Abstract
The lack of an appropriate in vitro infection system for the major human pathogen hepatitis B virus (HBV) has prevented a molecular understanding of the early infection events of HBV. We used the novel HBV-infectible cell line HepaRG and primary human hepatocytes to investigate the interference of infection by HBV envelope protein-derived peptides. We found that a peptide consisting of the authentically myristoylated N-terminal 47 amino acids of the pre-S1 domain of the large viral envelope protein (L protein) specifically prevented HBV infection, with a 50% inhibitory concentration (IC50) of 8 nM. The replacement of myristic acid with other hydrophobic moieties resulted in changes in the inhibitory activity, most notably by a decrease in the IC50 to picomolar concentrations for longer unbranched fatty acids. The obstruction of HepaRG cell susceptibility to HBV infection after short preincubation times with the peptides suggested that the peptides efficiently target and inactivate a receptor at the hepatocyte surface. Our data both shed light on the molecular mechanism of HBV entry into hepatocytes and provide a basis for the development of potent hepadnaviral entry inhibitors as a novel therapeutic concept for the treatment of hepatitis Β.
The human hepatitis B virus (HBV) causes acute and chronic liver infections in humans. Owing to the propensity of HBV to establish persistent infections, about 400 million people worldwide have an ≈100-fold higher risk of developing liver cirrhosis and hepatocellular carcinoma than uninfected people. As a consequence, about 1 million people die every year from HBV-related end-stage liver failure (27). Thus, regardless of the availability of a vaccine and the possibility to therapeutically interfere with genome replication in already infected cells, there is a vital need for the development of agents that protect healthy hepatocytes from infection (e.g., by interference with virus entry) and hence bear the potential to be curative (13).
HBV belongs to the family Hepadnaviridae, which includes small enveloped partially double-stranded DNA viruses infecting primates, rodents, and birds (7). Hepadnaviruses possess remarkable species specificities and preferentially target parenchymal liver cells of their respective natural hosts. Experimental in vitro HBV infections have so far only been successful in highly differentiated primary hepatocytes of humans (PHH) (9) and, surprisingly, Tupaia belangeri (8) or in the recently described HepaRG cell line (11). In vivo studies have been restricted to chimpanzees or, as alternatives of unclear relevance, the animal models Pekin ducks (19) and woodchucks (30), using the corresponding duck HBV (DHBV) and woodchuck HBV, respectively. Since the delivery (e.g., by transfection) of hepadnaviral genomes into nonsusceptible cell lines of diverse origins results in the replication, assembly, and secretion of infectious particles (1, 5), it has been assumed that the described limitations are related to some early infection events (receptor recognition, coreceptor dependence, etc.). By the application of these transfection systems, substantial insights have been gained regarding the intracellular part of the hepadnaviral replication cycle, particularly the transcription of subgenomic and pregenomic RNAs, encapsidation of pregenomic RNA, synthesis of the viral DNA by reverse transcription, and establishment of an intracellular pool of covalently closed circular HBV DNA (27). In contrast, we lack an elementary understanding of HBV receptor binding, virus uptake, and membrane fusion, which are addressed by the functional analysis presented in this article.
The HBV envelope consists of the large (L), middle (M), and small (S) surface proteins. These proteins are encoded by a single open reading frame containing three in-phase start codons. The largely hydrophobic S domain serves as a membrane anchor and plays important roles in virus assembly (3) and possibly membrane fusion (2). An N-terminal extension of S by 55 amino acids (termed pre-S2) results in the M protein, while an additional 108 (genotype D) or 119 (genotypes A and C) N-terminal amino acid residues (termed pre-S1) define L. During synthesis and prior to translocation to the lumen of the endoplasmic reticulum (ER), the pre-S domain of the L protein becomes posttranslationally myristoylated at glycine 2 (23). This modification plays an important role early in the HBV life cycle, as the replacement of glycine-2 by alanine, preventing the addition of myristic acid by the cellular N-myristoyltransferase, renders the properly assembled mutated virus noninfectious for PHH (4, 10). Myristoylation is therefore important for HBV infectivity, although its role has not yet been elucidated at the molecular level.
To gain initial insights into the amino acid sequence requirements within the pre-S domain for HBV infection, Le Seyec et al. examined the infectivity of mutated HBV particles carrying continuous deletions of five amino acids in the pre-S1 and pre-S2 regions of the L protein (16, 17). Their results defined an extended sequence encompassing amino acids 2 to 77 of pre-S1 to be mandatory for infection and excluded an essential role of pre-S2 in the infection process. Consistent with these characteristics, we found that an N-terminally myristoylated peptide comprising this region (HBVpreS/2-78myr) blocked HBV infections of PHH and HepaRG cells, thus demonstrating the specific susceptibility of this novel cell line towards HBV infection (11). Notably, this part of the pre-S1 sequence includes epitopes for monoclonal antibodies (e.g., MA18/7 or 5a19) that have been described to block the binding of HBV particles to PHH (18) and HepG2 cells (21) and to neutralize HBV infections of primary hepatocytes from T. belangeri (8).
Based on these findings, in this report we describe acylated pre-S1-derived peptides and mutants thereof and an analysis of their ability to interfere with HBV infections of PHH and HepaRG cells. By using this approach, we have (i) defined amino acid sequence requirements for infection inhibition and hence receptor recognition, (ii) characterized the role of N-terminal acylation of pre-S1, and (iii) provided a model of infection interference by targeting a cellular receptor on the hepatocyte surface.
MATERIALS AND METHODS
Cell lines and primary cell cultures.
HepaRG cells were grown in William's E medium supplemented with 10% fetal calf serum (FCS), 100 U of penicillin/ml, 100 μg of streptomycin/ml, 5 μg of insulin/ml, and 5 × 10−5 M hydrocortisone hemisuccinate. One-fifth of the cells were passaged every 2 weeks by trypsinization. Two to three weeks before infection, cell differentiation was induced by adding 2% dimethyl sulfoxide (DMSO) to the maintenance medium. The medium was exchanged every 2 to 3 days. Primary human hepatocytes were isolated from patients undergoing hepatic resection for liver metastases. Access to this material was obtained in agreement with French laws and satisfied the requirements of the ethics committee of the institution. Hepatocytes were isolated as described previously (12) and were cultured in H medium supplemented with 3.5 × 10−6 M hydrocortisone hemisuccinate, 2% DMSO, 5% adult human serum, and 5% FCS.
Infection competition assays.
For an infectious inoculum, a 50-fold concentrated culture supernatant of HepG2 clone 2.2.15 cells was used because of its unlimited supply and constant quality. It was prepared from freshly collected supernatants by precipitating viral particles in the presence of 6% polyethylene glycol (PEG) 8000. The pellets were resuspended in phosphate-buffered saline containing 25% FCS. Aliquots were stored at −80°C. Differentiated HepaRG cells or PHH were incubated with the concentrated infectious source diluted 10-fold in culture medium supplemented with 4% PEG 8000 (Sigma) for 20 h at 37°C. At the end of the incubation, the cells were washed three times with the culture medium, maintained in the presence of 2% DMSO and 5 × 10−5 M hydrocortisone hemisuccinate, and harvested at the indicated times. Competition experiments were performed in 12-well plates. Approximately 106 cells were first preincubated for 30 min with chemically synthesized HBV-derived peptides and then were coincubated with peptides and virus for 20 h. All competition series were performed at least twice, and the results of one representative experiment are shown in each case.
Peptide synthesis, purification, and analysis.
Peptides were synthesized at the Department of Biomolecular Chemistry, Zentrum für Molekulare Biologie, Heidelberg, Germany, and by Peptide Specialty Laboratories, GmbH, Heidelberg, Germany. For increased stability, the C termini of all peptides were amidated. The raw products were purified by standard reverse-phase high-performance liquid chromatography. Fractions eluting at the expected retention times were lyophilized and analyzed by high-resolution mass spectrometry. Table 1 compares the theoretically calculated monoisotopic and average masses with those obtained experimentally. The resolution of the mass spectrometric analysis allowed the discrimination of peptides differing by only 2 mass units, which would be caused by the introduction of one double bond. For infection competition experiments, stock solutions of 100 μM DMSO were prepared and added to the medium at an appropriate concentration. Stock solutions were stored at −80°C.
TABLE 1.
Calculated and experimentally determined molecular masses of pre-S peptides
| Peptide | Monoisotopic mass (Da) | Calc. avg mass (Da) | Retention time (min) | Observed mass (Da) |
|---|---|---|---|---|
| WMpreS/1-48 | 5,320.52 | 5,323.86 | 14.368 | 5,324.0 |
| WMpreS/2-48myr | 5,400.69 | 5,404.03 | 20.094 | 5,403.0 |
| HepreS/2-44myr | 4,810.62 | 4,813.63 | 16.650 | 4,812.0 |
| DHBVpreS/2-41myr | 4,598.56 | 4,601.51 | 15.245 | 4,600.0 |
| HBVpreS/1-48 | ||||
| HBVpreS/2-68myr | ||||
| HBVpreS/2-48myr | 5,435.65 | 5,438.99 | 19.427 | 5,437.37 |
| HBVpreS/2-39myr | 4,467.18 | 4,469.92 | 16.705 | 4,468.0 |
| HBVpreS/2-28myr | ||||
| HBVpreS/2-18myr | 2,069.07 | 2,070.36 | 19.864 | 2,068.0 |
| HBVpreS/2-8myr | 9,15.56 | 916.10 | 23.525 | 915.4 |
| HBVpreS/2-48 | 5,225.45 | 5,228.63 | 13.575 | 5,227.64 |
| HBVpreS/2-48pent | 5,309.51 | 5,312.75 | 14.626 | 5,310.59 |
| HBVpreS/2-48oct | 5,351.56 | 5,354.82 | 15.830 | 5,352.02 |
| HBVpreS/2-48octadecanoyl | 5,491.71 | 5,495.10 | 22.871 | 5,493.59 |
| HBVpreS/2-48myrΔ20-21 | 5,223.57 | 5,226.78 | 19.450 | 5,223.94 |
| HBVpreS/2-48octadece-cis-9 | 5,489.70 | 5,493.08 | 22.019 | 5,491.98 |
| HBVpreS/2-48octadece-trans-9 | 5,489.70 | 5,493.08 | 21.637 | 5,491.92 |
| HBVpreS/2-48octadecadi-cis-cis-9-12 | 5,487.68 | 5,491.07 | 19.970 | 5,490.04 |
| HBVpreS/2-48myrP24-E24 | 5,408.59 | 5,411.92 | 19.833 | 5,410.92 |
| HBVpreS/2-48myrΔ23-27 | 4,846.35 | 4,849.34 | 19.999 | 4,847.85 |
| HBVpreS/2-48myrΔDPAF | 5,005.47 | 5,008.53 | 19.371 | 5,008.16 |
| HBVpreS/2-48palm | 5,461.67 | 5,465.03 | 20.945 | 5,465.48 |
Peptide labeling with Cy3.
Four hundred microliters of a 250 μM aqueous stock solution of HBVpreS/2-48myr (1.36 mg/ml) or HBVpreS/2-48myrΔ20-23 (1.25 mg/ml) was mixed with 100 μl of 0.5 M NaHCO3 to yield a final peptide concentration of 200 μM at pH 9.3. Coupling reactions were started by the addition of 10 μl of Cy3 monoreactive dye (Amersham Pharmacia). The mixture was incubated in the dark with mild shaking at room temperature for 2 h. Due to the specificity of the reactive dye, the blockade of the N-terminal amino group by myristic acid, and the sequence of the peptide, only lysines 38 and 46 served as substrates. For exchange of the alkaline buffer and removal of the unreacted free dye, the reaction mixture was applied to a PD10 column (Amersham Pharmacia) equilibrated with phosphate-buffered saline. The first 2 ml of the elution volume was discarded. The fluorescently labeled peptide fraction eluting between 2 and 3 ml was collected and used for labeling experiments.
RESULTS
HBV infection inhibition by myristoylated HBV pre-S1 peptides.
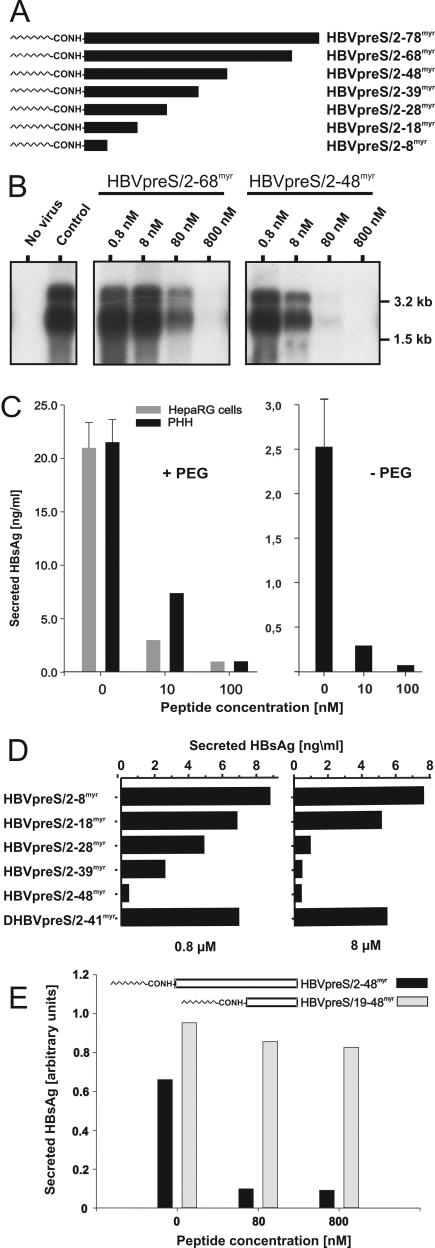
To investigate the amino acid sequence requirements for HBV infection inhibition, we synthesized the myristoylated HBV pre-S1-derived peptides depicted in Fig. 1A and tested them for the ability to interfere with HBV infection of HepaRG cells. As shown in Fig. 1B, HBVpreS/2-68myr and HBVpreS/2-48myr competed with HBV infections of HepaRG cells in a dose-dependent manner, with 50% inhibitory concentrations (IC50s) of ∼80 nM (HBVpreS/2-68myr) and, surprisingly, only ∼8 nM (HBVpreS/2-48myr), as determined by Northern blot analyses of genomic and subgenomic viral transcripts present in infected cells at 12 days postinfection. To indubitably rule out nonspecific inhibitory effects, we included a myristoylated peptide derived from the pre-S domain of the DHBV or heron HBV (HHBV) L protein (DHBVpreS/2-41myr or HHBVpres/2-44myr [32]) (see Fig. 3B) and free myristic acid (not shown in the competition experiment) as controls. Neither reagent influenced HBV infection at a concentration of 800 nM. Since we previously demonstrated that DHBVpreS/2-41myr blocks DHBV infection of primary duck hepatocytes, its failure to interfere with HBV infection revealed a species- or possibly genus-specific amino acid sequence requirement for infection competition. It also excluded a nonspecific effect caused by the simple presence of a hydrophobic side chain or a direct effect of the myristic acid present in the peptide, e.g., by direct binding to a viral or cellular target molecule. To test the efficiency of HBV infection in HepaRG cells in order to detect possible differences in the activities of acylated pre-S peptides, we performed all further experiments in parallel with PHH. In all of these experiments, no significant variations were observed. Figure 1C (left) shows one example of such a comparative inhibition of HBV infection by HBVpreS/2-48myr. For uncompeted infections, the amount of HBV secreted antigen (HBsAg) secretion from days 12 to 14 postinfection was determined to be 20 to 25 ng/ml. The competition was reduced to about 25% by the use of 10 nM HBVpreS/2-48myr and was complete at 100 nM. Note that the amounts of newly produced HBsAg in uncompeted infections of PHH as well as HepaRG cells were comparable to the amount of HBsAg secreted within 48 h by the stably transfected HBV-producing cell line HepG2.2.15 (1). This indicates that infection under the chosen conditions is efficient, as has been described previously (11). To rule out the possibility that PEG, which was present during the inoculation of hepatocytes with viral particles, influences infection inhibition, we performed a control experiment in its absence. As depicted in Fig. 1C (right), HBVpreS/2-48myr competed with an HBV infection of PHH at similar concentrations as in the presence of PEG. However, in accordance with previous observations, the efficiency of infection was about 10-fold lower.
FIG. 1.
Infection inhibition activity of terminally deleted myristoylated HBV pre-S1 peptides. (A) Schematic illustration of HBV L-protein-derived pre-S1 peptides used for infection inhibition. HBVpreS/2-78myr, consisting of the first 77 amino acids of the HBV pre-S1 domain (subtype ayw) as an N-terminal amide of myristic acid (11), and progressively C-terminally deleted myristoylated variants aredepicted on an equivalent scale. Numbers in the names of the peptides specify the first and last amino acids; “myr” indicates that the respective peptide is N-terminally myristoylated at glycine 2. (B) Competition of HBV infection by two myristoylated pre-S1 peptides, HBVpreS/2-68myr and HBVpreS/2-48myr. HepaRG cells were infected with a multiplicity of genome equivalents of ≈50 in the presence of 0.8, 8, 80, and 800 nM concentrations of the respective pre-S peptides. Fourteen hours later, the cells were washed twice with culture medium. At 12 days postinfection, cellular mRNAs were prepared and analyzed by Northern blotting as described previously (11). Both genomic and subgenomic RNAs are represented. The specificity of infection was controlled by an RNA analysis of uninfected HepaRG cells (left lane, no virus); the specificity of inhibition was tested by the addition of the myristoylated DHBV pre-S peptide DHBVpreS/2-41myr at 800 nM (control). RNA size markers are shown on the right. (C) PHH and HepaRG cells were infected overnight under comparable conditions in the presence (left) or absence (right) of PEG, with 0, 10, or 100 nM HBVpreS2/48myr as an inhibitor. After removal of the peptide, the medium was exchanged every 2 to 3 days and the amount of HBsAg was determined from days 12 to 14 postinfection. Error bars in the uncompeted infections represent the standard deviations for six independent infections. (D) HepaRG cells were infected in the presence of 0.8 μM (left) and 8 μM (right) HBVpreS/2-8myr, HBVpreS/2-18myr, HBVpreS/2-28myr, HBVpreS/2-39myr, and as controls, HBVpreS/2-48myr and DHBVpreS/2-41myr. The infectious inoculum and the peptides were incubated overnight, washed, and replaced with new medium. At 12 days postinfection, the collected cell culture supernatants from days 8 to 12 were analyzed for secreted HBsAg by use of a quantitative commercially available ELISA. The results obtained are given in nanograms of HBsAg per milliliter of cell culture medium. (E) Comparative HBV infection assay with the two myristoylated pre-S1-derived peptides HBVpreS19-48myr, which lacks the first 17 pre-S1 amino acids and carries an artificial myristic acid residue at the N-terminal leucine 19, and HBVpreS/2-48myr. HepaRG cells were infected with HBV (MGE = 50) for 12 h in the presence of 8, 80, and 800 nM concentrations of the respective peptide. The medium was changed, and newly synthesized HBsAg was determined between days 8 and 12 postinfection. Values are given as relative units (optical densities) obtained from the ELISA reader.
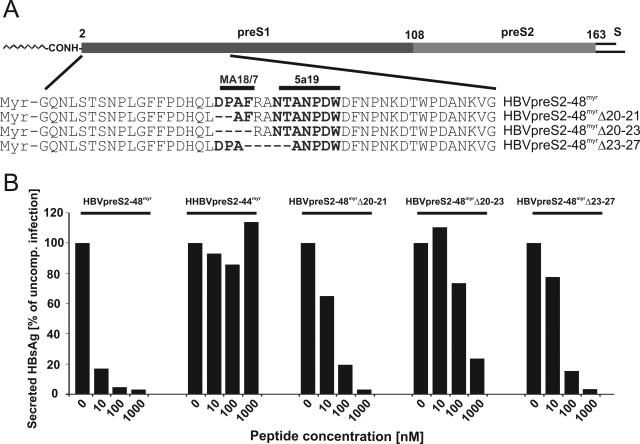
FIG. 3.
Epitopes of neutralizing anti-pre-S1 monoclonal antibodies overlap with key amino acids required for efficient infection inhibition. (A) Schematic drawing of pre-S1 and pre-S2 domains of the HBV L protein (subtype ayw). Numbers at the top indicate the N-terminally myristoylated glycine 2 of pre-S1, the last amino acid of pre-S1 (108), and pre-S2 (163). The position of the inhibitory domain and the sequence of the wild-type inhibitory peptide HBVpreS/2-48myr are depicted below, with the recognition epitopes for the two neutralizing monoclonal antibodies MA18/7 (20-DPAF-23) and 5a19 (26-NTANPDW-32) highlighted in bold. The sequences of the deletion mutants HBVpreS/2-48myrΔ20-21, HBVpreS/2-48myrΔ20-23, and HBVpreS/2-48myrΔ23-27 are aligned with the HBVpreS/2-48myr peptide sequence. (B) Comparative HBV infection competition assay using the internal deletion mutants depicted in panel A. HepaRG cells were infected either in the absence (0 nM) or in the presence of 10, 100, and 1,000 nM HBVpreS/2-48myr, HHBVpreS/2-44myr (a heron hepatitis B virus-derived analogue that served as an additional control [32]), HBVpreS/2-48myrΔ20-21, HBVpreS/2-48myrΔ20-23, and HBVpreS/2-48myrΔ23-27. The infectious inoculum and the peptides were incubated overnight. After being washed, the cells were maintained for another 12 days to allow viral gene expression. Cell culture supernatants from days 8 to 12 were collected and analyzed for secreted HBsAg by use of a quantitative commercially available ELISA. HBsAg values from the respective uncompeted infection were set to 100%, and the degree of infection inhibition is given as a percentage of the uncompeted infection. The absolute mean value of HBsAg for the uncompeted infection was 6.3 ng/ml.
Compared with HBVpreS/2-48myr, the further C-terminally shortened peptides HBVpreS/2-39myr and HBVpreS/2-28myr displayed progressively reduced, but still clearly detectable, specific inhibitory activities. The two further shortened peptides HBVpreS/2-18myr and HBVpreS/2-8myr, however, were indistinguishable from the negative control DHBVpreS/2-41myr, even at a concentration of 8 μM (Fig. 1D). This led us to the conclusion that HBV infection inhibition by myristoylated pre-S peptides is pre-S sequence specific and requires amino acids 19 to 48 for efficient activity.
To examine if this sequence on its own is capable of blocking HBV infection, we synthesized a Leu-19 myristoylated HBVpreS/19-48 peptide and determined its activity relative to that of HBVpreS/2-48myr. As depicted in Fig. 1E, HBVpreS/19-48myr showed no detectable inhibition at a concentration of 800 nM. Hence, the N-terminal 18 amino acids of pre-S1, although not mediating significant inhibition on their own, are required for infection competition.
Species specificity of mammalian hepadnaviruses is not reflected in species-specific infection interference by myristoylated pre-S peptides.
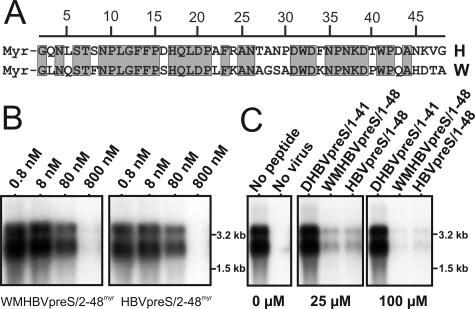
It has been reported that pseudotyped HBV particles enveloped with the woolly monkey HBV (WMHBV) L protein are not infectious in primary human hepatocytes (6) or HepaRG cells (not shown). This deficiency can be overcome by the replacement of the first 30 pre-S1 amino acids of the WMHBV L protein with the corresponding HBV sequence. To test whether host specificity is also reflected in a difference in the specific activity of a myristoylated WMHBV pre-S1 peptide, we compared the potentials of WMHBVpreS/2-48myr and HBVpreS/2-48myr (aligned in Fig. 2A) to interfere with HBV infection. As shown in Fig. 2B, we found no significant discrepancy in the activities of both peptides. This result was confirmed when we used the two unmyristoylated variants WMHBVpreS/1-48 and HBVpreS/1-48 at much higher concentrations (Fig. 2C). Both peptides significantly competed with HBV infection of HepaRG cells at 25 μM and almost completely blocked infection at 100 μM, as shown by a Northern blot analysis of newly synthesized intracellular viral RNAs. Inhibition by the nonmyristoylated hepadnaviral pre-S peptides was again species or genus specific since the DHBV-derived analogue DHBVpreS1-41 did not show an inhibitory effect at 100 μM. We concluded that (i) the two peptides address a common target with similar efficiencies, suggesting that the binding of viral particles to this target is not responsible for the differences in the observed species specificity; (ii) pre-S-specific infection inhibition is not absolutely dependent on myristoylation but is >100-fold more efficient with myristoylation; and (iii) the observed genus specificity indicates a principal difference in the entry pathways of avian and mammalian hepadnaviruses.
FIG. 2.
HBV infection inhibition by a pre-S-derived peptide of the WMHBV L protein. (A) Amino acid sequence alignment of the two pre-S1-derived peptides HBVpreS/2-48myr (H) and WMHBVpreS/2-48myr (W). Numbers above the sequences indicate amino acid positions; gray boxes denote perfect sequence accordance. The sequence identity within this pre-S sequence was 64%. Note that most of the remaining 36% amino acid deviations are nonconservative. (B) Comparative HBV infection competition assay with 0.8, 8, 80, and 800 nM WMHBVpreS/2-48myr (left) and HBVpreS/2-48myr (right). At 12 days postinoculation of virus and peptide, total cellular mRNAs were isolated and viral transcripts (genomic and subgenomic RNAs) were analyzed by Northern blot hybridization using an HBV-specific probe. Size markers are indicated on the right. (C) HBV infection competition using nonmyristoylated pre-S-derived peptides of DHBV (DHBVpreS/1-41), WMHBV (WMHBVpreS/1-48), and HBV (HBVpreS/1-48) at the elevated concentrations of 25 μM (middle) and 100 μM (right) in comparison to an uncompeted infection (left lane in left panel) and a mock infection (right lane in left panel). Cells were incubated with HBV in the presence of the indicated peptide concentrations for 16 h. Twelve days after the removal of the inoculum, the cells were washed twice, RNAs were prepared, and viral transcripts were detected by Northern blot hybridization.
Important sequences for peptide activity overlap with epitopes recognized by neutralizing anti-pre-S1 antibodies.
Epitope mapping of neutralizing anti-pre-S1 antibodies had revealed that the corresponding antigen binding site is located within the first third of the HBV pre-S1 sequence (8, 18, 22). Since these epitopes partially overlap with the sequence determined above as being important for inhibition (residues 19 to 48), we investigated the effect of short internal deletions within the recognition sites of the two neutralizing monoclonal antibodies MA18/7 (20-DPAF-23) (15) and 5a19 (26-NTANPDW-32) (24). Figure 3A schematically depicts the pre-S region of the HBV L protein and shows the sequences of HBVpreS/2-48myr and the mutant peptides HBVpreS/2-48myrΔ20-21, lacking the aspartic acid and proline of the MA18/7 recognition sequence, HBVpreS/2-48myrΔ20-23, lacking the whole DPAF motif, and HBVpreS/2-48myrΔ23-27, lacking parts of the 5a19 recognition site and rendering a respective pseudotyped mutant virus noninfectious (17). Figure 3B shows the results of an infection competition experiment with the HBV pre-S-derived peptide variants compared to an additional negative control, myristoylated HHBV pre-S, or HHBVpreS/2-44myr. Consistent with the results obtained with the terminal deletion constructs described above, none of the internal mutants was completely inactive, emphasizing again that an extended pre-S region and not just a short sequential motif is required for effective infection inhibition. However, all deletions led to a significant reduction in infection interference compared with wild-type HBVpreS/2-48myr. The strongest effect, leading to an approximately 100-fold reduction in activity, was observed for the Δ20-23 deletion. The HBVpreS/2-48myrΔ23-27 and HBVpreS/2-48myrΔ20-21 peptides, however, displayed only a 10-fold lower activity than the unaltered peptide. This indicated an important functional role for amino acids 20 to 27 of HBV pre-S. In addition, this observation suggests that neutralization by both pre-S1 antibodies and the peptides addresses an identical step in infection, presumably, as shown below, receptor interaction.
HBVpreS/2-48myr targets a cell-associated factor on the surfaces of hepatocytes.
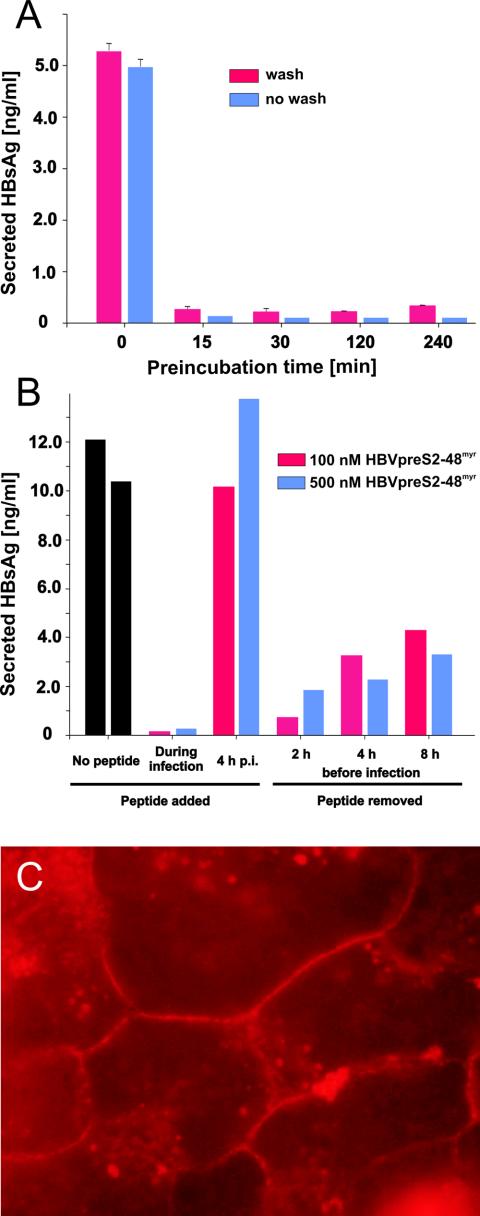
To address the question of whether infection inhibition by HBV pre-S-derived peptides is caused by inactivation of the virus by the peptides or by functional neutralization of a hepatocyte-specific cell surface receptor, we treated HepaRG cells for different time periods with HBVpreS/2-48myr (800 nM), removed the inhibitor, washed the cells, and incubated them for an additional 12 h with HBV. As depicted in Fig. 4A, a pretreatment of HepaRG cells with HBVpreS/2-48myr for only 15 min was sufficient to nearly completely abolish the susceptibility of the cells to HBV infection. Continuing the incubation of the peptide together with the virus on the cells showed a comparable effect.
FIG. 4.
HBVpreS/2-48myr addresses a cell-associated factor on the surfaces of hepatocytes. (A) HBV infection inhibition after preincubation of HepaRG cells with HBVpreS/2-48myr. HepaRG cells were incubated with HBVpreS/2-48myr (800 nM) for 0, 15, 30, 120, and 240 min. Subsequently, the peptide was either removed (red bars) or lefton the cells (blue bars) for the duration of the HBV infection (an additional 12 h with ≈50 genome equivalents per cell). After being washed, the cells were incubated for an additional 12 days to allow the expression of viral genes. For quantification, cell culture supernatants were collected between days 8 and 12 postinfection and then subjected to a quantitative HBsAg ELISA. Experiments were done in duplicate; standard deviations are indicated by error bars. (B) Effect of HBVpreS/2-48myr on HBV infection when administered before or after the establishment of infection. HepaRG cells were inoculated overnight with ≈50 genome equivalents of HBV in the absence or presence of 100 nM (red bars) or 500 nM (blue bars) HBVpreS/2-48myr. In contrast, HBVpreS/2-48myr was added just 4 h after removal of the infectious inoculum and was then left on the cells for an additional 16 h. Alternatively, HBVpreS/2-48myr was incubated with HepaRG cells at the indicated concentrations (incubation time, 16 h). After removal of the peptide, the cells were cultivated for another 2, 4, or 8 h at 37°C. Subsequently, the cells were infected with HBV in the absence of any inhibitor (12 h; MGE, ≈50) and then washed, and virus replication was allowed to occur for an additional 12 days. As described above, newly synthesized HBsAg was determined by quantitative HBsAg ELISA. (C) Direct visualization of HBVpreS/2-48myr binding to PHH. HBVpreS/2-48myr was labeled with Cy3 as described in Materials and Methods. The labeled peptide was allowed to bind PHH for 1 h. After removal of the peptide, the cells were fixed and analyzed by fluorescence microscopy.
To gain insight into the kinetics of inactivation, we performed pre- and postexposure experiments (Fig. 4B). HepaRG cells were preincubated with HBVpreS/2-48myr (100 and 500 nM) for 16 h. The cells were washed, incubated for another 0, 2, 4, or 8 h at 37°C, and subsequently infected for 16 h at a multiplicity of genome equivalents (MGE) of 50. As a control, we infected untreated cells and added the peptide 4 h after virus inoculation. Again, preincubation of the cells with HBVpreS/2-48myr led to an almost complete loss of susceptibility to infection (Fig. 4B). In contrast, the addition of the peptide 4 h after infection had no detectable effect on virus replication. However, different time periods between the removal of free peptide and virus inoculation resulted in a time-dependent recovery of susceptibility to HBV, with >50% inhibition still remaining after 8 h of recovery time. These results indicate that HBVpreS/2-48myr addresses a cellular factor present on the surfaces of hepatocytes and is ineffective after virus binding and uptake have occurred.
Based on this functional evidence, we attempted to directly visualize the localization of HBVpreS/2-48myr. To this end, we labeled HBVpreS/2-48myr with the fluorescent dye Cy3 and incubated the peptide for 1 h with PHH. As shown in Fig. 4C, the incubation of labeled HBVpreS/2-48myr with PHH resulted in specific staining of the plasma membrane. Taken together, our results suggest that specific cell surface targeting of the peptide results in very efficient functional inactivation.
Replacement of myristic acid with other hydrocarbon moieties allows the modulation of peptide-specific activities.
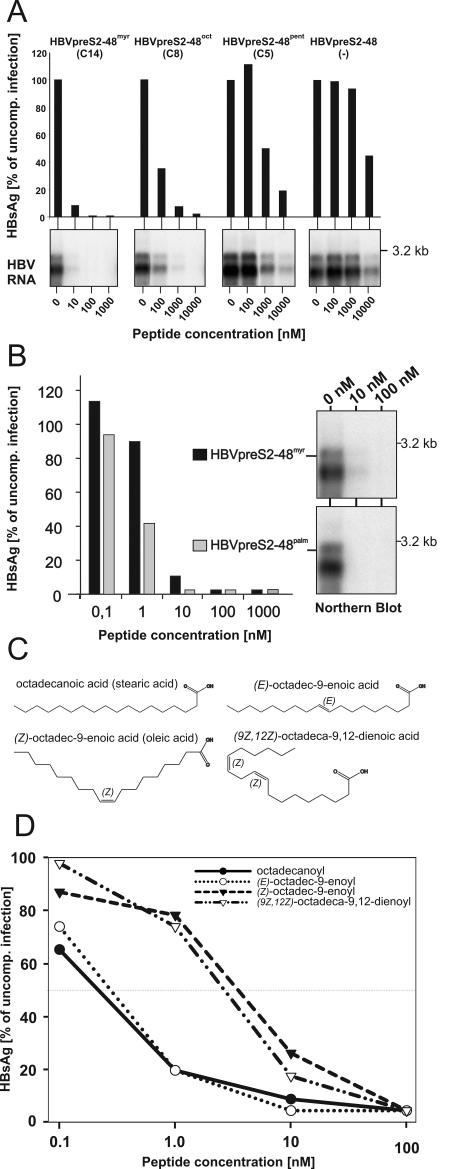
To clarify the role of myristoylation in infection inhibition, we replaced the C14-myristic acid chain with the other hydrophobic moieties listed in Table 1. In a first set of experiments, we replaced the myristoyl moiety with two saturated shortened hydrocarbonic acid residues, octanoyl (C8) and pentanoyl (C5), giving rise to the peptides HBVpreS/2-48oct and HBVpreS/2-48pent. In addition, we synthesized and tested the unmodified peptide HBVpreS/2-48. As shown by Northern blot analysis and HBsAg enzyme-linked immunosorbent assay (ELISA) (Fig. 5A), none of the changes resulted in a complete loss of infection interference of the peptides. However, we observed remarkable differences in their specific activities, namely a chain-length-dependent decrease in IC50s (∼100 nM for HBVpreS/2-48oct, 1 μM for HBVpreS/2-48pent, and ∼10 μM for the unmodified peptide). To confirm and clarify the pivotal role of the hydrophobic residue, we tested peptide variants containing saturated acyl chains with increased hydrocarbon chain lengths, specifically palmitic acid (C16) (Fig. 5B) and stearic acid (C18) (Fig. 5D). Both peptides competed with HBV infection at least ∼10-fold more efficiently than HBVpreS/2-48myr. The IC50s were determined to be ∼800 pM for HBVpreS/2-48palm and ∼400 pM for HBVpreS/2-48stearoyl, with a potency for blocking infection at <10 nM. The introduction of artificially extended acyl moieties thus allowed an increase in the specific infection inhibition activity of the pre-S-derived peptides.
FIG. 5.
Influence of the nature of the N-terminal acyl moiety on pre-S-specific HBV infection inhibition. (A) HBV infection inhibition assay using HBVpreS/2-48 variants without or with shortened hydrocarbon acid chain lengths, i.e., HBVpreS/2-48, pentanoyl-HBVpreS/2-48 (HBVpreS/2-48pent [C5]), octanoyl-HBVpreS/2-48 (HBVpreS/2-48oct [C8]), and as a reference, myristoyl-HBVpreS/2-48 (HBVpreS/2-48myr [C14]). HepaRG cells were infected overnight in the presence of the indicated concentrations of the respective acyl HBV pre-S inhibitor. After removal of the inoculum, the cells were further cultivated and the expression of newly synthesized HBsAg between days 8and 12 (bar diagrams) and of viral transcripts on day 12 (autoradiograms) was analyzed by ELISA and Northern blot hybridization. Note the 10-fold higher concentration used for the inhibition of shortened variants. (B) Comparative HBV infection inhibition assay using HBVpreS/2-48myr and its derivative palmitoyl-HBVpreS/2-48 (HBVpreS/2-48palm [C16]) with a lengthened hydrocarbon acid chain. HepaRG cells were infected in parallel for 12 h in the presence of 0.1, 1, 10, 100, and 1,000 nM concentrations of the two peptides. After being washed, the cells were cultivated for another 12 days and the expression of HBsAg between days 8 and 12 was quantified by ELISA (bar diagram). Values are displayed as percentages of the corresponding uncompeted control infection. In addition to HBsAg secretion, viral RNAs prepared at 12 days postinfection were subjected to Northern blot analysis (autoradiograms). Data for genomic and subgenomic RNAs are shown. (C) Structural formulas of octadecanoic (stearic) acid, (Z)-octadec-9-enoic (oleic) acid, (E)-octadec-9-enoic acid, and (9Z,12Z)-octadeca-9,12-dienoic acid, which were used for the synthesis of the corresponding HBVpreS/2-48 peptides analyzed below. Note the steric differences of the two Z derivatives compared to the saturated and singly unsaturated E variants. (D) Comparative HBV infection competition assay using the four acylated pre-S2-48 derivatives depicted in panel C. HepaRG cells were inoculated overnight with HBV (≈50 genome equivalents) in the presence of 0.1, 1, 10, and 100 nM concentrations of the respective peptide derivatives. Cells were washed, and newly synthesized HBsAg (days 8 to 12) was quantified by ELISA. Values are given as percentages of the corresponding uncompeted control infection.
To determine whether the configuration of the hydrophobic residue influenced its potentiating role, we introduced double bonds in cis and trans configurations in the C18 hydrocarbon chain, resulting in the peptides (E)-octadec-9-enoyl-HBVpreS/2-48, (Z)-octadec-9-enoyl-HBVpreS/2-48, and (9Z,12Z)-octadeca-9,12-dienoyl-HBVpreS/2-48 (Fig. 5C). Fig. 5D shows the results of a direct comparison of these four substances in an infection competition assay. We did not observe a complete loss of peptide activity for any of the mutants. However, peptide variants containing one or two cis double bonds in the C18 acyl chain displayed significantly reduced activities. In contrast, the trans isomer HBVpreS/2-48-octadecenoyl-trans-9 had a comparable specific activity to that of the saturated HBVpreS/2-48-octadecanoyl variant.
DISCUSSION
For this study, we took advantage of the recently described human hepatoma cell line HepaRG and demonstrated its reliability and comparability to PHH as an in vitro infection system for HBV. Previous observations indicated the importance of the first 77 pre-S1 amino acids for HBV infectivity and the requirement of N-terminal L-protein myristoylation. We described here acylated pre-S1 peptides derived from this part of L and showed that they interfere with the infection process with extraordinary efficiencies. Mutational analyses addressing the primary sequence and the nature of the N-terminal hydrophobic modification revealed that (i) similar to the case for DHBV (31), a rather extended pre-S region enclosing amino acids 2 to 48 defines the infection interference site (IIS) that is required for efficient inhibition; (ii) amino acids 20 to 27 within this sequence play an important role; and (iii) acylation is not mandatory for inhibition but allows the modulation of the peptides' specific activities by about 5 orders of magnitude. Lastly, we provided evidence that the pre-S1-mediated elimination of HBV infection is presumably caused by the specific targeting and inactivation of a yet unidentified receptor on the surfaces of hepatocytes.
Pseudotyped HBV particles with deletions in the HBV L protein are noninfectious if the integrity of the N-terminal 77 amino acids of pre-S1 is disrupted (17). Consistent with this finding is the fact that a synthetic myristoylated peptide representing this part of L (HBVpreS/2-78myr) can block HBV infection when it is added during infection. Our observation of specific inhibition ruled out the possibility that the presence of PEG during the infection process could promote a receptor-independent fusion of viral particles with the hepatocyte membrane. Since inhibition was also observed in the absence of PEG, we concluded that PEG does not qualitatively influence infection but may enhance the accessibility of the virus to the cells. The observation that C-terminal shortening of HBVpreS/2-78myr up to amino acid 48 did not eliminate, but instead increased, the inhibitory activity suggests assorted roles of pre-S1 in HBV entry that are related to distinct regions (amino acids 2 to 48 and 49 to 78) serving diverse functions. Accordingly, the reduced activity of HBVpreS/2-78myr compared to HBVpreS/2-48myr may be explained by the assumption that amino acids 49 to 78 limit the exposure of the IIS by intramolecular masking. This might be important for allowing the secretion of viral particles that would otherwise bind intracellularly to the IIS target molecule.
The failure of myristoylated pre-S peptides of the avian hepadnaviruses DHBV and HHBV to interfere with HBV infection and vice versa (32) unequivocally excluded nonspecific inhibitory effects of acylated peptides per se and suggested that avian and mammalian hepadnaviruses possibly use diverse entry pathways. This raises the question of how more distantly related hepadnaviral pre-S peptides from woodchuck HBV or ground squirrel HBV act on HBV or DHBV infection.
In contrast to the observed genus specificity of inhibition, the lack of species-specific infection inhibition, as demonstrated by the comparable activities of WMHBVpreS/2-48myr and HBVpreS/2-48myr, indicates that WMHBV and HBV utilize at least one common step in cell entry, i.e., the one addressed by the peptides. This is remarkable when one considers that replacement of the HBV L protein with its WMHBV counterpart leads to the production of pseudotyped virions with a very limited infectivity. Moreover, the approximately 30% amino acid divergence in both pre-S sequences as well as the observation of the gradual loss of specific activities through successive C-terminal deletions up to amino acid 19 and the only partial disruption of the peptide's inhibitory activity through the introduction of internal deletions into the IIS raises the issue of the mode of interaction with a postulated target molecule. Nevertheless, the >100-fold reduced activity of HBVpreS/2-48Δ20-23myr compared to HBVpreS/2-48myr indicates a major role for amino acids 20-DPAF-23 in the infection process. Interestingly, this sequence constitutes a highly immunogenic pre-S epitope that is present on either complete hepadnaviral particles (28) or recombinant pre-S polypeptides (unpublished data). Antibodies directed against those antigens neutralize infection (8, 26; unpublished data), possibly by preventing virus binding to its cellular receptor.
For human immunodeficiency virus, influenza A virus, and several paramyxoviruses, it is well known that peptides derived from the respective viral fusion proteins (gp41, HA-2, and F) can block infection through interference with the formation of a six-helix bundle intermediate driving membrane fusion (14, 25, 29). Although this is attractive as a model, the peptides described here most likely act by a different mechanism, namely, the sequestering and inactivation of a yet unknown cellular receptor on the surface of the hepatocyte. This conclusion is based on our observation that already short preincubation times of HepaRG cells with HBVpreS/2-48myr blocked HBV infection for at least 12 h. The slow recovery of susceptibility (t1/2 ≈ 12 h) indicated the general reversibility of this process by either dissociation of the peptide-receptor complex, degradation of the peptide, or substitution of the sequestered target molecule by dynamic turnover, e.g., trafficking. Moreover, the possibility of visualizing the plasma membrane-anchored HBVpreS/2-48myr peptide after Cy3 labeling provided direct evidence of efficient cell targeting. Lastly, in an elegant parallel study using primary hepatocytes from T. belangeri as a model for HBV infection, Glebe et al. demonstrate that HBVpreS/2-48myr specifically abolishes the binding of highly purified pre-S1 containing HBsAg (D. Glebe, personal communication).
Although our results suggest the direct interaction of the myristoylated N-terminal pre-S1 peptide with a cellular receptor, an additional interaction with a viral protein cannot be excluded and may contribute to the inhibitory effect. One hypothesis is that the peptide interacts with another subdomain(s) within the L or S protein, which could explain the hypothesized masking effect.
Replacement of the myristoyl group with shorter or longer saturated or unsaturated acyl moieties did not result in a loss of peptide activity but allowed modulation of the IC50 by a factor of about 10,000 (IC50 for HBVpreS/2-48, ≈10 μM; for HBVpreS/2-48octadecanoyl, ≤1 nM). Many cellular and viral proteins become posttranslationally myristoylated. Myristic acid has been described to mediate membrane associations, but it can also participate in direct protein-protein interactions. Both of these hypotheses are compatible with our results. However, spontaneous anchoring in the membrane is rather unlikely since it should allow the binding of HBVpreS/2-48myr to any kind of cell, a prediction that has never been observed in our experiments. So far, only hepatocytes of human origin have been specifically stained with fluorescently labeled acylated peptides (P. Gripon, unpublished data). This observation favors the hypothesis that the acyl moiety participates directly, in concert with the IIS, in the interaction with the cellular receptor. Such an interaction has been studied in detail for calmodulin and CAP-22/NAP-22 (20).
Although the N-terminal region of positions 2 to 18 per se is not sufficient to support a strong inhibitory activity, it is essential for the peptide activity. This was concluded from the observation that the deletion of amino acids 2 to 18 combined with an artificial fusion of myristic acid to Leu-19 led to an inactive peptide. Taking into account that HBVpreS/2-18myr is poorly active, this region either participates directly in receptor recognition or provides a spacer function, maintaining the correct distance between the IIS and the hydrocarbon chain.
So far, all available therapeutics to treat chronic hepatitis B virus infections interfere with intracellular replication steps. The peptides described here offer a new strategy by efficiently blocking an essential pathway during the entry process. Particular applications might be related to the prevention of reinfection after liver transplantation, interference in mother-child transmission during birth, or postexposure prophylaxis. The suitability of this strategy for the treatment of chronic infections, however, will depend on future insights regarding to what extent new rounds of infection are required for the maintenance of chronicity. Since the blockade can still be achieved with WMHBV-derived peptides, it is unlikely that escape mutants occur. Moreover, the generation of antibodies during possible therapy may enhance the therapeutic goal since they are expected to be neutralizing.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (UR 72/1-3) and a fellowship from the H. and C. Schaller Stiftung to S.U. and by funding from the Institute National de la Santé et de la Recherche Médicale (INSERM) and the Association pour la Recherche contre le Cancer to P.G.
We thank the Biological Resource Centre (BRC) of Rennes for the supply of isolated human hepatocytes. Part of this work was performed in the laboratory of Heinz Schaller, ZMBH, whom we thank for continuous support. We are also grateful to Hans Ulrich Schairer for financial help and many discussions. We appreciate the technical assistance of Stephanie Held and thank Ralf Bartenschlager, Wolfram H. Gerlich, and Jacques Le Seyec for critical readings of the manuscript.
Footnotes
In memory of Peter Hans Hofschneider.
REFERENCES
- 1.Acs, G., M. A. Sells, R. H. Purcell, P. Price, R. Engle, M. Shapiro, and H. Popper. 1987. Hepatitis B virus produced by transfected HepG2 cells causes hepatitis in chimpanzees. Proc. Natl. Acad. Sci. USA 84:4641-4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berting, A., C. Fischer, S. Schaefer, W. Garten, H. D. Klenk, and W. H. Gerlich. 2000. Hemifusion activity of a chimeric influenza virus hemagglutinin with a putative fusion peptide from hepatitis B virus. Virus Res. 68:35-49. [DOI] [PubMed] [Google Scholar]
- 3.Bruss, V., and D. Ganem. 1991. The role of envelope proteins in hepatitis B virus assembly. Proc. Natl. Acad. Sci. USA 88:1059-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruss, V., J. Hagelstein, E. Gerhardt, and P. R. Galle. 1996. Myristylation of the large surface protein is required for hepatitis B virus in vitro infectivity. Virology 218:396-399. [DOI] [PubMed] [Google Scholar]
- 5.Chang, C. M., K. S. Jeng, C. P. Hu, S. J. Lo, T. S. Su, L. P. Ting, C. K. Chou, S. H. Han, E. Pfaff, J. Salfeld, et al. 1987. Production of hepatitis B virus in vitro by transient expression of cloned HBV DNA in a hepatoma cell line. EMBO J. 6:675-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chouteau, P., J. Le Seyec, I. Cannie, M. Nassal, C. Guguen-Guillouzo, and P. Gripon. 2001. A short N-proximal region in the large envelope protein harbors a determinant that contributes to the species specificity of human hepatitis B virus. J. Virol. 75:11565-11572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganem, D., and R. J. Schneider. 2001. Hepadnaviridae: the viruses and their replication, p. 2923-2969. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 8.Glebe, D., M. Aliakbari, P. Krass, E. V. Knoop, K. P. Valerius, and W. H. Gerlich. 2003. Pre-S1 antigen-dependent infection of Tupaia hepatocyte cultures with human hepatitis B virus. J. Virol. 77:9511-9521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gripon, P., C. Diot, N. Theze, I. Fourel, O. Loreal, C. Brechot, and C. Guguen-Guillouzo. 1988. Hepatitis B virus infection of adult human hepatocytes cultured in the presence of dimethyl sulfoxide. J. Virol. 62:4136-4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gripon, P., J. Le Seyec, S. Rumin, and C. Guguen-Guillouzo. 1995. Myristylation of the hepatitis B virus large surface protein is essential for viral infectivity. Virology 213:292-299. [DOI] [PubMed] [Google Scholar]
- 11.Gripon, P., S. Rumin, S. Urban, J. Le Seyec, D. Glaise, I. Cannie, C. Guyomard, J. Lucas, C. Trepo, and C. Guguen-Guillouzo. 2002. Infection of a human hepatoma cell line by hepatitis B virus. Proc. Natl. Acad. Sci. USA 99:15655-15660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guguen-Guillouzo, C., and A. Guillouzo. 1986. Methods for preparation of adult and fetal hepatocytes, p. 1-12. In A. Guillouzo and C. Guguen-Guillouzo (ed.), Isolated and cultured hepatocytes. Les Éditions INSERM Paris, John Libbey and Co., Ltd., London, United Kingdom.
- 13.Gumina, G., G. Y. Song, and C. K. Chu. 2001. Advances in antiviral agents for hepatitis B virus. Antivir. Chem. Chemother. 12(Suppl. 1):93-117. [PubMed] [Google Scholar]
- 14.He, Y., R. Vassell, M. Zaitseva, N. Nguyen, Z. Yang, Y. Weng, and C. D. Weiss. 2003. Peptides trap the human immunodeficiency virus type 1 envelope glycoprotein fusion intermediate at two sites. J. Virol. 77:1666-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heermann, K. H., F. Kruse, M. Seifer, and W. H. Gerlich. 1987. Immunogenicity of the gene S and pre-S domains in hepatitis B virions and HBsAg filaments. Intervirology 28:14-25. [DOI] [PubMed] [Google Scholar]
- 16.Le Seyec, J., P. Chouteau, I. Cannie, C. Guguen-Guillouzo, and P. Gripon. 1998. Role of the pre-S2 domain of the large envelope protein in hepatitis B virus assembly and infectivity. J. Virol. 72:5573-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Seyec, J., P. Chouteau, I. Cannie, C. Guguen-Guillouzo, and P. Gripon. 1999. Infection process of the hepatitis B virus depends on the presence of a defined sequence in the pre-S1 domain. J. Virol. 73:2052-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maeng, C. Y., C. J. Ryu, P. Gripon, C. Guguen-Guillouzo, and H. J. Hong. 2000. Fine mapping of virus-neutralizing epitopes on hepatitis B virus preS1. Virology 270:9-16. [DOI] [PubMed] [Google Scholar]
- 19.Mason, W. S., G. Seal, and J. Summers. 1980. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J. Virol. 36:829-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsubara, M., T. Nakatsu, H. Kato, and H. Taniguchi. 2004. Crystal structure of a myristoylated CAP-23/NAP-22 N-terminal domain complexed with Ca2+/calmodulin. EMBO J. 23:712-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neurath, A. R., S. B. Kent, N. Strick, and K. Parker. 1986. Identification and chemical synthesis of a host cell receptor binding site on hepatitis B virus. Cell 46:429-436. [DOI] [PubMed] [Google Scholar]
- 22.Neurath, A. R., B. Seto, and N. Strick. 1989. Antibodies to synthetic peptides from the preS1 region of the hepatitis B virus (HBV) envelope (env) protein are virus-neutralizing and protective. Vaccine 7:234-236. [DOI] [PubMed] [Google Scholar]
- 23.Persing, D. H., H. E. Varmus, and D. Ganem. 1987. The pre-S1 protein of hepatitis B virus is acylated at its amino terminus with myristic acid. J. Virol. 61:1672-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pizarro, J. C., B. Vulliez-le Normand, M. M. Riottot, A. Budkowska, and G. A. Bentley. 2001. Structural and functional characterization of a monoclonal antibody specific for the preS1 region of hepatitis B virus. FEBS Lett. 509:463-468. [DOI] [PubMed] [Google Scholar]
- 25.Russell, C. J., T. S. Jardetzky, and R. A. Lamb. 2001. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. EMBO J. 20:4024-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryu, C. J., P. Gripon, H. R. Park, S. S. Park, Y. K. Kim, C. Guguen-Guillouzo, O. J. Yoo, and H. J. Hong. 1997. In vitro neutralization of hepatitis B virus by monoclonal antibodies against the viral surface antigen. J. Med. Virol. 52:226-233. [DOI] [PubMed] [Google Scholar]
- 27.Seeger, C., and W. S. Mason. 2000. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 64:51-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shouval, D., Y. Ilan, R. Adler, R. Deepen, A. Panet, Z. Even-Chen, M. Gorecki, and W. H. Gerlich. 1994. Improved immunogenicity in mice of a mammalian cell-derived recombinant hepatitis B vaccine containing pre-S1 and pre-S2 antigens as compared with conventional yeast-derived vaccines. Vaccine 12:1453-1459. [DOI] [PubMed] [Google Scholar]
- 29.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 30.Summers, J., J. M. Smolec, and R. Snyder. 1978. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc. Natl. Acad. Sci. USA 75:4533-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urban, S., C. Schwarz, U. C. Marx, H. Zentgraf, H. Schaller, and G. Multhaup. 2000. Receptor recognition by a hepatitis B virus reveals a novel mode of high affinity virus-receptor interaction. EMBO J. 19:1217-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urban, S., and P. Gripon. 2002. Inhibition of duck hepatitis B virus infection by a myristoylated pre-S peptide of the large viral surface protein. J. Virol. 76:1986-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]