Abstract
Objective
The heterogeneous human frontal pole has been identified as a node in the dysfunctional network of major depressive disorder. The contribution of the medial (socio-affective) versus lateral (cognitive) frontal pole to major depression pathogenesis is currently unclear. The present study performs morphometric comparison of the microstructurally informed subdivisions of human frontal pole between depressed patients and controls using both uni- and multivariate statistics.
Methods
Multi-site voxel- and region-based morphometric MRI analysis of 73 depressed patients and 73 matched controls without psychiatric history. Frontal pole volume was first compared between depressed patients and controls by subdivision-wise classical morphometric analysis. In a second approach, frontal pole volume was compared by subdivision-naive multivariate searchlight analysis based on support vector machines.
Results
Subdivision-wise morphometric analysis found a significantly smaller medial frontal pole in depressed patients with a negative correlation of disease severity and duration. Histologically uninformed multivariate voxel-wise statistics provided converging evidence for structural aberrations specific to the microstructurally defined medial area of the frontal pole in depressed patients.
Conclusions
Across disparate methods, we demonstrated subregion specificity in the left medial frontal pole volume in depressed patients. Indeed, the frontal pole was shown to structurally and functionally connect to other key regions in major depression pathology like the anterior cingulate cortex and the amygdala via the uncinate fasciculus. Present and previous findings consolidate the left medial portion of the frontal pole as particularly altered in major depression.
Keywords: voxel-based morphometry, human frontal pole, major depression disorder, cytoarchitecture, JuBrain, machine learning, support vector machines
Introduction
Major depressive disorder is characterized by affective, cognitive and vegetative symptoms. These interfere with a person’s ability to work, sleep, eat and enjoy once pleasurable activities. Major depressive disorder thus critically impairs tasks in every-day life. Given its high prevalence, it also represents one of the biggest health challenges and will probably be one of the worldwide leading causes of disability and burden by 20201.
It has frequently been proposed that the human frontal pole, coinciding with Brodmann Area (BA) 102, may subserve an integratory role for higher-order social, emotional and cognitive processes3, 4. The frontal pole seems to contribute to behavioral disturbances in depression related to introspective evaluation5, self-relevant reflection6 and rumination occurrence7. Corroborating the functional considerations, which implicate a close relationship to depression-related behavioral disturbances, there are reports about structural alterations of the human frontal pole in major depressive disorder. Consistently, variations in the neurophysiological metabolism of receptors with significance to depression, particularly pronounced in the serotoninergic system, have already been described for the human frontal pole8–10. There is moreover evidence for morphological alterations in depression, such as reduced gray matter volume, within the prefrontal cortex, putatively including area 1011–13. A recent deep brain stimulation study with treatment-resistant depressed patients showed a response to the treatment only in those patients in which the activation volumes affected the uncinate fasciculus14. Since it is the frontal pole which represents the termination of this fiber bundle, possible structural changes within that part of the cortex may represent an important pathophysiological aspect of major depressive disorder.
To date, however, depression research has dedicated much attention to the anterior cingulate and orbitofrontal cortices (i.e., regions neighboring the frontal pole). Given broad consensus within the field that the pathology of depression relates to a larger dysfunctional network, this calls for extension to other potentially affected nodes, i.e., regions with evidence for affection, such as the frontal pole.
Importantly, the functional organization of the frontal pole and surrounding regions still remains under-researched. The nomenclature in this part of the brain is inconsistent and vague labels such as “anterior prefrontal cortex” are frequently applied in a non-quantitative fashion. Recent histological examination demonstrated the presence of two distinct cytoarchitectonic areas on the human frontal pole: Area frontopolaris 1 (Fp1, lateral) and Area frontopolaris 2 (Fp2, medial)15. These are clearly distinct in function and connectivity3, 4, 15. The medial frontal pole (area Fp2) plays a central role in limbic processes such as emotional and social cognition16–18. The lateral frontal pole (area Fp1), in turn, is related to cognitive functions such as working memory and perception3, 4, 15. This raises the questions whether the frontal pole is altered in major depressive disorder as well as whether any of these two structurally and functionally distinct regions are differentially affected by this disorder.
To test this, we combined volumetric analysis of structural magnetic resonance imaging (MRI) data with cytoarchitectonic maps of the human frontal pole and statistical learning algorithms. In a multi-center setting, we included 73 depressed patients and 73 well-matched healthy controls. In a univariate approach, region-based volumetric analysis was based on the microstructural maps of Fp1 and Fp2 in standard space15 as a reliable neuroanatomical prior. In a multivariate statistical approach, we then tested whether, and where, a searchlight analysis covering the combined extent of areas Fp1 and Fp2 can locate morphological patterns that allow for classification of a previously unseen subject as patient or control. The combination of these approaches thus provides a comprehensive assessment of the involvement of the cytoarchitectonically defined areas Fp1 and Fp2 in major depression disorder.
Methods
Our volume of interest was derived from a recent cytoarchitectonic mapping of the human frontal pole in ten human post-mortem brains15, which yielded the areas Fp1 and Fp2 (Fig 1) as new parts of the JuBrain atlas19, 20.
Figure 1. Maps of area Fp1 and area Fp2.
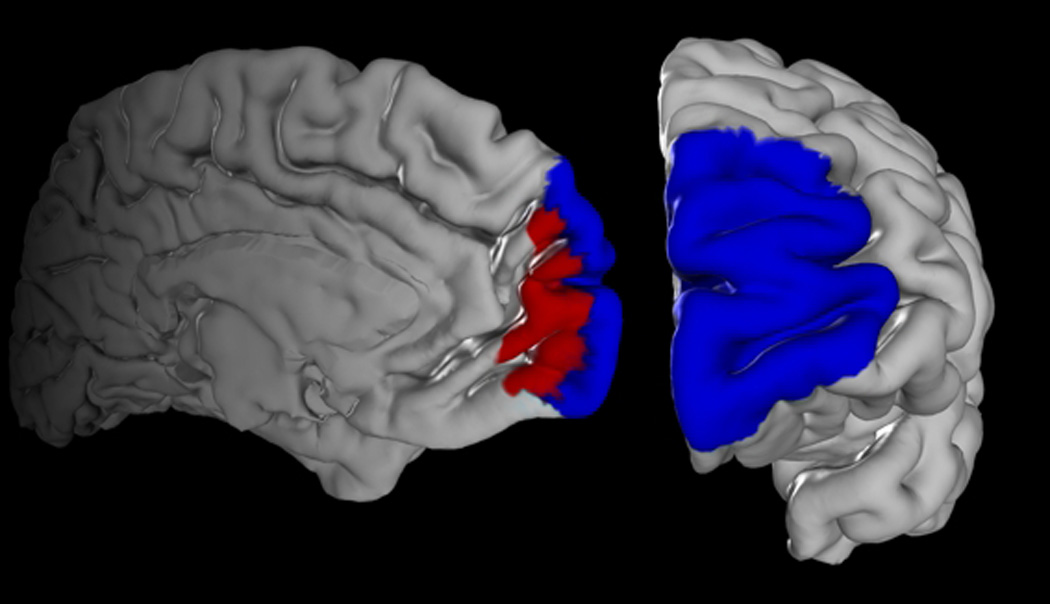
Maximum probability maps of the human frontal pole were used as seed regions for microanatomically informed voxel-based morphometry. (Fp1: blue; Fp2: red)
Participants
Patients with major depression were recruited from the university clinics in Aachen, Göttingen and Munich, Germany. For all patients, diagnosis was based on clinical examination by the attending psychiatrist in accordance with the International Classification of Diseases (ICD-10) supplemented by the Beck Depression Inventory (BDI-II) as a self-reported measure of symptom severity. Control participants were recruited in the respective local communities. All subjects gave written consent to participate in the study as approved by the local ethics committees. Data pooling and joint analysis was approved by the ethics committee of the Heinrich-Heine University of Düsseldorf. In total, 73 patients in a current depressive episode and 73 healthy controls were included in the present study (Table 1). In the patient group, current substance abuse was no exclusion criteria, but was specifically recorded. 16 patients were classified as suffering from substance abuse disorders (3 patients F10.1, 2 patients F12.1, 11 patients F17.1 according to the ICD-10). Importantly, patients and controls were not only matched at the (overall) group level but also on a site level. That is, each site not only investigated an equal number of patients and controls but, also, within each site patients and controls were matched with respect to age and gender to avoid potential confounding influences (Table 1). Among the patients, 44 had previous episodes and were hence classified as suffering from “recurrent depressive disorder” (F33 according to the ICD-10). For 4 patients, there was no information available about their current medication. Two patients received no medication at all, and one received lithium. The remaining patients received their regular medication as prescribed by the attending psychiatrist. While most were prescribed SSRIs or SSNRIs, there was a considerable variability in the administered drugs and combination therapies were frequently used. We thus did not perform sub-analyses between patients with different medication regime, but rather regarded differences in medication and their potential effects on brain structure as a non-systematic source of variance in the patient group. We note that variability introduced by heterogeneous medication should make it harder for the statistical analysis to identify consistent differences between patients and controls (due to increased variance in the patient group). Therefore, our analysis represents a more conservative approach to identifying structural aberrations in depression than a setting of homogeneous medication. In addition, this more natural setting in combination with the multi-site approach should better reflect the overall population of patients with major depression disorder.
Table 1.
Dataset of T1-weighted MRI scans from depressed patients and age- and gender-matched controls.
| Controls (N=73) |
Patients (N=73) |
|||
|---|---|---|---|---|
| Gender [f/m]: | 40/33 | 40/33 | ||
| Mean | SD | Mean | SD | |
| Age: | 38.5 | 12.5 | 41.7 | 12.9 |
| Education [Years]: | 14.8 | 2.9 | 14.6 | 3.2 |
| Beck Depression Inventory : | 1.1 | 1.9 | 21.9 | 8.2 |
| Clinical data: | Mean | SD | ||
| Onset [Age]: | 26.6 | 10.7 | ||
| Duration [Years]: | 13.6 | 11.1 | ||
| Episodes: | 5.0 | 3.1 | ||
All healthy controls were free of any current or past neurological or psychiatric disorder or substance abuse disorder and psychotropic medication.
Preprocessing: Voxel-based morphometry
Whole-brain T1-weighted structural MRI scans were acquired for all participants on 3.0T scanners at the respective sites. Similar acquisition protocols and a common voxel resolution of 1×1×1mm3 were used at all sites (Table 2). Subsequently, all scans were jointly processed with the same pipeline using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html). Here we used the standard setting for all steps including bias-field correction, segmentation into gray and white matter and cerebrospinal fluid, adjustment for partial volume effects, spatial normalization into Montreal Neurological Institute (MNI) space and non-linear modulation21. As we used non-linearly modulated gray matter maps for further analysis, the voxel values of the ensuing gray matter volume maps reflect the local volume of gray matter, i.e., the absolute amount of tissue corrected for individual brain sizes. Consequently, we did not include total brain volume as an additional covariate in any of the subsequent analyses, since inter-individual differences in brain volume were directly accounted for in the preprocessing.
Table 2.
MRI acquisition parameters for used TFE/MPRAGE sequences per site
| Site | number of subjects (patients/controls) |
echo time [ms] |
repetition time [ms] |
flip angle |
Voxel[mm3] |
|---|---|---|---|---|---|
| University of Aachen, Siemens TrioTim 3T scanner |
21/21 | 2.52 | 1900 | 9° | 1×1×1 |
| University of Goettingen, Siemens TrioTim 3T scanner |
27/27 | 3.26 | 2250 | 9° | 1×1×1 |
| University of Munich, Achieva, Philips 3T scanner |
25/25 | 4 | 3000 | 8° | 1×1×1 |
Univariate morphometric comparison
For this analysis, the four cytoarchitectonically defined volumes of interest in standard space were used as individual regions of interest. First, for each subject voxel-wise gray matter volume values were summed up across all voxels of each of the four histological masks (representing Fp1 and Fp2, separately for each hemisphere). This yielded the volume for each of these areas in every subject (corrected for total brain size, as we used non-linearly modulated images). No additional smoothing was performed as we aimed at integrating over histologically defined areas rather than to replace each voxel with a weighted mean of its surrounding voxels. Prior to statistical comparison, we removed variance in the data attributable to between-site differences. This was done by first regressing out the variance from the confounding factor ‘site’ and using the ensuing residuals for all subsequent analyses. That is, in addition to a close matching between patients and controls within each site, effects that could be explained by the measurement site were removed from the data. Volume differences between the two groups were then assessed for each of the four volumes of interest by a non-parametric label-exchange procedure using Monte-Carlo simulations (p<0.05, False Discovery Rate (FDR) corrected for multiple comparisons). In a subsequent step, we tested for an association between the areas' volumes and the severity of symptoms in the patient group as reflected in the Beck depression inventory summary score, the number of previous episodes and disease duration using rank correlations.
Multivariate morphometric comparison
Besides the “classical” univariate analysis, the gray matter volume maps of the entire frontal pole (i.e., Fp1 and Fp2) were fed into an multivariate pattern classification analysis22 using linear support vector machines23. Prior to this classification, we preprocessed the gray matter volume map by scaling and mean-centering (across the entire sample) for each voxel. We then used support vector machines in a four-fold cross-validation framework. In particular, a linear statistical function was first learned on the training data and subsequently applied to previously unseen test data. Note that structural data from each participant has thus been in the test data only once. This framework is the gold standard to obtain an unbiased estimate of a trained classifier to generalize beyond the subject sample at hand24.
To apply this procedure in regional neighborhoods, we performed a searchlight analysis25. For each voxel in the frontal pole mask provided by the combined maps of Fp1 and Fp2, we first collected the (pre-processed) gray matter volume values of the immediate neighborhood (radius 2 to 7 voxels). In each such searchlight, we shrunk the voxel pool to the most varying 33% using an F-test26. We then trained a linear support vector machine per voxel in one part of the voxel-based morphometry images (training set) and subsequently determined the prediction accuracy in the remaining images (test set). Finally, the mean classification accuracy across all permutation folds was mapped to the center of the sphere. We moved the searchlight through the volumes of interest until each seed voxel had once been the center of voxel of the searchlight. This yielded a voxel-wise classification accuracy map for the entire frontal pole. Regarding its software implementation, the multivariate searchlight analysis has been performed using nilearn - a scientific computing Python package for machine-learning in neuroimaging datasets. It is freely accessible online (http://github.com/nilearn/nilearn).
This type of map of the frontal pole (defined by the combined maps for Fp1 and Fp2) thus identifies the locations where the morphological patterns are most discriminative between depressed patients and controls. The resulting accuracy maps were transformed into p-value maps using the binomial test and corrected for multiple comparisons using Bonferroni's method. As a post-hoc analysis, we then tested the hypothesis that the significantly discriminative voxels correspond to distinct cytoarchitectonic areas Fp1 and Fp2 by anatomical assignment using the SPM Anatomy toolbox27.
Results
Univariate morphometric comparison
We first tested for gray matter volume differences between patients and controls within individual left and right Fp1 and Fp2 (i.e., four regions). These analyses revealed a significant decrease of volume only for left area Fp2 in depressed patients (Fig. 2, p<0.05; FDR corrected). The other three regions did not show any significant gray matter volume difference between the two groups.
Figure 2. Region based gray matter volume analysis in major depressive disorder using cytoarchitectonic maps of the human frontal pole.
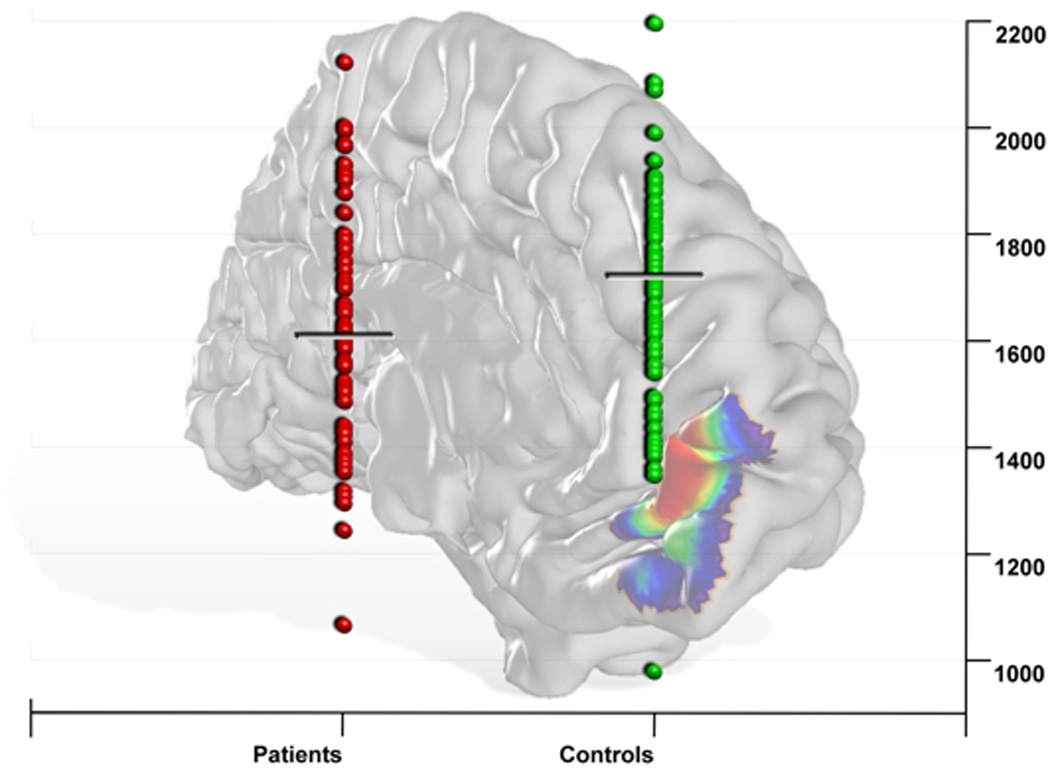
Significant decrease of volume only for the left medial part of the frontal pole (left area Fp2) in depressed patients compared to controls (p<0.05; FDR corrected).
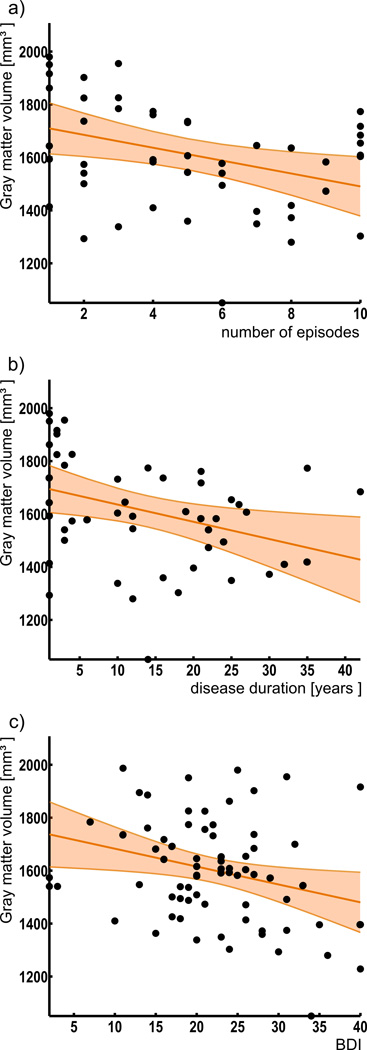
In the next step, we correlated the individual gray matter volume of left Fp2 with disease severity and chronicity in the patient group. This analysis revealed a significant negative correlation between left Fp2 gray matter volume and the Beck Depression Inventory scores (r=−0.26, p<0.05). Furthermore, the number of previous episodes (r=−0.34, p<0.05) as well as disease duration (r=−0.35, p<0.05) correlated negatively with gray matter volume of left Fp2 in the patient group (Fig. 3). In an exploratory analysis, we additionally assessed potential correlations between the volumes of right Fp2 and bilateral Fp1. i.e., those areas that did not show a significant main effect of diagnosis, with the respective clinical information. None of these regions revealed any significant correlation; not even at an uncorrected level. In summary, we found that left Fp2 was not only significantly smaller in major depressive disorder, but that this atrophy was also more pronounced in more severely or chronically affected patients.
Figure 3.
Relation between areal volumes and clinical parameters
Significant negative correlation between the volume of left Fp2 and Beck Depression Inventory (BDI) scores (r=−0.26), number of previous episodes (r=−0.34) and disease duration (r=−0.35) in depression.
Multivariate morphometric comparison
After (individual) univariate analysis of gray matter volume differences between patients and controls for each of the histologically defined frontal pole areas, we performed multivariate searchlight analysis for each voxel of the entire frontal pole (that is, naive to either Fp1 or Fp2). This structural pattern recognition using support vector machine based searchlight analysis congruently revealed the left medial aspects of the human frontal pole to carry the most discriminative morphological features. This was indicated by an extended discriminative cluster with disease prediction success between 65% and 73%, depending on the chosen radius. This accuracy clearly exceeded chance level even when correcting for multiple testing across all voxels in the frontal pole (Fig. 4). Of note, the location of the cluster conveying the most diagnostic morphological properties was identical throughout the searchlight analyses with a radius of 2 to 7 voxels, attesting to the robustness of these findings. Across analyses the medial portion of the left prefrontal cortex demonstrated regional clustering of high prediction accuracies. No other part of the search-region exhibited similar prediction success in accuracy score or regional extent. In a post-hoc cytoarchitectonic assessment, the SPM Anatomy toolbox assigned this cluster to the left Fp2. This demonstrates that local volumes in the left medial frontal pole allow a classification of previously unseen subjects as patients and controls.
Figure 4.
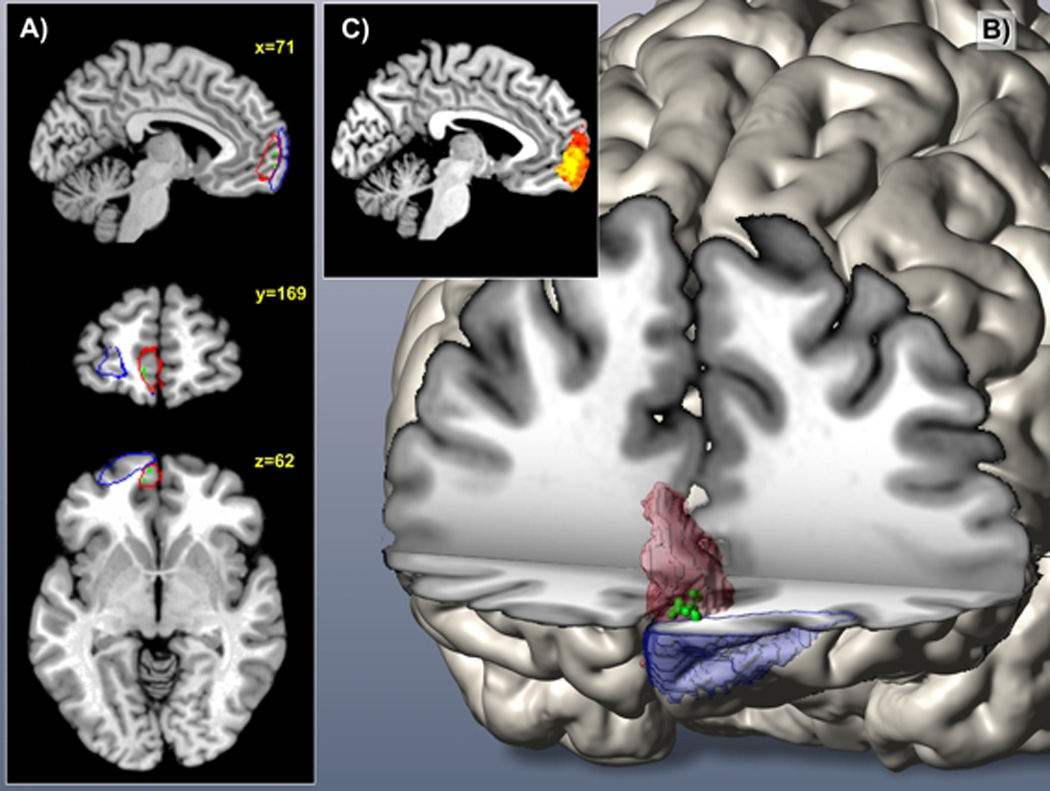
A, B) 3D reconstruction of area Fp1 and area Fp2 in MNI reference space
A, B) Green voxels located mainly in area Fp2 represent Bonferroni corrected voxels (p<0.05) conveying the most diagnostic morphological properties. (Fp1: blue; Fp2: red) C) The miniature sagittal slice depicts color-coded regional difference in prediction success (heat map range: 50 to 65% accuracy)
Discussion
Recent cytoarchitectonic work has shown that the human frontal pole consists of two distinct areas15. These feature different functional connectivity and functions, with area Fp2 associated to emotional and social cognition while Fp1 is associated with working memory and perception3, 4, 15. We here investigated structural differences of the frontal pole between 73 patients and a matched healthy control group. We used two different approaches to assess whether, and where, the frontal pole is structurally altered in depressed patients. We found converging evidence pointing to a specific morphological alteration of only left medial region Fp2 in major depressive disorder. Notably, decreased gray matter volume in this area was not only related to clinical depression severity and chronicity, but also allowed classification of individual subjects as depressed or healthy at a statistically significant level.
Structural alterations of the human frontal pole in major depressive disorder
Our results relate well to previously described neurobiological alterations in the human frontal pole observed in depressed patients, including differences in serotonin-receptor densities9, 10. Other histological studies demonstrated a marked decrease in the density of glial cells, an enlargement of glial cell sizes, as well as reductions in the neuronal density, predominantly in layer III, of the prefrontal cortex in depressed patients28.
Additionally, significant gray matter volume reductions in depressed patients were described for the human prefrontal cortex including the frontal pole12, 13. Those former results match well with our results, nevertheless reports about bilateral volume reductions might be caused by the usage of different respectively non-probabilistic atlases. This might lead to a slightly different assignment of region specific volume alterations. Encapsulating areas Fp1 and Fp2 in such broader region12, 29, 30, however, has raised the question, whether and where the frontal pole is affected in major depressive disorder. Since the anterior cingulate cortex is described as a key region in the pathophysiologic network of major depressive disorder31, most of those results were interpreted in the context of being mainly associated with the cingulate cortex12. Including cytoarchitectonically specific maps of areas Fp1 and Fp2 in the current analytic framework, however, provided a unique opportunity for assessing the spatial arrangement and the degree of volume loss in the frontal pole and thus a much more specific assessment of regional atrophy than possible by macroanatomical definitions.
Region specific contribution of areas Fp1 and Fp2 to the pathophysiology of major depressive disorder
We found a depression-related volumetric atrophy specifically associated with medial frontopolar area Fp2. Interestingly, this area is predominantly associated with social-affective processes, whereas the lateral frontal pole, i.e., area Fp1, is predominantly associated with more cognitive functions including working memory and perception4, 15.
Previous pathophysiological considerations drew attention to the medial part of BA10, comparable to area Fp2, as being part of the limbic-cortical dysregulation network of major depression16–18. One important anatomical structure of this dysregulation network is the uncinate fasciculus, which connects the frontal pole with other key regions in the pathophysiology of depression, e.g., with the amygdala35, 36. Furthermore, the uncinate fasciculus contributes to frontal-subcortical neuronal circuits, which are involved in emotional and cognitive processing37, 38, making them particularly relevant to depression. A recent deep brain stimulation study with depressed patients showed that alterations of the uncinate fasciculus provides a good measurement to discriminate between responders and non-responders, and postulated that targeting subcallosal cingulate and medial BA10 cortex might be sufficient for antidepressant treatment14. This likewise pointed to a potentially heterogeneous contribution of areas Fp1 and Fp2 to depression, which we confirmed in the present work. Additionally, and in line with its association to social-affective processes4, 15, it should be noted that it is the medial part of the human frontal pole which maintains the strongest functional association with the anterior cingulate cortex39 and the amygdala15.
Interestingly, the present study also revealed a correlation with disease severity exclusively in left area Fp2. This is in line with previous studies reporting structural connectivity deficits between limbic structures and frontal areas connected via the uncinate fasciculus in depressed patients specifically for the left uncinate fasciculus40. Taken together, this indicates a possible correlation between an altered structural composition, either of the cortex or the white matter of the left medial frontal pole, and the pathophysiology of major depressive disorder.
Limitations
The multi-site setting of the current study is challenging, since it required an age and gender matching per site (which was realized in the current study in addition to the removal of any variance related to the different measurement sites) and might involve possible different standard medication approaches within patient groups. On the other hand, it provides a more realistic representation of patients with major depressive disorder in Germany. Those factors could only be minimized by strict matching and statistical confound adjustment, but could not systematically be eliminated.
Secondly, since all but two patients within our study received standard anti-depressive medication, we cannot exclude medication-induced effects. Moreover, medication-specific subgroup analyses were not possible due to highly variable medication regimens as prescribed by the attending psychiatrists. While the rating of depression severity using (only) a self-rated measure may be considered a drawback of the present study, we would argue that it actually does provide a benefit in the employed multi-site setting. In particular, we would like to note that noise variance in Beck Depression Inventory scores should be more or less randomly distributed, as it depends on inter-individual differences in introspective abilities, repression, honesty etc. In turn, assessment by an observer-rated inventory should be more susceptible to site or rater-dependent systematic biases. As both, the uni- and multivariate morphometric analysis congruently detected the morphological properties of only medial area Fp2 as most informative for the disease status, our results can be regarded as being robust. Could they be medication related? We would argue that the specific location to only one region in one hemisphere provides evidence to the contrary, as drug-induced gray matter alterations would be expected to affect both hemispheres in a comparable way. To our knowledge there are no reported volumetric effects caused by medication specifically for one hemisphere of one region.
Finally, we note that various structural brain atlases based on different features of regional brain organization, from gyral anatomy to gene-expression and diffusion-weighted imaging, coexist in the field of systems neuroscience (for review see Amunts et al., 2014). The JuBrain histological atlas, however, is currently the only atlas based on microstructural criteria obtained from a group of subjects, yielding a reliable definition of cortical microstructure, which in turn should represent a main constraint to functional organization19.
Conclusions
In the presented analyses of multi-site voxel-based morphometric data we found converging evidence for structural alterations of the left medial frontal pole area Fp2 in major depressive disorder across both uni- and multivariate statistical approaches. These complementary analyses confirmed that the volume of left Fp2 was smaller in depressed patients than in healthy controls. Additionally this regional atrophy also correlated negatively with disease severity and duration, and allowed the discrimination between patients and controls. In line with earlier functional decoding of areas Fp1 and Fp215, this highlights social-affective processes as associated with atrophy in the left medial frontal pole in depressed patients. This potential implication of socio-emotional processing in the pathophysiology of major depressive disorder is closely related to its clinical hallmarks, including mood disturbances and rumination.
Supplementary Material
Acknowledgments
This study was in part supported by the National Institute of Mental Health (R01-MH074457, SBE), the German Research Foundation (EI 816/4-1 SBE; LA 3071/3-1 SBE; EI 816/6-1 SBE, DB) and the German National Merit Foundation (DB). The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 604102, HBP (KA, SE).
Footnotes
The authors declare no conflict of interest.
References
- 1.Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS medicine. 2013;10(11):e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodmann K. Vergleichende Lokalisationslehre der Großhirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Verlag von Johann Ambrosius Barth; 1909. [Google Scholar]
- 3.Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci. 2007;11(7):290–298. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert SJ, Gonen-Yaacovi G, Benoit RG, Volle E, Burgess PW. Distinct functional connectivity associated with lateral versus medial rostral prefrontal cortex: a meta-analysis. Neuroimage. 2010;53(4):1359–1367. doi: 10.1016/j.neuroimage.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: evidence for a rostrocaudal heirarchical organisation within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- 6.Johnson MK, Nolen-Hoeksema S, Mitchell KJ, Levin Y. Medial cortex activity, self-reflection and depression. Social cognitive and affective neuroscience. 2009;4(4):313–327. doi: 10.1093/scan/nsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JD, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cognitive, affective & behavioral neuroscience. 2005;5(2):156–168. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons AS, Scarr E, McLean C, Sundram S, Dean B. Decreased muscarinic receptor binding in the frontal cortex of bipolar disorder and major depressive disorder subjects. Journal of affective disorders. 2009;116(3):184–191. doi: 10.1016/j.jad.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szewczyk B, Albert PR, Burns AM, et al. Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int J Neuropsychopharmacol. 2009;12(2):155–168. doi: 10.1017/S1461145708009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shelton RC, Sanders-Bush E, Manier DH, Lewis DA. Elevated 5-HT 2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience. 2009;158(4):1406–1415. doi: 10.1016/j.neuroscience.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Molecular psychiatry. 1998;3(3):220–226. 190–221. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- 12.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Human brain mapping. 2009;30(11):3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. Widespread reductions in gray matter volume in depression. NeuroImage. Clinical. 2013;3:332–339. doi: 10.1016/j.nicl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riva-Posse P, Choi KS, Holtzheimer PE, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biological psychiatry. 2014;76(12):963–969. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bludau S, Eickhoff SB, Mohlberg H, et al. Cytoarchitecture, probability maps and functions of the human frontal pole. Neuroimage. 2014;93(Pt 2):260–275. doi: 10.1016/j.neuroimage.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. British medical bulletin. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 17.Seminowicz DA, Mayberg HS, McIntosh AR, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22(1):409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain structure & function. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zilles K, Amunts K. Centenary of Brodmann's map--conception and fate. Nature reviews. Neuroscience. 2010;11(2):139–145. doi: 10.1038/nrn2776. [DOI] [PubMed] [Google Scholar]
- 20.Amunts K, Hawrylycz MJ, Van Essen DC, et al. Interoperable atlases of the human brain. Neuroimage. 2014;99:525–532. doi: 10.1016/j.neuroimage.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Ashburner J. Computational anatomy with the SPM software. Magnetic resonance imaging. 2009;27(8):1163–1174. doi: 10.1016/j.mri.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Ashburner J, Kloppel S. Multivariate models of inter-subject anatomical variability. Neuroimage. 2011;56(2):422–439. doi: 10.1016/j.neuroimage.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson SJ, Halchenko YO. Brain Reading Using Full Brain Support VectorMachines for Object Recognition: There Is No “Face” Identification Area. Neural Comput. 2008;20:486–503. doi: 10.1162/neco.2007.09-06-340. [DOI] [PubMed] [Google Scholar]
- 24.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning. Heidelberg, Germany: Springer Series in Statistics; 2011. [Google Scholar]
- 25.Kriegeskorte N, Goebel R, Bandettini P. Information-based functional brain mapping. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(10):3863–3868. doi: 10.1073/pnas.0600244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saeys Y, Inza I, Larranaga P. A review of feature selection techniques in bioinformatics. Bioinformatics. 2007;23(19):2507–2517. doi: 10.1093/bioinformatics/btm344. [DOI] [PubMed] [Google Scholar]
- 27.Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32(2):570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- 28.Rajkowska G. Depression: what we can learn from postmortem studies. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2003;9(4):273–284. doi: 10.1177/1073858403252773. [DOI] [PubMed] [Google Scholar]
- 29.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. The American journal of psychiatry. 2000;157(1):115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 30.Frodl T, Schaub A, Banac S, et al. Reduced hippocampal volume correlates with executive dysfunctioning in major depression. Journal of psychiatry & neuroscience : JPN. 2006;31(5):316–323. [PMC free article] [PubMed] [Google Scholar]
- 31.Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 32.Neubert FX, Mars RB, Thomas AG, Sallet J, Rushworth MF. Comparison of human ventral frontal cortex areas for cognitive control and language with areas in monkey frontal cortex. Neuron. 2014;81(3):700–713. doi: 10.1016/j.neuron.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Tsujimoto S, Genovesio A, Wise SP. Frontal pole cortex: encoding ends at the end of the endbrain. Trends Cogn Sci. 2011;15(4):169–176. doi: 10.1016/j.tics.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. The Journal of comparative neurology. 1996;371(2):179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 35.Thiebaut de Schotten M, Dell'Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex; a journal devoted to the study of the nervous system and behavior. 2012;48(1):82–96. doi: 10.1016/j.cortex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain : a journal of neurology. 2013;136(Pt 6):1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cummings JL. Frontal-subcortical circuits and human behavior. Archives of neurology. 1993;50(8):873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 38.Marchand WR. Cortico-basal ganglia circuitry: a review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain structure & function. 2010;215(2):73–96. doi: 10.1007/s00429-010-0280-y. [DOI] [PubMed] [Google Scholar]
- 39.Liu H, Qin W, Li W, et al. Connectivity-based parcellation of the human frontal pole with diffusion tensor imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(16):6782–6790. doi: 10.1523/JNEUROSCI.4882-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor WD, MacFall JR, Gerig G, Krishnan RR. Structural integrity of the uncinate fasciculus in geriatric depression: Relationship with age of onset. Neuropsychiatric disease and treatment. 2007;3(5):669–674. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.