [DOI] [PubMed] [Google Scholar]
- 23.Freeman MC, Chard AN, Nikolay B, Garn JV, Okoyo C, Kihara J. Associations between school- and household-level water, sanitation and hygiene conditions and soil-transmitted helminth infection among Kenyan school children. Parasit Vectors. 2015;8:412–412. doi: 10.1186/s13071-015-1024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sangaré I, Bamba S, Cissé M, Zida A, Bamogo R, Sirima C. Prevalence of intestinal opportunistic parasites infections in the University hospital of Bobo-Dioulasso, Burkina Faso. Infect Dis Poverty. 2015;4:32–32. doi: 10.1186/s40249-015-0065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehlhorn H, Al-Quraishy S, Al-Rasheid KA, Jatzlau A, Abdel-Ghaffar F. Addition of a combination of onion (Allium cepa) and coconut (Cocos nucifera) to food of sheep stops gastrointestinal helminthic infections. Parasitol Res. 2011;108:1041–1046. doi: 10.1007/s00436-010-2169-3. [DOI] [PubMed] [Google Scholar]
- 26.Stepek G, Curtis RH, Kerry BR, Shewry PR, Clark SL, Lowe AE. Nematicidal effects of cysteine proteinases against sedentary plant parasitic nematodes. Parasitology. 2007;134:1831–1838. doi: 10.1017/S0031182007003289. [DOI] [PubMed] [Google Scholar]
- 27.Suryatinah Y, Andiarsa D, Hairani B. Pengaruh sistein terhadap aktivitas proteolitik papain kasar pada kematian cacing Ascaridia galli in vitro. J Buski. 2013;4:188–191. [Google Scholar]
- 28.Shaziya B, Goyal PK. Anthelmintic effect of natural plant (Carica papaya) extract against the gastrointestinal nematode, Ancylostoma caninum in mice. ISCA J Biol Sci. 2012;1:2–6. [Google Scholar]
- 29.Mursof EP, He S. A potential role of papaya latex as an anthelmintic against patent Ascaridia galli infection chicken. Hemera Zoa. 1991;74:11–20. [Google Scholar]
- 30.Hansson A, Veliz G, Naquira C, Amren M, Arroyo M, Arevalo G. Preclinical and clinical studies with latex from Ficus glabrata HBK, a traditional intestinal anthelminthic in the Amazonian area. J Ethnopharmacol. 1986;17:105–138. doi: 10.1016/0378-8741(86)90053-x. [DOI] [PubMed] [Google Scholar]
- 31.Powers KG, Wood IB, Eckert J, Gibson T, Smith HJ. World Association for the Advancement of Veterinary Parasitology (W A.A.V.P.) guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine and ovine) Vet Parasitol. 1982;10:265–284. doi: 10.1016/0304-4017(82)90078-4. [DOI] [PubMed] [Google Scholar]
- 32.Kanthal LK, Mondal P, De S, Jana S, Aneela S, Satyavathi K. Evaluation of anthelmintic of Carica papaya latex using Pheritima posthuma. Int J Life Sci Pharma Res. 2012;2:10–12. [Google Scholar]
- 33.Krychak-Furtado S, Negrelle RB, Miguel OG, Zaniolo SR, Kapronezai J, Ramos SJ. Efeito de Carica papaya L (Caricaceae) e Musa parasidiacaLinn. (Musaceae) sobre o desenvolvimento de ovos de nematódeos gastrintestinais de ovinos. Arq Inst Biol São Paulo. 2005;72:191–197. [Google Scholar]
- 34.Camurça-Vasconcelos AL, Morais SM, Santos LF, Rocha MF, Bevilaqua CM. Validação de plantas medicinais com atividade anti-helmíntica. Rev Bras Plantas Med. 2005;7:97–106. [Google Scholar]


