Abstract
Objective:
The objective of this study was to assess the efficacy of tamsulosin and finasteride monotherapies, and their combination in men with benign prostatic hyperplasia (BPH).
Materials and Methods:
This is a prospective single-blind randomized study of ninety men with BPH who were managed using drugs. The International Prostate Symptom Score (IPSS), peak urinary flow rate, and prostate volume were measured as parameters for assessment at the beginning, 3 months, and 6 months of the study.
Results:
The mean age of patients was 61.65 with a range of 44–81 years. There was a progressive and sustained improvement in the IPSS score in all patient groups with mean decrease at 3 months of 7.24 (42.59%), 7.60 (41.85%), and 7.24 (40.61%) and at 6 months of 8.14 (47.88%), 10.33 (56.88%), and 11.1 (62.25%) in the tamsulosin, finasteride, and combination groups, respectively. There was an increase in peak urinary flow rate in all groups with mean increase at 3 months of 0.98, 0.05, and 3.55 (ml/s) and at 6 months of 4.11, 0.87, and 3.74 (ml/s) in the tamsulosin, finasteride, and combination groups, respectively. There was a reduction in the prostate volume in the finasteride and combination groups at 6 months of 6.8 and 6.32 cm3, respectively, while the tamsulosin group recorded an increase.
Conclusion:
At the end of 6 months, tamsulosin monotherapy and combination therapy appear to be equally effective in the treatment of lower urinary tract symptoms BPH while finasteride monotherapy appears to be the least effective. Bothersome, side effects were more in patients taking finasteride alone or as combination therapy.
Keywords: Benign prostatic hyperplasia, combination, finasteride, tamsulosin
Introduction
Benign prostatic hyperplasia (BPH) is a benign disease of the prostate characterized by proliferation of prostatic tissue in the periurethral zone. It causes various symptoms due to urethral obstruction and changes in the bladder musculature.[1] Various drugs are used in the medical treatment for BPH used singly or in combination. Alpha-adrenergic blockers, five-alpha-reductase inhibitors (5ARI), anticholinergics, and recently beta-3 adrenergic agonist are available in the armamentarium of the urologist for the management of patients with BPH.[2,3] Alpha-blocker and 5ARI have been shown to be beneficial in other population groups, but there is paucity of studies in the Nigerian population. Previous studies from Nigeria studied the effect of alpha-blocker monotherapy.[4,5] We sought to evaluate the effect of alpha-blocker and five alpha-reductase used singly or in combination over a 6-month period. To our knowledge, this is the first study that assesses the effect of 5ARI monotherapy and the combination therapy in the management of BPH in Nigerian men.
Materials and Methods
The study is a single-blind, randomized prospective study of the effects of tamsulosin and finasteride monotherapy or their combination on the International Prostate Symptom Score (IPSS), urine flow rates, and prostate volume. Ethical approval was obtained from the ethics committee of the hospital and written informed consent was obtained from each patient
The patients were randomized into three groups by simple random sampling. Group A (tamsulosin monotherapy), Group B (finasteride monotherapy), and Group C (combination therapy of tamsulosin and finasteride). The patients were blinded to the specific group they were in. Group A had tamsulosin 0.4 mg daily; Group B had 5 mg of finasteride daily while the combination group had both drugs. Patients on any of the drugs before this time were given a washout period of 2 weeks. The IPSS and maximum urine flow rate (Qmax) were assessed before commencement of therapy and after 3 and 6 months of therapy. The primary endpoint of the study is statistically significant improvement in IPSS, maximum urine flow rate, and reduction in prostate volume at the end of 6 months. A patient is deemed to have met a surrogate endpoint in the study if he develops acute urinary retention or withdraws from the study before the complete duration of the study. The Qmax was determined using a rotating-disc uroflowmetry by Urodyn by Mediwatchref-U2A1002-UK. The prostate volume was assessed using transabdominal ultrasound technique at the beginning, 3 months and 6 months of the study using a 3.5 MHz convex probe of Belson 200 machine by a single sonologist. The sonologist was blinded to the specific group of the patient. The prostate was measured in three dimensions (length, breadth, and height) and this is multiplied together using the prolate ellipsoid formula for volume multiplying with a factor of 0.52.
The sample size was calculated using the following formula:[6]
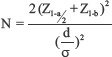
σ = Standard deviation = 36
α = 5% level of significance = 0.05
1−β = Power at 90%
d = difference observed in prostate volume[7] of groups = 41
When α = 0.05, β =0.10 then (z1−α/2 + z1−β)2 = 10.51
Sample size was 16 by calculation but increased to 30 per group and 90 patients in all to account for attrition.
Statistical analysis was done with the aid of SPSS 20 (IBM corporation, Armonk, NewYork, United states of America). The IPSS score, prostate volume, postvoid residual (PVR) urine volume, and peak flow rate were computed and presented in table and graphical format. The categorical data were cross-tabulated and Chi-square determined for significance. For numeric data, descriptive statistics were analyzed and thereafter analysis of variance done to determine significant difference in the mean between the different groups. Student's t-test was done to determine significant difference within each group at completion of the study. A P < 0.05 was considered statistically significant.
Inclusion criteria
All men with diagnosis of clinical BPH who gave informed consent were included in the study. Clinical BPH was defined by the presence of lower urinary tract symptoms (LUTS) in a patient with an enlarged prostate detected by digital rectal examination (DRE) and imaging studies.
Exclusion criteria included patients with known allergy or contraindication to any of the drugs and the following: Patients with supine blood pressure <90/70 mmHg, histologically confirmed prostate cancer, men with suspicion of prostate cancer by DRE findings, ultrasound findings, or very high prostate-specific antigen (above 10 ng/ml) with negative biopsy. Others include men with prior prostate or bladder surgeries, history of chronic prostatitis, or two or more episodes of acute urinary retention requiring catheterization within a year of the study
Results
A total of ninety patients were recruited and randomized into the three groups with thirty patients in each group.
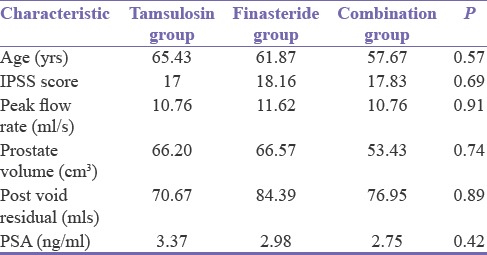
The age range of the patients was 44–81 years with a mean age of 61.7 years for the study. There was no statistically significant difference in the baseline IPSS, peak flow rate, prostate volume, and PVR in all the groups [Table 1].
Table 1.
Baseline characteristics of study population
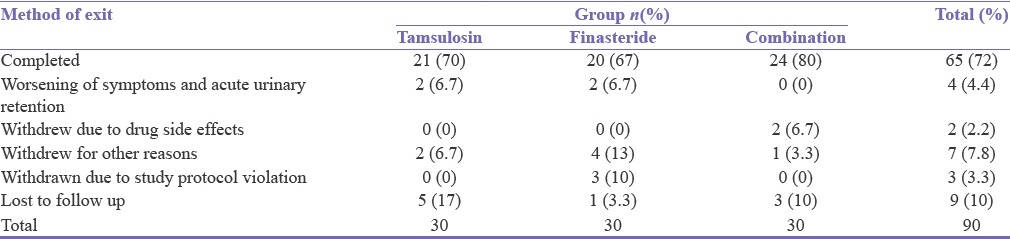
Sixty-five (72%) patients completed the study, 4 (4.44%) patients had acute urinary retention, 12 (13.33%) patients withdrew, and 9 (10%) were lost to follow-up [Table 2].
Table 2.
Method of patient exit from the study
Four patients who developed acute urinary retention were catheterized and subsequently had open prostatectomy. Two patients (2.22%) in the Group C who withdrew from the study due to erectile dysfunction were managed with alfuzosin and tadalafil.
Ten patients (11.11%) withdrew for varied reasons. Two patients relocated to their village. A patient (1.11%) claimed that he had spontaneous improvement of symptom after the 3rd month. Three patients (3.33%) were withdrawn due to study protocol violation (use of herbal concoction or other active drugs). Four patients (4.44%) withdrew from the finasteride group due to perceived ineffectiveness. They were given alpha-blocker as rescue medication.
International Prostate Symptoms Score
There was progressive improvement in the IPSS scores in the three groups. When compared to baseline, each of the groups achieved statistically significant improvement in IPSS (P < 0.01 for Group A, P < 0.01 for Group B, and P < 0.01 for Group C). There was no difference in the efficacy of the drugs when compared to each other at 3 and 6 months [P = 0.72 at 3 months and P = 0.97 at 6 months, Figure 1].
Figure 1.
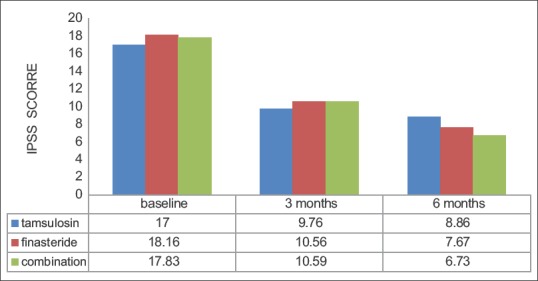
Change in international prostate symptom score
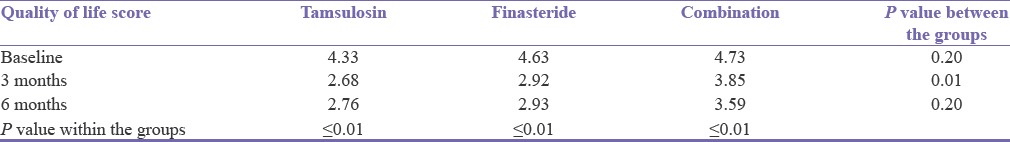
Each group recorded a statistically significant reduction in quality of life score compared to the baseline. The greatest reduction in quality of life score was in the Group A while the least reduction was observed in Group C [Table 3].
Table 3.
Change in quality of life score
Flow rate
There was improvement in the flow rate in all groups compared to baseline. This achieved statistical significance in the Group A (P < 0.001) and Group C (P < 0.001). The Group B, however, failed to achieve statistically significant improvement in flow rate (P = 0.07). The mean change in flow rate at 3 months was 0.98, 0.05, 3.55 and at 6 months 4.11, 0.87, 3.74 (ml/s) for Groups A, B, and C, respectively [Figure 2].
Figure 2.
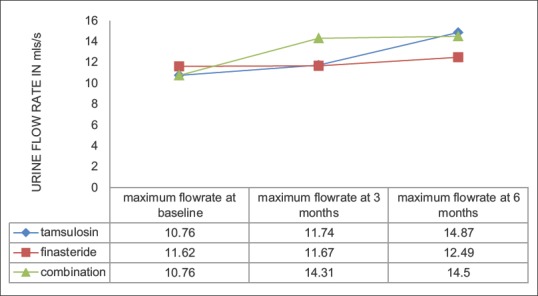
Change in urine flow rate
Prostate volume
There was no statistically significant difference in prostate volume at 6 months in each group compared to baseline values (P = 0.17 for Group A, P = 0.49 for Group B, and P = 0.13 for Group C). There was an increase in prostate volume at 6 months for Group A while Group B and C experienced a reduction in prostate volume. Group C experienced the greatest percentage reduction in prostate volume (11.83%).
This difference at 6 months was significantly different between Group C and Group A (P = 0.006). Group C and Group B achieved similar reduction in prostate volume at 6 months (P = 0.14). This result shows that tamsulosin had no effect on prostate volume while finasteride and combination therapy reduced it [Figure 3].
Figure 3.
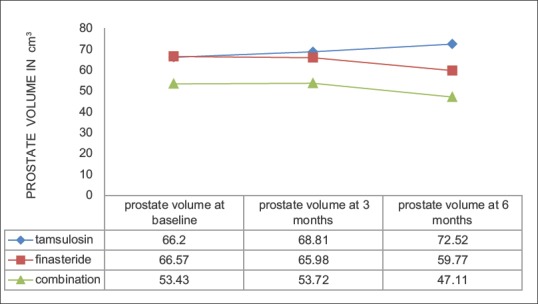
Change in prostate volume
Side effects
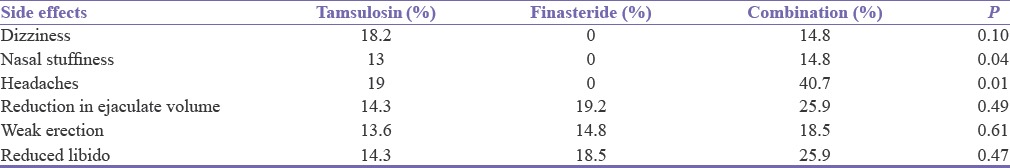
All the patients in all the three groups experienced some side effects. Only two patients in Group C withdrew from the study because of erectile dysfunction [Table 4].
Table 4.
Side effects experienced by patients
Discussion
BPH is a common cause of LUTS in the aging male. For a long period, surgery was the only effective modality of treatment, but medical therapy has now been established as an effective alternate treatment.
Alpha-adrenergic blockers (A1RB) are known to cause smooth muscle relaxation in the prostate stroma and thereby relieve the dynamic cause of obstruction in BPH.[8] The effect of dihydrotestosterone on prostatic tissue is blocked using 5ARI. Castration prevents the development of BPH and androgen suppression causes regression of established BPH.[9]
Previous studies in Nigerian men have evaluated the efficacy of A1RB.[4,5] These studies, however, utilized doxazosin a nonuroselective alpha-blocker unlike the present study where the uroselective tamsulosin was used. Furthermore, the role of the 5ARI finasteride and its combination with tamsulosin was evaluated in the present study.
There was an overall improvement in IPSS in all groups studied. This is consistent with report of other workers (at 6 months) who had utilized similar agents.[7,10,11] The percentage reduction in IPSS at 3 months is 42.59%, 41.85%, 40.61% and at 6 months is 47.88%, 56.88%, 62.25% in the Group A, B, and C, respectively. This reduction in IPSS was statistically significant within each group (P < 0.01). This observation suggests that each form of therapy was effective in the reduction of IPSS. Even though no single group was better than the other; there was no statistically significant difference between the three group in efficacy of reduction in IPSS (3 and 6 months P = 0.72 and 0.97, respectively). The reduction in IPSS was most noticeable in the combination arm of the study which may represent synergism in the effect of A1RB and 5ARI.
Patients in Groups A and B experienced comparable improvement in their quality of life. Group C patients did not achieve a similar level of improvement in the quality of life scores despite achieving better reduction in IPSS. A possible explanation for this may be the higher incidence of side effects in the group.
There was progressive improvement in the flow rate in all groups at 3 and 6 months. The change in flow rate was most noticeable in Group C at 3 months and this improvement was maintained at 6 months. This early improvement may be due to the synergistic effect of the tamsulosin and finasteride. The monotherapies had a gradual increase in flow rate at 3 and 6 months. Group A had the greatest increase in flow rate while Group B had the least increase at 6 months.
The mechanism of action of A1RB which causes rapid relaxation of smooth muscle in the prostate within 24 h compared with 5ARI causes a reduction in prostate volume over months by inhibiting the production of dihydrotestosterone may be responsible.[1] Other studies have also reported better flow rates in patients on combination therapy.[7,10] In this study, the flow rate was better in Group C at 3 months; however, they were similar in Group A and C at 6 months.
This study documented a reduction in prostate volume in the Group B and C at 6 months which is consistent with the report of others.[7,10] The short duration of this study may, however, be the reason for failure of this to achieve statistical significance [Figure 2]. Combination therapy group (Group C) achieved similar reduction in prostate volume as the finasteride monotherapy group (Group B); thereby reinforcing the belief that the reduction in prostate volume is being mediated by the 5ARI.
Finasteride is known to induce epithelial involution in the prostate gland and thereby cause a reduction in prostate volume. There was, however, a gradual increase in prostate volume in Group A at 3 and 6 months, this implies that tamsulosin does not cause involution of the prostate. This is in keeping with the known mechanism of action of A1RB in the prostate.
A study by Gormley et al.[12] demonstrated an 19% reduction in prostate volume over 12 months in patients given 5 mg of finasteride. He utilized magnetic resonance imaging for his measurements. Another study by Nacey et al.[13] recorded most of the reduction in the prostate volume occurring in the first 3 months; he demonstrated a reduction in prostate volume of 27% at 12 months in patients on 5 mg of finasteride. We have demonstrated a reduction in prostate volume in Groups B and C of 10.22% and 11.83%, respectively, at 6 months although further comparison to these other studies may be limited by the shorter duration of this study. We utilized transabdominal USS while other studies[7,10,13] have utilized transrectal USS. However, Malemo et al.[14] has demonstrated that transabdominal USS and transrectal USS have similar accuracy in the assessment of prostate volume. We elected to use transabdominal USS because it is easily available and more convenient for the patient.
Only 72% of patients completed the study. This high attrition rate is noted in similar studies although these were for longer periods.[7,10] Study protocol violation with concomitant use of herbal concoction was common in Nigerian patients. Although patients experienced various side effects only erectile dysfunction in two patients in the combination arm led to withdrawal from the study. This is similar to findings in a longer study where withdrawal due to drug-related side effects was more in the combination arm and erectile dysfunction was also noted more in the combination arm.[7]
Overall side effects including headaches, reduction in ejaculate volume, loss of libido, and erectile dysfunction were more in Group C. Similar observation was also reported in the CombAT study.[7] The CombAT study,[7] however, utilized dutasteride while finasteride was used in this study.
The conclusions drawn from the study may be limited by the fact that there was no placebo arm and the study duration was limited to 6 months. Therefore, only short-term effects could be assessed. We would recommend longer studies with a placebo arm in the future.
Conclusion
Tamsulosin monotherapy is recommended in men who want rapid improvement in flow rate but wish to avoid side effects of 5ARI. Combination therapy is advised in elderly men with need for improvement in flow rate who are not bothered by sexual side effects. Finasteride as a monotherapy is not recommended in the short-term.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Roehrborn CG. Benign prostatic hyperplasia: Etiology, pathophysiology, epidemiology, and natural history. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell Walsh Urology. 10th ed. United States of America: Saunders Elsevier; 2012. pp. 2570–694. [Google Scholar]
- 2.Athanasopoulos A, Chapple C, Fowler C, Gratzke C, Kaplan S, Stief C, et al. The role of antimuscarinics in the management of men with symptoms of overactive bladder associated with concomitant bladder outlet obstruction: An update. Eur Urol. 2011;60:94–105. doi: 10.1016/j.eururo.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 3.Otsuki H, Kosaka T, Nakamura K, Mishima J, Kuwahara Y, Tsukamoto T. ß3-Adrenoceptor agonist mirabegron is effective for overactive bladder that is unresponsive to antimuscarinic treatment or is related to benign prostatic hyperplasia in men. Int Urol Nephrol. 2013;45:53–60. doi: 10.1007/s11255-012-0343-5. [DOI] [PubMed] [Google Scholar]
- 4.Nwofor AM, Dogunro AS. Doxazosin in the treatment of elderly patients with benign prostatic hyperplasia. Afr J Urol. 2002;8:56–61. [Google Scholar]
- 5.Jeje EA, Dogunro AS, Ogunjimi MA, Tijani KH. Efficacy and safety of doxazosin (CARDURATM) in the management of benign prostatic hyperplasia. Niger J Surg. 2011;17:55–9. [Google Scholar]
- 6.Kirkwood RB, Sterne AC. Essential medical statistics. In: Kirkwood RB, Sterne AC, editors. Essential Medical Statistics. United State of America: Blackwell Publishing Company; 2003. p. 418. [Google Scholar]
- 7.Roehrborn CG, Siami P, Barkin J, Damião R, Major-Walker K, Nandy I, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol. 2010;57:123–31. doi: 10.1016/j.eururo.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 8.Lepor H, Lawson RK. Prostate diseases. Philadelphia: W.B Saunders; 1993. The role of alpha blockade in the therapy of benign prostatic hyperplasia; pp. 170–81. [Google Scholar]
- 9.McConnell JD. Benign prostatic hyperplasia. Hormonal treatment. Urol Clin North Am. 1995;22:387–400. [PubMed] [Google Scholar]
- 10.McConnell JD, Roehrborn CG, Bautista OM, Andriole GL, Jr, Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–98. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 11.Lepor H, Williford WO, Barry MJ, Brawer MK, Dixon CM, Gormley G, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. Veterans Affairs Cooperative Studies Benign Prostatic Hyperplasia Study Group. N Engl J Med. 1996;335:533–9. doi: 10.1056/NEJM199608223350801. [DOI] [PubMed] [Google Scholar]
- 12.Gormley GJ, Stoner E, Bruskewitz RC, Imperato-McGinley J, Walsh PC, McConnell JD, et al. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N Engl J Med. 1992;327:1185–91. doi: 10.1056/NEJM199210223271701. [DOI] [PubMed] [Google Scholar]
- 13.Nacey JN, Meffan PJ, Delahunt B. The effect of finasteride on prostate volume, urinary flow rate and symptom score in men with benign prostatic hyperplasia. Aust N Z J Surg. 1995;65:35–9. doi: 10.1111/j.1445-2197.1995.tb01744.x. [DOI] [PubMed] [Google Scholar]
- 14.Malemo K, Galukande M, Hawkes M, Bugeza S, Nyavandu K, Kaggwa S. Validation of supra-pubic ultrasonography for preoperative prostate volume measurement in sub-Saharan Africa. Int Urol Nephrol. 2011;43:283–8. doi: 10.1007/s11255-010-9844-2. [DOI] [PubMed] [Google Scholar]


