Abstract
The Gag protein of human immunodeficiency virus type 1 contains a 14-amino-acid region, termed SP1, between the capsid and downstream nucleocapsid sequences. Although SP1 is known to be indispensable for virus production, the mechanisms involved are mostly unclear. In this study, we demonstrate that an M368A mutation within SP1 severely diminished the ability of Gag to associate with cellular membranes. Although wild-type levels of membrane binding were restored to the M368A Gag by a second-site L20K mutation within matrix, the resultant Gag mutant L20K-M368A remained defective in virus production. This latter deficit was partially consequent to the binding of L20K-M368A Gag to nonraft membranes as opposed to raft association seen for wild-type Gag. Further analysis revealed that the majority of membrane-bound M368A Gag proteins were small oligomers, indicating a multimerization defect. In support of this observation, purified recombinant Gag derivatives containing the M368A mutation formed much lower amounts of high-molecular-weight complexes that were pelletable at 21,000 × g than did wild-type Gag. Based on the myristyl switch model, we propose that the M368A mutation inhibits Gag multimerization and, as a result, restricts the binding of Gag to cellular membranes.
The formation of human immunodeficiency virus type 1 (HIV-1) particles is driven by the Gag precursor (17). During or shortly after virus budding, Gag is cleaved by viral protease, giving rise to matrix (MA), capsid (CA), nucleocapsid (NC), and p6 as well as two short peptides named SP1 and SP2. This proteolytic event leads to the transformation of the virus particle from an immature form to a mature form. Within mature particles, the MA protein is layered underneath the viral envelope while CA constitutes the shell of the core structure in which NC is tightly associated with viral genomic RNA. In contrast to the immature particles that represent an assembly mode, mature particles are ready to disassemble and to initiate reverse transcription upon entering a new host cell (11).
Three major events take place during virus assembly: binding of Gag to the plasma membrane, Gag multimerization, and virus release from cells. Membrane binding is mediated by a myristyl group that is covalently attached to the second amino acid, glycine, of MA (19, 34). Together with a number of basic residues at the N terminus of MA, the myristyl moiety constitutes the membrane-binding (M) domain (46, 56). Virus release is directed by a tetrapeptide element, “P(T/S)AP,” that is located at the N terminus of p6 (18, 24). This short peptide motif recruits a cellular factor named TSG101 (tumor susceptibility gene 101) to the virus assembly site and, along with other cellular factors such as AIP1/ALIX, catalyzes virus budding from host cells (12, 16, 31, 47, 49, 50). The P(T/S)AP motif is thus designated the late-budding domain.
Gag multimerization involves both protein-RNA and protein-protein interactions. The NC sequence, particularly N-terminal basic residues, binds to RNA and profoundly promotes the clustering of Gag molecules (6-9, 25, 44, 54). The RNA that is bound by NC can be of either viral or cellular origin (37). Owing to its importance in Gag oligomerization, the relevant NC region is termed the interaction domain. This NC function can be enacted by heterogeneous protein sequences that are capable of nucleic acid binding (such as Bacillus subtilis MtrB protein) or by others that mediate protein-protein interactions (such as the leucine zipper sequence of yeast transcription factor GCN4) (2, 53).
Direct Gag-Gag interactions are mediated mainly by the MA and CA regions. MA can form trimers under certain conditions and thus contains a potential site for Gag-Gag contact (22). CA consists of two independently folded regions, termed the N-terminal domain (NTD) and the C-terminal domain (CTD), that are connected by a flexible linker (4, 13, 14, 35). Using electron microscopy and image reconstruction techniques, in vitro assembly experiments performed with various CA derivatives have shown that the NTD of CA forms hexameric rings through intermolecular interactions involving helices 1 and 2 (15, 28). The CTD is poorly resolved in these images, yet it is believed that strong molecular interactions must be initiated by the CTD in order to stabilize the hexameric arrangement. These CTD interactions, at least in part, involve a well-characterized dimer interface accommodated by helix 9. Formation of the hexagonal lattice may represent a basic principle not only for the construction of capsid cores within mature particles but also for the generation of immature virus by the Gag precursor (32, 33).
In addition to the three major domains of Gag, i.e., MA, CA, and NC, the 14-amino-acid SP1 region also plays an indispensable role in virus production. For instance, deletion of SP1 largely eliminates virus assembly (26). The results of computer modeling and genetic studies suggest that SP1 may function in the context of an α-helix across the CA-SP1 junction (1). In support of this notion, six residues of the 13 amino acids that comprise the above-mentioned helix are essential for generation of virus particles (29). However, it is unclear how SP1 affects the assembly of HIV-1 particles. In this study, we demonstrate that mutation of the CA-SP1 boundary sequence inhibits both the multimerization and membrane-binding activities of Gag.
MATERIALS AND METHODS
Plasmid construction.
The BH10 infectious HIV-1 cDNA clone was employed as starting material to generate the L20K mutation. This mutation was engineered by PCR using primers pBSSH-S and pL20K-A (5′-CTGAAGCGCGCACGGCAAGAGG-3′ and 5′-CCCCCTGGCCTCTTCCGAATTTTTTCCC-3′, respectively). The resultant PCR products were used as primers in a second round of PCR together with an antisense primer, pSph-A (5′-GGCCCTGCATGCACTGGATGC-3′). The final PCR products were digested with restriction enzymes BssHII and SphI and inserted into a BH10 protease-negative mutant [BH10(D25A) containing a D25A mutation in the protease sequence (36). Primers were purchased from Invitrogen (Burlington, Ontario, Canada).
The mini-Gag DNA construct lacks the MA (positions 8 to 131) and the N-terminal domain of CA (positions 132 to 277). To engineer this DNA construct, PCR was performed by using primers BssH-S (5′-CTGAAGCGCGCACGGCAAGAGG-3′) and DMA (5′-GCACATGCATG CTACTGACGCTCTCGCACCCATCTCTCTCC-3′) to delete the Gag sequence at positions 8 to 218. The PCR products were digested with BssHII and SphI and inserted into BH10 containing a protease-negative mutation D25A (36). To further remove Gag sequence at positions 219 to 277, a second PCR was conducted with primers DCA (5′-GCACATG CATGCAGCCCTACCAGCATTCTGGACATAAGACAAGG ACC-3′) and NC-A (5′-TTAGCCTGTCTCTCGTACAATC-3′), and the PCR DNA products were inserted into the protease-negative mutant BH10(D25A) following digestion with SphI and ApaI.
Cell culture and transfection.
COS-7 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. Transfection was performed by using Lipofectamine (Invitrogen) in accordance with the manufacturer's instructions.
Membrane flotation centrifugation.
Membrane flotation centrifugation experiments were performed as described previously (40). In brief, transfected COS-7 cells were washed three times with cold phosphate-buffered saline and suspended in ice-cold TNE buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM EDTA, 0.1% 2-mercaptoethanol, and protease inhibitor cocktails (Roche Diagnostics, Laval, Quebec City, Canada). After Dounce homogenization on ice, cell homogenates were clarified at 3,000 rpm for 30 min at 4°C in a Beckman GS-6R centrifuge to remove cell debris and nuclei. The supernatants were adjusted to 73% sucrose and loaded at the bottom of the ultracentrifuge tube. After being layered with 65 and 10% sucrose solution, the samples were centrifuged at 100,000 × g for 16 h at 4°C. Eight fractions were then collected from the top of the tube, and the membrane-associated materials at the 10%-65% interface were harvested as fraction 2.
The samples from fraction 2 were then mixed with 85.5% sucrose to a final 73% sucrose concentration. After being loaded at the bottom of a 12-ml ultracentrifugation tube, the samples were layered with 1.5 ml of 65% sucrose followed by 1.5 ml of 50%, 1.5 ml of 40%, 1.5 ml of 30%, 1.5 ml of 20%, and 0.75 ml of 10% sucrose solutions. Centrifugation was performed at 100,000 × g for 16 h at 4°C, after which 21 fractions were collected from the top of the gradient.
Immunoblots.
Samples were fractionated on sodium dodecyl sulfate-10% polyacrylamide gels. After proteins were transferred onto polyvinylidene difluoride membranes (Roche), Western blots were performed with the following antibodies: (i) mouse anti-p24 antibodies (ID Labs Inc., London, Ontario, Canada), (ii) mouse anti-HisG antibodies (Invitrogen), (iii) mouse anti-caveolin-1 antibodies (Sigma-Aldrich), and (iv) rabbit anti-transferrin receptor antibodies (Santa Cruz Biotechnology, Santa Cruz, Calif.).
Gag purification and in vitro assembly assay.
An HIV-1 Gag derivative containing coding sequences for the first two to seven amino acids of MA, the CTD of CA (positions 278 to 364), SP1 (positions 365 to 378), and NC (positions 379 to 433) was cloned into the expression vector Topo 100-D (Invitrogen) as described previously (43). This truncated form of Gag was termed His-mGag. After transformation into Escherichia coli BL21(DE3), expression of His-mGag was induced by adding IPTG (isopropyl-β-d-thiogalactopyranoside). Three hours postinduction, the His-mGag proteins were purified by using nickel-nitrilotriacetic acid resin (QIAGEN, Mississauga, Ontario, Canada) in accordance with the manufacturer's instructions. The purified His-mGag proteins were refolded through dialysis in a buffer containing 0.5 M Tris-HCl (pH 8.0), 50 mM NaCl, 10 μM ZnCl2, 5/0.5 mM reduced/oxidized glutathione, and 0.01% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} at 4°C for 3 h followed by centrifugation at 57,000 × g to remove any misfolded or aggregated Gag proteins.
An in vitro assembly assay was performed as described previously (7). Briefly, 100 μg of Gag proteins was incubated with 4 μg of 24-nucleotide oligonucleotides (5′-GGGCCCCAGTACTTACCAGGAAGG-3′) at room temperature for 3 h in 100 μl of buffer containing 0.1 M NaCl, 0.5% NP-40, and 10 mM dithiothreitol. The reaction mixtures were then centrifuged at 21,000 × g for 1 h at 4°C to pellet any oligomerized Gag. The supernatants and pelleted materials were analyzed by Western blot using anti-HisG antibodies (Invitrogen).
Confocal microscopy.
COS-7 cells were transfected with Gag-green fluorescent protein (GFP) followed by fixation in 4% paraformaldehyde (in phosphate-buffered saline) at room temperature for 20 min. After washing with phosphate-buffered saline, cells were directly visualized by using a Zeiss LSM 5 PASCAL laser-scanning confocal microscope. Images were recorded from laser-scanned cell layers with a thickness of 0.9 μm.
RESULTS
Mutation of SP1 diminishes membrane binding of Gag.
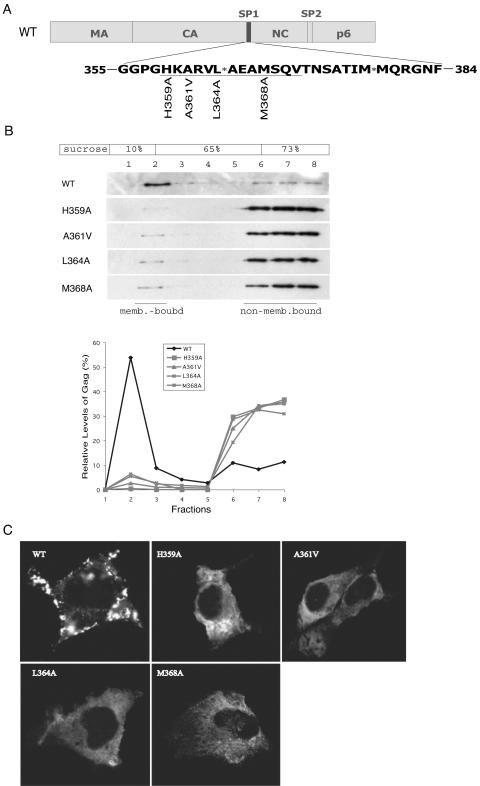
We have previously identified a series of point mutations within the CA-SP1 boundary region that impair HIV-1 production (29). To understand the basis for this severe deficit, we performed membrane flotation assays to measure the membrane-binding ability of several forms of mutated Gag proteins, including those containing H359A, A361V, L364A, and M368A mutations (Fig. 1A). In addition, the above-described mutants were tested in an HIV-1 DNA clone containing a protease-negative mutation, D25A, in order to better follow the migration of Gag precursor in the sucrose gradient (36). The results shown in Fig. 1B show that less than 5% of the mutated Gag proteins, as opposed to approximately 40% of wild-type Gag, were membrane associated. Consistent with this reduced membrane-binding ability, confocal microscopy revealed that the mutated Gag proteins exhibited a dispersed distribution pattern within the cytoplasm, whereas the wild-type Gag proteins were mostly associated with the plasma membrane in a punctate state (Fig. 1C). We conclude that mutagenesis of SP1 and adjacent CA sequences severely attenuated Gag-membrane association, a defect that likely accounted for the impairment in virus production.
FIG. 1.
The H359A, A361V, L364A, and M368A mutations diminish the membrane-binding capacity of HIV-1 Gag. (A) Schema of the H359A, A361V, L364A, and M368A mutations. The domain structures of Gag are illustrated at the top, while the sequences of the CA-SP1-NC junctions are shown at the bottom. WT, wild type. (B) Effects of the H359A, A361V, L364A, and M368A mutations on the membrane association of Gag. Cell homogenates were first clarified by centrifugation at 1,000 × g for 30 min at 4°C to remove cell debris and nuclei followed by membrane flotation centrifugation at 100,000 × g at 4°C for 16 h. Eight fractions were collected from the top of the sucrose gradient, and membrane-associated material was harvested from fraction 2. Gag proteins within each fraction were detected by Western blot using anti-p24 (capsid) antibodies. Relative levels of Gag were determined through use of the NIH Image program, and the results are summarized in the graph. (C) Subcellular distribution of wild-type and mutated Gag proteins within COS-7 cells. The GFP gene was appended to the C terminus of Gag (20), and fusion proteins were visualized by laser-scanning confocal microscopy. The images shown represent the most prevalent phenotypes obtained.
A second-site L20K mutation within MA restores wild-type levels of membrane binding to the SP1-mutated Gag proteins.
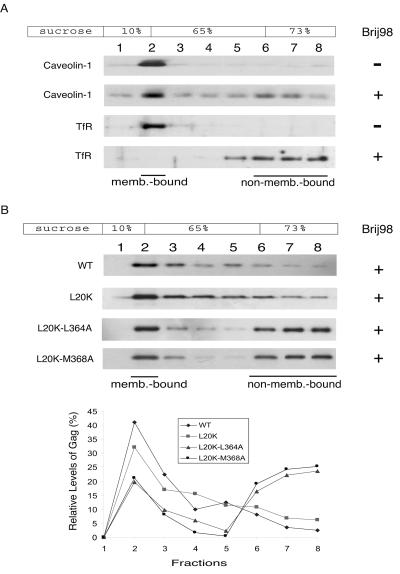
If the decreased membrane binding was the sole cause for the impairment in virus production, correction of the former deficit should restore viral assembly capacity. To test this concept, a second-site L20K mutation within MA was combined with either L364A or M368A mutations (Fig. 2A). L20K has been shown to increase the membrane binding of Gag to substantial levels (Fig. 2B) (40). Consistently, when L20K was combined with either L364A or M368A, the resultant Gag mutants L20K-L364A and L20K-M368A displayed nearly wild-type levels of membrane association as shown by the results of both membrane flotation assay and confocal microscopy (Fig. 2B and C). In contrast, substantially lower levels of virus than those of the wild type were produced when either the L20K-L364A or L20K-M368A mutations were present. (Fig. 2D). Therefore, the L20K-L364A and L20K-M368A mutations result in defective virus production, even though Gag proteins containing these changes can bind cellular membranes at wild-type levels.
FIG. 2.
The L20K mutation within MA restores wild-type (WT) membrane binding to the L364A- and M368A-containing Gag proteins. (A) Illustration of the L20K, L20K-L364A, and L20K-M368A Gag proteins. (B) The L20K-L364A and L20K-M368A Gag proteins bind cellular membranes as efficiently as wild-type Gag. Levels of membrane-associated Gag proteins were determined by performing membrane flotationcentrifugation. Relative amounts of Gag within each fraction are summarized in the graph. (C) Subcellular distribution of Gag mutants L20K-L364A and L20K-M368A. GFP was linked to the C terminus of Gag protein, and the location of fusion proteins was determined by confocal microscopy. The images shown represent the most prevalent phenotypes. (D) Virus production in transfection experiments performed with DNA constructs that yield wild-type and mutated Gag proteins. Levels of cell- and virion-associated Gag proteins were assessed by Western blot using anti-p24 (capsid) antibodies. The HIV-1 DNA constructs tested contain a protease-negative D25A mutation and thus only produce the Pr55Gag protein when transfected into COS-7 cells.
It has been reported that HIV-1 Gag binds to raft-like membrane domains (23, 30, 39, 41). We speculated that the L20K-L364A and L20K-M368A Gag mutants might have been targeted to nonraft membrane regions and were thus unable to produce virus particles in an efficient manner. To test this possibility, membrane-bound Gag proteins were isolated in a first round of flotation centrifugation followed by extraction with 0.5% Brij 98 at 37°C for 5 min prior to a second round of membrane flotation. It has been previously reported that Brij 98 treatment at 37°C is able to solubilize the nonraft membranes while preserving the raft domains (23). This finding is further shown by the opposing behaviors of a nonraft marker, i.e., transferrin receptor, and a raft marker, i.e., caveolin-1 (Fig. 3A). In contrast to the observation that the vast majority of wild-type as well as L20K Gag proteins remained membrane bound after Brij 98 extraction (Fig. 3B), greater than 75% of either L20K-L364A Gag or L20K-M368A Gag dissociated from the membranes (Fig. 3B). This latter observation can be due to either the association of the mutant Gag with nonraft membrane domains or the weak interactions between the mutant Gag molecules. Each of these two potential defects may lead to diminished virus production.
FIG. 3.
The membrane-bound L20K-L364A and L20K-M368A Gag proteins are sensitive to Brij 98 extraction. Membrane-associated materials were harvested in the first round of a membrane flotation experiment followed by incubation at 37°C with or without 0.5% Brij 98 for 5 min. The samples were then subjected to a second round of membrane flotation centrifugation. Western blots were performed to detect the distribution of caveolin-1, transferrin receptor (TfR), or Gag proteins in the sucrose gradient. (A) Caveolin-1, but not transferrin receptor, is resistant to Brij 98 treatment. (B) Substantial amounts of L20K-L364A and L20K-M368A Gag proteins dissociated from membranes after Brij 98 extraction. Relative levels of Gag in each fraction are shown in the graph. Incubation at 37°C in the absence of Brij 98 did not affect the stability of the membrane-associated wild-type (WT) and mutated Gag proteins (data not shown).
SP1-mutated Gag proteins form a membrane-associated complex of lower density than wild-type Gag.
The above-described results indicate that L364A- and M368A-containing Gag proteins displayed reduced affinity for cellular membranes and were targeted to Brij 98-sensitive nonraft membrane domains. To obtain further insights into these membrane-binding defects, we tried to separate membrane-bound Gag complexes through a 10%-20%-30%-40%-50%-65% multilayer sucrose gradient. The results of Fig. 4 show that wild-type Gag migrated from the 73% sucrose fraction at the bottom of the ultracentrifuge tube up through fractions 9 to 12 with densities of 1.17 to 1.23 g/ml. In contrast, M368A Gag was much more broadly distributed in the gradient, with approximately 35% of this molecule being located within fractions 4 to 8 at densities of 1.07 to 1.16 g/ml (Fig. 4). Similar findings were obtained for the Gag mutants H359A, A361V, and L364A (data not shown). Therefore, mutation of the CA-SP1 boundary leads to the formation of low-density membrane-associated Gag complexes that are rarely seen with wild-type Gag.
FIG. 4.
Separation of membrane-bound Gag proteins by multilayer sucrose gradient flotation centrifugation. Membrane-bound Gag proteins were first isolated by 10%-65%-73% sucrose gradient flotation centrifugation followed by incubation at 37°C with or without 0.5% Brij 98 for 5 min. The samples were then assessed in a second round of flotation using the 10%-20%-30%-40%-50%-65% sucrose gradient. Twenty-one fractions were collected from the top of each gradient, and the distribution of Gag was assessed by Western blot using anti-p24 (capsid) antibodies. Relative levels of Gag are shown in the graph. The density of each fraction was measured by refractometer (Carl Zeiss). WT, wild type.
Conceivably, formation of the low-density Gag-membrane complexes obtained with M368A might result either from the distinct composition of the membranes that were generated or from defective multimerization properties of the mutated Gag protein. The results of Fig. 3B demonstrate that significant amounts of the L20K-M368A mutant Gag proteins might be targeted to nonraft membranes, supporting the first of these alternate hypotheses. To verify that this membrane-targeting deficit of L20K-M368A Gag was caused solely by M368A, we treated the membrane-associated M368A Gag with Brij 98 prior to a further fractionation through the 10%-20%-30%-40%-50%-65% multilayer sucrose gradient. The results shown in Fig. 4 show that over 90% of wild-type Gag proteins were still able to float from the 73% sucrose fraction after such a treatment, and their distribution was now shifted to fractions of higher density. In contrast, approximately 50% of the mutant Gag molecules remained within the bottom fractions, i.e., fractions 17 to 21 (Fig. 4). The L20K-M368A Gag exhibited moderately higher ability to float in the sucrose gradient with Brij 98 treatment than the M368A Gag (Fig. 4), which is likely due to the wild-type association of L20K-M368A Gag with membranes (Fig. 2). Since the Brij 98-resistant rafts contain substantial levels of cholesterol and sphingolipids that are not present in the nonraft membranes, the absence of these particular lipid components may partially account for the low densities of the non-raft-associated M368A Gag complexes.
The M368A mutation inhibits Gag multimerization.
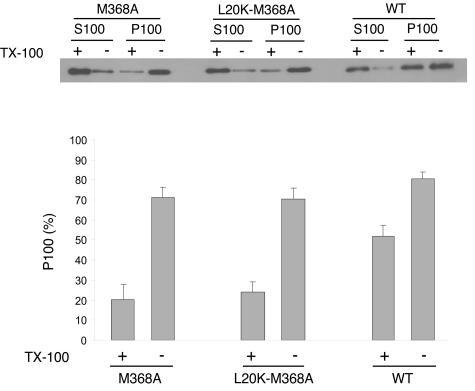
It is possible that the high-density wild-type Gag complexes observed after Brij 98 extraction shown in Fig. 4 represent the capsid structure depleted of membranes. In this context, the low proportion of Brij 98-treated M368A Gag observed in fractions 11 to 16 implicates defective Gag multimerization (Fig. 4). To test this possibility, we tried to determine the multimerization levels of membrane-associated Gag proteins. Membrane-associated materials were isolated by using the membrane flotation assay and treated with Triton X-100 at 37°C for 10 min in order to extract all membrane components. The resultant membrane-free Gag proteins were unable to float to the 10%-65% sucrose layer interface after flotation centrifugation (data not shown). The samples were then centrifuged at 100,000 × g to separate monomeric from multimerized Gag proteins. Figure 5 shows that more than 50% of wild-type Gag was recovered in the pellet fraction (P100), indicating that the majority of wild-type Gag proteins assembled into large complexes on cellular membranes. In contrast, less than 25% of membrane-associated M368A and L20K-M368A Gag were pelletable at 100,000 × g after Triton X-100 extraction (Fig. 5). Therefore, the M368A mutation significantly restricts the ability of Gag to multimerize on membranes, and the L20K mutation does not fix this defect.
FIG. 5.
Size determination of membrane-bound Gag complexes. Membrane-bound Gag proteins were first isolated by membrane flotation and extracted with 1% Triton X-100 at 37°C for 10 min to deplete membranes. Membrane-free Gag proteins were further centrifuged at 100,000 × g for 1 h at 4°C. The resultant supernatants (termed S100) and pellets (termed P100) were assayed by Western blot using anti-p24 (capsid) antibodies. Relative quantities of the M368A, L20K-M368A, and wild-type Gag proteins in the P100 fraction were assessed by using the NIH Image program, and the results are shown in the graph. WT, wild type.
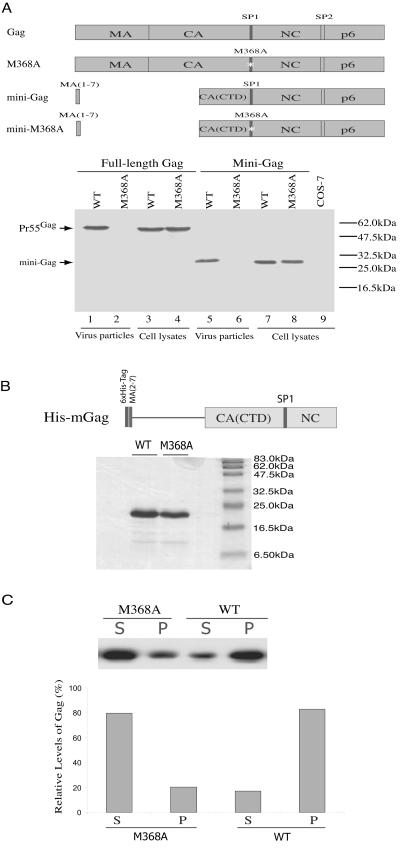
This multimerization defect of M368A Gag was further studied by using in vitro assembly assays. Previous studies have shown that a mini-Gag construct containing the first seven amino acids of MA, the CTD of CA, SP1, NC, and p6 is capable of efficient particle production (5). We further demonstrated that insertion of the M368A mutation into this mini-Gag sequence impeded virus production (Fig. 6A). Since the role of p6 is to catalyze virus budding, and since this function can be performed by different types of late domains such as PPPY from Rous sarcoma virus in the context of mini-Gag (2), it follows that the multimerization activity of mini-Gag must be associated with the CA (CTD)-SP1-NC sequences. On this basis, we constructed a His-tagged HIV-1 Gag derivative named His-mGag that contained the first two to seven amino acids of MA, the CTD of CA, SP1, and NC to study Gag multimerization in vitro (Fig. 6B). When wild-type His-mGag was purified and incubated together with DNA oligonucleotides under proper buffer conditions (7), over 80% of the proteins were able to form complexes that pelleted at 21,000 × g (Fig. 6C). In contrast, less than 20% of M368A His-mGag proteins were recovered in the pelletable fraction in a parallel experiment. This result demonstrates the adverse effect of the M368A mutation on Gag multimerization.
FIG. 6.
Effect of the M368A mutation on Gag multimerization. (A) The M368A mutation impedes particle production by the HIV-1 mini-Gag. The mini-Gag lacks Gag sequence at positions 8 to 277. Mini-M368A contains the M368A point mutation within SP1. The viral protease-negative BH10(D25A) DNA constructs containing either the wild type (WT) or the mutated Gag sequences were transfected into COS-7 cells. Cell lysates and virus particles in the culture supernatants were assessed by Western blot using anti-p24 (capsid) antibodies. (B) Depiction of the His-tagged HIV-1 Gag derivative termed His-mGag. The top depicts a truncated version of Gag protein containing the first two to seven amino acids of MA, the CTD of CA, SP1, and NC sequences, appended to a six-His tag. Purified wild-type His-mGag protein as well as a mutated version containing the M368A mutation were separated on sodium dodecyl sulfate-15% polyacrylamide gels and stained with Coomassie blue. The sizes of protein markers are indicated on the right. (C) Multimerization of purified Gag protein incell-free reactions. One hundred micrograms of Gag was incubated with the 24-nucleotide DNA oligonucleotides at room temperature for 2 h followed by centrifugation at 21,000 × g at 4°C for 1 h. Supernatants (S) and pellets (P) were assessed by Western blot to determine levels of multimerized Gag proteins. Relative amounts of Gag in the S and P fractions are shown in the graph.
DISCUSSION
Lentiviral Gag proteins contain a short spacer between their CA and NC domains which has been shown to play an indispensable role in the production of HIV-1 and bovine immunodeficiency virus (1, 20, 26, 29). In this study, we demonstrate that mutation of the CA-NC spacer sequence inhibits both the multimerization and membrane-binding activities of HIV-1 Gag, and we thus obtain further insight into the mechanism for the action of this short region in virus assembly.
The role of SP1 in HIV-1 Gag-membrane association is shown by the adverse effect of an M368A point mutation on this process (Fig. 1). SP1 is located far downstream of the M domain that resides at the N terminus of MA. Considering that Gag precursors are aligned underneath the plasma membrane in a lateral manner, with the N terminus attached to the membrane and the C terminus projecting away, SP1 is spatially separated from the M domain. It thus seems unlikely that SP1 directly interacts with the M domain. An alternative possibility is that SP1 may influence M domain conformation by promoting Gag multimerization.
Indeed, a correlation between Gag multimerization and Gag-membrane binding has been proposed in a “myristyl switch” model (21, 42, 45, 57). On the basis of this model, the myristyl group is buried inside the monomeric Gag molecule. Upon Gag multimerization, this myristate moiety is exposed and thus profoundly increases the affinity of Gag for cellular membranes. This hypothesis is directly supported by the nuclear magnetic resonance (NMR)-resolved structures of myristoylated Gag derivatives in which oligomerization of a Gag-like molecule (including amino acids 1 to 362) leads to an entropic switch of the myristate from a sequestered to an exposed state and thus significantly promotes Gag binding to membranes (48). This concept is further supported by the similar structures between MA and its N-myristoylated form as determined by NMR (52). We believe that the substantially decreased membrane-binding ability of M368A Gag is a consequence of its defective capacity for multimerization. Indeed, the data in Fig. 5 and 6 clearly show that this mutant Gag exhibits a severe impairment in multimerization.
Two structural features of SP1 may have contributed to its involvement in Gag multimerization, i.e., the ability to form an α-helix together with the adjacent CA sequence (1) as well as the structural flexibility in the context of the CTD of CA and NC (38, 51). Supporting the importance of this α-helix in Gag assembly, insertion of either a glycine or a proline residue into this region profoundly inhibits virus production (1). In particular, six amino acids within this short helical region, including H359A, K360, A361, L364, A367, and M368, are essential for the generation of virus particles (29), indicating that this α-helix is involved in sequence-specific interactions. We now provide direct evidence that a purified Gag derivative containing the M368A mutation is severely impaired in forming large complex (Fig. 6C). More recently, the NMR-resolved structure of a peptide containing the last 21 amino acids of CA and SP1 and the first few residues of NC showed that the hydrophobic residues, e.g., L364 and M368, are located on one face of the helix, while hydrophilic residues, e.g., H359, R362, and E366, are found on another face (N. Morellet et al., Abstr. 186, Proceedings of the 2004 Meeting on Retroviruses, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.). One implication of this structure is that residues within the CA-SP1 helix are capable of mediating Gag-Gag interactions through both hydrophobic and hydrophilic interactions.
On the other hand, the last 11 amino acids of CA together with the downstream SP1 sequence were observed as a disordered region in the crystal structure of CA146-231-SP1 as well as in the NMR structure of CA (CTD)-SP1-NC (38, 51). One interpretation of this finding is that SP1 has a high structural flexibility, and this feature may be required by Gag in order to construct a particle for the following reasons. The CTD of CA folds into a very compact and rigid structure (14, 35). This structure is involved in the formation of a hexagonal CA lattice that is employed to generate the core and possibly the immature particle as well (15, 28, 32, 33). In contrast to this highly ordered geometric structure, the downstream NC region assumes variable conformations when binding to different RNA sequences such as SL2 and SL3 packaging signals (3, 10). Since NC-RNA interactions can bring Gag molecules in close proximity and thus profoundly promote Gag multimerization, it follows that considerable constraints are involved in accommodating the regular CA lattice and downstream NC-RNA complexes of heterogeneous conformations within the restricted space of a virus particle. Presumably, SP1 together with the C-terminal 11 amino acids of CA can release these constraints by acting as a structurally flexible linker and thus represent a key element in Gag multimerization.
In summary, the results of this study demonstrate the indispensable roles of the SP1 sequence in HIV Gag multimerization as well as in Gag-membrane binding. In light of the recent success of discovering HIV inhibitors that act by delaying the cleavage of SP1 at the late stage of virus maturation (27, 55), understanding how SP1 participates in Gag assembly will help to identify novel compounds to antagonize HIV replication.
Acknowledgments
We thank Brian Udashkin for the assistance in performing confocal microscopy.
This research was supported by grants from the Canadian Institutes of Health Research (CIHR) and by the Fonds de la Recherche en Sante du Quebec (FRSQ). C.L. is a New Investigator of the CIHR and a Chercheur-Boursier of the FRSQ.
REFERENCES
- 1.Accola, M. A., S. Höglund, and H. G. Göttlinger. 1998. A putative α-helical structure which overlaps the capsid-p2 boundary in the human immunodeficiency virus type 1 Gag precursor is crucial for viral particle assembly. J. Virol. 72:2072-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accola, M. A., B. Strack, and H. G. Göttlinger. 2000. Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain. J. Virol. 74:5395-5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amarasinghe, G. K., R. N. De Guzman, R. B. Turner, K. J. Chancellor, Z. R. Wu, and M. F. Summers. 2000. NMR structure of the HIV-1 nucleocapsid protein bound to stem-loop SL2 of the psi-RNA packaging signal. Implications for genome recognition. J. Mol. Biol. 301:491-511. [DOI] [PubMed] [Google Scholar]
- 4.Berthet-Colominas, C., S. Monaco, A. Novelli, G. Sibai, F. Mallet, and S. Cusack. 1999. Head-to-tail dimers and interdomain flexibility revealed by the crystal structure of HIV-1 capsid protein (p24) complexed with a monoclonal antibody Fab. EMBO J. 18:1124-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borsetti, A., A. Ohagen, and H. G. Gottlinger. 1998. The C-terminal half of the human immunodeficiency virus type 1 Gag precursor is sufficient for efficient particle assembly. J. Virol. 72:9313-9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burniston, M. T., A. Cimarelli, J. Colgan, S. P. Curtis, and J. Luban. 1999. Human immunodeficiency virus type 1 Gag polyprotein multimerization requires the nucleocapsid domain and RNA and is promoted by the capsid-dimer interface and the basic region of matrix protein. J. Virol. 73:8527-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell, S., and A. Rein. 1999. In vitro assembly properties of human immunodeficiency virus type 1 Gag protein lacking the p6 domain. J. Virol. 73:2270-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell, S., and V. M. Vogt. 1995. Self-assembly in vitro of purified CA-NC proteins from Rous sarcoma virus and human immunodeficiency virus type 1. J. Virol. 69:6487-6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cimarelli, A., S. Sandin, S. Hoglund, and J. Luban. 2000. Basic residues in human immunodeficiency virus type 1 nucleocapsid promote virion assembly via interaction with RNA. J. Virol. 74:3046-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Guzman, R. N., Z. R. Wu, C. C. Stalling, L. Pappalardo, P. N. Borer, and M. F. Summers. 1998. Structure of the HIV-1 nucleocapsid protein bound to the SL3 psi-RNA recognition element. Science 279:384-388. [DOI] [PubMed] [Google Scholar]
- 11.Freed, E. O. 1998. HIV-1 Gag proteins: diverse functions in the virus life cycle. Virology 251:1-15. [DOI] [PubMed] [Google Scholar]
- 12.Freed, E. O. 2002. Viral late domains. J. Virol. 76:4679-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gamble, T. R., F. F. Vajdos, S. Yoo, D. K. Worthylake, M. Houseweart, W. I. Sundquist, and C. P. Hill. 1996. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell 87:1285-1294. [DOI] [PubMed] [Google Scholar]
- 14.Gamble, T. R., S. Yoo, F. F. Vajdos, U. K. von Schwedler, D. K. Worthylake, H. Wang, J. P. McCutcheon, W. I. Sundquist, and C. P. Hill. 1997. Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein. Science 278:849-853. [DOI] [PubMed] [Google Scholar]
- 15.Ganser, B. K., S. Li, V. Y. Klishko, J. T. Finch, and W. I. Sundquist. 1999. Assembly and analysis of conical models for the HIV-1 core. Science 283:80-83. [DOI] [PubMed] [Google Scholar]
- 16.Garrus, J. E., U. K. von Schwedler, O. W. Pornillos, S. G. Morham, K. H. Zavitz, H. E. Wang, D. A. Wettstein, K. M. Stray, M. Cote, R. L. Rich, D. G. Myszka, and W. I. Sundquist. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55-65. [DOI] [PubMed] [Google Scholar]
- 17.Gheysen, D., E. Jacobs, F. de Foresta, C. Thiriart, M. Francotte, D. Thines, and M. De Wilde. 1989. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell 59:103-112. [DOI] [PubMed] [Google Scholar]
- 18.Göttlinger, H. G., T. Dorfman, J. G. Sodroski, and W. A. Haseltine. 1991. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. USA 88:3195-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlinger, H. G., J. G. Sodroski, and W. A. Haseltine. 1989. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 86:5781-5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo, X., J. Hu, J. B. Whitney, R. S. Russell, and C. Liang. 2004. Important role for the CA-NC spacer region in the assembly of bovine immunodeficiency virus Gag protein. J. Virol. 78:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermida-Matsumoto, L., and M. D. Resh. 1999. Human immunodeficiency virus type 1 protease triggers a myristoyl switch that modulates membrane binding of Pr55gag and p17MA. J. Virol. 73:1902-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill, C. P., D. Worthylake, D. P. Bancroft, A. M. Christensen, and W. I. Sundquist. 1996. Crystal structures of the trimeric human immunodeficiency virus type 1 matrix protein: implications for membrane association and assembly. Proc. Natl. Acad. Sci. USA 93:3099-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holm, K., K. Weclewicz, R. Hewson, and M. Suomalainen. 2003. Human immunodeficiency virus type 1 assembly and lipid rafts: Pr55gag associates with membrane domains that are largely resistant to Brij98 but sensitive to Triton X-100. J. Virol. 77:4805-4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, M., J. M. Orenstein, M. A. Martin, and E. O. Freed. 1995. p6Gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J. Virol. 69:6810-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khorchid, A., R. Halwani, M. A. Wainberg, and L. Kleiman. 2002. Role of RNA in facilitating Gag/Gag-Pol interaction. J. Virol. 76:4131-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krausslich, H. G., M. Facke, A. M. Heuser, J. Konvalinka, and H. Zentgraf. 1995. The spacer peptide between human immunodeficiency virus capsid and nucleocapsid proteins is essential for ordered assembly and viral infectivity. J. Virol. 69:3407-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, F., R. Goila-Gaur, K. Salzwedel, N. R. Kilgore, M. Reddick, C. Matallana, A. Castillo, D. Zoumplis, D. E. Martin, J. M. Orenstein, G. P. Allaway, E. O. Freed, and C. T. Wild. 2003. PA-457: a potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing. Proc. Natl. Acad. Sci. USA 100:13555-13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, S., C. P. Hill, W. I. Sundquist, and J. T. Finch. 2000. Image reconstructions of helical assemblies of the HIV-1 CA protein. Nature 407:409-413. [DOI] [PubMed] [Google Scholar]
- 29.Liang, C., J. Hu, R. S. Russell, A. Roldan, L. Kleiman, and M. A. Wainberg. 2002. Characterization of a putative α-helix across the capsid-SP1 boundary that is critical for the multimerization of human immunodeficiency virus type 1 Gag. J. Virol. 76:11729-11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindwasser, O. W., and M. D. Resh. 2001. Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains. J. Virol. 75:7913-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin-Serrano, J., T. Zang, and P. D. Bieniasz. 2001. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 7:1313-1319. [DOI] [PubMed] [Google Scholar]
- 32.Mayo, K., D. Huseby, J. McDermott, B. Arvidson, L. Finlay, and E. Barklis. 2003. Retrovirus capsid protein assembly arrangements. J. Mol. Biol. 325:225-237. [DOI] [PubMed] [Google Scholar]
- 33.Mayo, K., M. L. Vana, J. McDermott, D. Huseby, J. Leis, and E. Barklis. 2002. Analysis of Rous sarcoma virus capsid protein variants assembled on lipid monolayers. J. Mol. Biol. 316:667-678. [DOI] [PubMed] [Google Scholar]
- 34.Mervis, R. J., N. Ahmad, E. P. Lillehoj, M. G. Raum, F. H. R. Salazar, H. W. Chan, and S. Venkatesan. 1988. The gag gene products of human immunodeficiency virus type 1: alignment within the gag open reading frame, identification of posttranslational modifications, and evidence for alternative gag precursors. J. Virol. 62:3993-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Momany, C., L. C. Kovari, A. J. Prongay, W. Keller, R. K. Gitti, B. M. Lee, A. E. Gorbalenya, L. Tong, J. McClure, L. S. Ehrlich, M. F. Summers, C. Carter, and M. G. Rossmann. 1996. Crystal structure of dimeric HIV-1 capsid protein. Nat. Struct. Biol. 3:763-770. [DOI] [PubMed] [Google Scholar]
- 36.Morin, N., E. Cherry, X. Li, and M. A. Wainberg. 1998. Cotransfection of mutated forms of human immunodeficiency virus type 1 Gag-Pol with wild-type constructs can interfere with processing and viral replication. J. Hum. Virol. 1:240-247. [PubMed] [Google Scholar]
- 37.Muriaux, D., J. Mirro, D. Harvin, and A. Rein. 2001. RNA is a structural element in retrovirus particles. Proc. Natl. Acad. Sci. USA 98:5246-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newman, J. L., E. W. Butcher, D. T. Patel, Y. Mikhaylenko, and M. F. Summers. 2004. Flexibility in the P2 domain of the HIV-1 Gag polyprotein. Protein Sci. 13:2101-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ono, A., and E. O. Freed. 1999. Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus. J. Virol. 73:4136-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ono, A., and E. O. Freed. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA 98:13925-13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paillart, J. C., and H. G. Gottlinger. 1999. Opposing effects of human immunodeficiency virus type 1 matrix mutations support a myristyl switch model of Gag membrane targeting. J. Virol. 73:2604-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roldan, A., R. S. Russell, B. Marchand, M. Gotte, C. Liang, and M. A. Wainberg. 2004. In vitro identification and characterization of an early complex linking HIV-1 genomic RNA recognition and Pr55Gag multimerization. J. Biol. Chem. 279:39886-39894. [Online.] [DOI] [PubMed] [Google Scholar]
- 44.Sandefur, S., R. M. Smith, V. Varthakavi, and P. Spearman. 2000. Mapping and characterization of the N-terminal I domain of human immunodeficiency virus type 1 Pr55Gag. J. Virol. 74:7238-7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spearman, P., R. Horton, L. Ratner, and I. Kuli-Zade. 1997. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J. Virol. 71:6582-6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spearman, P., J. J. Wang, N. Vander Heyden, and L. Ratner. 1994. Identification of human immunodeficiency virus type 1 Gag protein domains essential to membrane binding and particle assembly. J. Virol. 68:3232-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strack, B., A. Calistri, S. Craig, E. Popova, and H. G. Gottlinger. 2003. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114:689-699. [DOI] [PubMed] [Google Scholar]
- 48.Tang, C., E. Loeliger, P. Luncsford, I. Kinde, D. Beckett, and M. F. Summers. 2004. Entropic switch regulates myristate exposure in the HIV-1 matrix protein. Proc. Natl. Acad. Sci. USA 101:517-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.VerPlank, L., F. Bouamr, T. J. LaGrassa, B. Agresta, A. Kikonyogo, J. Leis, and C. A. Carter. 2001. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55Gag. Proc. Natl. Acad. Sci. USA 98:7724-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Schwedler, U. K., M. Stuchell, B. Muller, D. M. Ward, H. Y. Chung, E. Morita, H. E. Wang, T. Davis, G. P. He, D. M. Cimbora, A. Scott, H. G. Krausslich, J. Kaplan, S. G. Morham, and W. I. Sundquist. 2003. The protein network of HIV budding. Cell 114:701-713. [DOI] [PubMed] [Google Scholar]
- 51.Worthylake, D. K., H. Wang, S. Yoo, W. I. Sundquist, and C. P. Hill. 1999. Structures of the HIV-1 capsid protein dimerization domain at 2.6 Å resolution. Acta Crystallogr. D Biol. Crystallogr. 55:85-92. [DOI] [PubMed] [Google Scholar]
- 52.Wu, Z., J. Alexandratos, B. Ericksen, J. Lubkowski, R. C. Gallo, and W. Lu. 2004. Total chemical synthesis of N-myristoylated HIV-1 matrix protein p17: structural and mechanistic implications of p17 myristoylation. Proc. Natl. Acad. Sci. USA 101:11587-11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, Y., and E. Barklis. 1997. Effects of nucleocapsid mutations on human immunodeficiency virus assembly and RNA encapsidation. J. Virol. 71:6765-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, Y., H. Qian, Z. Love, and E. Barklis. 1998. Analysis of the assembly function of the human immunodeficiency virus type 1 Gag protein nucleocapsid domain. J. Virol. 72:1782-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou, J., X. Yuan, D. Dismuke, B. M. Forshey, C. Lundquist, K. H. Lee, C. Aiken, and C. H. Chen. 2004. Small-molecule inhibition of human immunodeficiency virus type 1 replication by specific targeting of the final step of virion maturation. J. Virol. 78:922-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou, W., L. J. Parent, J. W. Wills, and M. D. Resh. 1994. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J. Virol. 68:2556-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou, W., and M. D. Resh. 1996. Differential membrane binding of the human immunodeficiency virus type 1 matrix protein. J. Virol. 70:8540-8548. [DOI] [PMC free article] [PubMed] [Google Scholar]