Abstract
Background and Objectives:
Depression, especially in concurrence with chronic medical disorders, is highly prevalent worldwide. An average between 9.3% and 23% of patients with one or more chronic disease have co-morbid depression. This comorbid depression has the worst health scores of all the disease states. Despite this, patients with chronic medical disorders are not commonly screened for depression. Lack of objective screening by health-care providers as well as lack of infrastructure (time/space/personnel) probably contributes to gross underdiagnosis of depression. This issue can be addressed using short objective depression screening score (Patient Health Questionnaire-9 [PHQ-9]) (validated in native languages, e.g., Hindi) and paperless self-administered interface on handheld computer (tablet), which is the objective of the study.
Subjects and Methods:
One hundred consecutive patients with chronic medical disorders visiting our medicine outpatient department were screened for depression using tablets with PHQ-9 Hindi on a self-administered interface.
Results:
The overall prevalence of depression was found to be 25% (95% confidence interval 16.6–34.8). Nearly half of the patients with depression had moderate depression (PHQ-9 score 10–14) while rest had moderately severe or very severe depression (PHQ-9 score >14). Association of depression was not found to be statistically significant with age, duration of disease, gender, the type of disease, or the number of disease. Majority of patients rated ease of the use of tablet interface (on a visual analog scale) as very easy (approx 95%). All the patients were able to complete the tablet screener without assistance, answering all of the questions. The median time of completion with interquartile range was 4 (3–5) min. Majority of the patients (63%) completed the questionnaire within 5 min while rest completed it in 5–10 min.
Conclusions:
It is feasible to use tablets with PHQ-9 questionnaire in native language for screening depression in chronic medical disorders. With high prevalence of comorbid depression, any comprehensive care of patients with chronic medical disorders will not be possible, unless such patients are screened and treated for depression. A self-administered screening questionnaire for depression on handheld tablets can prove to be a handy tool to achieve above aim.
Keywords: Chronic medical disorders, depression, Patient Health Questionnaire-9, screening, tablet/handheld computers
Introduction
Depression, especially in concurrence with chronic medical disorders, has been found to be highly prevalent worldwide. In an analysis of the World Health Organization (WHO) World health survey (WHS),[1] an average between 9.3% and 23% of patients with one or more chronic disease had comorbid depression. The likelihood of having depression in the absence of chronic physical disease was significantly lower (3.2%) in comparison. The comorbid state of depression has worst health scores as compared to any disorder alone (depression/any chronic disease/any combination of chronic diseases). Some projections indicate that after heart disease, depression is expected to become the second-leading cause of disease burden by the year 2020.
Despite the high prevalence of comorbid depression, there is a glaring treatment gap, across the nations. Kohn et al.[2] have reviewed community-based psychiatric epidemiology studies and found that for depression, the median treatment gap is about 56.3%. According to Kohn et al., the gap reported is an underestimate due to the unavailability of community-based data from developing countries where services are scarcer. Lack of objective screening by health-care providers as well as lack of infrastructure (time/space/personnel) probably contributes to gross underdiagnosis of depression.
These indicate the urgency of addressing depression as a public health priority to reduce disease burden and disability and to improve the overall health of populations. In recognition of above, the WHO adopted the Mental Health Global Action Program in 2002.
Therefore, it can be concluded that one, the comorbid depression is highly prevalent with worst health scores and two that there is a large treatment gap that needs to be bridged. Any attempt to provide comprehensive health care to patients with chronic medical disorders will be inadequate if these patients are not screened for depressive illness and treated for it.
One of the root causes of nonimplementation of depression screening questionnaire is restraint of resources and lack of consultation in privacy in the outpatient department (OPDs) in most government hospitals.
This study attempts to address the depression screening by use of:
Short objective depression screening score (Patient Health Questionnaire-9 [PHQ-9]) (validated in native language, e.g., Hindi)
Paperless self-administered interface on handheld computer (tablet).
PHQ-9 is a validated tool for depression screening. A PHQ-9 score ≥10 has a sensitivity of 88% and a specificity of 88% for major depression.[3] PHQ-9 and its translation in 11 Indian languages (including Hindi) were undertaken by Kochhar et al.[4] This prospective study conducted at 18 sites, in psychiatric and general clinics involving 3000 participants, validated the screening questionnaire. PHQ-9 has been used in several Indian studies for depression screening.[5,6]
Subjects and Methods
A cross-sectional study was conducted to screen patients with chronic medical disorders for depression and to determine the feasibility of handheld computers (tablet) as interface. This study was carried out in the outpatient department of a tertiary level hospital in central India. Hundred consecutive patients with physician-diagnosed chronic medical disorders such as diabetes mellitus (DM), hypertension (HTN), rheumatoid arthritis (RA), chronic obstructive pulmonary disease (COPD), hypothyroidism, and cardiovascular diseases (CVD) were evaluated. They were selected on the basis of certain criteria, mentioned below. The study was carried out after due to approval from the Human Ethics Committee of the institute, and informed written consent was obtained from all the participants. These patients were explained about the study and the PHQ-9 individually.
Inclusion criteria were patients above age of 18 years with DM, HTN, RA, COPD, hypothyroidism, or CVD and their combinations for more than a year.
Exclusion criteria were patients exhibiting severe symptoms and/or medically unstable; known patients of psychiatric illnesses; physical and/or audiovisual impairment; cognitive defects or mental disorders; illiterate patients; pregnant women; or patients of age <18 years. This was assessed from past medical records as well as patients' self-reported history.
Patient's demographic details (employment, education, etc.,) and details of medical illness were recorded.
Patients were asked to rate the ease of the use of tablet interface after completion of questionnaire. This was done with the help of the visual analog scale (VAS) which was rated from 0 to 10, 0–3 being very hard and 7–10 being very easy. The score of 5–7 was labeled easy and 3–5 was labeled hard. The time taken to complete the questionnaire was recorded in the software.
Depression was screened in the patients by administering PHQ-9-Hindi version.
The 9-item questionnaire scores each of the 9 Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria as “0” (not at all) to “3” (nearly every day).
For the diagnosis of depression, clinically significant depression was defined as score more than 10 or more while score of 0–9 was clubbed as no or mild depression. Score 10–14 as moderate depression, 15–19 as moderately severe, and 20 or more as severe depression.
iSurvey software (after obtaining paid subscription) was downloaded on iPads (model – iPad mini 4 with 7.9-inch display with 326 pixel per inch resolution) to be handed to the patients [Figure 1]. iSurvey is a paid android/iPhone Operating System-based application, which allows configuration of survey questionnaire (PHQ-9 in this case) into its touch interactive interface, and the data from tablets are uploaded wirelessly later. The patients were instructed on how to use the tablet to answer the questionnaire. The questionnaire was answered independently by the patient. The software ensured that the filled questionnaire was not be submitted till all the 9 items had been answered, and more than one choice per question was not accepted. The feasibility of the use of the tablet in an OPD setting was observed.
Figure 1.

Screenshot of the questionnaire on the tablet
The data were entered and analyzed using Epi info (Version 7.1.5.2) software package, Centers for Disease Control and Prevention (CDC) Atlanta USA. Chi-square test was used to test the association of dependent variable with independent variables, P < 0.05 was considered significant. Where Chi-square was invalid, Fisher's exact values were used.
A failure to complete the screening process was defined as:
Failure to complete the screening process even after two trials of assisted counseling
Failure to complete the process in finite time (10 min).
The patients screened and found positive on the PHQ were referred to the psychiatry OPD.
Results
One hundred consecutive patients with chronic medical disorders were enrolled in the study after obtaining informed consent.
Distribution of patients
The study included 53% (53) females and 47% (47) males, with mean age 51.8 ± 11.6 years. The age-wise distribution of the patients was as follows: 18–30 years = 1, 30–45 years = 28, 45–60 years = 36, and 60–75 years = 35. Type 2 DM constituted 44% (44) of patients followed by HTN, 31% (31), hypothyroidism, 25% (25), RA, 17% (17), CVD, 7% (7), and COPD, 2% (2). This includes the instances when patients presented with two or more chronic disorders together.
The distribution of Patient Health Questionnaire-9 score
The overall prevalence of depression was found to be 25% (95% confidence interval 16.6–34.8). The prevalence of depression in females was 30.1% and in males was 23.6%. However, the difference in prevalence between male and female gender was not statistically significant (P > 0.05). Forty-eight percent (n = 12) of the patients with depression had moderate depression, 28% (n = 7) patients had moderately severe depression, and 24% (n = 6) had severe depression [Figure 2].
Figure 2.
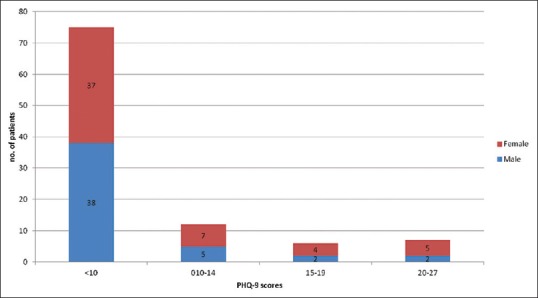
Distribution of the Patient Health Questionnaire-9 score in association with gender
The prevalence of depression in diabetes as a singular disorder was 20%. Similarly, the prevalence of depression for HTN was 8.3%, hypothyroidism (37.5%), COPD (50%), RA (20%), and CVD (41.6%) [Figure 3]. The patients with two chronic medical disorders were 21, of which 28.5% had depression on screening, while none of the patients suffering from three chronic medical disorders reported moderate to severe depression.
Figure 3.
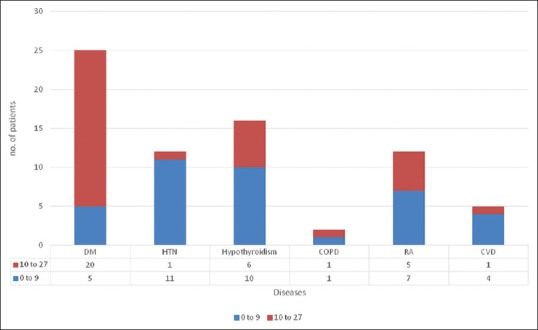
The number of patients with depression with only one chronic medical disorder
Age-wise distribution of patients with moderate to severe depression (PHQ-9 score 10–27) is depicted in Table 1. There was no statistically significant correlation between age and prevalence of depression. Association of depression was not found to be statistically significant with age, duration of disease, gender, the type of disease, or the number of disease.
Table 1.
Distribution of PHQ-9 scores in association with age (as discrete variable)

Ease of use
One hundred patients were approached for inclusion for checking the ease of use of the tablet, and 94 (94%) provided consent. Majority of patients (94.6%) (n = 89) rated ease of completion as very easy and rest (5.3%) (n = 5) as hard. All the patients were able to complete the tablet screener without assistance, answering all of the questions. The internal consistency of responses was moderate or acceptable (Cronbach alpha – 0.752). The median time of completion with interquartile range was 4 (3–5) min. Sixty-three percent (n = 59) patients completed the questionnaire within 5 min, and 35% (n = 33) of patients completed took 5–10 min to complete it [Figure 4]. However, these were found to be not statistically significant. Overall, the tablet had no malfunctions and operated normally throughout the study.
Figure 4.
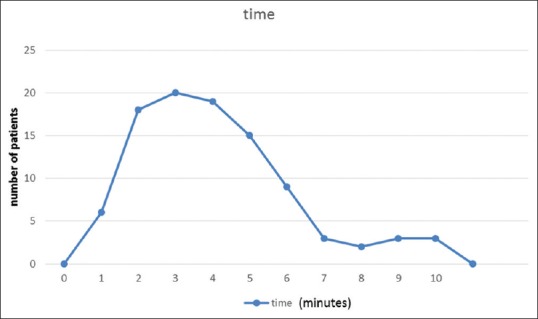
Time taken to complete the questionnaire
Discussion
In this study, we found that 25% of patients with chronic medical disorders (diabetes, hypothyroidism, HTN, COPD, RA, and cardiovascular disorders) had moderate to severe depression (PHQ-9 score10 or above). Out of 25 patients screened positive for depression, 48% (n = 12) had moderate depression; 28% (n = 7) had moderately severe depression; and 24% (n = 6) had severe depression. The prevalence of depression in diabetes as a singular disorder was 20%. Similarly, the prevalence of depression for HTN was 8.3%, hypothyroidism (37.5%), COPD (50%), RA (20%), and CVD (41.6%). No correlation was found between age, gender, duration of illness, or number of chronic medical disorders with depression.
According to the WHS,[1] an average of between 9.3% and 23% of participants with one or more chronic physical disease had comorbid depression. Many other Indian studies have also arrived at comparable figures. Kulkarni et al.[7] have found 29.1% of patients with chronic medical disorders to be depressed while in diabetic patients, Guruprasad et al.[8] have found 27.6% and Raval et al.[9] have 41% prevalence of depression. The studies were, however, inconsistent with the correlation between the duration of illness and depression severity.
There has been a surge in the use of handheld computers (tablets) and mobile-based applications in health care. The handheld computers have advantages of portability, automatic data upkeep, provision of audiovisual interaction interface, privacy of response, and consistency in response can be ensured.
It may have disadvantages in cost and maintenance, requirement of active mobile network or internet network, difficult with visually impaired respondents, or unacceptable to less technology savvy.
The handheld device with unassisted self-administered questionnaire can be expected to combine several advantages mentioned above with privacy of the patient. Patient can respond to issues which may have social stigma or are intrusive. This may be the case in assessment for depression, substance abuse, addictions, or sexual disorders.
In our study, majority of patients (63%) completed the questionnaire within 5 min while rest took 5–10 min. There was no statistically significant association between the time taken to complete the questionnaire and gender or time taken with age. Majority (94.1%) patients gave feedback of very easy to the use on VAS. The entire screening could be completed in the absence of Wifi/internet and data obtained safely uploaded later using internet. Hence, active internet connectivity in OPD area was not compulsory. There was no loss of information as entire data were uploaded to server. The software interface ensured that there was no incomplete questionnaire submission or duplication of choices for a select question.
Thus, screening of patients with chronic medical disorders in the OPD for depression using handheld computers (tablets) as interface and PHQ-9 as a tool appears to be feasible. Authors propose studies at larger scale at community and primary care clinics, to establish feasibility, and ease of use at large.
Lane et al.[10] have summarized trials which have compared handheld computers and pen-paper for data capture in clinical research. They had included trials, in which following outcomes was assessed: data accuracy; timeliness of data capture; and adherence to protocols for data collection. Handheld computers appear superior in timeliness of receipt and data handling (four of four studies) and are preferred by most participants (three of four studies). On the other hand, only one of the trials adequately compared adherence to instructions for recording and submission of data (handheld computers were superior).
Bernabe-Ortiz et al.[11] evaluated the quality of sexual behavior data collected with handheld computers in comparison with paper-based questionnaires. They found general agreement between data collected with paper format and handheld computers while the number of inconsistencies and missing values was significantly higher in paper questionnaires.
Weiner et al.[12] used an electronic tablet version of a screener for opioid prescription abuse potential. They found that 93% patients found interface very easy while majority (95%) were able to complete the survey within 5 min.
This study had limitations in that there was a degree of referral bias, and patients were part of urban population. There is a need to replicate above study in rural and primary health-care setting for assessing feasibility and acceptability. Furthermore, nonmedical contributory factors such as marital status and socioeconomic status were not analyzed.
Conclusions
It is feasible to use tablets with PHQ-9 questionnaire in native language for screening depression in chronic medical disorders. With high prevalence of comorbid depression, any comprehensive care of patients with chronic medical disorders will not be possible, unless such patients are screened and treated for depression. A self-administered screening questionnaire for depression on handheld tablets can prove to be a handy tool to achieve above aim.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank Dr. Krishna Prasad M, Assistant Professor, Department of Psychiatry, AIIMS, Bhopal, and Dr. Rajnish Joshi, Assistant Professor, Department of Medicine, AIIMS, Bhopal, India, for their contribution to this study.
References
- 1.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: Results from the World Health Surveys. Lancet. 2007;370:851–8. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 2.Kohn R, Saxena S, Levav I, Saraceno B. The treatment gap in mental health care. Bull World Health Organ. 2004;82:858–66. [PMC free article] [PubMed] [Google Scholar]
- 3.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochhar PH, Rajadhyaksha SS, Suvarna VR. Translation and validation of brief patient health questionnaire against DSM IV as a tool to diagnose major depressive disorder in Indian patients. J Postgrad Med. 2007;53:102–7. doi: 10.4103/0022-3859.32209. [DOI] [PubMed] [Google Scholar]
- 5.Dutta D, Bharati S, Roy C, Das G. Measurement of prevalence of 'major depressive syndrome' among Indian patients attending pain clinic with chronic pain using PHQ-9 scale. J Anaesthesiol Clin Pharmacol. 2013;29:76–82. doi: 10.4103/0970-9185.105808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De S. Prevalence of depression in stable chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci. 2011;53:35–9. [PubMed] [Google Scholar]
- 7.Kulkarni V, Chinnakali P, Kanchan T, Rao A, Shenoy M, Papanna MK. Psychiatric co-morbidities among patients with select non-communicable diseases in a Coastal City of South India. Int J Prev Med. 2014;5:1139–45. [PMC free article] [PubMed] [Google Scholar]
- 8.Guruprasad KG, Niranjan MR, Ashwin S. A study of association of depressive symptoms among the type 2 diabetic outpatients presenting to a tertiary care hospital. Indian J Psychol Med. 2012;34:30–3. doi: 10.4103/0253-7176.96153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raval A, Dhanaraj E, Bhansali A, Grover S, Tiwari P. Prevalence & determinants of depression in type 2 diabetes patients in a tertiary care centre. Indian J Med Res. 2010;132:195–200. [PubMed] [Google Scholar]
- 10.Lane SJ, Heddle NM, Arnold E, Walker I. A review of randomized controlled trials comparing the effectiveness of hand held computers with paper methods for data collection. BMC Med Inform Decis Mak. 2006;6:23. doi: 10.1186/1472-6947-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernabe-Ortiz A, Curioso WH, Gonzales MA, Evangelista W, Castagnetto JM, Carcamo CP, et al. Handheld computers for self-administered sensitive data collection: A comparative study in Peru. BMC Med Inform Decis Mak. 2008;8:11. doi: 10.1186/1472-6947-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner SG, Horton LC, Green TC, Butler SF. Feasibility of tablet computer screening for opioid abuse in the emergency department. West J Emerg Med. 2015;16:18–23. doi: 10.5811/westjem.2014.11.23316. [DOI] [PMC free article] [PubMed] [Google Scholar]


