Abstract
The zebrafish, Danio rerio, has become recognized as a valuable model for the study of development, genetics, and toxicology. Recently, the zebrafish has been recognized as a useful model for infectious disease and immunity. In this study, the pathogenesis and antiviral immune response of zebrafish to experimental snakehead rhabdovirus (SHRV) infection was characterized. Zebrafish 24 h postfertilization to 30 days postfertilization were susceptible to infection by immersion in 106 50% tissue culture infective doses (TCID50) of SHRV/ml, and adult zebrafish were susceptible to infection by intraperitoneal (i.p.) injection of 105 TCID50 of SHRV/ml. Mortalities exceeded 40% in infected fish, and clinical presentation of infection included petechial hemorrhaging, redness of the abdomen, and erratic swim behavior. Virus reisolation and reverse transcription-PCR analysis of the viral nucleocapsid gene confirmed the presence of SHRV. Histological sections of moribund embryonic and juvenile fish revealed necrosis of the pharyngeal epithelium and liver, in addition to congestion of the swim bladder by cell debris. Histopathology in adult fish injected i.p. was confined to the site of injection. The antiviral response in zebrafish was monitored by quantitative real-time PCR analysis of zebrafish interferon (IFN) and Mx expression. IFN and Mx levels were elevated in zebrafish exposed to SHRV, although expression and intensity differed with age and route of infection. This study is the first to examine the pathogenesis of SHRV infection in zebrafish. Furthermore, this study is the first to describe experimental infection of zebrafish embryos with a viral pathogen, which will be important for future experiments involving targeted gene disruption and forward genetic screens.
Recently, zebrafish have been recognized for their value as a model for infectious disease. Genomic comparisons and the discovery of evolutionarily conserved host defense strategies, such as the interferon (IFN)-mediated antiviral response (3, 4) and Toll-like receptors (16, 33), suggest that studies of infectious disease in zebrafish will lead to a better understanding of nonspecific immunity and resistance. Few groups have been successful in identifying viral pathogens that can infect and cause disease in zebrafish. LaPatra et al. (27) reported that infectious hematopoietic necrosis virus (IHNV) and infectious pancreatic necrosis virus (IPNV) were able to replicate in adult zebrafish. Despite toxic effects in the hematopoietic tissue of the kidney in zebrafish, no mortalities occurred. Sanders et al. (40) described the susceptibility of adult zebrafish to experimental infection with spring viremia of carp virus (SVCV). Zebrafish infected with SVCV exhibited significant mortalities and histopathology, marked by necrosis of the gills, liver, and spleen. In a recent collaboration between our lab and the Leong lab (2), recombinant SHRV mutants were used to demonstrate the role of the SHRV nonvirion gene during infection in adult zebrafish; however, detailed pathogenesis and antiviral response were not reported.
Snakehead rhabdovirus (SHRV) was chosen for the present study because the virus affects warm-water fish and the optimal temperature range for SHRV replication is suitable for zebrafish maintenance. SHRV is an enveloped, nonsegmented, negative-sense RNA virus that belongs to the Rhabdoviridae family, genus Novirhabdovirus (20). The virus is chloroform, heat, and acid labile; replicates optimally between 24 and 30°C; and produces the highest titers in snakehead and carp cell lines (21, 21a). First isolated from the tissues of diseased snakehead fish (Ophicephalus striatus) during an epizootic ulcerative syndrome (EUS) outbreak in Thailand (50), the specific role of SHRV in EUS had not been determined (21). Experimental infection with EUS-associated rhabdoviruses was successful in snakehead fry and juveniles (12, 29), although the characteristic ulcerative disease was not observed. In the present study, we examine the ability of SHRV to cause disease and to stimulate an inflammatory immune response in embryonic and adult zebrafish.
When a viral pathogen is able to penetrate the external barriers of an organism and cause infection, initially a nonspecific inflammatory response is triggered. This immediate and vigorous response is mediated in part by antiviral response proteins IFN and Mx. IFN induces an antiviral state in which host cells block mRNA transcription and translation in order to prevent viral replication in infected cells (22). Genes for IFN have been identified in several species of mammals, birds, and fish, including zebrafish, channel catfish, carp, and Atlantic salmon (3, 19, 31, 39, 42, 44, 47, 55). IFN production also induces the expression of antiviral proteins such as Mx, double-stranded RNA-dependent protein kinase, and 2′-5′ oligoadenylate synthetase (22). Mx is a member of the GTPase family and was originally recognized for its antiviral activity against orthomyxoviruses (15). Antiviral activity of Mx has also been reported after infection of rhabdoviruses (32, 45), bunyaviruses (13), togaviruses (26), and paramyxoviruses (41). Mx genes have been identified in mammals, birds, and a variety of fish, including zebrafish (4, 17, 18, 28, 46, 49).
We report here the successful infection of zebrafish embryos and adults with SHRV, resulting in the induction of disease-related immune responses and pathology. Experimental infection of both embryonic and adult zebrafish with SHRV produced infection kinetics and histopathology that are indicative of acute infection. Analyses of IFN and Mx mRNA expression profiles revealed evidence of upregulation of the antiviral response in zebrafish embryos and adults as a result of SHRV infection. The results presented here provide the first evaluation of pathogenesis and antiviral response in zebrafish after experimental SHRV infection. We also describe here the first method for infecting zebrafish embryos with a viral pathogen, which will be important for forward genetic screens and targeted gene disruption experiments.
MATERIALS AND METHODS
Cells and virus.
SHRV was propagated in Epithelioma papulosum cyprini (EPC) cells (11) or zebrafish embryo fibroblast (ZF4) cells (8) at 28°C. EPC cells were grown in minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Carlsbad, Calif.). ZF4 cells were grown in Dulbecco modified eagle medium/F12 supplemented with 5% fetal bovine serum (Gibco). Propagation of virus was achieved by infecting 75%-80% confluent cultures at a multiplicity of infection (MOI) of 0.001. Cultures with 80 to 90% CPE were harvested and centrifuged 2000 × g for 10 min at 4°C, and the supernatants were stored at −80°C. Virus titers were quantified by determining 50% tissue culture infective doses (TCID50)/ml. EPC cells were used to determine virus titers for virus stocks propagated in EPC cells, and ZF4 cells were used to determine virus titers for virus stocks propagated in ZF4 cells.
Zebrafish care and maintenance.
AB inbred strain of zebrafish were reared in recirculating systems from Aquatic Habitats (Apopka, Fla.). The water temperature was maintained at 28°C with a flow rate of 150 liters/min. All adult fish (>3 months old) used in infection experiments were transferred to an isolated flowthrough system, a modified version of the recirculating system, and acclimated for several days before infection. Effluent from the flowthrough system was treated with chlorine. Zebrafish embryos were maintained at 28°C in egg water (60 μg of Instant Ocean sea salts/ml). Zebrafish were handled according to Institutional Animal Care and Use Committee guidelines.
Exposure by immersion.
At 24 h postfertilization (hpf), 3 days postfertilization (dpf), 7 dpf, and 30 dpf zebrafish embryos were exposed to 106 TCID50 of SHRV/ml by static immersion for 5 h. At 24 hpf embryos were manually dechorionated prior to infection. Control fish were exposed to phosphate-buffered saline (PBS). Exposures were carried out in triplicate with 20 fish per group per dose. Parallel groups for histology and reisolation sampling were exposed similarly. Zebrafish at 24 hpf, 3 dpf, 7 dpf, and 30 dpf were exposed to 106 TCID50 of SHRV/ml in total volumes of 2, 3, 3, and 100 ml, respectively. Adult fish ranging from 300 to 800 mg in weight (3 to 4 cm in length) were exposed to 103, 104, 105, or 106 TCID50 of SHRV/ml. After 5 h, each group of adult fish was moved to a 2.75-liter tank in the flowthrough system and maintained for 20 days. Mortalities were tallied daily. Fish from parallel groups were routinely sampled for reisolation of virus and histology.
Exposure by injection.
Adult fish were anesthetized in 160 μg of tricaine/ml and injected intraperitoneally (i.p.) with 105 TCID50 of SHRV/ml. Control groups were similarly anesthetized and injected with PBS. Exposures were conducted in triplicate with 20 fish per group per dose. Parallel groups for histology and reisolation sampling were infected accordingly. Mortalities were tallied daily. The fish from the parallel groups were routinely sampled for reisolation of pathogen and histology.
Virus confirmation.
Randomly selected adult zebrafish, ranging in weight from 300 to 800 mg, from the parallel sampling groups infected with SHRV, were euthanized in 4 mg of tricaine/ml, immediately frozen in liquid nitrogen, and placed in a plastic stomacher bag for storage at −80°C. Moribund and dead adult fish were collected in a similar fashion. Adult samples were processed by addition of 1:10 (wt/vol) serum-free MEM supplemented with 50 U penicillin, 0.05 mg of streptomycin, and 0.01 mg of neomycin and then homogenized. Infected embryos and juvenile fish from the parallel 24-hpf, 3-dpf, 7-dpf, and 30-dpf sampling groups were euthanized and diluted 1:100 (wt/vol) in serum-free MEM. Embryo and juvenile samples were homogenized in 1.5-ml microcentrifuge tubes; supernatants were collected, filtered, and stored at 4°C. Filtered samples were diluted 10-fold and added to cells in culture. Infected cells were monitored for 1 week, and TCID50/milliliter concentrations were calculated.
Histology.
Adult zebrafish sampled for histology were euthanized in 4 mg of tricaine/ml and placed in 10 ml of buffered 10% formalin. Fish samples were embedded in paraffin and sections were stained with hematoxylin-eosin. Zebrafish embryos sampled for histology were euthanized in 4 mg of tricaine/ml and placed in 0.5 ml of TEM fixative (0.2 M sodium cacodylate, 25% glutaraldehyde, 15% paraformaldehyde, 0.5% calcium chloride). Embryonic samples were postfixed with osmium tetroxide and embedded in Epon medium. Semithin sections were stained with toluidine blue.
Primer design and nucleotide sequences.
Primers were designed from their respective gene sequences by using PrimerQuest (Integrated DNA Technologies). Primer sequences and their expected fragment sizes for quantitative real-time PCR are listed in Table 1. Primers for the PCR analysis of the SHRV nucleocapsid gene were synthesized from the SHRV N-gene sequence (accession no. AF147498). The sense primer, 5′-ATTTATCCGCTGGAGAGGGATTGG-3′, and the antisense primer, 5′-GTTGAGCCCATAGGCCTTGAAGTA-3′, direct the amplification of 829-nucleotide portion of the SHRV nucleocapsid gene. Cycling parameters were 94°C for 30s, 55°C for 30s, and 72°C for 30s for a total of 35 cycles. The 829-nucleotide amplicon was subcloned into a pGEM-T Easy vector (Promega, Madison, Wis.) and submitted for sequencing to the University of Maine Sequencing Facility with an ABI 373 DNA sequencer (Applied Biosystems, Foster City, Calif.).
TABLE 1.
Quantitative real-time primer sequences and amplicon size
| Gene | Accession no. | Primer sequences | Product size (bp) |
|---|---|---|---|
| Zebrafish β-actin | AF025305 | 5′-ATGGATGAGGAAATCGCTG-3′ | 130 |
| 5′-ATGCCAACCATCACTCCCTG-3′ | |||
| Zebrafish Mx | AF533769 | 5′-ATAGGAGACCAAAGCTCGGGAAAG-3′ | 145 |
| 5′-ATTCTCCCATGCCACCTATCTTGG-3′ | |||
| Zebrafish IFN | AY135716 | 5′-GAATGGCTTGGCCGATACAGGATA-3′ | 136 |
| 5′-TCCTCCACCTTTGACTTGTCCATC-3′ |
RNA extraction and cDNA synthesis.
Zebrafish embryos and larvae were sampled at 6, 12, 24, 48, 72, and 96 h postinfection (hpi) for total RNA extraction. All sample fish were collected and placed in 200 μl of RNAlater (Ambion, Austin, Tex.) for storage. Exposures were conducted in triplicate with 10 fish per sample time point. Livers from adult fish injected i.p. with virus were removed from randomly selected fish and stored in 200 μl of RNAlater. Exposures were conducted in triplicate with two adult livers per sample time point. Total RNA was extracted from RNAlater preserved samples by using the MasterPure RNA Purification kit, according to the manufacturer's instructions (Epicentre, Madison, Wis.). Total RNA concentrations were determined by UV spectrophotometry. Reverse transcription (RT) reactions were performed to convert total RNA into cDNA. Briefly, ca. 1.0 μg of total RNA and 0.5 μg of random hexamers were combined and incubated at 70°C for 5 min, followed by a quick chill at 4°C for 5 min. To this mixture, Improm II 5× reaction buffer, 25 mM MgCl2, 10 mM deoxynucleoside triphosphate, 0.5 U of RNase inhibitor, and 1 μl of Improm II reverse transcriptase was added (Promega). The reaction mixture was incubated at 25°C for 5 min, 37°C for 1 h, and 72°C for 15 min. Reactions were brought up to 40 μl with RNase-free water.
Quantitative real-time PCR.
Quantitative real-time PCR was performed by using the iCycler iQ Detection System (Bio-Rad Laboratories, Hercules, Calif.). Gene-specific primers for quantitative real-time PCR were designed to generate single gene-specific amplicons of 125 to 200 nucleotides. The 96-well real-time PCR format included duplicate 10-fold dilutions of the linearized plasmid DNA standard ranging from 109 to 102 copies. Zebrafish β-actin primers were used to normalize the starting quantity of RNA. Zebrafish IFN and Mx were assayed in triplicate for each sample time point with appropriate standards. Reactions were performed in an iCycler iQ real-time PCR detection system (Bio-Rad) according to the manufacturer's instructions. Reactions were performed in a 20-μl volume comprised of 1 μl of cDNA reaction, 10 μl of 2x IQ SYBR Green Supermix (Bio-Rad), and 250 nM concentrations of each primer. The cycling parameters were 94°C for 15 min to activate the polymerase, followed by 40 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s. Fluorescence measurements were taken at each cycle during the 55°C annealing step. The copy number for each reaction was calculated by the iCycler software. Values were normalized to the corresponding β-actin values to determine the relative copy number. The relative copy number was then used to calculate the fold induction values of virus-induced samples over the control samples.
RESULTS
SHRV infection kinetics and gross pathology.
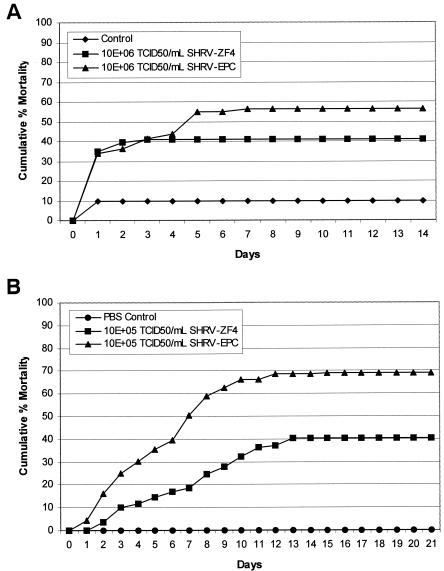
The infectivity of SHRV grown on ZF4 cells and EPC cells was determined for 24-hpf fish exposed to 106 TCID50 of SHRV/ml by immersion challenge (Fig. 1A) and for adult fish injected i.p. with 105 TCID50 of SHRV/ml (Fig. 1B). The 24-hpf fish exposed to SHRV showed average cumulative mortalities of 55% with virus isolated from EPC cells compared to mortalities of 40% with virus produced in ZF4 cells (Fig. 1A). Adult fish infected with SHRV showed average cumulative mortalities of 70% with SHRV grown on EPC cells and average cumulative mortalities of 40% with SHRV grown on ZF4 cells (Fig. 1B). Mortalities due to SHRV infection began 1 day postinfection (dpi) and continued through 10 dpi, although the majority of mortalities occurred between 2 and 8 dpi. Based upon these results, SHRV grown on EPC cells (SHRV-EPC) was more virulent and was used for all subsequent challenges.
FIG. 1.
Comparison of cumulative percent mortalities in zebrafish exposed to SHRV grown on EPC (SHRV-EPC) or ZF-4 cells (SHRV-ZF4). (A) Zebrafish embryos at 24 hpf were exposed to 106 TCID50 of SHRV/ml by immersion and then monitored for 14 days. (B) Adult zebrafish were injected i.p. with 105 TCID50 of SHRV/ml and monitored for 21 days. The data are representative of multiple independent challenges run in triplicate.
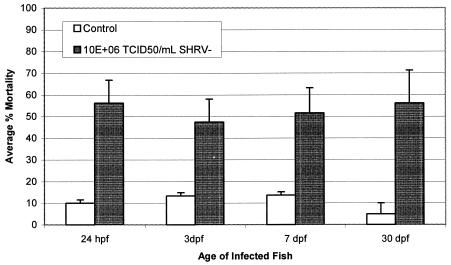
In order to determine the effect on overall fish survival, embryonic and juvenile fish of ages 24 hpf and 3, 7, and 30 dpf were exposed by immersion to 106 TCID50 of SHRV-EPC/ml and monitored daily for 14 days (Fig. 2). The 24-hpf fish exposed to virus showed average cumulative mortalities of 55% during the 14-day challenge period compared to 10% mortality in control fish. The 3-dpf fish showed average cumulative mortalities of 47% by 14 dpi after exposure to SHRV compared to 11% mortality in control fish. The 7-dpf fish exposed to virus showed average cumulative mortalities of 51% at 14 dpi compared to 11% mortality in control fish. The 30-dpf fish exposed to virus showed mortalities of 57% during the challenge period compared to 5% mortality in control fish. Mortalities in control fish were comparable to the proportion of mortalities in typical healthy clutches of eggs from routine breeding. Mortalities associated with immersion infection in the embryonic and juvenile fish began as early as 1 dpi and continued through 12 dpi. Infected fish appeared lethargic prior to death, with subepidermal petechial hemorrhaging observed in the fish infected at 30 dpf. Adult fish exposed to virus by immersion appeared to be refractory to infection and showed inconsistent mortalities of <20% over a period of 21 days (data not shown).
FIG. 2.
Cumulative percent mortalities in zebrafish infected at 24 hpf and at 3, 7, and 30 dpf with SHRV. Zebrafish were exposed to 106 TCID50 of SHRV/ml by immersion and then monitored for 14 days. The final percent mortalities were calculated and recorded for each age group. The data are representative of multiple challenges run in triplicate.
In a separate experiment, adult zebrafish were i.p. injected with 105 TCID50 of SHRV/ml and monitored for 21 days to determine whether they were susceptible to infection by this route. Injection of 105 TCID50 of SHRV/ml produced average cumulative mortalities of 69% (Fig. 1B). Injection of PBS caused no mortalities. Mortalities in adult fish after exposure to SHRV began at 1 dpi and continued through 13 dpi, after which there were no further deaths. Adult zebrafish injected with SHRV exhibited severe petechial hemorrhages on the abdomen as soon as 2 dpi (Fig. 3). Moribund fish exhibited erratic swimming patterns and lingered near the surface of the water. Control fish exhibited no abnormal behaviors or lesions.
FIG. 3.
Gross pathology of adult zebrafish injected i.p. with SHRV. Fish were infected with SHRV and examined at 2 dpi for signs of viral infection and clinical disease. (A) Control fish were injected with 10 μl of PBS. (B) Infected fish were injected i.p. with 105 TCID50 of SHRV/ml.
SHRV reisolation and confirmation.
Juvenile and adult fish exposed to virus by immersion or i.p. injection were randomly sampled for virus titers at intervals during infection. The 24 hpf fish yielded a virus titer that increased from 3.2 × 107 TCID50/ml at 1 dpi to a maximum of 4.7 × 109 TCID50/ml at 3 dpi (Table 2). Virus titers in the 24-hpf fish decreased to 3.2 × 108 TCID50/ml by 6 dpi and continued to decline to 1.0 × 106 TCID50/ml by 10 dpi. The 3-dpf fish showed a virus titer of 3.2 × 107 TCID50/ml at 1 dpi that increased to 4.7 × 108 TCID50/ml by 3 dpi. The 3-dpf fish virus titer declined to 3.2 × 107 TCID50/ml by 6 dpi and reached 3.2 × 106 TCID50/ml by 10 dpi. The 7-dpf fish showed a virus titer of 3.2 × 107 TCID50/ml at 1 dpi that increased to 4.7 × 107 TCID50/ml by 3 dpi. At 6 dpi, the virus titer had dropped to 3.2 × 107 TCID50/ml, and by 10 dpi the virus titer had decreased to 3.2 × 106 TCID50/ml. SHRV immersion challenges with 30-dpf fish showed a slightly different pattern in virus titer with 3.2 × 106 TCID50/ml at 1 dpi and a maximum of 1.0 × 109 TCID50/ml by 2 dpi. However, virus titers in the 30-dpf fish decreased to 1.0 × 108 TCID50/ml by 6 dpi and to 4.2 × 106 TCID50/ml by 10 dpi.
TABLE 2.
Virus titers from fish exposed to SHRV via immersion or i.p. injection
| Sample | Virus titer at:
|
|||||
|---|---|---|---|---|---|---|
| 1 dpi | 2 dpi | 3 dpi | 6 dpi | 8 dpi | 10 dpi | |
| 24 hpf | 3.2 × 107 | 1.0 × 109 | 4.7 × 109 | 3.2 × 108 | 1.0 × 107 | 1.0 × 106 |
| 3 dpf | 3.2 × 107 | 3.2 × 108 | 4.7 × 108 | 3.2 × 107 | 1.0 × 107 | 3.2 × 106 |
| 7 dpf | 3.2 × 107 | 1.0 × 107 | 4.7 × 107 | 3.2 × 107 | 3.2 × 107 | 3.2 × 106 |
| 30 dpf | 3.2 × 106 | 1.0 × 109 | 1.0 × 109 | 1.0 × 108 | 3.2 × 108 | 4.2 × 106 |
| Adult (i.p. injected) | 4.7 × 103 | 3.2 × 107 | 4.7 × 107 | 3.2 × 107 | 3.2 × 107 | 3.2 × 106 |
| Adult (immersion) | 1.0 × 104 | 3.2 × 103 | 1.0 × 102 | 1.0 × 102 | 1.0 × 102 | 0 |
| Controls | 0 | 0 | 0 | 0 | 0 | 0 |
Virus titers were determined for i.p.-injected adult fish and showed a virus titer of 4.7 × 103 TCID50/ml at 1 dpi and a maximum virus titer at 3 dpi of 4.7 × 107 TCID50/ml (Table 2). The levels of SHRV remained elevated at 3.2 × 107 TCID50/ml at 6 and 8 dpi. By 10 dpi, the virus titer in the sampled fish injected i.p. had fallen to 3.2 × 106 TCID50/ml. As seen in the immersion challenges with the young fish, the decrease in virus titers at 10 dpi corresponded to a decrease in overall cumulative fish mortalities.
To better understand the inconsistent mortality data observed when adult fish were infected with SHRV by immersion, adult fish were sampled at intervals for reisolation of virus. Adult fish immersed in SHRV showed a maximum virus titer of 1.0 × 104 TCID50/ml by 1 dpi (Table 2). The virus titers in the adults immersed in SHRV were 3.2 × 103 TCID50/ml by 2 dpi and decreased to 1.0 × 102 TCID50/ml by 3 dpi, where it remained through 8 dpi. By 10 dpi, there was no detectable virus titer in sampled adult fish. Due to the refractory nature of adult infection by immersion, very few adult fish died; however, fish that died were sampled and had virus titers of 3.2 × 107 TCID50/ml (data not shown). Uninfected control fish produced no virus titers in cell culture in any of the infected age groups (Table 2).
To further confirm infection by SHRV, primers for PCR analysis were synthesized from the sequence encoding the nucleocapsid (N-gene) of SHRV (accession no. AF147498). An amplicon of 829 nucleotides spanning nucleotides 47 to 876 of the SHRV N-gene was observed in all virus-infected samples by 1 dpi, confirming the cell culture data. The amplicon continued to be present through 10 dpi in the juvenile fish immersed in SHRV and in livers of the adult fish injected i.p. In the adult fish infected by immersion in SHRV, the amplicon was undetectable in isolated fish livers at 3 dpi. The corresponding amplicon for the SHRV N-gene was absent in all uninfected control samples (data not shown).
Histopathology of SHRV infection.
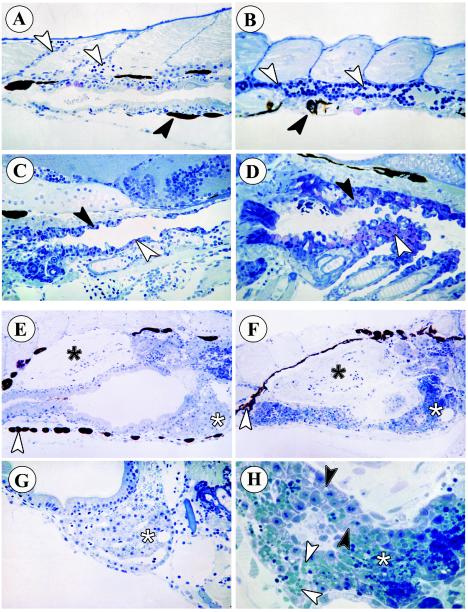
For each of the developmental stages, fish from parallel infected and control groups were sampled daily for histological examination. Similar histopathology was observed in all developmental stages of fish exposed to SHRV by immersion. Figure 4 shows representative histopathology, comparing the blood vessels (Fig. 4A and B), branchial regions (Fig. 4C and D), and livers (Fig. 4E to 4H) of control and infected embryos and juveniles. The histopathology observed in zebrafish infected with SHRV included high numbers of monocytes in the blood vessels of the perianal region and a marked absence of erythrocytes (Fig. 4B, white arrows). Compared to the control (Fig. 4C), virus-infected fish displayed a higher number of mucus cells in the buccopharyngeal epithelium (Fig. 4D, white arrow). The pharyngeal epithelium itself became necrotic, displaying a rough structure and epithelial cells sloughing into the lumen (Fig. 4D, black arrow). The pigment cells of the infected fish also appeared to be irregular in shape (Fig. 4B, black arrow, and Fig. 4F, white arrow) compared to the pigment cells in the control fish (Fig. 4A, black arrow, and Fig. 4E, white arrow). The black asterisk in Fig. 4E indicates the lumen of the swim bladder of the control fish. In the infected fish the lumen of the swim bladder was congested with cell debris (Fig. 4F, black asterisk). The white asterisk in Fig. 4E and F indicates the liver tissue. The dark staining liver of the infected fish contained necrotic cells and fat droplets not present in control fish. Upon closer examination, the liver tissue displayed cytoplasmic vacuolization and pyknosis of hepatocytic nuclei, indicating toxic conditions (Fig. 4H, black arrows). Moreover, hepatocytes were filled with fat droplets that, after the cells disintegrated, appeared in the extracellular space (Fig. 4H, white arrows). In addition, several obstructed hepatic ducts were visible in the infected fish (Fig. 4H) in comparison with the liver of the control fish (Fig. 4G).
FIG. 4.
Histopathology of zebrafish embryos infected with SHRV by immersion. (A) Perianal region of control fish. White arrowheads indicate normal blood vessels. The black arrowhead indicates a normal pigment cell. Magnification, ×400. (B) Perianal region of infected fish. The white arrowheads indicate a blood vessel filled with monocytes. The black arrowhead indicates an irregularly shaped pigment cell. Magnification, ×400. (C) Branchial region of control fish, with normal mucus cells of the branchial epithelia (white arrowhead). Magnification, ×400. The black arrowhead points to the upper pharyngeal epithelium. (D) Branchial region of infected fish. The upper pharyngeal epithelium has a rough structure and contains many proliferating cells (black arrowhead). The white arrowhead indicates numerous pink mucus cells. Magnification, ×400. (E) Liver (white asterisk) and swim bladder (black asterisk) of control fish. The white arrowhead indicates normal pigment cells. Magnification, ×250. (F) Dark staining liver (white asterisk) and congested swim bladder (black asterisk) of infected fish. The white arrowhead indicates irregularly shaped pigment cells of infected fish. magnification, ×250. (G) Higher magnification of liver tissue (white asterisk) from control fish. Magnification, ×400. (H) Higher magnification of liver tissue (white asterisk) from infected fish. Black arrowheads indicate intracellular vacuoles. White arrowheads indicate glycogen vesicles in the extracellular space. Magnification, ×1000.
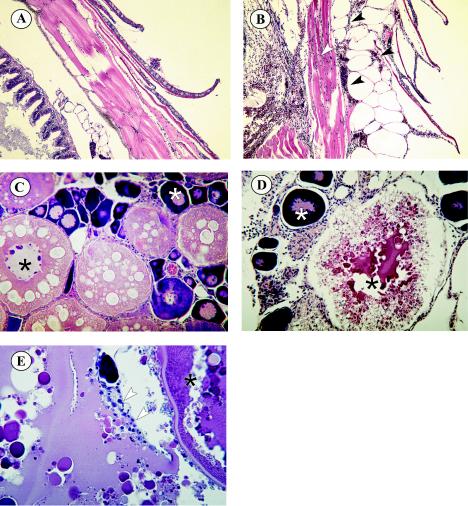
Figure 5 presents comparisons of the scales and epidermis (Fig. 5A and B) and ovaries (Fig. 5C to E) of control and SHRV i.p.-injected adult zebrafish. Histopathology of SHRV-infected adult fish included subepidermal petechial hemorrhages and edema near the site of injection (Fig. 5B), whereas PBS-injected control fish showed no signs of infection or inflammation. These findings agree with the gross pathology observed in SHRV-infected adult fish, as seen in Fig. 3. Infected females also exhibited degeneration of secondary oocytes (Fig. 5D and E). Disruption of the yolks of secondary oocytes and subsequent reabsorption of the yolk by neighboring epithelial granulosa cells were most notable. Some of the infected fish displayed fluid and inflammatory cell accumulation in the abdominal cavity. All other major organs appeared to be unaffected by infection with SHRV.
FIG. 5.
Histopathology of adult zebra fish infected with SHRV by i.p. injection. (A) Normal scales and epidermis of control fish. Magnification, ×100. (B) Scales and epidermis of infected fish. Black arrowheads indicate subdermal edema and hemorrhaging. The white arrowhead indicates hemorrhaging in the underlying muscle tissue. Magnification, ×100. (C) Ovaries of control fish showing normal egg development, with generations of ova in different developmental stages. The white asterisk indicates a primary oocyte, the black asterisk indicates a secondary oocyte, and the black arrowhead indicates the epithelial granulosa (nursing) cells. Magnification, ×200. (D) Degenerating secondary oocyte of SHRV-infected fish (black asterisk). Primary oocytes seem to be unaffected (white asterisk). Magnification, ×200. (E) Epithelial granulosa cells (white arrows) reabsorbing remaining yolk from secondary oocyte.
IFN and Mx expression.
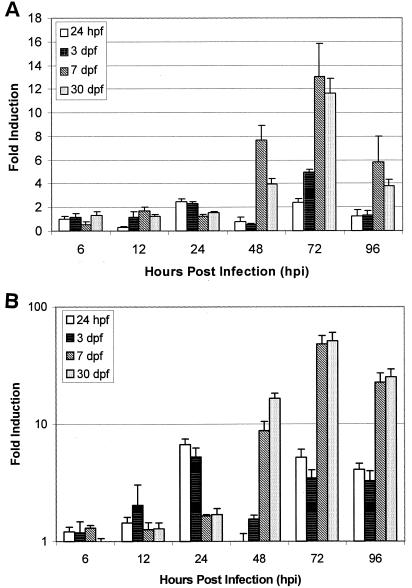
The mRNA expression levels of two immune effector molecules, IFN and Mx, were examined in control and SHRV-infected zebrafish samples by using quantitative real-time PCR analysis. In order to understand the effects of SHRV infection throughout the development of the zebrafish immune system, fish were infected by immersion at 24 hpf and at 3, 7, and 30 dpf. Figure 6A compares IFN mRNA induction levels in fish at 24 hpf and at 3, 7, and 30 dpf at selected times after infection with SHRV. Expression of IFN transcripts in the 24-hpf fish remained at basal levels through 12 hpi and increased 2.5-fold over controls at 24 hpi and 2.4-fold over controls at 72 hpi (Fig. 6A). IFN induction by SHRV in the 3-dpf fish also remained at basal levels of expression through 12 hpi but increased 2.5-fold over controls at 24 hpi and 5.0-fold over controls at 72 dpi. In contrast to the 24-hpf and 3-dpf fish, the 7- and 30-dpf fish displayed a different pattern of IFN mRNA expression. The 7-dpf fish exposed to SHRV showed basal levels of IFN mRNA expression through 24 hpi and an increase of 7.7-fold over controls at 48 hpi. IFN mRNA in the 7-dpf fish reached a maximum expression of 18.0-fold over controls at 72 hpi and decreased to 14.8-fold by 96 hpi. Similar to the 7-dpf fish, the 30-dpf fish remained at basal levels of IFN mRNA expression through 24 hpi and showed an increase of 4.0-fold over controls by 48 hpi. IFN induction increased to a maximum of 11.6-fold in the 30-dpf fish at 72 hpi and decreased to 3.8-fold induction over controls by 96 hpi. These data indicate that SHRV can induce IFN in zebrafish and demonstrates a difference in the antiviral response during zebrafish development.
FIG. 6.
Quantitative real-time PCR analysis of zebrafish IFN (A) and Mx (B) mRNA expression after immersion for 5 h in 106 TCID50 of SHRV/ml. Zebrafish were exposed at 24 hpf and at 3, 7, and 30 dpf. Total RNA was extracted from selected samples through 96 hpi. The data are representative of three independent exposures. Each bar represents the mean fold induction of SHRV-infected samples over corresponding controls. Expression values were normalized to zebrafish β-actin.
SHRV-induced Mx mRNA expression closely paralleled the expression of IFN during infection and development (Fig. 6B). The 24-hpf fish infected with SHRV showed basal levels of Mx mRNA expression through 12 hpi, with increases in Mx expression of 6.7-fold at 24 hpi and 5.2-fold at 72 hpi. By 96 hpi, Mx mRNA levels in the 24-hpf fish began to decrease but remained 4.1-fold greater than the control levels. In 3-dpf fish, Mx remained at basal expression through 6 hpi and increased only 2.0-fold at 12 hpi. At 24 and 72 hpi, the Mx levels in the 3-dpf fish increased by 5.2- and 3.5-fold, respectively. Similar to the 24-hpf fish, Mx levels the 3-dpf fish remained elevated at 3.3-fold through 96 hpi. As was observed in the IFN expression patterns, the Mx expression patterns for the 24-hpf and 3-dpf fish differed from the pattern observed in the 7- and 30-dpf fish. Mx expression in the 7-dpf fish remained at basal levels through 12 hpi and increased only slightly by 1.6-fold over controls at 24 hpi. By 48 hpi, Mx mRNA in the 7-dpf fish had increased by 8.8-fold and continued to increase to a maximum at 72 hpi of 48.0-fold over controls. Expression remained elevated in the 7-dpf fish through 96 hpi, with a 22.7-fold increase in Mx transcripts. The fish samples infected at 30 dpf also remained at basal levels of Mx expression through 12 hpi and showed only a minor increase at 24 hpi of 1.7-fold. Again, Mx expression increased 16.5-fold by 48 hpi in the 30-dpf fish and reached a maximum of 51.0-fold at 72 hpi. Levels decreased in the 30-dpf fish by 96 hpi, but remain elevated at 25.2-fold above controls. As observed with the expression of IFN, the Mx expression pattern and intensity varied with the developmental age of the fish. Mx transcripts increased to higher levels in fish older than 7 dpf, suggesting a more robust antiviral response.
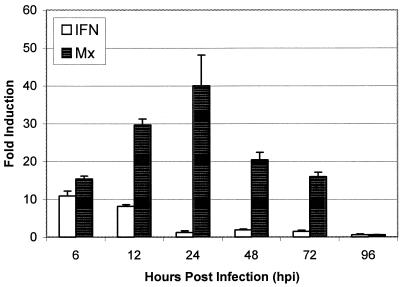
Adult fish exposed to SHRV by i.p. injection were used to examine the antiviral response in the mature zebrafish. Injection of adult fish i.p. with 105 TCID50 of SHRV/ml showed a more rapid antiviral response compared to the 24 hpf and 3-, 7-, and 30-dpf fish exposed by immersion to SHRV. IFN mRNA levels in the adult fish injected i.p. reached a maximum of 10.9-fold by 6 hpi, followed by a rapid return to basal levels of expression by 24 hpi (Fig. 7). Mx mRNA transcripts were elevated 15.4-fold by 6 hpi, and a maximum of 40.0-fold over PBS injected controls was achieved by 24 hpi. Mx expression decreased to 20.5-fold induction by 48 hpi but remained elevated at 16.0-fold through 72 hpi. Transcripts of Mx returned to basal levels of expression by 96 hpi.
FIG. 7.
Quantitative real-time PCR analysis of zebrafish IFN and Mx mRNA expression in the livers of adult fish injected i.p. with 105 TCID50 of SHRV/ml. The data are representative of three independent exposures. Each bar represents the mean fold induction of SHRV-infected samples over corresponding controls. Expression values were normalized to zebrafish β-actin.
DISCUSSION
We describe here experimental infection of zebrafish with SHRV and the ensuing pathology and antiviral response to infection. Zebrafish were exposed to SHRV at various stages of development via immersion or i.p. injection. Embryonic and juvenile fish ages 24 hpf to 30 dpf were susceptible to infection by immersion with SHRV. Adult zebrafish were only susceptible to infection with SHRV by i.p. injection and were refractory to infection by immersion. Observation of gross pathology and examination of histological sections of infected fish showed substantial evidence of viral disease and necrosis. Quantitative analysis of IFN and Mx mRNA levels in infected juvenile zebrafish showed an increase in IFN and Mx expression in response to SHRV infection compared to controls.
Natural SHRV infection has only been associated with the complex disease EUS; however, experimental infection with SHRV has been successful in snakehead fry and juveniles and in zebrafish (2, 12, 21, 29). This is the first study to examine, in detail, the pathogenesis and antiviral response in zebrafish associated with SHRV infection. In infected embryos and juveniles, the histopathology indicates the virus was able to invade and spread throughout the vasculature and adjacent tissues. The marked accumulation of monocytes in the blood vessels of infected embryos indicates the presence of a primary immune response in the infected fish (43). The accumulation of cell debris in the swim bladder and the necrosis of the pharyngeal epithelium and liver cells all indicate toxic conditions (38). Alterations in the pharyngeal epithelium and hepatic necrosis have been observed in rainbow trout, zebrafish, and carp in connection with other acute rhabdoviral diseases, including VHSV, IHNV, and SVCV (5, 37, 38, 53, 54).
The difference in virulence of the two virus stocks derived from separate host cells is likely due to the selective pressures placed upon the virus during replication in the specific cell line. Single cell lines often prevent microvariant virus particles from replicating and select for those virus particles that will replicate most efficiently in the given cell line. This selective pressure can affect virulence factors and the overall pathogenicity of the virus. Therefore, although the virus stocks were used at the same titer for infections, the pathogenicity of the stocks is significantly different, so that different mortalities were observed after challenge.
Pathogenesis due to SHRV infection by injection in adult fish appeared confined to the abdominal cavity at the site of viral injection. The absence of viral infection involving other major organ systems, as seen in the immersion challenges, may be due to the route of infection in the adult i.p. injection challenges. Infection by immersion may mimic a more natural route of infection and allow the virus to spread throughout the fish. However, adult zebrafish were refractory to infection by immersion in SHRV, whereas fish at 24 hpf and at 3, 7, and 30 dpf succumb to infection by immersion in SHRV. This change in susceptibility to SHRV infection by immersion may reflect an alteration in receptor-mediated entry of the virus or development of an innate immune barrier in the adult zebrafish that prevents viral penetration into the host. Neely et al. found that adult zebrafish were refractory to infection by immersion with Streptococcus iniae and Streptococcus pyogenes (35). These authors observed that removal of scales and abrasion of the dermis rendered the fish susceptible to infection by immersion. A similar method of challenge was used with SHRV in adult zebrafish but did not produce mortalities (data not shown).
Real-time quantitative PCR was used to examine the antiviral immune response to SHRV by comparing the expression levels of IFN and Mx in embryo, juvenile, and adult zebrafish infected by SHRV. Transfection of zebrafish cells with zebrafish IFN in vitro has demonstrated that zebrafish IFN has antiviral activity against SHRV (3). In addition, our lab has previously shown that Mx can be induced by SHRV and poly(I-C) in cell culture (4). In the zebrafish embryos and juveniles in the present study, SHRV induces IFN and Mx expression at various levels, and patterns that appear dependent upon the age of the fish age and route of infection. Fish at 24 hpf and 3 dpf displayed minor increases in IFN and Mx expression at 24 and 48 hpi, whereas fish older than 7 dpf showed a single maximum increase in IFN and Mx expression at 72 hpi. Furthermore, a more robust increase in IFN and Mx expression was observed in the older fish.
The differences in cytokine response in fish at 24 hpf and 3 dpf and in fish 7 and 30 dpf may be attributable to changes in physiological or immunological development. During zebrafish development, the pharyngeal arch structures that give rise to the gills begin to develop during the 24- to 48-hpf pharyngula period, and gill slits do not form in the branchial arches until the 48- to 72-hpf hatching period (23). The primordium of the liver along the gastrointestinal tract also begins to develop during the 24- to 48-hpf pharyngula period (10, 24). Previous work conducted with the related fish rhabdoviruses IHNV, SVCV, and VHSV demonstrates the importance of the gills and gastrointestinal tract as initial routes of entry and replication (1, 9, 36). Therefore, potentially important routes of entry for SHRV are not fully developed in the 24-hpf and 3-dpf fish at the time of infection. Nevertheless, the histopathology of SHRV infection clearly showed extensive involvement of the branchial regions and livers of infected zebrafish at all stages of development. The histopathology data, in conjunction with the cumulative percent mortality and virus isolation data, indicate that whereas possible entry routes for SHRV are not mature in the 24-hpf and 3-dpf fish, the organ primordia for the gills and gastrointestinal tract may provide sufficient binding sites for SHRV to invade zebrafish of these ages and cause infection.
In addition to differences in physiological development, the maturity of the immune system may also be playing a role in the antiviral response. Prior reports indicate expression of zebrafish rag1, rag2, T-cell receptor α, and immunoglobulin M transcripts as early as 4 dpf (6, 7, 51, 52). Experiments performed by Lam et al. (25) support earlier findings showing the zebrafish immune system requires 4 to 6 weeks postfertilization to become morphologically and functionally mature. The complement system in zebrafish may also contribute to the observed differences in antiviral cytokine response between fish at 24 hpf and 3 dpf versus fish at 7 and 30 dpf. Complement is produced by the liver and serves as an important mediator to amplify the immune response to pathogens by activating monocytes, macrophages, neutrophils, and dendritic cells (14, 48). In mammals, activated macrophages and dendritic cells then produce IFN-α/β to induce an antiviral state in host cells (14). Fish contain a highly developed complement system that is similar to the complement system of mammals, and zebrafish have several important C3 and factor B genes that, in mammals, act as an inflammatory mediator and a serine protease, respectively (48). Since the livers of zebrafish embryos begin to develop at 3 dpf, the mature liver may be enhancing the antiviral response observed in the 7- and 30-dpf fish. Therefore, we may be detecting increased levels of IFN and Mx due to a more mature immune system in 7- and 30-dpf fish.
Livers from adults infected by i.p. injection displayed a rapid induction of IFN by 6 hpi and of Mx by 24 hpi, possibly due to the introduction of virus directly into the body cavity of the fish. Expression studies of Mx mRNA in Japanese flounder leukocytes showed approximately threefold increases in response to intramuscular injection of Hirame rhabdovirus at 72 hpi (28). At 4 dpi, Jensen et al. (17) found that livers from Atlantic salmon injected i.p. with poly(I-C) had three- to eightfold increases in Mx expression compared to controls. Similar experiments in Atlantic halibut demonstrated that Mx mRNA expression is induced when fish are injected i.p. with either poly(I-C) or IPNV (18). Our results for the 24-hpf and 3-dpf SHRV-infected fish correlate well with previous reports describing the expression of Mx after viral infection in other fish species such as Japanese flounder, Atlantic salmon, and Atlantic halibut. However, the data for the 7-dpf, 30-dpf, and adult liver samples show much more dramatic increases in Mx induction. Previous studies examined Mx mRNA expression in fish through the use of Northern blot and RT-PCR analysis (18, 28). Northern blot and RT-PCR analysis lack the sensitivity of quantitative real-time PCR for determining relative levels of mRNA. Therefore, the discrepancies between our Mx mRNA expression data and previously reported Mx mRNA expression data may be a result of increased sensitivity using quantitative real-time PCR experiments to quantify Mx mRNA levels.
Few zebrafish disease models for viral infection have been documented. LaPatra et al. found that IHNV and IPNV were able replicate in zebrafish (26). Although no mortalities occurred in fish exposed by immersion or injection of either virus, histological analysis revealed that both viruses produced similar toxic effects in erythroid kidney cells. Recently, Sanders et al. described a viral pathogen model in adult zebrafish with SVCV (40). Histological and gross pathological evaluation of SVCV-infected fish showed branchial necrosis, hepatic and splenic necrosis, an increase in melanomacrophages, and epidermal petechial hemorrhages. The average percent mortalities were shown to increase as infected adult zebrafish were maintained at colder temperatures of 15 and 20°C, well below the optimal temperature for zebrafish. A major drawback to the zebrafish SVCV model is the length of time involved for the experimental infection. The fish needed to acclimate to colder temperatures, and the water temperature had to be gradually increased throughout the challenge. In addition, SVCV infection was only examined in adult zebrafish. LaPatra et al. and Sanders et al. did not describe the use of zebrafish embryos in any of their viral challenge experiments. The ability to infect zebrafish embryos will be important for future studies involving genetic modulation and forward genetic screening to identify immunological mutations.
Zebrafish have become an established model in biomedical research and have great potential for studies involving vertebrate immune system development and function. The proliferation of mutant strains and completion of the zebrafish genome project will provide new opportunities for investigating vertebrate biology and immune function. As more immune function-related genes are identified in the zebrafish, biological and functional assays will be needed to elucidate their function. For example, antisense morpholino technology has been used to inhibit gene translation in developing embryos (34). This method of targeted gene disruptions can be used in conjunction with pathogen challenge to alter immunity to infection. Differences in mortality rates, pathogenesis, and gene expression may provide clues about the role of genes linked to immunity. The methods and results of the present study comprise an essential foundation on which future research on host-pathogen interactions and innate immunity in the zebrafish model can be built.
Acknowledgments
We thank Steve Altmann, Con Sullivan, and Paul Millard for helpful comments on the manuscript. We also thank Nick Stasulis for excellent technical support.
This study was supported by National Institutes of Health grant R15 AI49237-01 and funds administered through the Maine Agricultural and Forest Experiment Station.
Footnotes
Maine Agricultural and Forest Experiment Station publication 2651.
REFERENCES
- 1.Ahne, W. 1978. Uptake and multiplication of spring viraemia of carp virus in carp, Cyprinus carpio L. J. Fish Dis. 1:265-268. [Google Scholar]
- 2.Alonso, M., C. H. Kim, M. C. Johnson, M. Pressley, and J. A. Leong. 2004. The NV gene of snakehead rhabdovirus (SHRV) is not required for pathogenesis, and a heterologous glycoprotein can be incorporated into the SHRV envelope. J. Virol. 78:5875-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altmann, S. M., M. T. Mellon, D. L. Distel, and C. H. Kim. 2003. Molecular and functional analysis of an interferon gene from the zebrafish, Danio rerio. J. Virol. 77:1992-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altmann, S. M., M. T. Mellon, M. C. Johnson, B. H. Paw, N. S. Trede, L. I. Zon, and C. H. Kim. 2004. Cloning and characterization of an Mx gene and its corresponding promoter from the zebrafish, Danio rerio. Dev. Comp. Immunol. 28:295-306. [DOI] [PubMed] [Google Scholar]
- 5.Amend, D. F., W. T. Yasutake, and R. W. Mead. 1969. A hematopoietic virus disease of rainbow trout and sock-eye salmon. Trans. Am. Fish. Soc. 98:796-804. [Google Scholar]
- 6.Danilova, N., V. S. Hohman, F. Sacher, T. Ota, C. E. Willett, and L. A. Steiner. 2004. T cells and the thymus in developing zebrafish. Dev. Comp. Immunol. 28:755-767. [DOI] [PubMed] [Google Scholar]
- 7.Danilova, N., and L. A. Steiner. 2002. B cells develop in the zebrafish pancreas. Proc. Natl. Acad. Sci. USA 99:13711-13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driever, W., and Z. Rangini. 1993. Characterization of a cell line derived from zebrafish (Brachydanio rerio) embryos. In Vitro Cell Dev. Biol. Anim. 29A:749-754. [DOI] [PubMed] [Google Scholar]
- 9.Drolet, B. S., J. S. Rohovec, and J. C. Leong. 1994. The route of entry and progression of infectious hematopoietic necrosis virus in Oncorhynchus mykiss (Walbaum): a sequential immunohistochemical study. J. Fish Dis. 17:337-347. [Google Scholar]
- 10.Field, H. A., E. A. Ober, T. Roeser, and D. Y. Stainier. 2003. Formation of the digestive system in zebra fish. I. Liver morphogenesis. Dev. Biol. 253:279-290. [DOI] [PubMed] [Google Scholar]
- 11.Fijan, N. 1983. Some properties of the Epithelioma papulosum cyprini (EPC) cell line from common carp Cyprinus carpio. Ann. Virol. 134E:207-220. [Google Scholar]
- 12.Frerichs, G. N., S. D. Millar, and S. Chinabut. 1993. Clinical response of snakeheads (Ophicephalus striatus) to experimental infection with snakehead fish rhabdovirus and snakehead cell line retrovirus. Aquaculture 116:297-301. [Google Scholar]
- 13.Frese, M., G. Kochs, H. Feldmann, C. Hertkorn, and O. Haller. 1996. Inhibition of bunyaviruses, phleboviruses, and hantaviruses by human MxA protein. J. Virol. 70:915-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldsby, R., T. Kindt, and B. Osborne. 2001. Kuby immunology, 4th ed. W. H. Freeman & Co., New York, N.Y.
- 15.Horisberger, M. A., P. Staeheli, and O. Haller. 1983. Interferon induces a unique protein in mouse cells bearing a gene for resistance to influenza virus. Proc. Natl. Acad. Sci. USA 80:1910-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jault, C., L. Pichon, and J. Chluba. 2004. Toll-like receptor gene family and TIR-domain adapters in Danio rerio. Mol. Immunol. 40:759-771. [DOI] [PubMed] [Google Scholar]
- 17.Jensen, I., A. Albuquerque, A. I. Sommer, and B. Robertsen. 2002. Effect of poly I:C on the expression of Mx proteins and resistance against infection by infectious salmon anaemia virus in Atlantic salmon. Fish Shellfish Immunol. 13:311-326. [DOI] [PubMed] [Google Scholar]
- 18.Jensen, V., and B. Robertsen. 2000. Cloning of an Mx cDNA from Atlantic halibut (Hippoglossus hippoglossus) and characterization of Mx mRNA expression in response to double-stranded RNA or infectious pancreatic necrosis virus. J. Interferon Cytokine Res. 20:701-710. [DOI] [PubMed] [Google Scholar]
- 19.Johansen, A., B. Collet, E. Sandaker, C. J. Secombes, and J. B. Jorgensen. 2004. Quantification of Atlantic salmon type-I interferon using an Mx1 promoter reporter gene assay. Fish Shellfish Immunol. 16:173-184. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, M. C., B. E. Simon, C. H. Kim, and J. A. Leong. 2000. Production of recombinant snakehead rhabdovirus: the NV protein is not required for viral replication. J. Virol. 74:2343-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasornchandra, J., C. N. Lannan, J. S. Rohovec, and J. L. Fryer. 1991. Characterization of a rhabdovirus isolated from the snakehead fish (Ophicephalus striatus, p. 175-182. In Second International Symposium on Viruses of Lower Vertebrates, Corvallis, Oreg.
- 21a.Kasornchandra, J., H. M. Engelking, C. N. Lannan, J. S. Rohovec, and J. L. Fryer. 1992. Characteristics of three rhabdoviruses from snakehead fish Ophicephalus striatus. Dis. Aquat. Org. 13:89-94. [Google Scholar]
- 22.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 23.Kimmel, C. B., W. W. Ballard, S. R. Kimmel, B. Ullmann, and T. F. Schilling. 1995. Stages of embryonic development of the zebrafish. Dev. Dyn. 203:253-310. [DOI] [PubMed] [Google Scholar]
- 24.Korzh, S., A. Emelyanov, and V. Korzh. 2001. Developmental analysis of ceruloplasmin gene and liver formation in zebrafish. Mech. Dev. 103:137-139. [DOI] [PubMed] [Google Scholar]
- 25.Lam, S. H., H. L. Chua, Z. Gong, T. J. Lam, and Y. M. Sin. 2004. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 28:9-28. [DOI] [PubMed] [Google Scholar]
- 26.Landis, H., A. Simon-Jodicke, A. Kloti, C. Di Paolo, J. J. Schnorr, S. Schneider-Schaulies, H. P. Hefti, and J. Pavlovic. 1998. Human MxA protein confers resistance to Semliki Forest virus and inhibits the amplification of a Semliki Forest virus-based replicon in the absence of viral structural proteins. J. Virol. 72:1516-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaPatra, S. E., L. Barone, G. R. Jones, and L. I. Zon. 2000. Effects of infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus infection on hematopoietic precursors of the zebrafish. Blood Cells Mol. Dis. 26:445-452. [DOI] [PubMed] [Google Scholar]
- 28.Lee, J. Y., I. Hirono, and T. Aoki. 2000. Cloning and analysis of expression of Mx cDNA in Japanese flounder, Paralichthys olivaceus. Dev. Comp. Immunol. 24:407-415. [DOI] [PubMed] [Google Scholar]
- 29.Lio-Po, G. D., L. J. Albright, G. S. Traxler, and E. M. Leano. 2001. Pathogenicity of the epizootic ulcerative syndrome (EUS)-associated rhabdovirus to snakehead Ophicephalus striatus. Fish Pathol. 36:57-66. [Google Scholar]
- 30.Lio-Po, G. D., G. S. Traxler, L. J. Albright, and E. M. Leano. 2000. Characterization of a virus obtained from snakeheads Ophicephalus striatus with epizootic ulcerative syndrome (EUS) in the Philippines. Dis. Aquat. Organ. 43:191-198. [DOI] [PubMed] [Google Scholar]
- 31.Long, S., M. Wilson, E. Bengten, L. Bryan, L. W. Clem, N. W. Miller, and V. G. Chinchar. 2004. Identification of a cDNA encoding channel catfish interferon. Dev. Comp. Immunol. 28:97-111. [DOI] [PubMed] [Google Scholar]
- 32.Meier, E., G. Kunz, O. Haller, and H. Arnheiter. 1990. Activity of rat Mx proteins against a rhabdovirus. J. Virol. 64:6263-6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meijer, A. H., S. F. Gabby Krens, I. A. Medina Rodriguez, S. He, W. Bitter, B. Ewa Snaar-Jagalska, and H. P. Spaink. 2004. Expression analysis of the Toll-like receptor and TIR domain adaptor families of zebrafish. Mol. Immunol. 40:773-783. [DOI] [PubMed] [Google Scholar]
- 34.Nasevicius, A., and S. C. Ekker. 2000. Effective targeted gene “knockdown” in zebrafish. Nat. Genet. 26:216-220. [DOI] [PubMed] [Google Scholar]
- 35.Neely, M. N., J. D. Pfeifer, and M. Caparon. 2002. Streptococcus-zebrafish model of bacterial pathogenesis. Infect. Immun. 70:3904-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neukirch, M. 1984. An experimental study of the entry and multiplication of a viral haemorrhagic septicemia virus in rainbow trout, Salmo gairdneri Richardson, after water-borne infection. J. Fish Dis. 7:231-234. [Google Scholar]
- 37.Noga, E. J. 2000. Fish disease: diagnosis and treatment. Iowa State University Press, Ames, Iowa.
- 38.Roberts, R. J. (ed.). 1978. Fish pathology. Baillire Tindall, London, England.
- 39.Roberts, R. M., L. Liu, Q. Guo, D. Leaman, and J. Bixby. 1998. The evolution of the type I interferons. J. Interferon Cytokine Res. 18:805-816. [DOI] [PubMed] [Google Scholar]
- 40.Sanders, G. E., W. N. Batts, and J. R. Winton. 2003. Susceptibility of zebrafish (Danio rerio) to a model pathogen, spring viremia of carp virus. Comp. Med. 53:514-521. [PubMed] [Google Scholar]
- 41.Schnorr, J. J., S. Schneider-Schaulies, A. Simon-Jodicke, J. Pavlovic, M. A. Horisberger, and V. ter Meulen. 1993. MxA-dependent inhibition of measles virus glycoprotein synthesis in a stably transfected human monocytic cell line. J. Virol. 67:4760-4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schultz, U., J. Kock, H. J. Schlicht, and P. Staeheli. 1995. Recombinant duck interferon: a new reagent for studying the mode of interferon action against hepatitis B virus. Virology 212:641-649. [DOI] [PubMed] [Google Scholar]
- 43.Secombes, C. J., and T. C. Fletcher. 1992. The role of phagocytes in the protective mechanisms of fish. Annu. Rev. Fish Dis. 2:53-71. [Google Scholar]
- 44.Sekellick, M. J., A. F. Ferrandino, D. A. Hopkins, and P. I. Marcus. 1994. Chicken interferon gene: cloning, expression, and analysis. J. Interferon Res. 14:71-79. [DOI] [PubMed] [Google Scholar]
- 45.Staeheli, P., and J. Pavlovic. 1991. Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J. Virol. 65:4498-4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staeheli, P., Y. X. Yu, R. Grob, and O. Haller. 1989. A double-stranded RNA-inducible fish gene homologous to the murine influenza virus resistance gene Mx. Mol. Cell. Biol. 9:3117-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suresh, M., K. Karaca, D. Foster, and J. M. Sharma. 1995. Molecular and functional characterization of turkey interferon. J. Virol. 69:8159-8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Traver, D., P. Herbomel, E. E. Patton, R. D. Murphey, J. A. Yoder, G. W. Litman, A. Catic, C. T. Amemiya, L. I. Zon, and N. S. Trede. 2003. The zebrafish as a model organism to study development of the immune system. Adv. Immunol. 81:253-330. [PubMed] [Google Scholar]
- 49.Trobridge, G. D., and J. A. Leong. 1995. Characterization of a rainbow trout Mx gene. J. Interferon Cytokine Res. 15:691-702. [DOI] [PubMed] [Google Scholar]
- 50.Wattanavijarn, W., J. Tangtronpiros, and K. Wattanodorn. 1986. Viruses of ulcerative diseased fish in Thailand, Burma, and Laos, p. 121. In First International Conference on the Impact of Viral Diseases on the Development of Asian Countries, Bangkok, Thailand.
- 51.Willett, C. E., J. J. Cherry, and L. A. Steiner. 1997. Characterization and expression of the recombination activating genes (rag1 and rag2) of zebrafish. Immunogenetics 45:394-404. [DOI] [PubMed] [Google Scholar]
- 52.Willett, C. E., A. G. Zapata, N. Hopkins, and L. A. Steiner. 1997. Expression of zebrafish rag genes during early development identifies the thymus. Dev. Biol. 182:331-341. [DOI] [PubMed] [Google Scholar]
- 53.Wolf, K. 1984. Diseases of pisces: diseases caused by microorganisms. Agents: viral, p. 17-113. In O. Kinne (ed.), Diseases of marine animals, vol. IV, part 1. Biologische Anstallt Helgoland, Hamburg, Germany.
- 54.Yasutake, W. T., and D. F. Amend. 1972. Some aspects of pathogenesis of infectious hematopoietic necrosis (IHN). J. Fish Biol. 4:261-264. [Google Scholar]
- 55.Zhang, Y. B., and J. F. Gui. 2004. Identification and expression analysis of two IFN-inducible genes in crucian carp (Carassius auratus L.). Gene 325:43-51. [DOI] [PubMed] [Google Scholar]