Abstract
The objective of this study was to compare the efficacy and mechanism of lyophilized black raspberries (BRB) versus the combination of celecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor, and S,S′-1,4-phenylene-bis(1,2-ethanediyl)bis-isothiourea (PBIT), a selective inducible nitric oxide synthase (iNOS) inhibitor in inhibition of carcinogen-induced esophageal squamous cell carcinoma in rats. Our data indicated that tumor multiplicity and histologic grade of esophageal precancerous lesions were reduced in animals fed BRB compared to those fed celecoxib + PBIT. The mechanistic studies showed that BRB and its major anthocyanin suppressed cell proliferation and oncogenic signaling. Our findings demonstrated that dietary BRB is superior to the combination of two pharmaceutical drugs in esophageal cancer prevention. These observations suggest the potential value of translational studies using BRB food products for esophageal cancer prevention in humans, particularly those with high-risk premalignant lesions.
Keywords: black raspberries, anthocyanin, esophageal cancer, cancer prevention
1 Introduction
Esophageal cancer is the 6th most common malignant neoplasm worldwide with more than 90% of all cases being esophageal squamous cell carcinoma (SCC) (Siegel, Ma, Zou, & Jemal, 2014; Souza, 2002). Esophageal carcinogenesis is a multistage process characterized by morphological changes from normal esophagus to basal cell hyperplasia, dysplasia, carcinoma in situ and esophageal SCC. Epidemiologic and clinical studies demonstrate that dysplasia is a premalignant lesion with a significant risk of progression to esophageal SCC. About 25% of patients with mild dysplasia and 50% of patients with moderate dysplasia will develop esophageal SCC over the subsequent decades (Wang et al., 2005).
Tobacco use and alcohol consumption are the major risk factors for esophageal SCC. Intake of food contaminated with various mycotoxins, nutrition-related deficiencies, diets low in fruit and vegetables, and thermal injuries due to the consumption of hot beverages are all associated with this disease (Freedman et al., 2007; Gao et al., 2011; Stoner & Gupta, 2001). The continued global expansion of alcohol and tobacco consumption coupled with high-risk dietary patterns makes esophageal SCC a major public health threat for decades to come (Trivers, Sabatino, & Stewart, 2008). The overall 5-year survival rate of esophageal SCC in the United States is only 13%, similar to that in many countries, as a result of presentation with advanced and metastatic disease at the time of diagnosis (Layke & Lopez, 2006). These characteristics strongly suggest that lifestyle changes such as promotion of anticancer dietary patterns, smoking cessation, and alcohol moderation may dramatically impact its risk. Furthermore, screening strategies coupled with the identification of high–risk cohorts for chemoprevention or dietary prevention may also reduce the public health burden from this disease.
Our laboratory has employed the N-nitrosomethylbenzylamine (NMBA)-induced animal model of esophageal SCC to identify putative chemopreventive agents and natural food components that are worthy of translation to human investigation (Carlton et al., 2002; Chen, Nines, Peschke, Kresty, & Stoner, 2004a; Chen & Stoner, 2004b; Shi et al., 2014; Stoner et al., 2005). In our previous studies, we found increased expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) in NMBA-induced esophagus SCC in rats (Carlton et al., 2002; Chen & Stoner, 2004b). We subsequently demonstrated that L-748706, a selective COX-2 inhibitor, and S,S′-1,4-phenylene-bis(1,2-ethanediyl)bis-isothiourea (PBIT), a selective iNOS inhibitor, significantly inhibit NMBA-induced rat esophageal tumorigenesis (Chen et al., 2004a; Stoner et al., 2005).
In parallel to the preventive strategy focusing on chemically pure agents, we have developed a food-based approach based upon the principle that multiple bioactive phytochemicals may have additive or synergistic activities with greater overall efficacy and safety in esophageal cancer prevention. Among the foods with potential anticancer activities, we have evaluated black raspberries (BRB) in NMBA-rat esophageal cancer model and suggested the preventive potential of BRB supported by mechanistic investigations (Chen, Hwang, Rose, Nines, & Stoner, 2006a; Chen, Rose, Hwang, Nines, & Stoner, 2006b).
In the present study, we tested a combination of selective COX-2 inhibitor plus iNOS inhibitor in NMBA-induced esophageal SCC in rats in comparison to a diet enriched with freeze-dried BRB. A direct comparison of the two approaches may suggest the priorities for future translational efforts in humans.
2 Materials and methods
2.1 Chemicals and reagent kits
NMBA was obtained from Ash Stevens (Detroit, MI) and determined to be > 98% pure by high-performance liquid chromatography (HPLC). Dimethyl sulfoxide (DMSO) was purchased from Sigma Chemical, Co. (St. Louis, MO). PBIT were obtained from Cayman Chemical Co. (Ann Arbor, MI). Celecoxib was purchased from LKT Laboratory Inc. (St. Paul, MN). The High-Capacity cDNA Reverse Transcription Kit and Fast SYBR Green Master Mix Kit were obtained from Applied Biosystems (Foster City, CA). KYSE-270 cell line was purchased from DSMZ (Braunschweig, Germany). The anthocyanin cyanidin-3-glucoside (C3G), cyanidin-3-rutinoside (C3R), cyaniding-3-sambubioside (C3S), pelargonidin-3-rutinoside (P3R), anthocyanin metabolite protocatechuic acid (PCA) and 4-hydroxybenzoic acid (4HBA) were purchased from Indofine Chemical Company (Hillsborough, NJ). The primary and secondary antibodies used in Western blot analysis were bought from Cell Signaling Technology (Beverly, MA).
2.2 Animals
Male F344 rats, 4 to 5 weeks old, were obtained from Harlan Sprague-Dawley (Indianapolis, IN). The animals were housed three per cage under standard conditions (20 ± 2°C; 50 ± 10% relative humidity; 12-hour light/dark cycles). Two weeks later, rats were randomized into four experimental groups and received different treatments as described below in experimental design. Food and water were freely available. Hygienic conditions were maintained by twice weekly cage changes and routine cleaning of the animal rooms. All animal cares and experimental procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of The Ohio State University (Protocol No. 2009A0054-R2).
2.3 Diets
The animals were fed a modified AIN-76A synthetic diet containing 20% casein, 0.3% D,L-methionine, 52% cornstarch, 13% dextrose, 5% cellulose, 5% corn oil, 3.5% AIN salt mixture, 1% AIN vitamin mixture, and 0.2% choline bitartrate (Dyets, Inc., Bethlehem, PA). The diet was stored routinely at 4°C before the preparation of experimental diets.
It is crucial that the experimental diet used is chemically and biologically uniform. To ensure a consistent agent, BRB (Rubus occidentalis) were obtained from the same farm in southern Ohio to eliminate area and growing differences (Stokes Berry Farm, Wilmington, OH). The berries provided for this study were mechanically harvested as a single lot, immediately washed, and frozen at −20°C on the farm property within 2 hours of harvesting. They were then shipped frozen to Van Drunen Farms (Momence, IL) for freeze-drying and subsequent grinding into a powder. BRB was shipped frozen to The Ohio State University (OSU) and kept at −20°C until used. OSU investigators have shown that the amounts of major anthocyanins vary from 10% – 20% over a 5-year period; and 26 micronutrients in freeze-dried berries remain within 10% – 20% of the original measurements when the berries are stored at −20°C (Stoner, 2009).
AIN-76A diet containing 300 ppm celecoxib + 50 ppm PBIT or 5% BRB was prepared fresh weekly and stored at 4°C. BRB or celecoxib + PBIT was mixed into the diet for 25 minutes with a Hobart mixer (Troy, OH). Fresh experimental and control diets were placed in glass feeding jars weekly and fed to the rats.
2.4 Experimental design
Rats in Group 1 were injected s.c. with 0.2 ml of a solution of 20% dimethyl sulfoxide (DMSO) in water (the solvent for NMBA) three times per week for five weeks. Rats in Groups 2, 3 and 4 were injected s.c. with 0.2 ml of NMBA (0.30 mg/kg body weight) in 20% DMSO:H2O three times per week for five weeks. Three days following the final NMBA injection, rats in Group 3 and 4 were fed AIN-76A diet containing 300 ppm celecoxib + 50 ppm PBIT or 5% BRB. At 35 weeks, all rats were euthanized by CO2 asphyxiation and subjected to gross necropsy. The esophagus of each rat was excised and opened longitudinally. Tumors larger than 0.5 mm in a single dimension were counted, mapped and the esophageal tissues were collected as previously described (Chen & Stoner, 2004).
2.5 HPLC-MS/MS analysis
To characterize the bioactive components in BRB that was used in this animal study, BRB phytochemicals were identified with a combination of high performance liquid chromatography/tandem mass spectrometry (HPLC-MS/MS), accessible standards, UV-vis and reported mass as described previously (Shi et al., 2015). Briefly, BRB sample (100 mg) was first extracted prior to HPLC-MS/MS analysis. For identification of compounds in BRB, HPLC instrumentation (2695 HPLC, Waters, Milford, MA) equipped with column heater set at 35°C, autosampler and PDA detector (Waters Corp, Milford, MA) was used and combined with a Waters Q-Tof Premier (Micromass MS Technologies, Manchester, UK). Phenolics in BRB were quantified using the same LCMS system monitoring at 260, 355 and 500 nm.
2.6 Histopathology of NMBA-induced epithelial lesions in esophagus
One-half of each formalin-fixed esophagus was cut into thirds and embedded in paraffin with the epithelium uppermost. Serial 4-μm sections were cut and mounted on superfrost plus slides (Histotechniques Laboratories, Powell, OH). The slides were stained with H&E for all animals and graded under the Eclipse 80i microscope (Nikon, Northvale, NJ) according to a previously reported standard with minor modifications (Shi et al., 2014). Each viewing field was scanned at 200× magnification and classified into one of the five histological categories: normal epithelium, epithelial hyperplasia, low-grade dysplasia, high-grade dysplasia and papilloma as described previously (Shi et al., 2014). The whole esophagus was examined, counted and the percentage of the categorized cells under each view was averaged.
2.7 Cell treatment
The human esophageal SCC KYSE-270 cells were cultured in RPMI-1640 medium with 10% fetal bovine serum, in a 37°C, 5% CO2 environment. HET-1A cells were obtained from American Type Culture Collection and cultured in LCH medium as described previously (Shi et al., 2014). To assess whether these agents could modulate COX-2, iNOS and associated cell signaling, KYSE-270 cells were treated with C3R, C3G, C3S, P3R, PCA, 4HBA, celecoxib and PBIT alone or in combination. Protein was extracted from the cells after 24 hours and used for Western blot analysis.
2.8 Cell proliferation assay
The proliferation of cells was assessed using the WST-1 kit (Cayman, Ann Arbor, Michigan). KYSE-270 cells were seeded in a 96-wells plate at a density of 1500 cells/well in 100 μl medium for 4 days. Cells were incubated with 10μl WST-1 reagent at 37°C in a 5% CO2 incubator for 2 hours. Cell viability was then detected at 0, 48 and 72 hours by plate reading at 550 nm using Omega Microplate Reader (BMC Labtech., Offenburg, Germany).
2.9 Western blot analysis
Protein was extracted from the frozen esophagus or KYSE 270 cells and quantitated using a DC Protein Assay Kit (Bio-Rad, Hercules, CA) according to the manufacturer’s recommendations before separation. The Western blot was carried on XCellSureLock® Mini-Cell and XCell II™ Blot Module (Invitrogen, Carlsbad, CA). The immunoreactive bands were detected with an Immun-star™ WesterCTm Kit (Bio-Rad Laboratories) using Molecular Imager ChemiDoc XRS (Bio-Rad Laboratories).
2.10 Real-time reverse transcriptase-PCR
Total cellular RNA was isolated from frozen esophagus using RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. High capacity cDNA Reverse transcription Kit (Applied Biosystems, Foster City, CA) was used to reverse-transcribe RNA into cDNA. Fast SYBR Green Master Mix (Applied Biosystems) was chosen for the amplification on a 7900HT Fast Real-Time PCR System (Applied Biosystems) and data were analyzed by Sequence Detection Systems Software version 2.3. Primers for the genes of interest were: COX-2 (Forward 5′-TGTATGCTACCATCTGGCTTCGG-3′, Reverse 5′-GTTTGGAACAGTCGCTCGTCATC-3′), iNOS (Forward 5′-TTGGGTCTTGTTAGCCTAGTC-3′, Reverse 5′-TGTGCAGTCCCAGTGAGGAAC-3′), and GAPDH (Forward 5′-TATTGGGCGCCTGGTCACCA-3′, Reverse 5′-CCACCTTCTTGATGTCATCA-3′).
2.11 Prostaglandin E2 enzyme immunoassay
COX-2 activity in frozen tissues was analyzed using the Prostaglandin E2 (PGE2) EIA Kit (Cayman Chemical Co.) according to the manufacturer’s instructions. Briefly, frozen samples were homogenized in phosphate buffer solution (PBS) (pH 7.5) with 0.02 mol/L EDTA and 5 mg/mL indomethacin. Total protein concentration for supernatant of each tissue homogenate was determined using the DC Protein Assay (Bio-Rad). PGE2 was collected, purified and detected by a PGE2 antibody using a colorimetric method. Absorbance was measured at 450 nm using the Omega Microplate Reader (BMC Labtech., Offenburg, Germany) for the calculation of PGE2 concentration.
2.12 Nitrate/nitrite colorimetric assay
The production of total nitrate and nitrite in frozen tissues was measured using the Nitrate/Nitrite Colorimetric Assay Kit (Cayman Chemical Co.). Frozen tissues were homogenized in PBS, and total protein concentration of the supernatant of each tissue homogenate was detected using the DC Protein Assay (Bio-Rad). Samples were then incubated with nitrate reductase. Griess reagent [sulfanilamide and N-(1-naphthyl)ethylenediamine] was added and the absorbance was measured at 550 nm using the Omega Microplate Reader (BMC Labtech.). The final concentration was the sum of the nitrite plus the reduced nitrate, which reflected the iNOS activity in each sample.
2.13 Statistical analysis
Tumor incidence was analyzed by χ2 test. Tumor multiplicity, expression and/or activity of COX-2, iNOS, p-NFκB, p-p38 MAPK, p-ERK and p-AKT were examined by one-way analysis of variance (ANOVA). For histopathological analysis, the number of field for each tissue type was first normalized to the total number of field in each esophagus, and then a linear mixed effects model was used to analyze the correlations among NMBA-control animals and animals fed experimental diets. Holm’s procedure was used to adjust for multiple comparisons to strongly controlled type I error at 0.05 (Holm, 1979). All statistical analysis was carried out using SAS 9.2 (SAS Institute Inc., NC, USA).
3. Results
3.1 Characterization and quantification of phytochemicals in BRB
In order to identify individual components potentially contributing to the biological effects of dietary BRB powder, we characterized a number of components by HPLC-MS/MS. The HPLC run was displayed at three wavelengths (260, 355 and 500 nm), those at which the various species were quantified (Figure 1). We identified and quantified 20 components classified to four categories: anthocyanins, ellagitannin, ellagic acid and derivatives, and flavonols. Anthocyanins are the major phenolic components in BRB by dry weight (84.2%). The non-anthocyanin phenolics include ellagitannins (11.5%), ellagic acid and derivatives (0.7%) and flavonols (3.6%). C3R is the main anthocyanin in BRB (58.2%). The other anthocyanins identified in BRB include cyaniding-3-xylorutinoside (C3X; 18.2%), C3G (4.9%), C3S; 2.1% and P3R (0.8%). The detailed quantitative results are summarized in Table 1.
Figure 1.
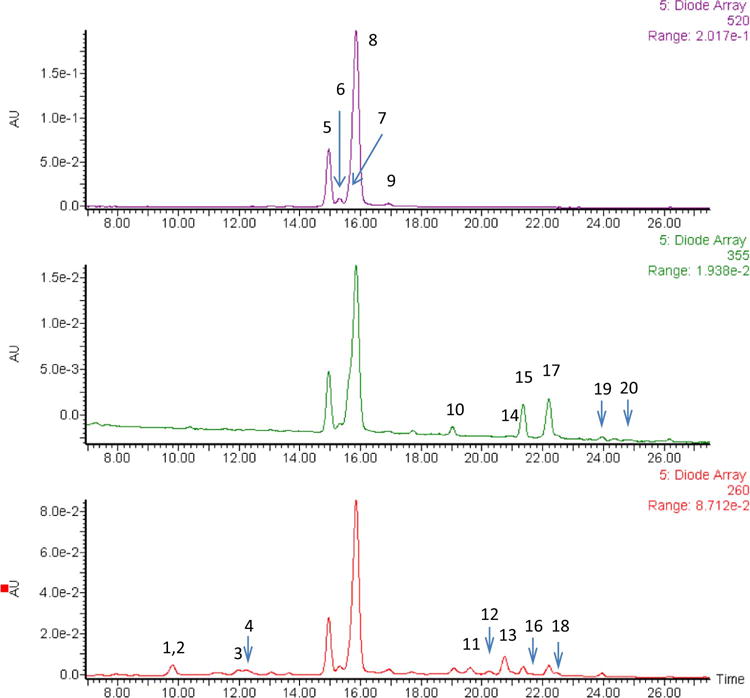
HPLC chromatogram of black raspberries separated on Zorbax SBCN 4.6 × 250 mm, 5 μm column with 1% (v/v) formic acid in water versus 1% (v/v) formic acid in acetonitrile mobile phase gradient. The peak identities were established using accurate mass MS results as well as UV-vis features and literature precedent for black raspberry analysis.
Table 1.
Bioactive phenolic compounds identified in lyophilized black raspberries.
| Peak No. | Retention Time (min) | [M-H]− MSe1a | [M-H]− MSe2b | Peak assignment | mg/100g | % by mg |
|---|---|---|---|---|---|---|
| Anthocyanins | ||||||
| 5 | 15 | 725 | 284/285 | cyanidin xylorutinoside | 916.3 | 18.2 |
| 6 | 15.4 | 579 | 284/285 | cyanidin sambubioside | 103.5 | 2.1 |
| 7 | 15.7 | 447 | 284/285 | cyanidin glucoside | 245.1 | 4.9 |
| 8 | 15.9 | 593 | 284/285 | cyanidin rutinoside | 2924.7 | 58.2 |
| 9 | 17 | 577 | 268/269 | pelargonidin rutinoside | 38.3 | 0.8 |
|
|
||||||
| Total | 84.2 | |||||
|
Ellagitannins | ||||||
| 1 | 9.7 | 933 | 301.0 | ellagitannin 933-1 | 50.5 | 1.0 |
| 2 | 9.8 | 783 | 301.0 | ellagitannin 783-1 | 101.1 | 2.0 |
| 3 | 12 | 933 | 301.0 | ellagitannin 933-2 | 75.8 | 1.5 |
| 4 | 12.2 | 783 | 301.0 | elagitannin 783-2 | 75.8 | 1.5 |
| 11 | 19.8 | 935 | 301.0 | ellagitannin 935-1 | 62.8 | 1.3 |
| 16 | 21.8 | 935 | 301.0 | ellagitannin 935-2 | 7.9 | 0.2 |
| 12 | 20.5 | (1401)2− | 935, 633 | Lambertiannin | 31.3 | 0.6 |
| 13 | 20.9 | (934)2− | 1567 | Sanguiin H6 | 173.2 | 3.4 |
|
|
||||||
| Total | 11.5 | |||||
|
Ellagic acid/ellagic acid derivatives | ||||||
| 14 | 21.1 | 433 | 301.0 | ellagic acid rhamnoside | 5.8 | 0.1 |
| 18 | 22.7 | 301.0 | ellagic acid (EA) | 9.3 | 0.2 | |
| 19 | 24.1 | 447.1 | 315, 300.0 | methyl ellagic acid pentoside | 16.0 | 0.3 |
| 20 | 25 | 479 | 317, 301.0 | myricetin hexoside,EA derivative (coelution) | 3.8 | 0.1 |
|
|
||||||
| Total | 0.7 | |||||
|
Flavonols | ||||||
| 10 | 19.2 | 741 | 301.0 | quercetin xylorutinoside | 24.2 | 0.5 |
| 15 | 21.5 | 609 | 301.0 | rutin (quercetin rutinoside) | 75.4 | 1.5 |
| 17 | 22.4 | 477 | 301.0 | quercetin hexuronide | 82.2 | 1.6 |
|
|
||||||
| Total | 3.6 | |||||
MSe1 = MS scans with low collision energy.
MSe2 = MS scans with high collision energy to induce fragmentation and produce MS/MS spectra.
3.2 BRB exhibits more potent inhibition on tumor formation and the growth of premalignant lesions compared to celecoxib + PBIT
BRB or celecoxib + PBIT treatment significantly reduced tumor incidence and multiplicity compared to animals fed the control diet (Table 2). Moreover, the tumor multiplicity in animals fed BRB was significantly lower than those fed celecoxib + PBIT (P < 0.05). The growth of premalignant lesions in the esophagus was assessed by microscopic examination (Figure 2). The animals fed BRB or celecoxib + PBIT showed a greater percentage of area exhibiting normal and esophageal hyperplasia compared to those fed the control diet (Figure 2B). The BRB- and celecoxib + PBIT- treated animals had fewer areas of low-grade dysplasia, high-grade dysplasia and papilloma compared to NMBA-control animals.
Table 2.
Effects of black raspberries (BRB) and celecoxib + S,S′-1,4-phenylene-bis(1,2-ethanediyl)bis-isothiourea (PBIT) on N-nitrosomethylbenzylamine (NMBA)-induced tumorigenesis in the rat esophagus at 35 weeks.
| Group | NMBA (mg/kg b.w.) | Diet | No. of Rats | Tumor Incidence (%) | Tumor Multiplicity (Mean + S.E.) |
|---|---|---|---|---|---|
| 1 | – | Control | 15 | 0 | 0 |
| 2 | 0.30 | Control | 25 | 100 | 4.73 ± 0.45 |
| 3 | 0.30 | 300 ppm celecoxib + 50 ppm PBIT | 20 | 80a | 2.00 ± 0.24b |
| 4 | 0.30 | 5% BRB | 20 | 80a | 1.44 ± 0.26b,c |
Significantly lower than Group 2 as determined by χ2 test (P < 0.05).
Significantly lower than Group 2 as determined by analysis of variance (P < 0.05).
Significantly lower than Group 3 as determined by analysis of variance (P < 0.05).
Figure 2.
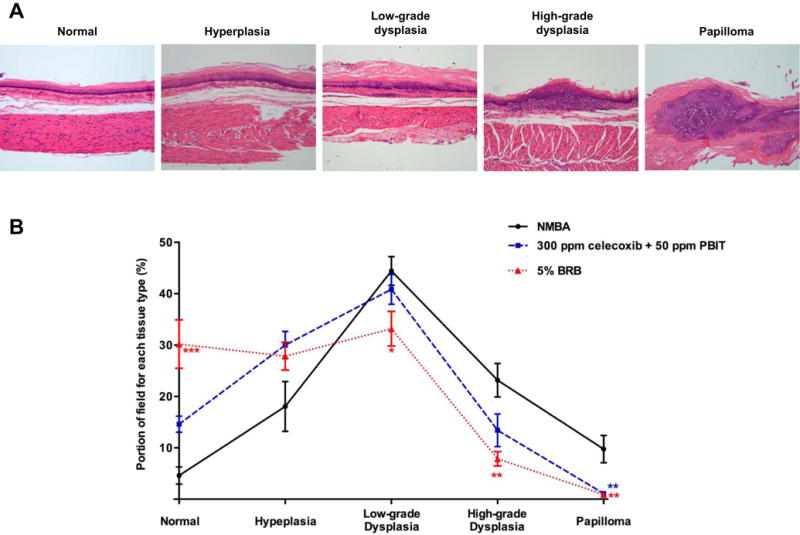
Histopathology of N-nitrosomethylbenzylamine (NMBA)-induced epithelial lesions in rat esophagus. A, H&E sections representative of normal, hyperplasia, low-grade dysplasia, high-grade dysplasia and papilloma (magnification × 200) and B, percentage of each histological type of rat esophageal tissues. * P < 0.05; ** P < 0.01; *** P < 0.001.
3.3 BRB exhibits more potent suppression of COX-2 and iNOS compared to celecoxib + PBIT
BRB and celecoxib + PBIT significantly inhibit expression and activity of COX-2 (Figures 3A and 3B). Moreover, BRB significantly suppresses expression and activity of iNOS (P < 0.001; Figures 3C and 3D).
Figure 3.
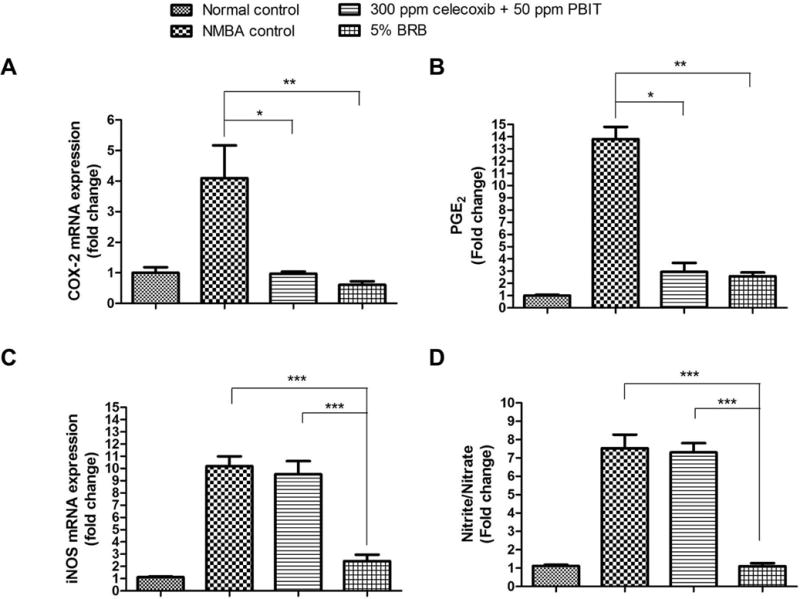
mRNA expression and activity of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) modulated by black raspberries (BRB) or celecoxib + S,S′-1,4-phenylene-bis(1,2-ethanediyl)bis-isothiourea (PBIT). A and C, the mRNA expression of COX-2 and iNOS in rats fed control diet, diet containing celecoxib + PBIT or 5% BRB; B and D, the productions of prostaglandin E2 (PGE2) and nitrite/nitrate in rats fed control diet, diet containing celecoxib + PBIT or 5% BRB. The values are expressed as mean; bars, ± SE; * P < 0.05; ** P < 0.01; *** P < 0.001 versus animals treated with N-nitrosomethylbenzylamine (NMBA) fed control diet.
3.4 BRB decreases protein expression of COX-2 and iNOS and phosphorylation of NFκB, MAPK and AKT
BRB downregulates the protein expression of COX-2 and iNOS (Figure 4, P < 0.01). Moreover, the phosphorylation of nuclear factor kappa B (NFκB), mitogen-activated protein kinase (MAPK; P < 0.05) and AKT (P < 0.01) are suppressed by BRB (Figure 4). Celecoxib + PBIT did not exhibit any effects on these signaling pathways.
Figure 4.
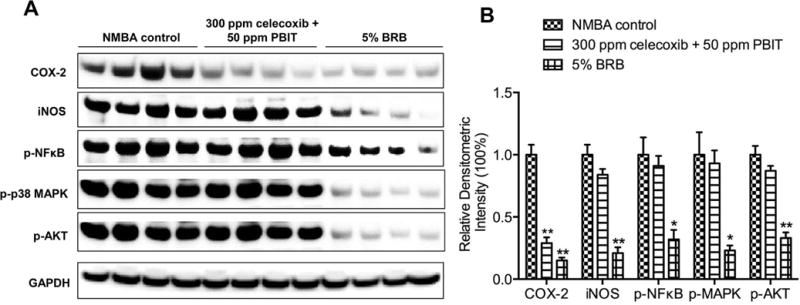
Western blot analysis of cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS) and phosphorylation of nuclear factor kappa B (NFκB), mitogen-activated protein kinases (MAPK) and AKT. Samples were probed with antibodies against COX-2, iNOS, p-NFκB, p-p38 MAPK or p-AKT. The Western blot membranes were stripped and reprobed for GADPH as an internal control to confirm equal loading. A, representative blots from one of three separate experiments and B, relative densitometric intensity. The values are expressed as mean; bars, ± SE; * P < 0.05, ** P < 0.01 versus animals treated with N-nitrosomethylbenzylamine (NMBA) fed control diet.
3.5 BRB anthocyanins, metabolites, celecoxib and PBIT inhibit cell proliferation in vitro
As indicated in Figures 5A to 5C, BRB anthocyanins (C3R, C3G, C3S and P3R), cyanidin and pelargonidin anthocyanin metabolites (PCA and 4HBA), celecoxib and PBIT, inhibit KYSE-270 cell proliferation in a dose-dependent manner. C3R, celecoxib and celecoxib + PBIT significantly inhibit cell proliferation (Figure 5D).
Figure 5.
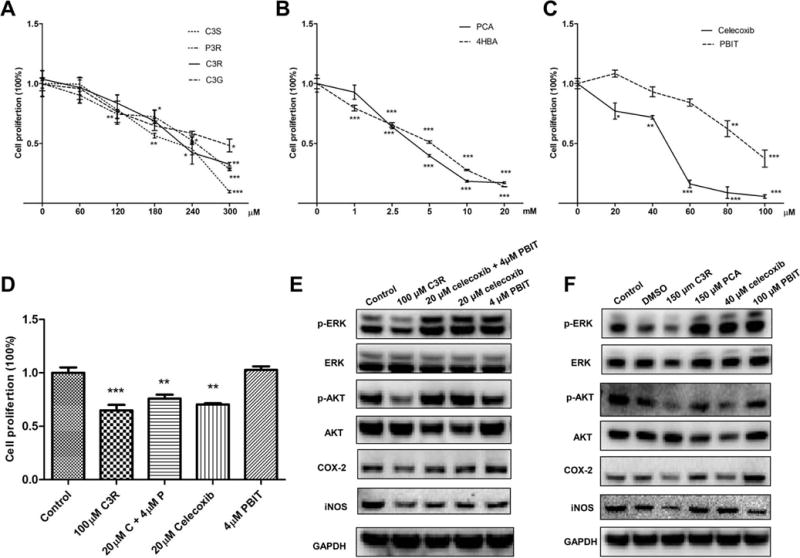
In-vitro mechanistic study of black raspberry anthocyanins, anthocyanin metabolites, celecoxib and S,S′-1,4-phenylene-bis(1,2-ethanediyl)bis-isothiourea (PBIT) in human esophageal squamous cell carcinoma KYSE-270 cells. A to D, WST-1 analysis of the growth of KYSE-270 cells that were treated with cyaniding-3-rutinosides (C3R), cyanidin-3-glucoside (C3G), cyaniding-3-sambubioside (C3S), pelargonidin-3-rutinoside (P3R), protocatechuic acid (PCA), 4-hydroxybenzoic acid (4HBA), celecoxib, PBIT or celecoxib + PBIT; E, Western blot analysis of cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and phosphorylation of extracellular signal-regulated kinases (ERK) and AKT modulated by C3R, celecoxib, PBIT or celecoxib + PBIT; and F, Western blot analysis of COX-2, iNOS, and phosphorylation of ERK and AKT modulated by C3R, celecoxib, PBIT or PCA at dose of IC50 value. Samples were probed with antibodies against COX-2, iNOS, p-ERK or p-AKT. The Western blot membranes were stripped and reprobed for GADPH as an internal control to confirm equal loading. Representative blots were from one of three separate experiments. The values are expressed as mean; bars, ± SE; * P < 0.05, ** P < 0.01, *** P < 0.001.
3.6 BRB anthocyanin suppresses oncogenic signaling in vitro
As indicated in Figure 5E, C3R downregulates COX-2 and iNOS protein, and suppresses the activation of extracellular receptor kinase (ERK) and AKT. The combination of celecoxib + PBIT shows slightly inhibition on COX-2 and iNOS but not on ERK or AKT. At the IC50 value shown in Figure 5F, C3R decreases the protein expression levels of p-ERK, p-AKT, COX-2 and iNOS. We did not observe the similar effects of PCA.
4 Discussion
Our results demonstrate that dietary administration of lyophilized BRB is a more potent cancer preventive strategy than the combination of the selective COX-2 and iNOS inhibitors in NMBA-induced esophageal SCC in rats. We showed that the lyophilized BRB employed in our study consists of numerous polyphenol phytochemicals with potential bioactivities. We examined several of them in esophageal SCC cells and found that they targeted multiple molecular and cellular processes associated with esophageal cancer initiation, promotion and progression. Our results suggest that the multiple bioactives found in lyophilized BRB may act in concert to reduce esophageal carcinogenesis with a potency exceeding that of two purified chemopreventive agents in combination.
The overexpression of COX-2 is associated with cell proliferation, angiogenesis, suppression of apoptosis and immune surveillance (Dubois et al., 1998; Wu, Gu, Ji, Li, & Xu, 2003). Celecoxib, a selective COX-2 inhibitor, has been widely evaluated in numerous preclinical studies and human clinical trials for potential chemopreventive activity for cancers of colon, bladder, head and neck, skin and esophagus (Bertagnolli et al., 2006; Elmets et al., 2010; Limburg et al., 2005; Sabichi et al., 2011; Shin et al., 2013). Unfortunately, the potential for toxicity, particularly cardiac, may limit the use of COX-2 inhibitors for chemoprevention with the exception of the most high risk cohorts (Solomon et al., 2005). A chemoprevention trial of 200 mg celecoxib was not effective for esophageal precancerous and malignant lesions in a study conducted in China, a region with more than 50% esophageal SCC cases in the world (Limburg et al., 2005). The lack of efficacy of celecoxib may be a result of the relatively low dose employed in this trial. The potential of low dose of celecoxib with reduced risk of toxicity combined with second effective drug exhibiting no cross-reactive toxicity may be a strategy to improve efficacy and safety. In this study, we use celecoxib plus PBIT, a selective iNOS inhibitor which has been identified as a chemopreventive agent in our previous investigation (Chen et al., 2004a). Our data indicates that combination of celecoxib and PBIT reduces tumor incidence and tumor multiplicity through the downregulation of COX-2 and iNOS, which supports further exploration of combinational chemoprevention for esophageal SCC.
The mechanisms whereby BRB prevent esophageal SCC are likely multifactorial. Previously, we showed that BRB significantly inhibited COX-2, iNOS, c-Jun and vascular endothelial growth factor, which were correlated with the reduction of PGE2, nitrate/nitrite and microvessel density, respectively, in preclinical animal studies (Chen, Hwang, et al., 2006a; Chen, Rose, et al., 2006b). To investigate the bioactive components in the lyophilized BRB employed in our study, we first conducted HPLC-MS/MS analysis and defined the polyphenol content, indicating that the anthocyanins are the major phenolic components in BRB (84.2% by dry weight). Single anthocyanin compound and anthocyanin extracts from fruits and vegetables have been reported to inhibit cell proliferation and cell signaling in vitro (Fan et al., 2015; Lu et al., 2015; Zikri et al., 2009). Recently, a rodent study showed that anthocyanin-enriched fraction derived from BRB, and anthocyanin metabolite PCA inhibited NMBA-induced esophageal tumorigenesis (Peiffer et al., 2014). C3R is the most abundant anthocyanin in BRB (58.2% by dry weight). In this study, we found that C3R inhibits cell proliferation, downregulates COX-2 and iNOS, and suppresses the activation of ERK and AKT in KYSE-270 cells. Celecoxib (20 μM) + PBIT (4 μM), however, showed little impact on COX-2, iNOS, ERK and AKT in KYSE-270 cells. We observed a reduction on protein expression of COX-2 and iNOS by celecoxib and PBIT when its dose was increased to IC50 value. Its impact on ERK and AKT, however, was still minimal.
MAPK is activated by a range of stimuli and mediates a number of physiological processes, and its activation is also noted during carcinogenesis (Mohamed et al., 2002). NFκB, a pleiotropic transcription factor, is present in almost all cell types and involved in inflammation, cell differentiation and tumorigenesis (Hayden & Ghosh, 2008). Alterations in the expression of MAPK and NFκB are observed during esophageal SCC tumor promotion and progression (Chen et al., 2012; Zhou et al., 2013). AKT signaling plays important role in multiple cellular processes including cell proliferation, survival, motility and angiogenesis. AKT and ERK signaling also promote cancer progression and metastasis. Thus, dual inhibition of these pathways is a logical strategy for cancer chemoprevention (Ye, Cai, Zheng, Evers, & She, 2014). Induction of cell survival by AKT is mediated through NFκB signaling (Madrid, Mayo, Reuther, & Baldwin, 2001). Activation of the AKT and ERK pathways acts in a synergistic manner to promote the mechanistic target of rapamycin signaling, which controls NFκB activity (Dan et al., 2008). Although we found that BRB powder (whole food) inhibits activation of NFκB in vivo, we did not observe a similar effect of BRB anthocyanin C3R and anthocyanin metabolite PCA in KYSE-270 SCC cells in vitro. The bioactivity of BRB for the inhibition of esophageal carcinogenesis may due to the co-action of multiple BRB phytochemicals and their metabolic intermediates. In Figure 5, we depict potential interactive mechanisms of BRB, C3R, celecoxib and PBIT against NMBA-induced tumor development in rat esophagus.
To our knowledge, this is the first study to conduct parallel investigation of BRB and combination of celecoxib and PBIT in esophageal cancer prevention. We observed a superior preventive effect of BRB over the two-drug combination. Our data demonstrates that BRB, with a diverse array of bioactive phytochemicals, is predicted to suppress carcinogenesis through multiple and potentially interactive mechanisms of action. Our study provides leads for further translational studies of BRB food products either alone or potentially in combination with chemopreventive agents for esophageal cancer prevention in humans.
Figure 6.
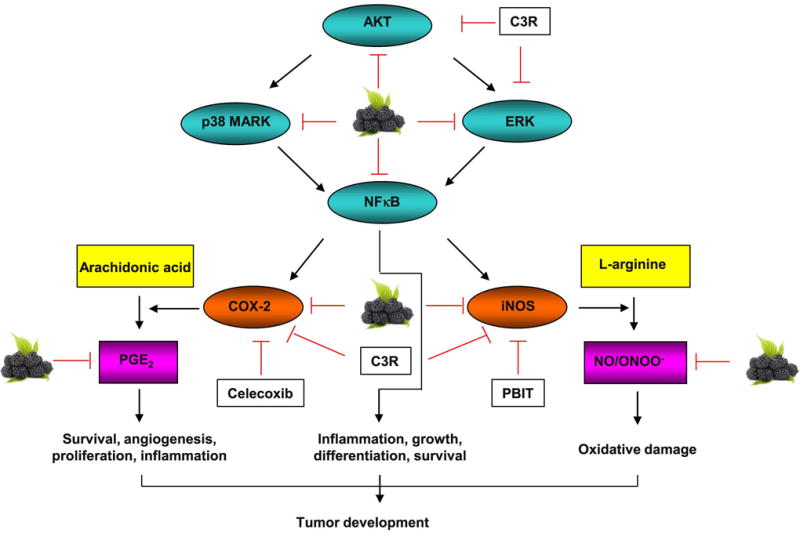
Possible interactive mechanisms of inhibition of N-nitrosomethylbenzylamine (NMBA)-induced esophageal squamous cell carcinoma in rats by black raspberries (BRB), cyaniding-3-rutinosides (C3R), celecoxib and S,S′-1,4-phenylene-bis(1,2-ethanediyl)bis-isothiourea (PBIT).
HIGHLIGHTS.
Black raspberries are more effective than drug combination in cancer prevention.
Black raspberries could inhibit activation of NFκB, MAPK and AKT.
Black raspberry anthocyanin could suppress oncogenic signaling in vitro.
Acknowledgments
This work was supported by National Cancer Institute Grant (R01 CA131073). The authors thank The Ohio State University Comprehensive Cancer Center Nutrient & Phyotochemical Analytics shared resource.
Abbreviations
- SCC
squamous cell carcinoma
- iNOS
inducible nitric oxide synthase
- COX-2
cyclooxygenase-2
- PBIT
S,S′-1,4-phenylene-bis(1,2-ethanediyl)bis-isothiourea
- BRB
black raspberries
- NMBA
N-nitrosomethylbenzylamine
- C3R
cyaniding-3-rutinosides
- C3G
cyanidin-3-glucoside
- C3S
cyaniding-3-sambubioside
- P3R
pelargonidin-3-rutinoside
- PCA
protocatechuic acid
- 4HBA
4-hydroxybenzoic acid
- PGE2
prostaglandin E2
- NFκB
nuclear factor kappa B
- MAPK
mitogen-activated protein kinases
- ERK
extracellular signal-regulated kinases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
No potential conflicts of interest were disclosed.
Chemical compounds:
Chemical compounds studied in this article: NMBA (PubChem CID: 13643); PBIT (PubChem CID: 935415); Celecoxib (PubChen CID: 2662).
References
- Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, Hawk ET. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- Carlton PS, Gopalakrishnan R, Gupta A, Liston BW, Habib S, Morse MA, Stoner GD. Piroxicam is an ineffective inhibitor of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus. Cancer Res. 2002;62(15):4376–4382. [PubMed] [Google Scholar]
- Chen T, Nines RG, Peschke SM, Kresty LA, Stoner GD. Chemopreventive effects of a selective nitric oxide synthase inhibitor on carcinogen-induced rat esophageal tumorigenesis. Cancer Research. 2004a;64:3714–3717. doi: 10.1158/0008-5472.CAN-04-0302. [DOI] [PubMed] [Google Scholar]
- Chen T, Stoner GD. Inducible nitric oxide synthase expression in N-nitrosomethylbenzylamine (NMBA)-induced rat esophageal tumorigenesis. Molecular Carcinogenesis. 2004b;40:232–240. doi: 10.1002/mc.20035. [DOI] [PubMed] [Google Scholar]
- Chen T, Hwang H, Rose ME, Nines RG, Stoner GD. Chemopreventive properties of black raspberries in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis: down-regulation of cyclooxygenase-2, inducible nitric oxide synthase, and c-Jun. Cancer Research. 2006a;66:2853–2859. doi: 10.1158/0008-5472.CAN-05-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Rose ME, Hwang H, Nines RG, Stoner GD. Black raspberries inhibit N-nitrosomethylbenzylamine (NMBA)-induced angiogenesis in rat esophagus parallel to the suppression of COX-2 and iNOS. Carcinogenesis. 2006b;27:2301–2307. doi: 10.1093/carcin/bgl109. [DOI] [PubMed] [Google Scholar]
- Chen T, Yan F, Qian J, Guo M, Zhang H, Tang X, Chen F, Stoner GD, Wang X. Randomized phase II trial of lyophilized strawberries in patients with dysplastic precancerous lesions of the esophagus. Cancer Prevention Research (Philadelphia, Pa) 2012;5:41–50. doi: 10.1158/1940-6207.CAPR-11-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-kappa B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22(11):1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12(12):1063–1073. [PubMed] [Google Scholar]
- Elmets CA, Viner JL, Pentland AP, Cantrell W, Lin HY, Bailey H, Gordon GB. Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst. 2010;102(24):1835–1844. doi: 10.1093/jnci/djq442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan MJ, Wang IC, Hsiao YT, Lin HY, Tang NY, Hung TC, Chung JG. Anthocyanins from black rice (Oryza sativa L.) demonstrate antimetastatic properties by reducing MMPs and NF-kappaB expressions in human oral cancer CAL 27 cells. Nutr Cancer. 2015;67(2):327–338. doi: 10.1080/01635581.2015.990576. [DOI] [PubMed] [Google Scholar]
- Freedman ND, Park Y, Subar AF, Hollenbeck AR, Leitzmann MF, Schatzkin A, Abnet CC. Fruit and vegetable intake and esophageal cancer in a large prospective cohort study. Int J Cancer. 2007;121(12):2753–2760. doi: 10.1002/ijc.22993. [DOI] [PubMed] [Google Scholar]
- Gao Y, Hu N, Han XY, Ding T, Giffen C, Goldstein AM, Taylor PR. Risk factors for esophageal and gastric cancers in Shanxi Province, China: a case-control study. Cancer Epidemiol. 2011;35(6):e91–99. doi: 10.1016/j.canep.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics. 1979;6(2):65–70. [Google Scholar]
- Layke JC, Lopez PP. Esophageal cancer: a review and update. Am Fam Physician. 2006;73(12):2187–2194. [PubMed] [Google Scholar]
- Limburg PJ, Wei W, Ahnen DJ, Qiao Y, Hawk ET, Wang G, Dawsey SM. Randomized, placebo-controlled, esophageal squamous cell cancer chemoprevention trial of selenomethionine and celecoxib. Gastroenterology. 2005;129(3):863–873. doi: 10.1053/j.gastro.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Lu JN, Lee WS, Nagappan A, Chang SH, Choi YH, Kim HJ, Hong SC. Anthocyanins From the Fruit of Vitis coignetiae Pulliat Potentiate the Cisplatin Activity by Inhibiting PI3K/Akt Signaling Pathways in Human Gastric Cancer Cells. J Cancer Prev. 2015;20(1):50–56. doi: 10.15430/JCP.2015.20.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276(22):18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- Mohamed AA, Jupp OJ, Anderson HM, Littlejohn AF, Vandenabeele P, MacEwan DJ. Tumour necrosis factor-induced activation of c-Jun N- terminal kinase is sensitive to caspase-dependent modulation while activation of mitogen-activated protein kinase (MAPK) or p38 MAPK is not. Biochem J. 2002;366(Pt 1):145–155. doi: 10.1042/BJ20020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer DS, Zimmerman NP, Wang LS, Ransom BW, Carmella SG, Kuo CT, Stoner GD. Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prev Res (Phila) 2014;7(6):574–584. doi: 10.1158/1940-6207.CAPR-14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabichi AL, Lee JJ, Grossman HB, Liu S, Richmond E, Czerniak BA, Lerner SP. A randomized controlled trial of celecoxib to prevent recurrence of nonmuscle-invasive bladder cancer. Cancer Prev Res (Phila) 2011;4(10):1580–1589. doi: 10.1158/1940-6207.CAPR-11-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N, Clinton SK, Liu Z, Wang Y, Riedl KM, Schwartz SJ, Chen T. Strawberry phytochemicals inhibit azoxymethane/dextran sodium sulfate-induced colorectal carcinogenesis in Crj: CD-1 mice. Nutrients. 2015;7(3):1696–1715. doi: 10.3390/nu7031696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N, Jin F, Zhang X, Clinton SK, Pan Z, Chen T. Overexpression of human beta-defensin 2 promotes growth and invasion during esophageal carcinogenesis. Oncotarget. 2014;5(22):11333–11344. doi: 10.18632/oncotarget.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Zhang H, Saba NF, Chen AY, Nannapaneni S, Amin AR, Chen ZG. Chemoprevention of head and neck cancer by simultaneous blocking of epidermal growth factor receptor and cyclooxygenase-2 signaling pathways: preclinical and clinical studies. Clin Cancer Res. 2013;19(5):1244–1256. doi: 10.1158/1078-0432.CCR-12-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Bertagnolli M. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352(11):1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- Souza RF. Molecular and biologic basis of upper gastrointestinal malignancy–esophageal carcinoma. Surg Oncol Clin N Am. 2002;11(2):257–272, viii. doi: 10.1016/s1055-3207(02)00003-0. [DOI] [PubMed] [Google Scholar]
- Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001;22:1737–1746. doi: 10.1093/carcin/22.11.1737. [DOI] [PubMed] [Google Scholar]
- Stoner GD, Qin H, Chen T, Carlton PS, Rose ME, Aziz RM, Dixit R. The effects of L-748706, a selective cyclooxygenase-2 inhibitor, on N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis. Carcinogenesis. 2005;26:1590–1595. doi: 10.1093/carcin/bgi111. [DOI] [PubMed] [Google Scholar]
- Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prevention Research (Philadelphia, Pa) 2009;2:187–194. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers KF, Sabatino SA, Stewart SL. Trends in esophageal cancer incidence by histology, United States, 1998–2003. Int J Cancer. 2008;123(6):1422–1428. doi: 10.1002/ijc.23691. [DOI] [PubMed] [Google Scholar]
- Wang GQ, Abnet CC, Shen Q, Lewin KJ, Sun XD, Roth MJ, Dawsey SM. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54(2):187–192. doi: 10.1136/gut.2004.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AW, Gu J, Ji JF, Li ZF, Xu GW. Role of COX-2 in carcinogenesis of colorectal cancer and its relationship with tumor biological characteristics and patients’ prognosis. World J Gastroenterol. 2003;9(9):1990–1994. doi: 10.3748/wjg.v9.i9.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Cai W, Zheng Y, Evers BM, She QB. ERK and AKT signaling cooperate to translationally regulate survivin expression for metastatic progression of colorectal cancer. Oncogene. 2014;33(14):1828–1839. doi: 10.1038/onc.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Ye W, Shao Q, Qi Y, Zhang M, Liang J. Prognostic significance of XIAP and NF-kappaB expression in esophageal carcinoma with postoperative radiotherapy. World J Surg Oncol. 2013;11:288. doi: 10.1186/1477-7819-11-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikri NN, Riedl KM, Wang LS, Lechner J, Schwartz SJ, Stoner GD. Black raspberry components inhibit proliferation, induce apoptosis, and modulate gene expression in rat esophageal epithelial cells. Nutr Cancer. 2009;61(6):816–826. doi: 10.1080/01635580903285148. [DOI] [PMC free article] [PubMed] [Google Scholar]


