Abstract
OBJECTIVE
CALGB 140503 is an ongoing multi-center randomized trial assessing whether sub-lobar resection is equivalent to lobectomy for the treatment of stage IA non-small cell lung cancer (NSCLC) ≤ 2 cm in diameter. The objective of this report is to determine the reasons precluding intra-operative randomization.
METHODS
From June 15, 2007 to March 22, 2013, 637 patients were preregistered to the trial. Three hundred eighty-nine were successfully randomized (61%) and 248 patients were not randomized (39%). We analyzed the reasons for non-randomization among a subset of the non-randomized patients (208) for which additional data were available.
RESULTS
Of these 208 patients, undiagnosed benign nodules (n=104, 16% of all registered patients) and understaging of NSCLC (n=45, 7% of all registered patients) were the dominant reasons precluding randomization. Granulomas represent one-quarter of the benign nodules. The understaged patients had unsuspected nodal metastases (n=28) or other more advanced NSCLC. The rate of randomization was significantly higher in those patients who had a preoperative biopsy (p<0.001)
CONCLUSIONS
In a carefully monitored cohort of patients with suspected small NSCLC ≤2 cm, a substantial number are misdiagnosed (benign nodules) or understaged. These patients may not have benefited from a thoracic surgical procedure. Preoperative biopsy significantly increased the rate of correct diagnosis. Preoperative biopsy of small suspected NSCLC will reduce the number of non-therapeutic or unnecessary thoracic procedures. Accuracy in preoperative diagnosis is increasingly important as more such small nodules are discovered through lung cancer screening.
INTRODUCTION
Cancer and Leukemia Group B (CALGB) 140503 is a large, multicenter randomized trial designed to test the hypothesis that sublobar resection (wedge resection or segmentectomy) for peripheral NSCLC ≤ 2 cm in size results in equivalent disease-free survival compared to lobectomy (CALGB is now part of the Alliance for Clinical Trials in Oncology). Randomization (1:1) to lobectomy or sublobar resection is done after pre-operative or intra-operative confirmation of both diagnosis and absence of metastasis in at least three nodal stations.
During trial design, we estimated that 30% of patients would be ineligible for randomization due to understaging or misdiagnosis. The actual percentage of registered patients who were unable to go on to randomization was about 40%. This rate of inaccuracy in the assessment of small nodules presumed to be clinical T1a NSCLC indicates that even in an era of improved imaging and staging methods, numerous patients are still subjected to thoracic surgery who may not benefit from it. We hypothesized that knowing the reasons why so many patients could not be randomized would allow corrective action to lower this rate and avoid unnecessary surgery with its attendant risks and costs.
METHODS
CALGB 140503 is a non-inferiority study that will determine whether a limited lung resection is equivalent to a standard lobectomy for treatment of early stage I NSCLC. Patients are recruited who could tolerate lobectomy, and whose tumor is ≤ 2 cm in diameter and peripheral (outer third of the lung) in location. The surgeon must believe that the tumor is resectable by wedge resection or segmentectomy and that the patient can tolerate lobectomy. All evaluations for distant disease, including pathologic examination of mediastinal and hilar lymph nodes at the time of operation, must be negative. Intra-operative randomization takes place only after the frozen sections of the required nodal stations nodes are negative and the surgeon confirms that both lobectomy and sublobar resection would be technically feasible. The surgeon may decide at any time to withdraw the patient from the assigned arm due to technical or patient safety reasons.
Approval for CALGB 140503 was granted by the CIRB on May 4, 2007 and subsequently by the institutional review board (IRB) at each participating institution. The study is registered with ClinicalTrials.gov (NCT 00499330) as of July 10, 2007. Approval for the sub-study (A081202: Ancillary Analysis of Unrandomized Patients on CALGB 140503: A Phase III Randomized Trial of Lobectomy versus Sublobar resection for Small ≤ 2 cm Peripheral Non-small Cell Lung Cancer) was granted by the Alliance for Clinical Trials in June 2012.
Informed Consent: The protocol specifies that “the patient must be aware of the neoplastic nature of his/her disease and willingly consent after being informed of the procedure to be performed, side effects, risks, and discomforts. Human protection committee approval of this protocol and a consent form is required.” A consent form approved by CALGB/Alliance, the CIRB and each institution’s IRB was provided to and discussed with each patient by the site study team. A signed copy is on file at each site and the Alliance data center.
Pre-registration Eligibility Criteria for parent study, CALGB 140503:
Peripheral NSCLC ≤ 2 cm as determined by CT scan. Patients with pure ground glass opacities or pathologically confirmed N1 or N2 disease are not eligible.
Tumor location suitable for either lobar or sublobar resection (wedge or segment).
ECOG performance status of 0–2.
No prior malignancy within 5 years (later changed to 3 years) other than non-melanoma skin cancer or superficial bladder cancer or carcinoma in situ of the cervix.
No prior neoadjuvant therapy for this malignancy.
Intra-operative Randomization Eligibility Criteria
Histologic confirmation of NSCLC (if not already obtained).
Confirmation of N0 status by frozen section examination of nodal levels 4, 7, and 10 on the right side and 5, 6, 7 and 10 on the left side.
The Principle Investigator requested additional records from all 248 consented patients on CALGB 140503 who were registered but not randomized between study opening in 2007 (first patient registered 10/17/2007) and a cut-off date of 3/22/2013. Requests were sent to both the enrolling thoracic surgeon and the clinical research associate at each site for the following documents: operative report, pathology report, discharge summary, and pre-op PET and CT reports. The data were sent by the study sites to the Alliance Statistics and Data Center. Although the consent signed by all registered participants covered the sharing of this information, and they are routinely collected on all randomized patients, the protocol did not require their submission once a patient was withdrawn from the study due to non-randomization.
Statistical Methods
Core statistical team members from the Alliance Statistics and Data Center at Duke University Cancer Institute, who are investigators on this study, performed the analysis. The data were locked on April 29, 2016. We summarized various reasons for non-randomization from the study database. Frequencies and percentages of the reasons were tabulated and depicted graphically. P- values comparing randomized versus un-randomized patients were calculated from t-test for continuous variable (age) or from chi-square test for categorical variables.1–4
RESULTS
At the time of data acquisition for this report, 637 patients had been registered to CALGB 140503, 389 (61%) had been successfully randomized and 248 patients were not randomized (39%). Over time, the rate of successful randomization in the study as a whole increased steadily from 56% to 73% (Figure 1). We analyzed the reasons for non-randomization among a subset of the unrandomized patients (208, 84% of the unrandomized) for whom the reason for not randomizing was available. Thirty-nine of 59 participating institutions which accrued patients during the study interval contributed to the 208 patient subset. Eleven institutions, comprising 29 registered patients, randomized all their patients so did not contribute to this analysis. Twenty-six institutions (134 patients) submitted data on all nonrandomized patients. Nine institutions (16 patients) did not submit data on any of their nonrandomized patients. The other 13 institutions (74 patients) submitted data on 13% – 94% of their nonrandomized patients. The reasons for failure to submit data were largely related to change in data management personnel over time at the institutions, and the lack of resource capacity at the sites to search patient records for these documents.
Figure 1.
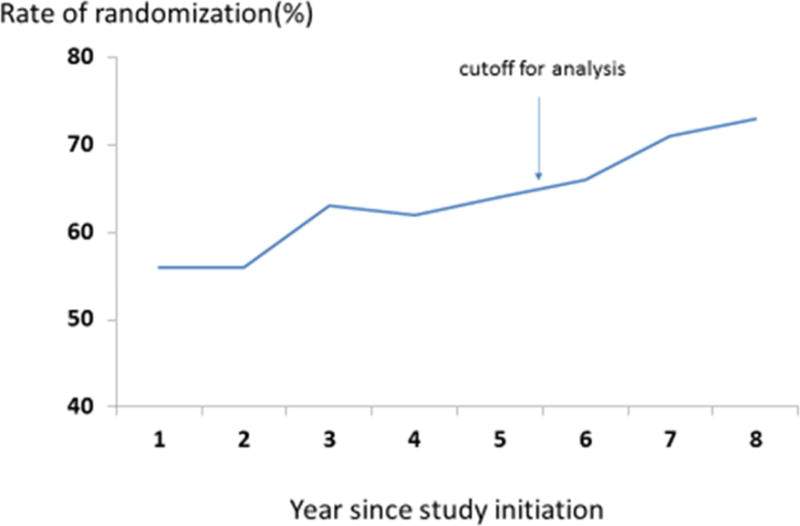
Rate of randomization over time
Demographics and nodule characteristics for these patients are shown in Table 1. Unrandomized patients are younger and more likely to be never-smokers than randomized patients. Most notably, the unrandomized patients (both in the 208-patient subset and the entire cohort of 248 unrandomized patients) had a rate of pre-operative biopsy only half that of the successfully randomized patients. Reasons for non-randomization (Table 2) include benign nodules, more advanced NSCLC (clinical under-staging), small cell lung cancer, metastatic disease from other sites and technical or administrative reasons.
Table 1.
Demographics and nodule characteristics (Percentages based on number of patients with data available for analysis)
| All Registered Patients (n=637) |
Randomized (n=389) |
Unrandomized (n=248) |
Included in analysis (n=208) |
p-value comparing randomized vs unrandomized | |
|---|---|---|---|---|---|
| Age (yr) – mean (SD) | 66 (10) | 68 (9) | 64 (10) | 64 (10) | < 0.001 |
| Female – n (%) | 359 (56%) | 228 (59%) | 131 (53%) | 114 (55%) | 0.151 |
| Location of nodule – n (%) | 0.572 | ||||
| RUL | 216 (36%) | 141 (37%) | 75 (36%) | 75 (36%) | |
| LUL | 162 (27%) | 107 (28%) | 55 (27%) | 55 (27%) | |
| RLL | 82 (14%) | 50 (13%) | 32 (15%) | 32 (15%) | |
| LLL | 93 (16%) | 65 (17%) | 28 (14%) | 28 (14%) | |
| RML | 39 (7%) | 22 (6%) | 17 (8%) | 17 (8%) | |
| Pre-op biopsy done – n (%) | 238 (41%) | 192 (50%) | 46 (23%) | 46 (23%) | < 0.001 |
| Never smoker – n (%) | 79 (14%) | 44 (11%) | 35 (18%) | 35 (18%) | 0.036 |
Table 2.
Reasons for non-randomization among 208 patients with data available
| Reason | # Patients (%) (N = 208) | |
|---|---|---|
| Not NSCLC | 120 (57.7%) | |
|
| ||
| Benign | 104 (50.0%) | |
|
| ||
| Granuloma | 24 (11.5%) | |
|
| ||
| Hamartoma1 | 9 (4.3%) | |
|
| ||
| Infection | 11 (5.3%) | |
|
| ||
| Other2,3 | 60 (28.8%) | |
|
| ||
| Other malignancy | 16 (7.7%) | |
|
| ||
| Small cell lung cancer | 3 (1.4%) | |
|
| ||
| Carcinoid | 4 (1.9%) | |
|
| ||
| Lymphoma | 5 (2.4%) | |
|
| ||
| Metastatic, other site | 4 (1.9%) | |
|
| ||
| NSCLC but ineligible (more advanced) | 47 (22.6% of unrandomized, 10.7% of all registered NSCLC) | |
| Stage IA (T1b) | 6 | |
| Stage IIA | 6 | |
| Stage IIB | 6 | |
| Stage IIIA | 25 | |
| Stage IV (M1a) | 2 | |
| Unknown | 2 | |
|
| ||
| Positive nodes - (4) | 28 (13.5% of unrandomized, 6.4% of all registered NSCLC) | |
| N2 20 | ||
| N1 6 | ||
| Not specified 2 | ||
|
| ||
| Satellite nodule | 6 (2.9%) | |
|
| ||
| 2nd cancer in other lobe | 3 (1.4%) | |
|
| ||
| Pleural effusion | 2 (1.0%) | |
|
| ||
| Tumor > 2 cm | 6 (2.9%) | |
|
| ||
| Other (multiple lesions) | 2 (1.0%) | |
|
| ||
| Technical Reasons | 12 (5.8%) | |
|
| ||
| Unable to sample nodes | 3 (1.4%) | |
|
| ||
| Unsuitable for sublobar resection | 6 (2.9%) | |
|
| ||
| Hemorrhage | 1 (0.5%) | |
|
| ||
| Difficult anatomy | 2 (1.0%) | |
|
| ||
| Administrative/ Other/Unknown | 29 (13.9%) | |
|
| ||
| Patient refusal/withdrawal | 12 (5.8%) | |
|
| ||
| Surgeon decision | 7 (3.4%) | |
|
| ||
| Unknown | 10 (4.8%) | |
one was also unsuitable for wedge
one patient had eligible NSCLC but a suspected lymphoma in another lobe which turned out to be benign
Two cases of chronic inflammation, one each emphysema, inflammatory nodule, ischemic necrosis, scar tissue, sarcoidosis. Remainder unspecified.
one N2 case pathology was small cell
DISCUSSION
We analyzed the reasons for non-randomization in patients participating in CALGB 140503, a multi-institutional randomized study of lobectomy vs. sublobar resection for clinical Stage IA NSCLCs ≤ 2cm in diameter who will not be included in the primary analysis. We found that despite all contemporary forms of imaging in an era of easily available and safe transthoracic needle biopsy and invasive staging techniques, a substantial number of clinical T1a NSCLC actually have benign disease masquerading as cancer, more advanced NSCLC or other malignancies. Our most notable finding was that randomized patients had a much higher rate of preoperative biopsy confirming their diagnosis (p<0.001). This supports recommending preoperative biopsy for all patients with suspected small lung cancers to avoid unnecessary thoracic surgery for those who have a benign nodule or a metastasis from another primary cancer. Most pulmonary nodules found incidentally and on screening are benign, especially in areas with endemic granulomatous disease.5 Single institution reviews of surgery for patients with pulmonary nodules range from 86% benign in a group of solitary pulmonary nodules (SPN) treated by video-assisted thoracic surgery (VATS) excision without preoperative biopsy6 to 9% in a group of patients from the histoplasmosis belt, a very high percentage of which were granulomas.5 DeCamp and colleagues7 found 40% of nodulectomies benign. Our results fall within the published range. Benign nodules less than 2 cm in diameter rarely require excision for treatment or palliation, and most patients in CALGB 140503 with benign lesions probably had unnecessary surgery which could have been avoided by preoperative biopsy in most cases.
A 6.4% rate of nodal upstaging is considerably lower than noted in a review of all Stage I NSCLC from the STS Database, where it was 14.3% for thoracotomy and 11.6% for VATS8 and in the Danish Lung Cancer Registry (18.6%).9 A recent review comparing robotic and VATS lobectomy found that among cT1a patients, 3.5% had pN1 and 4.9% had pN2, comparable to our findings.10 Meyers et al did a cost-effectiveness analysis of routine mediastinoscopy in patients with clinical stage I lung cancer by CT and PET and concluded that such patients benefit little from mediastinoscopy unless the prevalence of N2 disease exceeds 10%.11 We do not have information on how many patients in CALGB 140503 had false-negative mediastinoscopy (if it was positive, they would not have been registered for this trial) or other forms of mediastinal nodal biopsy such as endobronchial ultrasound (EBUS) but since our rate of N2 upstaging by mediastinal biopsy at VATS or thoracotomy was only 4.6%, it is unlikely that mediastinoscopy would have detected many additional cases not detected by direct biopsy of three mediastinal nodal stations. There does not seem to be a compelling case for additional preoperative mediastinal staging in patients with suspected T1a NSCLC.
Only 11 patients were excluded from randomization because of a malignant second nodule of NSCLC pathology found at surgery (6 satellite nodules, 3 cancers in another lobe and 2 with multiple lesions). This is consistent with a study by Stiles et al, where the incidence of secondary nodules in patients having surgery for NSCLC was 57% but only 13% were malignant.12 Patients with known or suspected multiple cancers would not have been eligible for this study.
Younger patients and never-smokers are considered at lower risk for lung cancer, although the rate of never-smokers with cancer is rising and one estimate puts it at 15% currently.13 Our rate of never-smokers was higher in the unrandomized than the randomized groups (18% vs. 11%, P=0.036) and the unrandomized group was also younger (mean 64 years vs 68, p<0.001) which could indicate a lower chance of malignancy and perhaps should favor even more strongly a less invasive approach, such as needle biopsy, in those with a lower predicted incidence of malignancy.
Biopsy for diagnosis of small lung nodules can be accomplished via transthoracic image guided needle biopsy (fine needle aspiration or core biopsy) or bronchoscopic biopsy, including navigational bronchoscopy. The patients in this trial had peripheral nodules suitable for sublobar resection, likely more often indicating transthoracic biopsy. The diagnostic success of trans-thoracic image-guided fine needle aspiration or core needle biopsy is now more than 90%14,15,16,17 and the risk of severe complications is low (0.75% in one large series).18
On the other hand, in the Dutch-Belgian randomized lung cancer screening trial (NELSON) 43% of patients who had thoracotomy for nodules which turned out to be benign had at least one minor complication, and 3 of 47 patients (6%) had a major complication.19 The Surgical Group of the International Association for the Study of Lung Cancer opines that “the use of CT-guided biopsy for suspicious nodules should be encouraged and will in many cases facilitate the surgical decision process.”20 Most benign nodules ≤2 cm in diameter will not require excision for symptoms or disease management and surgery can be completely avoided once a benign diagnosis has been confirmed by biopsy.
Although the current study is not directly comparable to a screening group (we do not have information about how many of these participants had their nodules detected on screening), comparison of rates of surgery for benign conditions in screening cohorts is useful since the type of lesion in CALGB 140503 is similar to those frequently encountered in screen-detected nodules (≤2cm). Utilization of a robust algorithm for evaluation and surveillance of dominant and secondary nodules, such as those employed in experienced screening programs should assure that interventions are appropriate and limited to those with a high pre-test probability of malignancy.
Documented benefits of CT scan screening for lung cancer began with the 1999 publication of ELCAP results.21 The ELCAP evaluation protocol dictated careful attention to imaging details and adherence to follow up regimens. No patient had surgery for benign disease, the vast majority of biopsies revealed cancer, and no complications occurred as a consequence of any of the biopsy procedures, even for the small non-calcified nodules. Flores and colleagues updated the I-ELCAP results in 2014.22 Among 31,646 baseline and 37,861 annual repeat screenings performed at US sites, 492 patients underwent surgical resection, of which 437 (89%) were diagnosed with lung cancer. Among these 437, 91% had pathologic stage I disease. Of clinical Stage I lung cancers, 7% had more advanced disease diagnosed at operation, identical to our finding. Among the 492 surgical resections, 230 had a preoperative diagnosis of lung cancer. Among the remaining 262 patients, who did not have a preoperative biopsy, 20% had a non-malignant diagnosis (higher than our rate of 15%). These results again emphasize the importance of preoperative biopsy to avoid unnecessary surgery.
There are other, smaller randomized trials of CT scan screening for lung cancer either completed or in progress. They show a wide range of surgery for benign nodules: DANTE trial: 0.9% surgery for benign lesions,23 COSMOS trial: 12% surgery for benign nodules24 and NELSON trial: 24% surgery for benign nodules.19 The NELSON trial also showed that radiologist expertise can reduce the number of false-positive results.25 Strict adherence to a diagnostic protocol such as I-ELCAP26 as well as increasing the rate of preoperative nodule biopsy in highly suspicious lesions should reduce these numbers so that fewer patients are subject to surgery which may not benefit them, with the associated risks and costs.
Our finding that in a large well-controlled study of clinical stage IA NSCLC, 26% (120 not NSCLC plus 47 more advanced NSCLC out of total registered of 637) had a different diagnosis not discovered until VATS or thoracotomy, and half of these (104 benign of 208 analyzed, 16% of 637 registered patients) were benign also has implications for the appropriateness of local therapy such as stereotactic body radiotherapy or radiofrequency ablation when done without preoperative confirmation of diagnosis. Core biopsies are diagnostic in over 90% of nodules with a low rate of complications14–17 and should be routine for suspected lung cancer, especially younger patients and never-smokers.
Study Limitations
The 208 patients included in this analysis represent 84% of unrandomized patients at cut-off date, and may not be representative of the entire cohort, noting that some participating institutions are not represented among these 208 patients. However, we believe the chance of systematic bias in only one direction coming from 13 different institutions is rather slight. We have also based our analysis on the entire 248 patients in the unrandomized group. Some of these categories might be different if we knew the reason for nonrandomization in the remaining 16%.
The ability of PET scan to improve accuracy will be assessed in the imaging component of the parent trial and having a PET scan may be correlated with rate of randomization. We do not have the data yet to investigate this possibility.
There may be a difference between the rate of under- and over-staging in this trial subset and that encountered in the non-study situation. In particular, surgeons motivated to accrue patients to clinical trials may enter them even if the clinical suspicion for lung cancer is low. Or, there may be a different practice pattern or threshold for pre-operative biopsy in patients considered for a clinical trial versus standard practice. Either of these, if true, would make the rate of patients subject to unnecessary operation different in the current analysis than in common practice.
Multidisciplinary lung cancer clinics or tumor boards, accreditation by the Commission on Cancer and adherence to National Comprehensive Cancer Network or other guidelines may also influence biopsy rates. Local availability and expertise in techniques such as transthoracic image guided biopsy, EBUS and navigational bronchoscopy as well as diagnostic radiologic and pathologic expertise or participation in a formal screening protocol may also contribute to institutional variation.
CONCLUSION
In a carefully monitored cohort of patients with suspected small NSCLC ≤2 cm, a substantial number are misdiagnosed (benign nodules) or understaged. These patients may not have benefited from a thoracic surgical procedure. Preoperative biopsy significantly increased the rate of correct diagnosis and will reduce the number of non-therapeutic or unnecessary thoracic procedures. The unrandomized patients were younger and more frequently never-smokers, favoring even more strongly a biopsy before proceeding to surgical resection in such patients since their likelihood of cancer is lower. Accuracy in preoperative diagnosis is increasingly important as more such small nodules are discovered through lung cancer screening.
The parent trial for this sub-set analysis, CALGB 140503, is an ambitious and successful randomized controlled trial addressing the question of whether sublobar resection, including mediastinal lymph node dissection, is equivalent to pulmonary lobectomy in terms of cancer-related outcomes. We look forward to the primary analysis of this landmark trial.
CENTRAL MESSAGE.
Preoperative confirmation of diagnosis by biopsy in suspected small NSCLC will reduce unnecessary and non-therapeutic lung resections.
Central Picture.
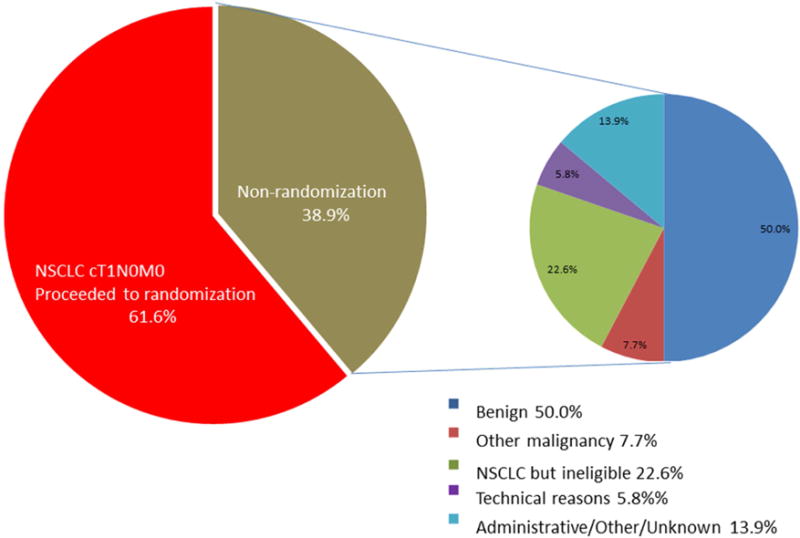
Diagnoses in suspected cT1A NSCLC
PERSPECTIVE STATEMENT.
CALGB 140503 compares sub-lobar resection to lobectomy for small NSCLC. Pre-operative diagnosis and staging are correct in only 61%, much higher in patients with preoperative biopsy. The number of patients subject to unnecessary thoracic surgery for small benign nodules will be reduced by increasing the rate of preoperative biopsy.
Acknowledgments
Cathy Spinelli for assistance with images, surgeons and clinical research professionals at all participating institutions for entering patients and providing additional data.
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA033601, U10CA031946, U10CA007968, and U10CA021060. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Leslie Kohman receives support for research unrelated to this paper from CareFusion. Nasser Altorki received support from AstraZeneca for clinical trial research and from Genentech for clinical trial participation.
Glossary of Abbreviations
- CALGB
Cancer and Leukemia Group B
- CIRB
Central Institutional Review Board
- cm
Centimeter
- CT
Computed tomography
- ECOG
Eastern Cooperative Oncology Group
- ELCAP
Early Lung Cancer Action Project
- I-ELCAP
International Early Lung Cancer Action Project
- IRB
Institutional Review Board
- LLL
Left lower lobe
- LUL
Left upper lobe
- n
Number
- NSCLC
Non-small cell lung cancer
- PET
Positron Emission Tomography
- RLL
Right lower lobe
- RML
Right middle lobe
- RUL
Right upper lobe
- VATS
Video-assisted thoracic surgery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Other authors have nothing to disclose.
Clinical Trial Registry Number: ClinicalTrials.gov Identifier: NCT00499330. It was approved by the Central Institutional Review Board (CIRB) on May 4, 2007
References
- 1.Agresti A. An Introduction to Categorical Data Analysis. 2nd. New York: John Wiley & Sons; 2007. [Google Scholar]
- 2.Stokes ME, Davis CS, Koch GG. Categorical Data Analysis Using SAS. 3rd. Cary, NC: SAS Institute Inc; 2012. [Google Scholar]
- 3.Steel RGD, Torrie JH. Principles and Procedures of Statistics. 2nd. New York: McGraw-Hill; 1980. [Google Scholar]
- 4.Wellek S. Testing Statistical Hypotheses of Equivalence. Boca Raton, FL: Chapman & Hall/CRC; 2003. [Google Scholar]
- 5.Smith MA, Battafarano RF, Meyers BF, Zoole JB, Cooper JD, Patterson GA. Prevalence of benign disease in patients undergoing resection for suspected lung cancer. Ann Thorac Surge. 2006;81:1824–9. doi: 10.1016/j.athoracsur.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Cardillo G, Regal M, Sera F, DiMartino M, Carbone L, Facciolo F, et al. Videothoracoscopic management of the solitary pulmonary nodule: a single-institution study on 429 cases. Ann Thorac Surg. 2003;75:1607–1611. doi: 10.1016/s0003-4975(02)04827-0. [DOI] [PubMed] [Google Scholar]
- 7.DeCamp MM, Jr, Jaklitsch MT, Mentzer SJ, Harpole DH, Jr, Sugarbaker DJ. The safety and versatility of video-thoracoscopy: A prospective analysis of 895 consecutive cases. J Am Coll Surg. 1995 Aug;181(2):113–20. [PubMed] [Google Scholar]
- 8.Boffa DJ, Kosinski AS, Paul S, Mitchell JD, Onaitis M. Lymph node evaluation of open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surge. 2012;94:347–53. doi: 10.1016/j.athoracsur.2012.04.059. [DOI] [PubMed] [Google Scholar]
- 9.Licht PB, Jorgenson OD, Ladegaard L, Jakobsen E. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg. 2013;96:943–50. doi: 10.1016/j.athoracsur.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Wilson JL, Louie BE, Cerfolio RJ, Park BJ, Vallières E, Aye RW, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg. 2014;97:1901–6. doi: 10.1016/j.athoracsur.2014.01.064. [DOI] [PubMed] [Google Scholar]
- 11.Meyers BF, Haddad F, Siegel BA, Zoole JB, Battafarano RJ, Veeramachaneni N, et al. Cost-effectiveness of routine mediastinoscopy in computed tomography- and positron emission tomography-screened patients with stage I lung cancer. J Thorac Cardiovasc Surg. 2006;131:822–9. doi: 10.1016/j.jtcvs.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 12.Stiles BM, Schulster M, Nasar A, Paul S, Lee PC, Port JL, et al. Characteristics and outcomes of secondary nodules identified on initial computed tomography scan for patients undergoing resection for primary non-small cell lung cancer. J Thorac Cardiovasc Surg. 2015;149:19–24. doi: 10.1016/j.jtcvs.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 13.Pelosof L, Ahn C, Horn A, Madriales J, Cox J, Roberts JN, et al. Increasing incidence of never smokers in non small cell lung cancer (NSCLC) patients. Abstracts from Proceedings, 16th World Conference on Lung Cancer; Denver CO. Sept 8 2015; ORAL22.01. [Google Scholar]
- 14.Ocak S, Duplaquet F, Jamart J, Pirard L, Weynand B, Delos M, et al. Diagnostic Accuracy and Safety of CT-Guided Percutaneous Transthoracic Needle Biopsies: 14-Gauge versus 22-Gauge Needles. J Vasc Interv Radiol. 2016;27:674–81. doi: 10.1016/j.jvir.2016.01.134. [DOI] [PubMed] [Google Scholar]
- 15.Sangha BS, Hague CJ, Jessup J, O’Connor R, Mayo JR. Transthoracic Computed Tomography-Guided Lung Nodule Biopsy: Comparison of Core Needle and Fine Needle Aspiration Techniques. Can Assoc Radiol J. 2016;67:284–9. doi: 10.1016/j.carj.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Jiao D, Yuan H, Zhang Q, Han X. Flat detector C-arm CT-guided transthoracic needle biopsy of small (≤2.0 cm) pulmonary nodules: diagnostic accuracy and complication in 100 patients. Radiol Med. 2016;121:268–78. doi: 10.1007/s11547-015-0604-3. [DOI] [PubMed] [Google Scholar]
- 17.DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis. 2015:S304–16. doi: 10.3978/j.issn.2072-1439.2015.12.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomiyama N, Yasuhara Y, Nakajima Y, Adachi S, Arai Y, Kusumoto M, et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol. 2006 Jul;59:60–4. doi: 10.1016/j.ejrad.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Van’t Westeinde SC, Horeweg N, De Leyn P, Groen HJ, Lammers JW, Weenink C, et al. Complications following lung surgery in the Dutch-Belgian randomized lung cancer screening trial. Eur J Cardiothorac Surg. 2012;42:420–9. doi: 10.1093/ejcts/ezs081. [DOI] [PubMed] [Google Scholar]
- 20.Field JK, Smith RA, Aberle DR, Oudkerk M, Baldwin DR, Yankelevitz D, et al. International Association for the Study of Lung Cancer computed tomography screening workshop 2011 report. J Thorac Oncol. 2012;7:10–19. doi: 10.1097/JTO.0b013e31823c58ab. [DOI] [PubMed] [Google Scholar]
- 21.Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 22.Flores R, Bauer T, Aye R, Andaz S, Kohman L, Sheppard B, et al. Balancing curability and unnecessary surgery in the context of computed tomography screening for lung cancer. J Thorac Cardiovasc Surg. 2014;147:1619–26. doi: 10.1016/j.jtcvs.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Infante M, Cavuto S, Lutman FR, Passera E, Chiarenza M, Chiesa G, et al. Long-Term Follow-up Results of the DANTE Trial, a Randomized Study of Lung Cancer Screening with Spiral Computed Tomography. Am J Respir Crit Care Med. 2015;191:1166–75. doi: 10.1164/rccm.201408-1475OC. [DOI] [PubMed] [Google Scholar]
- 24.Veronesi G, Maisonneuve P, Rampinelli C, Bertolotti R, Petrella F, Spaggiari L, et al. Computed tomography screening for lung cancer: results of ten years of annual screening and validation of cosmos prediction model. Lung Cancer. 2013;82:426–30. doi: 10.1016/j.lungcan.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 25.Heuvelmans MA, Oudkerk M, de Jong PA, Mali WP, Groen HJ, Vliegenthart R. The impact of radiologists’ expertise on screen results decisions in a CT lung cancer screening trial. Eur Radiol. 2015;25:792–9. doi: 10.1007/s00330-014-3467-4. [DOI] [PubMed] [Google Scholar]
- 26.Henschke CI. I-ELCAP protocol. http://www.ielcap.org/sites/default/files/ielcap.pdf. Accessed August 28, 2016.


