Abstract
Background:
Oxidative stress has become a real entity in etiopathogenesis of Type 2 diabetes mellitus (DM). It may result from steady flux of free radicals and lipid peroxides in vivo. Malondialdehyde (MDA) is a stable end product of lipid peroxidation. Accumulative evidences suggest that hyperglycemia in Type 2 DM can produce major changes in nitric oxide (NO) production as well as in its action. Alteration in metabolism of trace elements is also observed in DM.
Objective:
To evaluate oxidative stress, status of NO, and trace elements zinc (Zn) and magnesium (Mg) in type 2 DM and to correlate these parameters with disease process.
Materials and Methods:
Ninety-two cases with diabetes were included in the study, out of which 51 were type 2 DM without any complication and 41 were type 2 DM with complications. Fifty-one nondiabetic healthy controls from hospital staff were selected for the study. Blood samples were collected after an overnight fast for estimation of fasting plasma glucose, postprandial glucose, glycated hemoglobin (HbA1c), lipid profile, trace element status, MDA, and NO.
Results:
Study revealed a rise in MDA levels in both uncomplicated and complicated cases with diabetes (2.47 ± 0.53, 3.98 ± 0.42 nmol/ml, respectively) as compared to controls (1.43 ± 0.23 nmol/ml), which was statistically significant (P < 0.05). The mean levels of NO, Zn, and Mg were significantly lower in both the diabetic groups than the control group (P < 0.05). MDA showed a significant positive correlation with plasma glucose, lipid profile parameters (except high-density lipoprotein cholesterol), and significant negative correlation with Zn (r = −0.44, P < 0.05) and Mg (r = −0.31, P < 0.05). NO levels were correlated significantly with plasma glucose, dyslipidemia, and HbA1c (P < 0.05). The effects of glycemic status on trace element concentrations were evident from a significant negative correlation between Zn and Mg with fasting plasma glucose and HbA1c.
Conclusion:
Findings of the present study may establish the role of hyperglycemia, oxidative stress, impaired NO, and trace elements in pathogenesis and long-term vascular complications of type 2 DM.
Keywords: Lipid peroxidation, malondialdehyde, nitric oxide, oxidative stress, trace element
Introduction
Type 2 diabetes mellitus (DM) is a global health problem affecting more than 200 million individuals worldwide.[1] It is characterized by absolute or relative deficiencies in insulin secretion and/or action. Insulin resistance (IR), considered to be the major contributor to pathogenesis of Type 2 DM, plays a key role in associated metabolic abnormalities such as hyperglycemia, dyslipidemia, and hypertension. There is a widespread understanding that hyperglycemia in Type 2 DM leads to overproduction of free radicals and reactive oxygen species (ROS).[2] Oxidative stress has become a real entity in the etiopathogenesis of DM, which may play an important role in advanced glycation end product (AGE) formation by nonenzymatic glycosylation of proteins.[2] AGEs accumulate in biological system and can cause damage to biological membranes and endothelium. Moreover, ROSs interact with lipid bilayer of cell membrane resulting in lipid peroxidation.[3] Malondialdehyde (MDA) is a stable end product of lipid peroxidation.[4] Thus, excess free radicals and the resultant oxidative stress along with elevated MDA explain the long-term vascular complications in DM, which is a major cause of mortality and morbidity.[5]
Endothelial dysfunction is considered to be an intrinsic element in the pathogenesis of diabetes complications. The free radical nitric oxide (NO), accounting to the biological activity of endothelium-derived relaxing factor, is well recognized for its association with vascular disease.[6] There is accumulating evidence that hyperglycemia in Type 2 DM can produce major change in NO production as well as in its action.[7] Moreover, a complex interaction exists between NO and ROS within the microenvironment of vessel wall, thereby contributing to the reduced bioavailability of NO leading to the development of long-term diabetic complications.[8]
Metal ions are known to play an essential role in living systems, both in growth and metabolism. Emerging evidences suggest an alteration of metabolism of several trace elements and their association in metabolic dysfunction in DM.[9] It is still unclear whether it is the consequence of DM or can lead to expression of DM.[10] Moreover, several studies have also documented a possible role of these minerals in the pathogenesis and progress of the disease.[11] Zinc (Zn), one of the essential trace elements, has been found to be associated with DM in a compromised state.[12] Similarly, several researchers have reported a compelling association between diabetes and hypomagnesemia as magnesium (Mg) plays an important role in enzymatic reactions of glucose metabolism.[13]
Taking into consideration of the above facts, the present study was undertaken with an objective to assess the oxidative stress (MDA), NO level, and trace element status (Zn and Mg) in patients with Type 2 diabetes. In addition, it investigated the possible association between these parameters in the disease process, which might have utility to delay progression of diabetes complications and in the treatment of this complex disorder.
Materials and Methods
This case–control study is single center and conducted in a tertiary health-care hospital over a period of 3 months between October and December 2015. As per sample size calculation, a total number of ninety-two patients with Type 2 diabetes aged between 40 and 65 years were selected for the study. Cases were diagnosed on the basis of history, physical examination, and biochemical investigation as per WHO criteria.[14] Out of 92 cases, 51 cases were Type 2 DM without complications and 41 cases were Type 2 DM with complications such as retinopathy, nephropathy, neuropathy, and atherosclerosis.
Exclusion criteria for cases
Patients taking antioxidants, anticonvulsants, steroid, and thyroxine
Patients with acute metabolic complications such as hypoglycemia, ketoacidosis, cerebro vascular accident, and acute infection
Primary hypertensives
Smokers and alcoholics.
A total number of 51 age- and sex-matched apparently healthy nondiabetic participants with normal blood sugar level from hospital staff, not receiving any kind of trace element and antioxidant supplementation, were taken as control. They were nonhypertensive, nonsmoker, and nonalcoholics. The Institutional Ethics Committee approval was obtained before study setup.
Blood sample collection and processing
Under aseptic condition, after overnight fasting, 5 ml of ethylenediaminetetraacetic acid (EDTA) venous blood samples in purple vacutainers, 1 ml of oxalate-fluoride samples in gray vacutainers, and 2 ml of samples in red vacutainers were collected from all study participants after taking informed written consent. Each sample was centrifuged at 4000 rmp for 10 min to collect plasma and serum, respectively. It was stored at −20°C in capped aliquots with wax tape around them for airtight packing until analysis. All the samples were analyzed on the same day of collection for the following biochemical laboratory investigations:
Plasma MDA which was estimated by Satoh.[15] It is a spectrophotometric assay. The lipid peroxidation product MDA formed a characteristic pink-chromogenic adduct with thiobarbituric acid and trichloroacetic acid which as measured colorimetrically after butanol extraction with a Systronics spectrophotometer at 532 nm. Reference range: 0.3–1.3 nmol/ml
Plasma NO which was estimated by Ding et al.[16] NO is immediately oxidized to its stable end products nitrite and nitrate. This assay was based on nitrite levels. Analysis was done by reaction of nitrite with a mixture of sulphanilamide and Napthyl ethylenediamine dihydrochloride to give a color complex, which was measured by a spectrophotometer at 570 nm. Reference range: 11–15 μmol/L
Zn and Mg were analyzed using a Varian Spectra model AA-220 atomic absorption spectrophotometer.[17] Reference range for Zn: 55–110 μg/dL and for Mg: 0.9–2.2 mg/dL
Fasting plasma glucose (FPG), 2 hourly postprandial plasma glucose (2hr PPPG), and lipid profile (triglyceride [TG], total cholesterol [TC], high-density lipoprotein cholesterol [HDL-C], and low-density lipoprotein cholesterol [LDL-C]) were done by Cobas-400 fully automated analyzer by respective kits from Roche Diagnostics
Glycated hemoglobin (HbA1c) was estimated with EDTA blood sample by Bio-Rad D-10 high-performance liquid chromatography.[18]
For adequate quality control of results, both normal and abnormal reference control serum solutions and calibrator were run at the beginning of each shift. That apart, initial calibration and routine maintenance were carried out periodically as recommended by manufacturer.
Statistical analysis
Results were expressed as mean ± standard deviation (SD) and were statistically analyzed using software SPSS 16.0 software (Chicago II, USA) and Microsoft Excel. Student's unpaired t-test was used to analyze comparison between the groups. Relationships between the variables were evaluated using Pearson's correlation coefficient. A P < 0.05 was considered to be statistically significant.
Results
Studied patients were divided into two groups: Group 1 consisting of 51 uncomplicated cases (37 males and 14 females) and Group 2 consisting of 41 complicated cases (32 males and 9 females). The mean duration of the disease was 3.15 ± 2.7 years (6 months to 7 years) in Group 1 patients and 7.89 ± 2.72 years (3–12 years) in Group 2 patients. Mean age was 47.54 ± 6.99, 52.68 ± 7.43, and 63.04 ± 5.71 years in controls, Group 1, and Group 2, respectively. Details of the mean values along with the SDs for various biochemical parameters in the two diabetic groups as well as in the control are shown in Table 1. A significant rise was noted in FPG, 2hr PPPG, TG (P < 0.001), and TC (P < 0.05) in both the diabetic group in comparison to control indicating a chronic hyperglycemia and dyslipidemia state. There was highly statistical significant difference in HbA1c, MDA, NO, Zn, and Mg among the three groups [Table 1]. Plasma MDA noted to be raised significantly in both uncomplicated and complicated DM as compared to healthy controls. While NO, Zn, and Mg showed a significant decrease (P < 0.05) in both the study groups as compared to control as shown in Table 1. Correlation of MDA and NO with various parameters was shown in Table 2. MDA was observed to document a statistically positive correlation with FPG, TC, TG, and HbA1c. Similarly, MDA registered a significant negative correlation with NO, Zn, and Mg. That apart, NO depicted a significant negative correlation with FPG (P < 0.001), TC and TG (P < 0.05). Table 3 revealed a significant negative correlation between Zn and Mg with other parameters such as FPG, HbA1c, and MDA.
Table 1.
Comparison of biochemical parameters in study group and control
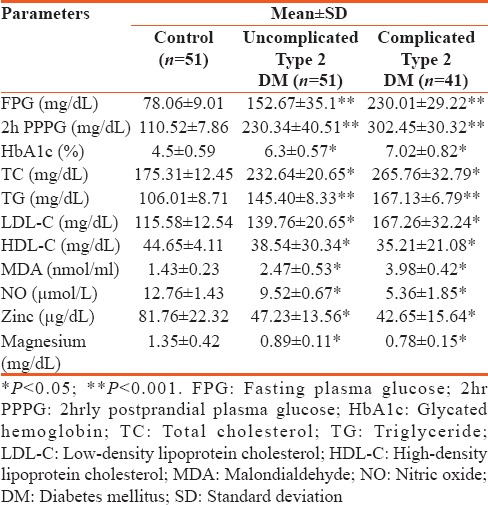
Table 2.
Correlation of malondialdehyde and nitric oxide with biochemical parameters
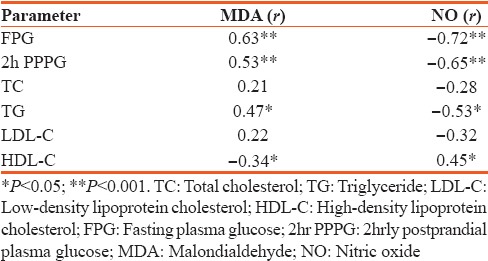
Table 3.
Correlation of zinc and magnesium with biochemical parameters
Discussion
The present study was undertaken with an objective to evaluate plasma MDA, NO, and trace elements (Zn and Mg) status and its association with the disease process in Type 2 DM patients. Hyperglycemia documented by a highly significantly raised FPG and 2hr PPPG (P < 0.001) in both the cases with diabetes Type 2 along with an increased HbA1c level (P < 0.05), and marked dyslipidemia was noted in the study population which is a quintessential feature of Type 2 DM. These metabolic derangements may be contributed by impaired insulin secretion, IR in adipose tissues, and increased hepatic glucose production along with increased free fatty acid flux from adipocytes resulting in increased lipid synthesis in hepatocytes.[19] Significant rise in HbA1c, a long-term index of glycemia, was found to be still higher in complicated DM, suggesting a long-term hyperglycemia. This agrees to the findings by other authors in this regard.[20] MDA showed a significant increase (P < 0.05) in both uncomplicated and complicated DM as compared to the control group [Table 1], explaining the generation of free radicals with increased lipid peroxidation during the course of disease process. This finding may be supported by Kumawat et al.,[21] who found out highly significantly increased (P < 0.001) MDA levels in Type 2 DM patients with and without complications. Hence, it can be stated that extent of oxidative stress and occurrence of complication in Type 2 diabetes are dependent on metabolic control of diabetes. This is supported by another study by Aydin et al. who reported similar significantly elevated MDA in poor glycemic control diabetic participants as compared to controls.[8] The contributory role of hyperglycemia in glucose autoxidation, free radical production, generation of toxic ROS, and genesis of oxidative stress has been demonstrated by a significant positive association (P < 0.001) of MDA with FPG, PPPG [Figure 1], and HbA1c in the present study. We also registered a statistically significant correlation of MDA with several lipid parameters [Table 2], which strengthens the fact that dyslipidemia might lead to increased lipid peroxidation resulting in genesis of ROS, responsible for long-term vascular complications in DM.[3]
Figure 1.
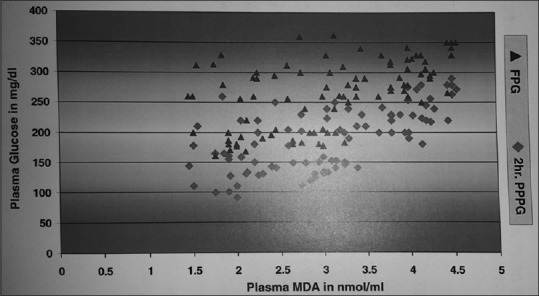
Correlation between malondialdehyde with plasma glucose in patients with Type 2 diabetes. Statistically significant correlation indicating the role of hyperglycemia causing oxidative stress in patients with diabetes
Plasma nitrite, the end product of NO, was noticed to be lower in both the study group of diabetes, more so in complicated group as compared to controls. This potentiates the reduced bioavailability NO due to decreased NO synthesis and increased NO inactivation, contributing to endothelial dysfunction in DM.[22] This argument may be strengthened the finding of Ghosh et al., who reported significant difference in NO, lipid profile parameters between healthy controls, and patients with diabetes in Sikkim population.[23] Another study by Vanizor et al. observed decreased NO end products (P < 0.01) in patients with diabetes Type 2 without any complication.[24] Significant negative correlation between NO and plasma glucose in our study explains the quenching effect of NO by hyperglycemia-induced AGEs [Table 2 and Figure 2]. This may be attributed to impaired NO synthase (NOS) activity and so reduced NO generation due to IR.[25] These findings are in agreement with Tessari et al., who stated that hyperglycemia may also play a role in decreased NO production by inhibiting endothelial NOS activity in glomeruli through protein kinase C-associated mechanism.[26] The present data revealed a statistically significant (P < 0.05) association of NO with lipid parameters [Table 2]. This is in harmony with the finding that dyslipidemia and oxidation of lipids may lead to diminished NO synthesis, thus leading to increased oxidative stress and vascular complications in diabetes. The observed statistically significant negative correlation between MDA and NO (r = −0.84, P < 0.001) in our study [Figure 3] reveals the quenching phenomena of ROS on NO to form peroxynitrite with its decreased bioavailability.[8] Thus, reduced NO production and raised MDA due to hyperglycemia, dyslipidemia, and oxidative stress in diabetes may exacerbate endothelial dysfunction and accelerate vascular complications in Type 2 DM.[2]
Figure 2.
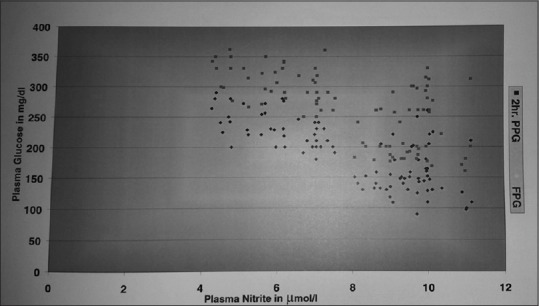
Correlation between nitric oxide with plasma glucose in patients with diabetes. Significant negative correlation explains the quenching effect of nitric oxide by hyperglycemia in patients with diabetes
Figure 3.
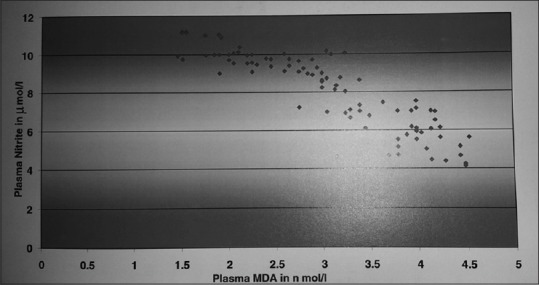
Correlation study between malondialdehyde and nitric oxide in patients with Type 2 diabetes mellitus. Above significant negative correlation justifies reduced bioavailability of nitric oxide due to the generation of reactive oxygen species in patients with diabetes
The present data revealed a significantly low Zn and Mg level in study cases (P < 0.05) as compared to healthy controls as shown in Table 1. These results were consistent with those obtained in other studies, conforming their role in the development of DM as well as the influence of glycemic status on them.[27,28,29] Slightly lower levels of the above parameters have been observed in patients with vascular complications when compared with patients with diabetes without complications. These results may indicate the role of trace elements as an additional risk factor in the progress of disease contributing to the complications. As regards Zn, it can be stated that hyperglycemia-induced decreased Zn level might be attributed to increased urinary loss and decreased active transport to tubular cell.[27] The present study revealed a negative correlation was noted with FPG, HbA1c, TC, TG, and LDL-C while with HDL-C, it was a positive correlation. This agrees with Al-Maroof and Al-Sharbatti who found a definite improvement in glycemic status after Zn supplementation to patients with diabetes Type 2.[30] As Zn has an important role in insulin synthesis, so it has been postulated to affect glucose tolerance and IR.[31] Zn supplementation plays an important role in preventing free radical-induced oxidative damage and chronic complications of DM.[32] This finding was supported by a statistically negative correlation between Zn and MDA (r = −0.44, P < 0.05) in the present study. This finding is in agreement with Salem et al., who stated similar statistically significant correlation (P < 0.05), concluding the role of impaired Zn metabolism in contributing the progression of diabetic complications.[33] Furthermore, it might be supported by the presence of Zn in many antioxidant enzymes such as catalase, peroxidase, and dismutase. Hence, Zn deficiency might lead to oxidative stress, which mediates most of the complications of diabetes.
As regards Mg, the present results observe a statistically significant negative correlation with FPG, HbA1c, and MDA (r = −0.41, r = −0.36, and r = −0.31, respectively). These results are consistent with the findings of Kamal et al.,[34] who stated that deficiency of trace elements may play a role in pathogenesis of DM. Furthermore, above findings may be attributed to the association of Mg deficiency with IR, carbohydrate intolerance, and dyslipidemia.[27] Thus, the impaired trace element metabolism of the present work may have a role in pathogenesis of DM by enhancing lipid peroxidation and oxidative stress, which might lead to vascular complications of diabetes.
Limitation of the study
Our study is limited by single center and small sample size. Additional parameters such as total antioxidant status, AGE product, and other trace elements such as copper and selenium will be added in our future research work, which might help elucidate the underlying mechanism.
Conclusion
Hyperglycemia in Type 2 DM is associated with accelerated nonenzymatic glycation, oxidative stress, and reduced bioavailability of NO and altered trace element metabolism. All these parameters might profoundly influence metabolic dysfunction, pathogenesis, and vascular complications of diabetes. Hence, there may be a place for trace element supplementation as an effective therapeutic intervention, in preventing complications in Type 2 DM along with control of other risk factors. Future research and adequately powered randomized placebo-controlled double-blind studies with trace element supplementation are required to explore the effect. Although several mechanisms represent a common pathway in the development of long-term complications in DM, there is a need for future longitudinal study to understand the casual relationship between oxidative stress, trace element, NO, and vascular complications in diabetes. Nevertheless, we hope the mentioned findings will provide a better insight into pathogenesis of DM and help in detecting complications at an early stage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Quinn L. Behavior and biology: The prevention of type 2 diabetes. J Cardiovasc Nurs. 2003;18:62–8. doi: 10.1097/00005082-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Cheeseman KH, Slater TF. An introduction to free radical biochemistry. Br Med Bull. 1993;49:481–93. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 3.West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000;17:171–80. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–14. [PubMed] [Google Scholar]
- 5.Wolff SP, Jiang ZY, Hunt JV. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med. 1991;10:339–52. doi: 10.1016/0891-5849(91)90040-a. [DOI] [PubMed] [Google Scholar]
- 6.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–12. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 7.McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, et al. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:771–6. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- 8.Aydin A, Orhan H, Sayal A, Ozata M, Sahin G, Isimer A. Oxidative stress and nitric oxide related parameters in type II diabetes mellitus: Effects of glycemic control. Clin Biochem. 2001;34:65–70. doi: 10.1016/s0009-9120(00)00199-5. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Seif MA, Youssef AA. Evaluation of some biochemical changes in diabetic patients. Clin Chim Acta. 2004;346:161–70. doi: 10.1016/j.cccn.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Karahan SC, Deger O, Orem A, Uçar F, Erem C, Alver A, et al. The effects of impaired trace element status on polymorphonuclear leukocyte activation in the development of vascular complications in type 2 diabetes mellitus. Clin Chem Lab Med. 2001;39:109–15. doi: 10.1515/CCLM.2001.019. [DOI] [PubMed] [Google Scholar]
- 11.Walter RM, Jr, Uriu-Hare JY, Olin KL, Oster MH, Anawalt BD, Critchfield JW, et al. Copper, zinc, manganese, and magnesium status and complications of diabetes mellitus. Diabetes Care. 1991;14:1050–6. doi: 10.2337/diacare.14.11.1050. [DOI] [PubMed] [Google Scholar]
- 12.Chausmer AB. Zinc, insulin and diabetes. J Am Coll Nutr. 1998;17:109–15. doi: 10.1080/07315724.1998.10718735. [DOI] [PubMed] [Google Scholar]
- 13.Barbagallo M, Dominguez LJ, Galioto A, Ferlisi A, Cani C, Malfa L, et al. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol Aspects Med. 2003;24:39–52. doi: 10.1016/s0098-2997(02)00090-0. [DOI] [PubMed] [Google Scholar]
- 14.Diabetes mellitus. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1985;727:1–113. [PubMed] [Google Scholar]
- 15.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 16.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediate and reactive oxygen intermediates from mouse peritoneal macrophages. J Immunol. 1988;141:2407–12. [PubMed] [Google Scholar]
- 17.Evenson ME. Spectrophotometric techniques. In: Burtis CA, Ashwood ER, editors. Teitz Textbook of Clinical Chemistry. 3rd ed. Philadelphia: WB Saunders Co.; 1999. pp. 75–93. [Google Scholar]
- 18.Davis JE, McDonald JM, Jarett L. A high-performance liquid chromatography method for hemoglobin A1c. Diabetes. 1978;27:102–7. doi: 10.2337/diab.27.2.102. [DOI] [PubMed] [Google Scholar]
- 19.Powers AC. Diabetes mellitus. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson J, Loscalzo J, editors. Harrison's Principle of Internal Medicine. 18th ed. New York: McGraw Hill; 2012. pp. 2968–9. [Google Scholar]
- 20.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27:1761–73. doi: 10.2337/diacare.27.7.1761. [DOI] [PubMed] [Google Scholar]
- 21.Kumawat M, Sharma TK, Singh I, Singh N, Ghalaut VS, Vardey SK, et al. Antioxidant enzymes and lipid peroxidation in type 2 diabetes mellitus patients with and without nephropathy. N Am J Med Sci. 2013;5:213–9. doi: 10.4103/1947-2714.109193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991;87:432–8. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh A, Sherpa ML, Bhutia Y, Pal R, Dahal S. Serum nitric oxide status in patients with type 2 diabetes mellitus in Sikkim. Int J Appl Basic Med Res. 2011;1:31–5. doi: 10.4103/2229-516X.81977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanizor B, Orem A, Karahan SC, Kiran E, Erem C, Aliyazicioglu R, et al. Decreased nitric oxide end-products and its relationship with high density lipoprotein and oxidative stress in people with type 2 diabetes without complications. Diabetes Res Clin Pract. 2001;54:33–9. doi: 10.1016/s0168-8227(01)00281-9. [DOI] [PubMed] [Google Scholar]
- 25.Tsai DC, Chiou SH, Lee FL, Chou CK, Chen SJ, Peng CH, et al. Possible involvement of nitric oxide in the progression of diabetic retinopathy. Ophthalmologica. 2003;217:342–6. doi: 10.1159/000071349. [DOI] [PubMed] [Google Scholar]
- 26.Tessari P, Cecchet D, Cosma A, Vettore M, Coracina A, Millioni R, et al. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Diabetes. 2010;59:2152–9. doi: 10.2337/db09-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Praveen S, Pasula S, Sameera K. Trace elements in diabetes mellitus. J Clin Diag Res. 2010;7:1863–5. doi: 10.7860/JCDR/2013/5464.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wälti MK, Zimmermann MB, Spinas GA, Hurrell RF. Low plasma magnesium in type 2 diabetes. Swiss Med Wkly. 2003;133:289–92. doi: 10.4414/smw.2003.10170. [DOI] [PubMed] [Google Scholar]
- 29.Viktorinova A, Toserova E, Krizko M, Durackova Z. Altered metabolism of Copper, Zinc, and Magnesium is associated with increased levels of glycated hemoglobin in patients with diabetes mellitus. Diabetes Mellitus. 2009;58:1477–82. doi: 10.1016/j.metabol.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 30.Al-Maroof RA, Al-Sharbatti SS. Serum zinc levels in diabetic patients and effect of zinc supplementation on glycemic control of type 2 diabetics. Saudi Med J. 2006;27:344–50. [PubMed] [Google Scholar]
- 31.Hashemipour M, Kelishadi R, Shapouri J, Sarrafzadegan N, Amini M, Tavakoli N, et al. Effect of zinc supplementation on insulin resistance and components of the metabolic syndrome in prepubertal obese children. Hormones (Athens) 2009;8:279–85. doi: 10.14310/horm.2002.1244. [DOI] [PubMed] [Google Scholar]
- 32.Anderson RA, Roussel AM, Zouari N, Mahjoub S, Matheau JM, Kerkeni A. Potential antioxidant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus. J Am Coll Nutr. 2001;20:212–8. doi: 10.1080/07315724.2001.10719034. [DOI] [PubMed] [Google Scholar]
- 33.Salem M, Kholoussi S, Kholoussi N, Fawzy R. Malondialdehyde and trace element levels in patients with type 2 diabetes mellitus. Arch Hellenic Med. 2011;28:83–8. [Google Scholar]
- 34.Kamal M, Salem M, Kholousi N, Ashmawy K. Evaluation of trace elements and malondialdehyde levels in typr 2 diabetes mellitus. Diabetes Metab Syndr Clin Res Rev. 2009;3:214–8. [Google Scholar]


