Abstract
Background:
Antepartum hemorrhage (APH) contributes significantly to maternal and perinatal morbidity and mortality globally, particularly in the developing world like ours. Prevention, early detection, and prompt management cannot be overemphasized to significantly reduce the morbidity and mortality associated with this condition.
Objectives:
The study is aimed at determining the prevalence, etiology, sociodemographic characteristics, and the fetomaternal outcome of pregnancies complicated by APH in Aminu Kano Teaching Hospital, Kano.
Materials and Methods:
A 5 years retrospective study of all pregnancies complicated by APH at Aminu Kano Teaching Hospital, Kano, Nigeria, between January 1, 2009, and December 31, 2013, was conducted.
Results:
A total of 224 cases of APH were recorded out of the 18,273 cases admitted for delivery during the study period, giving an institutional prevalence rate of 1.2%. Two hundred and eighteen folders were retrieved and analyzed giving a retrieval rate of 97.3%. The mean gestational age at presentation was 35.3 ± 2 weeks and the most common causes were abruptio placenta and placenta previa constituting 68.3% and 30.0%, respectively. Sociodemographic characteristics associated with the occurrence of APH included age, booking status, parity, and socioeconomic status. The peak prevalence of APH was observed in the 35–39 year age group accounting for 33.0%. There were 123 live births and 92 stillbirths. The cesarean section rate was 53.5%. Major complications were intrauterine fetal deaths in 42.8%, postpartum hemorrhage in 24.2% of cases, and anemia necessitating blood transfusion in 61.5%. There were three maternal deaths all due to abruptio placentae during the study period giving a case specific fatality rate of 2%.
Conclusion:
The prevalence of APH in our setting is high. The major causes were abruptio placenta and placenta previa. The major fetal complication was intrauterine fetal death, and the major maternal complications were postpartum hemorrhage and anemia with consequent high blood transfusion rate. Early detection, provision of antenatal care, and emergency obstetric care services can reduce the negative effects of APH.
Keywords: Aminu Kano Teaching Hospital, antepartum hemorrhage, fetomaternal outcome, prevalence
Introduction
Antepartum hemorrhage (APH) is a major cause of maternal and perinatal morbidity and mortality even in modern day obstetrics and is one of the most frequent emergencies in obstetrics.[1,2] APH is defined as bleeding from the genital tract from the time of viability of pregnancy for extrauterine survival to the delivery of the baby.[3]
APH complicates 0.5–5% of pregnancies which varies with sociodemographic variables.[1,4,5] The main causes of APH are placenta previa and abruptio placentae; however, the exact cause of bleeding in some cases may be undetermined.[5] In a small proportion where placenta previa and abruption have been excluded, the cause may be related to local lesions of the cervix and vagina, e.g., cervicitis, cervical erosion, genital tumors, vulvar varicosities, ruptured vasa previa, and heavy show.[5,6]
The overall prevalence in a study from Qatar was found to be 15.3%[7] and poor education, family history of hypertension, G6PD, and Down's syndrome were found to be significantly associated with increased APH in that study.[7] However, in a study from Osun, South-Western Nigeria, the prevalence was 1.5% and the major cause of antepartum hemorrhage was found to be placenta previa followed by abruption and lastly by unknown causes.[8] In Lagos, Nigeria, an incidence of 3.5% was reported and placenta previa constituted 58.4% of the cases, while placental abruption was a factor in 35.6%.[9]
In a comparison of maternal risk factors, research reports[5] concluded that abruption is more likely to be related to conditions occurring during pregnancy (preeclampsia, abdominal trauma, intrauterine infections, prelabor rupture of membranes, polyhydramnios elevated maternal serum alpha-fetoprotein, smoking, and substance abuse) and placenta previa related to conditions existing prior to the pregnancy (uterine scar, manual removal of placenta, curettage, advanced maternal age, multiparity, and previous placenta previa).[5] The precise cause of abruption is unknown; however, hypertension is the most consistent predisposing factor.[10] In a study conducted at the University of Oslo, age was studied as a significant sociodemographic characteristic, with mothers over the age of 40 years being significantly more likely to have severe hemorrhage.[1] On the other hand, maternal characteristics associated with lower sociodemographic status, namely low education was the main variable associated with APH in a study from Qatar.[7]
Maternal complications of APH include hypovolemic shock, disseminated intravascular coagulation, and acute renal failure.[11] It also includes higher rates of cesarean sections as high as 83.3% for placenta previa, peripartum hysterectomies (2.1%), and postoperative anemia (7.3%) in a study from Sokoto, Nigeria.[12] The maternal mortality rate was 1% in both studies.[11,12]
Fetal complications are premature delivery, low birth weight, birth asphyxia, and intrauterine fetal death.[1] Up to one-fifth of very preterm babies are born in association with APH,[5] and the known association of APH with cerebral palsy can be explained by preterm delivery. A retrospective observational study from Australia found that women with unexplained APH are at greater risk of preterm delivery, and their babies are more likely to develop hyperbilirubinemia.[5] Furthermore, women with unexplained APH were more likely to have smaller babies, and this difference remained statistically significant when the birth weight was adjusted for gestational age at delivery and other confounders.[5]
As APH stands out as a serious, life-threatening condition resulting in significant maternal and perinatal morbidity and mortality, it is particularly important to appraise the pattern of this condition in a developing country for better maternal health-care services. Thus, this study is aimed at determining the prevalence, etiology, sociodemographic characteristics, and fetomaternal outcome of APH at Aminu Kano Teaching Hospital (AKTH) Kano.
Materials and Methods
This was a retrospective study carried out in the Department of Obstetrics and Gynaecology of AKTH, Kano, over a 5 years period. Permission was obtained from the Institution's Ethics and Research Committee prior to the commencement of the study. A list of all patients that had APH from January 1, 2009, to December 31, 2013, was compiled from labor ward and obstetrics theater records, and the case notes were then retrieved from the Medical Records Department of the hospital. APH is defined as bleeding from the genital tract from the time of viability of pregnancy (from 28 weeks of gestation and beyond in this study) for extrauterine survival to the delivery of the baby.[3] The names and hospital numbers were carefully cross-checked to ensure there was no repetition. Patients whose folders could not be traced were excluded from the study. The total number of deliveries during the study period was also obtained from the statistics unit of the Records Department. Data relating to etiology, age, educational status, parity, booking status, gestational age at presentation, baby's sex, birth weight, mode of delivery, and the maternal and fetal outcome were extracted and entered into a questionnaire designed for the study. The data were analyzed using (version 12.21, Minitab Inc., State College, PA, USA) for frequencies and Chi-square at 95% confidence interval. P < 0.05 was considered significant. Results were presented in tabular form.
Results
A total of 224 patients were diagnosed with APH during the study period and a total of 18,273 deliveries were similarly recorded during the same period giving an institutional prevalence of 1.2%, with abruptio placentae having 0.82%, placenta previa 0.36, while unknown causes had 0.02%. Two hundred and eighteen folders were retrieved and analyzed, giving a retrieval rate of 97.3%. The mean age of the patients was 32.8 ± 5.5 years with a range of 20–44 years. Table 1 shows the causes of APH. The major causes were abruptio placentae in 150 (68.8%) cases, and placenta previa accounted for 65 (29.9%), while 3 (1.3%) were of unknown cause. Table 2 shows the comparison of sociodemographic characteristics of the patients and their relative occurrence among patients with abruptio placentae and placenta previa. All the patients were married. It is seen that the occurrence of abruptio placentae was significantly higher in the younger (P = 0.002) and primigravida mothers (P = 0.000), while placenta previa was significantly higher among older (P = 0.002) and multiparous patients (P = 0.000). APH occurred more in the 35–39 year age group accounting for 33.0% and the higher parity mothers accounting 70.7%. Significantly, more abruptio placentae are seen among the unbooked patients (0.010) when compared with the booked with 124 out of 150 developing abruptio placentae while placenta previa was more common in the booked patients. There was also statistically significant occurrence of abruptio placentae among homemakers (0.042), while gainful employment is significantly associated (0.042) with placenta previa. Religion however did not show any statistically significant influence on the occurrence of placental abruption over placenta previa and vice versa. Other factors such as marital status and place of residence did not appear to affect APH. Not shown in the table are the three unknown causes of APH which constituted about 1.3%.
Table 1.
Causes of antepartum hemorrhage

Table 2.
Comparison of sociodemographic factors between abruptio placenta and placenta previa
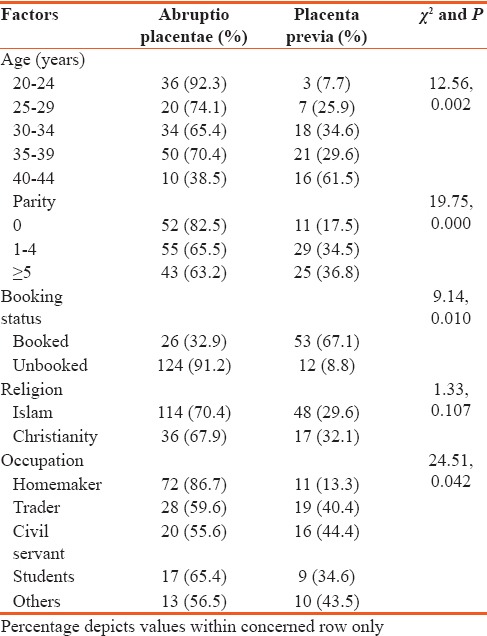
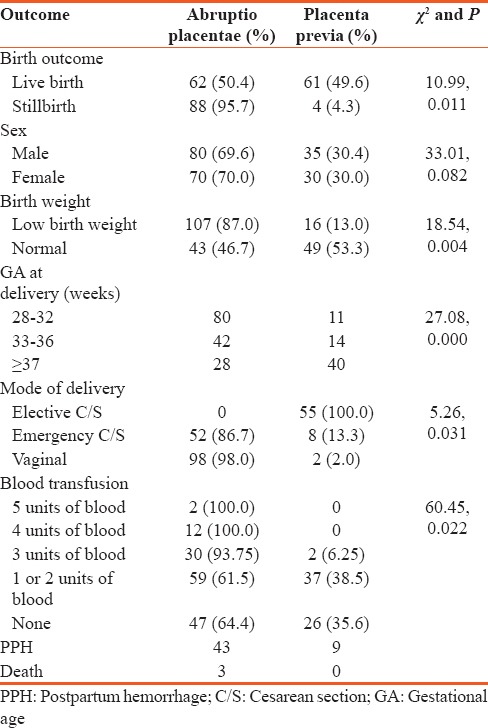
Table 3 shows the comparison of fetomaternal outcome between abruptio placentae and placenta previa. The mean gestational age at presentation was 35.3 ± 2.0 weeks. Most, 68.8%, of the patients presented preterm between gestational ages of 33 and 36 weeks which was significantly lower for abruptio placentae (P = 0.000); 80 out of 91 of those delivering at 28–32 had abruptio placentae, while 40 out of 68 of those delivering at 37 weeks and beyond had placenta previa. The fetal outcome was generally better in placenta previa group (P = 0.001). Significantly more babies had good Apgar scores of 7–10, while 136 out of 150 were either asphyxiated or stillborn in the abruptio placentae group. Up to 87% of the babies in the abruptio placental group were of low birth weight 107 out of 150 (P = 0.004). More than half; 53.5% of the patients were delivered via cesarean section; however, significantly more patients with placenta previa (P = 0.031) had cesarean section compared to those with abruptio placentae. Majority (82.7%) of the patients with abruptio placentae had PPH compared to 17.3% with placenta previa. Anemia severe enough to necessitate blood transfusion occurred in 65.3% of the patient with APH. Significantly more patients with abruptio placentae (P = 0.022) had blood transfusion compared to those with placenta previa. There were three maternal deaths all due to abruptio placentae giving a case-specific fatality rate of 2%. Sex of the baby did not have any statistical influence on the occurrence of APH.
Table 3.
Comparison of fetomaternal outcome between patients with abruptio placentae and placenta previa
Discussion
The prevalence of APH was 1.2% in this study which is comparable to 1.5% reported from Oshogbo.[8] It is however lower than 5.4% documented in Pakistan[1] and 15.3% from Qatar.[7] Other studies have also reported higher values 2–5%, 3.01%,[4] and 2.53%[1] when compared to our result. The lower figure found in our study may be an underestimate of the actual figure as many patients with APH fail to reach the hospital in time or a multitude of cases may not report to the hospital at all. The low prevalence may also reflect the sociocultural and economic factors in the study environment that do not allow most women to seek medical attention unless in dire need.[13]
The leading cause of antepartum hemorrhage in this study was found to be abruptio placenta followed by placenta previa as opposed to findings in Southwestern Nigeria[14] in which placenta previa was found to be the leading cause. Hypertension has also been found to be the most consistent predisposing factor associated with abruptio placentae.[10] The finding of advanced maternal age is similar to findings by other authors. It is also similar to the finding from a study in Enugu (33.3%)[15] and Niger (38.2%).[16] This maybe because up to one-third (33%) of our patients in the study were within the 35–39 year age group and probably age-related chronic medical conditions such as hypertension might have set in.[17]
The prevalence of APH in this study is higher in multipara (70.7%) when compared to patients with lower parity (29.3%). This agrees with reports of other studies.[18,19] Hence being a problem of multiparity, reduction in family size and contraception are highly recommended if the prevalence and associated morbidity and mortality are to be reduced.
Up to 77% of unbooked patients suffered APH in a study reported from Hyderabad.[1] Most of the patients (63.3%) who suffered APH in our study were similarly unbooked. This clearly shows the importance of antenatal care in the prevention and early detection of patients with risk factors for APH to reduce morbidity and mortality. Late presentation with complications as shown above was probably due to illiteracy, culture, and poverty which tends to prevent women from coming to the hospital except in life-threatening conditions.[20] The higher incidence of unbooked patients may also be related to their socioeconomic class as up to 38.6% of patients in our study were found to be homemakers and probably not gainfully employed. However, a study from Peru[21] found no association between placental abruption and socioeconomic status although the study was conducted among low socioeconomic women. On the other hand, a study conducted in the USA found that women on support were more likely to have placental abruption.[22] In general, the literature has noted an effect of sociodemographic characteristics on placental abruption but not on placenta previa.[23]
Pregnancy outcome among women with APH is associated with both maternal and neonatal complications, and APH has been implicated as a major cause of perinatal death, which is in agreement with other studies in developed settings.[8] This study confirmed the established finding that those with APH were more likely to have an adverse neonatal outcome,[8] such as low birth weight, low Apgar score, stillbirths, and preterm deliveries which were up to 57.2%, 25.6%, 42.8%, and 68.4%, respectively, in our study. In most cases, delivery was iatrogenic in maternal interest.[1] The stillbirth rate was 42.8% in our study, while other studies have quoted a stillbirth rate of 50.2% and 22.2%[1,8] in cases of antepartum hemorrhage. This may likely be related to the rate at which timely intervention is instituted as hemorrhage exposed the fetuses to hypoxia and ultimately death[1] and this again proves the importance of early and rigorous management of APH.
The maternal complications and/or outcome observed from this study included a cesarean section rate of 53.5% which is similar to the cesarean section rate of 57.1% in Hyderabad[1] and 53.1% in Oshogbo.[8] Postpartum hemorrhage was found in 24.2% of the women, more than 7.1% found in Oshogbo,[8] and 19% in Hyderabad.[1] This may be related to the significant number of multiparous patients in our study. A transfusion rate of 66.0% was found to be slightly lower than 77.4% in Hyderabad.[1] Anemia severe enough to warrant transfusion of 1–2 units of blood was found in 44.7% of our patients and up to 21.4% had more than 2 units transfused. This is a high value when the risks of blood transfusion including the potential risks of transmission of infectious agents are considered. On the contrary, a delay in the correction of hypovolemia can be fatal in cases of hemorrhage. Maternal mortality in this study was due to abruptio placentae and was found to be 2% which is comparable to the study by Pandelis[11] but lower than the 3% quoted by Sheikh and Khokhar.[1] This higher rate was attributed to late presentation of the patients and lack of prompt availability of blood and therapy for DIC in Sheikh's study.[1] The low mortality recorded in this study may be due to the higher number of patients in that study and on the other hand improved blood banking services and emergency services scheme provided by our hospital. On a general note, this cannot be said for most areas of the country where difficulties with transportation and restricted medical facilities ensure that APH continues to be responsible for many maternal deaths.[12]
Conclusion
APH is an obstetric emergency with a high prevalence in our environment, and it is one of the most common causes of significant maternal and perinatal morbidity and mortality. Clinical care should therefore concentrate on prevention, early detection, and prompt management. Furthermore, pregnant women with APH should be considered high risk and timely management should be offered by a trained team and women at risk of APH should be encouraged to book for antenatal care and be delivered in centers equipped for blood transfusion and operative delivery services. Further studies on APH are also recommended and there is still need for prompt blood banking services. The main limitation of the study was in its retrospective nature and difficulty in accessing the records.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sheikh F, Khokhar S. A study of antepartum haemorrhage: Maternal and perinatal outcomes. Med Chan. 2010;16:22. [Google Scholar]
- 2.Paterson-Brown S. Obstetetric emergencies. In: Edmunds DK, editor. Dewhurst Textbook of Obstetrics and Gynaecology for Postgraduates. UK: Blackwell Science; 2007. pp. 149–52. [Google Scholar]
- 3.Arulkumaran S, Regan L. Obstetrics and Gynaecology. 1st ed. New York City, USA: Oxford University Press; 2011. Antepartum haemorrhage; pp. 326–7. [Google Scholar]
- 4.Singhal S, Nymphaea, Nanda S. Maternal and Perinatal Outcome In Antepartum Haemorrhage: A study At A Tertiary Care Referral Institute. The Internet Journal Of Gynaecology and Obstetrics. 2007;9:1–4. [Google Scholar]
- 5.Green-top Guideline No. 63. London: RCOG; 2011. Royal College of Obstetricians and Gynaecologists. Antepartum Haemorrhage. [Google Scholar]
- 6.Kwakwume EY. Antepartum haemorrhage. In: Kwakwume EY, Emuveyan EE, editors. Comprehensive Obstetrics and Gynaecology in the Tropics. 1st ed. Accra, Ghana: Asante and Hitscher Printing Press; 2002. pp. 140–50. [Google Scholar]
- 7.Bener A, Saleh NM, Yousafzai MT. Prevalence and associated risk factors of ante-partum hemorrhage among Arab women in an economically fast growing society. Niger J Clin Pract. 2012;15:185–9. doi: 10.4103/1119-3077.97315. [DOI] [PubMed] [Google Scholar]
- 8.Adekanle A, Adeyemi A, Fadero F. Antepartum haemorrhage in LAUTECH Teaching Hospital, South-Western Nigeria. J Med Sci. 2011;2:1243–7. [Google Scholar]
- 9.Adegbola RO, Okunodo AA. Pattern of antepartum haemorrhage at the Lagos University Teaching Hospital. Niger Med Pract. 2009;5:6. [Google Scholar]
- 10.Giordano R, Cacciatore A, Cignini P, Vigna R, Romano M. Antepartum haemorrhage. J Prenat Med. 2010;4:12–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Pandelis K, Kardina A, Samina M. Antepartum haemorrhage. Obstet Gynaecol Reprod Med. 2012;1:21–5. [Google Scholar]
- 12.Borodo AT, Shehu CE. Placenta previae at Usmanu Danfodio University Teaching Hospital: A 5 year review. Sahel Med J. 2013;16:56–9. [Google Scholar]
- 13.Nwobodo EI. Obstetric emergencies as seen in a tertiary health institution in North-Western Nigeria: Maternal and fetal outcome. Niger Med Pract. 2006;49:54–5. [Google Scholar]
- 14.Ikechebelu JI, Onwusulu DN. Placenta praevia: Review of clinical presentation and management in a Nigerian teaching hospital. Niger J Med. 2007;16:61–4. doi: 10.4314/njm.v16i1.37283. [DOI] [PubMed] [Google Scholar]
- 15.Ozumba BC. Abruptio placentae at the University of Nigeria Teaching Hospital, Enugu: A 3-year study. Aust N Z J Obstet Gynaecol. 1989;29:117–20. doi: 10.1111/j.1479-828x.1989.tb01698.x. [DOI] [PubMed] [Google Scholar]
- 16.Nayama M, Tamakloé-Azamesu D, Garba M, Idi N, Djibril B, Kamayé M, et al. Abruptio placentae. Management in a reference Nigerien maternity. Prospective study about 118 cases during one year. Gynecol Obstet Fertil. 2007;35:975–81. doi: 10.1016/j.gyobfe.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Agboola A, editor. Textbook of Obstetrics and Gynaecology for Medical Students. Lagos: Heinemann Education Books Plc.; 2006. Antepartum haemorrhage; pp. 340–7. [Google Scholar]
- 18.Eniola AO, Bako AU, Selo-Ojeme DO. Risk factors for placenta praevia in Southern Nigeria. East Afr Med J. 2002;79:535–8. doi: 10.4314/eamj.v79i10.8816. [DOI] [PubMed] [Google Scholar]
- 19.Faiz AS, Ananth CV. Etiology and risk factors for placenta previa: An overview and meta-analysis of observational studies. J Matern Fetal Neonatal Med. 2003;13:175–90. doi: 10.1080/jmf.13.3.175.190. [DOI] [PubMed] [Google Scholar]
- 20.Adinma JI. Aetiology and management of obstetric haemorrhage. In: Okonofua A, Odunsi K, editors. Contemporary Obstetrics and Gynaecology in Developing Countries. Benin City, Nigeria: Women's Health and Action Research Centre; 2003. pp. 620–43. [Google Scholar]
- 21.Sanchez SE, Pacora PN, Farfan JH, Fernandez A, Qiu C, Ananth CV, et al. Risk factors of abruptio placentae among Peruvian women. Am J Obstet Gynecol. 2006;194:225–30. doi: 10.1016/j.ajog.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Arnold DL, Williams MA, Miller RS, Qiu C, Sorensen TK. Iron deficiency anemia, cigarette smoking and risk of abruptio placentae. J Obstet Gynaecol Res. 2009;35:446–52. doi: 10.1111/j.1447-0756.2008.00980.x. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee S, Bhide A. Antepartum haemorrhage. Obstet Gynaecol Reprod Med. 2008;18:335–9. [Google Scholar]


