Abstract
The placenta is a critical organ during pregnancy, essential for the provision of an optimal intrauterine environment, with fetal survival, growth, and development relying on correct placental function. It must allow nutritional compounds and relevant hormones to pass into the fetal bloodstream and metabolic waste products to be cleared. It also acts as a semipermeable barrier to potentially harmful chemicals, both endogenous and exogenous. Transporter proteins allow for bidirectional transport and are found in the syncytiotrophoblast of the placenta and endothelium of fetal capillaries. The major transporter families in the human placenta are ATP-binding cassette (ABC) and solute carrier (SLC), and insufficiency of these transporters may lead to deleterious effects on the fetus. Transporter expression levels are gestation-dependent and this is of considerable clinical interest as levels of drug resistance may be altered from one trimester to the next. This highlights the importance of these transporters in mediating correct and timely transplacental passage of essential compounds but also for efflux of potentially toxic drugs and xenobiotics. We review the current literature on placental molecular transporters with respect to their localization and ontogeny, the influence of fetal sex, and the relevance of animal models. We conclude that a paucity of information exists, and further studies are required to unlock the enigma of this dynamic organ.
Keywords: placenta, placental transport, human, ontogeny
Summary Sentence
This review summarises the existing knowledge of human placental molecular transporters (SLC and ABC superfamilies). We highlight areas where greater and more accurate knowledge is required and discuss weaknesses of animal models for the human.
Introduction
The placenta is an important, ephemeral organ, and optimal function is essential for normal fetal development and a successful pregnancy. It is well known that the placenta acts as a conduit to supply nutrients and remove metabolic waste from the fetus. The placenta also synthesizes and secretes hormones such as estrogens, progestogens, human chorionic gonadotrophin, and human placental lactogen which are essential for the establishment and maintenance of the pregnancy. Vitally, it acts as a gate keeper, regulating the flux of drugs and xenobiotic compounds in and out of the fetal compartment. It is the mother that ensures such compounds are present in the fetoplacental environment and their availability greatly depends on maternal lifestyle factors including but not limited to environmental chemicals (e.g., endocrine disruptors and environmental toxins), pharmaceuticals, illicit/recreational drugs, tobacco smoke constituents, and alcohol. It is important to note that such exposures may harm the fetus not only directly but also indirectly by perturbing placental function itself, impacting on fetal health without ever reaching the fetal compartment.
It is increasingly recognized that after parturition the placenta is not simply a waste by-product of pregnancy, but rather an organ that carries a fingerprint about important pregnancy parameters (e.g., pregnancy progression, substance exposure, fetal metabolism). This “placental fingerprint”, unique to every pregnancy, potentially encompasses biomarkers that can be used to make predictions about the offspring's health. For example, the shape and size of the term placenta can predict blood pressure in childhood, with reduced placentation (smaller placentas) resulting in higher systolic blood pressure due to undernutrition [1]. Pregnancy disorders such as intrauterine growth restriction, pre-eclampsia, and even miscarriage remain relatively poorly understood but their etiology is likely to include elements of placental dysfunction [2, 3]. In addition to perinatal adversities, the “Barker Hypothesis” (also known as “fetal origins of adult disease hypothesis”) postulates that long-term health problems can manifest due to perturbed fetal programming [4]. Since placental function is a determinant of both fetal development and postnatal health, a better understanding of placental molecular processes that define the placental fingerprint (i.e., which genetic/molecular/gross morphology markers the placenta has at term) will improve our understanding of these complications and will aid the detection of susceptible individuals.
The placenta is a highly dynamic structure throughout its 9-month lifespan, which not only grows in proportion to the fetus but also changes both structurally and morphologically. The demand for increased feto-maternal communication during gestation dictates branching and development of placental mesenchymal villi into terminal villi [5] thereby increasing the transport epithelial interface. In the later stages of the first trimester, the placenta is fully functional and serves the ever-increasing requirements of the fetus. Global gene expression is adjusted constantly and genes relating to metabolism are highly expressed in the first trimester whereas signal transduction pathways are more prominent in term placenta [6]. Due to a dynamic molecular environment, the profile of transcripts encoding molecular transporters adapts throughout the pregnancy as discussed below.
Transplacental transport
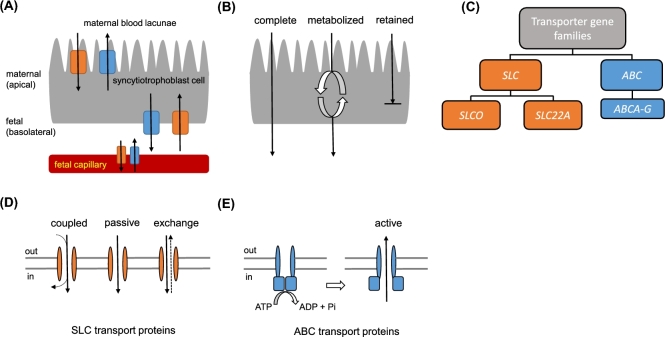
The placenta is the first fetal organ to be fully developed during pregnancy. At around 7–12 days postconception, the epithelium of the placenta, the syncytiotrophoblast, invades the endometrium [7]. The syncytiotrophoblast is the transporting epithelium of the human placenta and its formation initiates an active border between mother and fetus, enabling molecular exchanges between the two. It is composed of an apical brush border which is bathed in a maternal blood lacunae and a basolateral membrane which encircles branches of the fetal capillary tree (Figure 1A). For a molecule to translocate from mother to fetus and vice versa, it must cross the syncytiotrophoblast, where it may be metabolized (Figure 1B), and the endothelium of the fetal capillary. Transplacental transport involves passive diffusion, pinocytosis, and facilitated and active transport via molecular transporter proteins which also support the barrier function of the placenta. Suboptimal placental performance may, therefore, result by disruption of the mechanisms that control those three modes of molecular transportation. Active transport via molecular transport proteins controls the flux of a wide variety of compounds and can be tightly regulated by controlling expression, localization, and activity of these transport proteins. For instance, polarized localization of transporter proteins in the syncytiotrophoblast and the endothelium of the fetal capillary can control directionality of the molecular flux between the mother and the fetus.
Figure 1.
Transporting epithelium of the placenta and molecular transporter function. A molecule must be transported (or diffuse) through the syncytiotrophoblast and endothelium of the fetal capillary to access the fetal compartment. (A) Molecular transporter proteins are positioned in the maternal-facing apical and fetal-facing basolateral membranes and in the endothelium of fetal capillaries. (B) Possible pathways across the syncytiotrophoblast. A molecule can use transporter proteins, passive diffusion, or pinocytosis to cross the syncytiotrophoblast membranes. The molecule can be transported intact or may be metabolized first. The syncytiotrophoblast also retains certain molecules for its own use. (C) Superfamilies of genes coding for molecular transporters found in the placenta. SLC can be divided into gene subfamilies SLCO and SLC22A. ABC consist of seven gene subfamilies: ABCA, ABCB, ABCC, ABCD, ABCE, ABCF, ABCG although ABCE and ABCF are unlikely to have transporter function. A list of corresponding proteins can be found in Table 1. (D) Mechanism of transport for SLC proteins. Compounds can enter/exit the cell coupled to another molecule, passively down its concentration gradient or in exchange for another molecule. SLC transporters can be bidirectional although they mainly allow for placental uptake of molecules. (E) Mechanism of transport for ABC proteins. ABC transporters actively efflux compounds against their concentration gradient. ATP hydrolysis is required for a conformational change of the transporter protein to allow uptake and expulsion of the substrate from the cell.
Solute carriers (SLC) and ATP-binding cassette proteins (ABC) are two major superfamilies of mammalian transporters. A schematic diagram of the SLC and ABC gene families is shown in Figure 1C. SLC transporters are capable of bidirectional transport but mainly deal with influx of substrates (Figure 1D) and can be further classified into subfamilies SLCO and SLC22A. ABC transporters are involved in substrate efflux (Figure 1E) and include subcategories A–F although it is considered unlikely that E and F have transporter function as they do not encode transmembrane domains [8]. Both superfamilies are involved in drug transport with ABC proteins more likely to govern fetal protection due to their active efflux properties, which move harmful xenobiotics/drugs away from the fetal circulation. Although many SLC and ABC transporters have been found in the placenta, very few have been characterized in terms of quantity, localization, gestational expression, degree of functionality, and substrate preference within the placenta. Membrane localization of some transporters has been well defined in the human although there is disagreement in the literature about how expression changes during gestation (Table 1).
Table 1.
ABC and SLC transporters known to show expression in the human placenta.
| Transporter | Syncytiotrophoblast localization | Substrates/function | References |
|---|---|---|---|
| ABCA1 | Apical | Cholesterol, phospholipids | [99–101] |
| ABCB1 | Apical | Drug resistance (antibiotics, antiemetic, cardiac drugs, HIV protease inhibitors) | [15, 16, 28] |
| ABCB4 | Basolateral | Bile acids | [24, 38] |
| ABCC1 | Apical | Folate | [28, 32] |
| ABCC2 | Apical | Folate, bilirubin, role in chemoprotection and detoxification | [32, 34, 102, 103] |
| ABCC3 | Apical | Bilirubin | [102] |
| ABCC4 | Apical | Conjugated bile acids | [102] |
| ABCC5 | Basolateral | Cyclic nucleotides | [35] |
| ABCC7 | Apical | Chloride transport | [104] |
| ABCG1 | Basolateral | Cholesterol, phospholipids | [100] |
| ABCG2 | Apical | Drug resistance | [14, 105] |
| SLCO2A1 | Unknown | Prostaglandin | [48] |
| SLCO2B1 | Basolateral | Sulfated steroids (DHEAS), glutamate | [49, 50] |
| SLCO4A1 | Apical | Thyroid hormones | [51] |
| SLCO1A2 | Apical | Thyroid hormones, bile acids, conjugated steroid hormones | [46, 47] |
| SLCO1B1 | Apical | Bile acids | [24] |
| SLCO3A1 | Apical | Bile acids | [24] |
| SLC22A6 | Unknown | Drug xenobiotic eliminator | [56] |
| SLC22A11 | Basolateral | Sulfated steroids (DHEAS) | [57, 58] |
| SLC22A3 | Basolateral | Cationic compounds | [52] |
| SLC22A5 | Apical | Lactate, folate, carnitine | [53–55] |
| SLC22A4 | Apical | Lactate, folate | [59] |
Over- or underexpression of transporters would be expected to have effects on delivery and clearance of molecules at the maternal–fetal barrier impacting on fetal growth and health. For example, increased molecular transport, as seen in some diabetic mothers [9], could lead to an abnormally large fetus increasing the risk of metabolic syndromes later in life [10]. Conversely, malfunction or underexpression of transporters, or even competition for binding sites between nutrients and xenobiotics [11], could cause suboptimal delivery of nutrients to the fetus and may lead to growth restriction and concomitant health-associated risks for the neonate including cardiovascular risk [12] and type 2 diabetes [13]. These examples highlight the importance of appropriate and timely placental regulation of transporter expression. Recognizing transporter expression dynamics across fetal parameters such as gestation, sex, and maternal lifestyle has the potential to allow us to assess levels of protection offered by the placenta, and to identify instances of increased susceptibility, especially during critical periods such as organogenesis.
This review will discuss molecular transport proteins of the SLC and ABC gene superfamilies in the placenta, their localization and their expression profile across the trimesters. The review concentrates on data derived from studies using human tissue or human-derived cell lines due to the vast interspecies differences in placental structure and function, as discussed below.
ABC superfamily
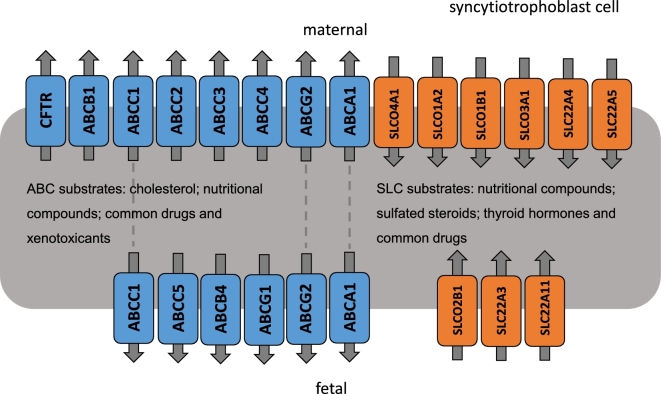
ABC proteins actively transport physiologically relevant compounds such as inorganic ions, glucose, amino acids, metal ions, cholesterol, and phospholipids. In addition to this, they have a protective function by acting as efflux pumps to move pharmaceuticals and xenotoxicants away from the fetus and into the maternal circulation and they are the most widely studied transporter family within the placenta. Over 50 ABC transporters have been identified in humans and three subfamilies are involved in drug/xenobiotic efflux: ABCB, ABCC, and ABCG proteins. Many of those transporters have been found in the human placenta at the transcript and/or protein level (Figure 2).
Figure 2.
Molecular transporter transcripts with confirmed syncytiotrophoblast location in the human placenta and their direction of transport. SLC (orange) and ABC (blue) are found in both apical (maternal-facing) and basolateral (fetal-facing) membranes. As seen by the schematic, understanding of complete transplacental routes for compound movement is incomplete. Grey dashed line indicates the transporter has been found to be expressed on both membranes. It is likely that many molecules must use a combination of transporters to cross the membrane and as many transporters have common substrates, this is entirely possible. Substrates mentioned here are generalized substrates of the superfamilies.
ABCB transporters
The ABCB subfamily is the most extensively studied of the ABC transporters due to its involvement in drug resistance. ABCB1 and its corresponding protein product (also known as P-glycoprotein (P-gp)) are of particular interest in the placenta as they are highly abundant [14]. So much so that ABCB1 is also known as the “ABC placental transporter” and was the first discovered to offer fetal protection against toxicity. The apical membrane of the syncytiotrophoblast is enriched with ABCB1 [15], which supports its function as a drug extrusion pump from the placenta back toward the maternal circulation. This avoids drug build-up in the placenta and escape toward the fetus. Known substrates of ABCB1 include cytotoxic, antiemetic and cardiac drugs, antibiotics, and HIV protease inhibitors [16]. It has been reported that ABCB1 is significantly upregulated (30%) in placentas that are exposed to methadone (with or without heroin and cocaine), a known substrate of this transporter [17]. Many studies have reported that mRNA and protein levels decrease significantly from the first to third trimesters in the human placenta [18–21]. A possible explanation for this marked reduction in human placental ABCB1 could be the early need for retrograde transport of drugs/chemicals during sensitive critical periods of organogenesis.
ABCB4 is involved in bile acid transport and is believed to show drug interaction profiles similar to those of ABCB1 [22]. Its basal location in syncytiotrophoblast cells [23] suggests that it moves molecules out of the placenta, thereby potentially protecting the placenta from chemotherapeutic drugs. ABCB4 transcript expression has been reported to be fourfold higher in the third trimester compared to first [24] although why expression increases towards term is unclear. The importance of this transporter is seen in cases where the mother carries an ABCB4 mutation, causing intrahepatic cholestasis of pregnancy and impaired transplacental bile acid transport which can sometimes result in fetal death [25].
ABCC transporters
The ABCC gene subfamily currently consists of seven multidrug resistance proteins [26] which transport conjugated and unconjugated organic ions and act as drug efflux pumps. Four of these proteins have been well characterized in the human placenta and ABCC1 is more abundant in placenta than in liver or kidney [27]. ABCC transporters are localized on opposing sides of the syncytium which may give us insight to their physiological roles. ABCC2 and ABCC3 can be found on the apical membrane, directing solutes toward the mother whilst ABCC1 and ABCC5 are localized basolaterally and would be expected to move molecules toward the fetus. This means that the latter two transporters would not be expected to play a role in reducing fetal exposure unless there was a change in expression levels. There is also evidence of ABCC1 localization on the apical membrane and high expression levels in the fetal capillary endothelium of both ABCC1 and ABCC3 [28]. These transporters also help in the clearance of biliary substances from fetal liver to maternal circulation, which is essential to avoid harmful build-up [29].
Another important substrate of the ABCC group is folate which is so essential to avoid neural tube defects in the fetus that women are advised to take extra supplementation during the periconceptual period [30]. ABCC1 and ABCC2 assist in folate transport across the maternal–fetal barrier. Currently, studies of ABCC1 expression levels during development are contradictory with levels reported to either increase [31] or decrease [32] with gestational age. If the age-related increase is correct, it could indicate increasing demand during a period of accelerated fetal growth and aligns with clinical guidelines for folic acid supplementation during the early stages of pregnancy. Conversely, if the reported decrease in ABCC1 is correct, this could suggest that this transporter is important in the earlier stages of pregnancy, during establishment of the placenta, and that folate is preferentially moved via other transporters later on. It should be noted that, either way, the fetus has chronic requirements for folic acid as there is evidence that it is required in other processes essential for a progressing pregnancy (e.g., angiogenesis) [33]. Nevertheless, the contradiction between the studies of [31] and [32] needs to be resolved in order to understand the role of ABCC1 in human placental function. ABCC2 is involved in the excretion of conjugated bilirubin and drugs, including anticancer pharmaceuticals [26]. ABCC2 has been shown by two groups to increase in the placenta during pregnancy with an associated increase in bilirubin expulsion, [32, 34]. ABCC5 transports cyclic nucleotides and is in a fetal-facing position on the syncytiotrophoblast; thus, it is unlikely to have a role in fetal protection. ABCC5 and its transcript, ABCC5, decrease across gestation [35].
ABCG transporters
Transporters in this family with identified functions in the placenta include ABCG1, involved in cholesterol transport, and ABCG2 (more commonly known as breast cancer resistance protein (BCRP)) involved with drug resistance. The placenta expresses higher levels of ABCG2 than any other organ [36]. ABCG2 has many exogenous and endogenous substrates and shares some of these with ABCB1. ABCG2 is localized to the apical membrane of syncytiotrophoblasts and the endothelium of fetal capillaries [37]. It has been hypothesized that ABCG2 is the placental “survival factor” during placental maturation and offers protection from cytokine-induced apoptosis [38]. ABCG2 may also be susceptible to maternal bisphenol A and para-nonylphenol exposure both of which are associated with downregulation of the protein [39].
Expression patterns of ABCG2 during pregnancy remain uncertain due to discrepancies in the literature [18, 21, 39–42]. More studies are needed to single out the functionally important reasons for these differences which may simply reflect cohort variation or may be due to differences in methodology. Variations in the normalizing genes used may also add to discrepancies in the literature as there are global, sex-dependent gene changes in the placenta across pregnancy and use of a stable combination of housekeeping genes is essential for the production of meaningful data [43]. Studies which correctly account for fetal sex, cover as many gestational weeks as possible and establish the most stable method of normalization are needed.
SLC superfamily
This superfamily is extremely large with 55 families and 300 members (SLC1-20 SLC22-47 then SLCO2-6 and uncoupling protein UCP1-3) [44] which can be categorized according to Figure 1C. Their positions within the syncytiotrophoblast can be seen in Figure 2. SLCs have been identified in almost every human epithelium and were exhaustively reviewed by Roth et al. in 2012 [45]. Not all of these are found, or are functional, in the human placenta, and this section will only discuss transporters relevant to the in utero environment. Previous studies have reported placental expression of SLCOs (SLCO1A2 [46, 47], SLCO1B1 [24], SLCO3A1 [24], SLCO2A1 [48], SLCO2B1 [49, 50], SLCO4A1 [51], and low expression of SLCO6A1) and SLC22As (SLC22A3 [52], SLC22A5 [53–55], SLC22A6 [56], SLC22A11 [57, 58] with SLC22A1, SLC22A2, SLC22A4 [59] showing weak expression) (see Table 1). The placenta uses these ATP-independent uptake transporters to import hydrophilic or charged molecules into the cell, some of which are then used as hormonal substrates. As previously mentioned, they mainly orchestrate uptake of molecules into the cell using coupled, passive, and exchanger transport (Figure 1B). Substrates of this family are extensive but include many nutritional compounds such as amino acids, glucose and sugars, vitamins, fatty acids, inorganic and organic ions, oligopeptides, sulfated steroids, and thyroid hormones [45]. Common drugs, such as NSAIDS, antibiotics, diuretics, and anti-cancer drugs are transported by SLC transporters [60]. Members of the family can be specific or polyspecific to these substrates and are also involved in drug resistance. Since they transport a wide range of toxins, common drugs, and nutritional compounds, these transporters are extremely important in placental function. Exogenous compounds have also been shown to overcome the maternal–fetal barrier by acting as transport substrates of SLC membrane transporter proteins (i.e., xenobiotics [61]), suggesting that placental expression of those transporters can control the exposure of the fetus to potentially harmful compounds.
Ontogeny of placental SLC transporters
Currently, data are limited with respect to the gestational expression of this family. The human-specific transporter SLC22A11 is found at high levels in kidney and at the basolateral membrane of the syncytiotrophoblast [57], where it imports sulfated dehydroepiandrosterone (DHEAS) into the placenta and away from the fetus [58] along with SLCO2B1, which has 10-fold lower affinity for DHEAS. This uptake is necessary for the placental synthesis of estrogens [62].
SLC22A4 and SLC22A5 are both expressed apically, although their gestational expression profiles are currently unknown. Both transporters import lactate and folate from the mother to the placenta, while SLC22A5 also uptakes L-arginine from the maternal side into the placenta and has been shown to import common drugs e.g., antibiotics and antidepressants [53]. SLC22A3 plays an important role in the transport of cationic compounds, but its full function within the placenta remains unknown although it may be placenta-specific as other tissues have very low expression [63]. SLC22A3 expression in pregnancy decreases between the first and third trimester [64].
Understanding the ontogeny of SLCO transporters is of intense interest due to their role in drug and essential hormone transport. Patel et al. [24] have reported that there are marked changes in SLCO transporter gene expression between the first and third trimester human placenta. Their data showed an 8-fold decrease in SLCO1A2 and a 17-fold decrease in SLCO3A1 (with no change in SLCO1B1 or SLCO4A1) during this period although the study was limited by a very small sample size (eight placentas at 9–12 weeks and six at term). Others have reported, in contrast, that SLCO1A2 increases toward term along with three other thyroid hormone transporters (SLC16A2, SLC16A10, SLC7A5) [65]. This latter study had a much larger sample size (n = 110) but the conflicting results highlight the need for further developmental studies on the placenta to be carried out. Prostaglandin (PG) transport is also mediated by SLCO proteins. SLCO2A1 is involved in the clearance of PG and was found to decrease with gestation [66].
Limitations in placental transport studies
Imperfect models for transport studies
Interspecies placental heterogeneity is considerable and consequently, a nonprimate (specifically non great ape) animal model that fully encompasses human placentation does not exist. This poses the question whether transporter expression and activity measured in an animal model has any relevance with respect to the human. Table 2 compares common animal models used for placental studies. There are obvious advantages to using animal models (in particular, it is just not possible to carry out many studies in the human for practical or ethical reasons), but there are also many drawbacks. For example, the syncytiotrophoblast in humans is not mimicked precisely by other animals, other than great apes, and therefore studies on transporter localization, or indeed time-dependent changes in expression, cannot be confidently extrapolated using nonhuman placental tissue. In addition, all animal models (and particularly rodents) have a shorter gestation than the human. In many cases, this means that they are not born at comparable stages of development so the dynamics of placental development is likely to be very different. Other differences are described in more detail below.
Table 2.
Comparing animal placentas used for comparative studies against the human placenta.
| Model | Days to term | Placental structure | Interhemal layers |
|---|---|---|---|
| Human | 266–280 | Discoid | 3 |
| Mouse/rat | 20–22 | Discoid | 5 |
| Guinea pig | 59–72 | Discoid | 3 |
| Sheep | 147 | Cotyledonary | 6 |
Comparison of gestational timing indicates that offspring are born at different stages of development. Placenta structure is classified according to gross shape and points of maternal contact. Interhemal layers is the number of membranes that separate the maternal and fetal circulations thus representing number of membranes that molecules must either diffuse or be transported across. This highlights difficulties of using animal models to understand transport processes in human placenta. All from maternal to fetal direction. Human: syncytiotrophoblast; cytotrophoblast; fetal capillary endothelium. Mouse/rat: syncytiotrophoblast; additional syncytial layer; sinusoidal giant cell layer. Guinea pig: syncytiotrophoblast; basal lamina; fetal endothelium. Sheep: fetal maternal endothelium; maternal stroma; uterine epithelium; trophoblast layer; fetal stroma; endothelium of fetal capillary.
Along with primates and rodents, the human placenta has a discoid shape and hemochorial nature (maternal blood is in direct contact with syncytiotrophoblast). However, significant differences can be seen in the gross structural morphology between species (Table 2). There are marked differences between human and mouse placenta as previously reviewed [67] but only the dissimilarities affecting transporter studies will be discussed here. Rodents have an additional inverted yolk sac placenta that exists alongside the normal placenta throughout the pregnancy and is essential for proper development of the embryo [68]. It is impossible to separate this secondary structure which can lead to questionable findings in feto-maternal transfer studies. For some of the transport proteins, there are no orthologs between human and rodent and transporters can also be differentially localized, move dissimilar substrates, can be regulated differently, and are differentially responsive to inhibitors [69]. The syncytiotrophoblast in mice follows the same pattern as humans but encompasses an additional syncytial layer and sinusoidal giant cell layer. In the architectural sense, they have a labyrinthine exchange barrier whilst human have a villous barrier. Trophoblast invasion also differs between the species; rats and mice have a single cotyledon and three trophoblast cell layers whilst humans have a cluster of cotyledons and a single syncytial layer [70]. Functionally, human syncytiotrophoblast cells support both endocrine and transporting function but in the mouse these roles are geographically separated into a junctional and labyrinth zone, respectively. One similarity between the mouse and the human is the thinning of the syncytial membranes which increases diffusion capacity toward term, making the late gestation rodent placenta comparable to the human in nutrient diffusion conductance studies (e.g., oxygen) [71].
The guinea pig is also commonly used as a model of placental function and, in particular, for nutrient transport studies in order to understand intrauterine growth restriction [72]. Anatomically, guinea pigs have the complication of a subplacenta [73] (a convoluted area of trophoblasts which is separated from main placenta by a junctional zone) and yolk sac that persists until term. However, guinea pigs have a lengthier gestation than rats and mice and give birth to precocial young and so the placenta must support further developmental processes compared to other rodents and their altricial young [74]. The guinea pig feto-maternal exchange interface is also similar to human with a single layer (hemomonochorial) syncytiotrophoblast (Table 2), although the cytotrophoblast does not persist into the second half of gestation.
The sheep has a similar cotyledon to the human with comparable fetal vasculature [75] and maturity at birth although it is structurally different. The syncytiotrophoblast differs with more maternal layers being retained, which increases the number of layers between maternal and fetal vasculature (Table 2). There is also no maternal blood lacunae so villi do not bathe directly in maternal blood. The syncytiotrophoblast of the sheep is only perfused maternally as fetal cells do not invade the syncytium, although they are interdigitated to aid diffusion. The sheep placenta is not as well studied as, for example, the guinea pig, since amino acid transporters have not been mapped onto the syncytial layers as yet [76].
These species-dependent differences in syncytiotrophoblast development, structure, and function create considerable problems when relating animal studies to an understanding of transfer and transporter activity in the human placenta. There are also other problems to consider when extrapolating to the human. For example, human endocrinology of pregnancy is unique due to the high levels of estrogen generated by placental aromatization of fetal adrenal dehydroepiandrosterone. Other species lack significant placental aromatase (CYP19A1) [67], and this lack of CYP19A1 will also change the metabolic profile of the placenta affecting toxicity/toxicological studies. There are also molecular features which are unique to human, affecting trophoblast invasion (Siglec-6 and IMUP-2) [67].
Overall, the shortcomings of animal models described here demonstrate the need for researchers to use human-derived tissue in studies relating to placentation, common pregnancy disorders, and toxicology. In order to minimize potentially misleading extrapolations from animal models, there have been human models established at both ex vivo and in vitro levels. The term placental perfusion model, although only a representation of the final pregnancy stage, can be used, for example, to study transfer of solutes between mother and fetus [77]. This has been useful, especially, in drug transport studies [78] as it accurately represents the syncytiotrophoblast in a physiological environment. Non-differentiated cell lines have also been used to represent placental function at all weeks of gestation. The BeWo cell line, established in 1968 by Pattillo and Gey [79], is established for placental studies while the Jeg-3 and JAr cell lines are also popular. These three immortalized cell lines are all derived from human choriocarcinoma cells but they differ with respect to proliferation and differentiation. It is vital, therefore, to characterize transporter expression in each cell line in order to make informed decisions about which one to use when studying the placental barrier [27].
Fetal age
There are, of course, a number of problems facing placental studies using human tissue. It is possible to collect tissues from normally progressing elective terminations (with a wide range of upper fetal age limits, from 12–20 weeks in different countries) and from term placentas but both require relevant ethical approvals which can be an arduous process. Even when tissues can be collected, what is generally missing are representative samples from the weeks between the latest elective termination date (after ∼20 weeks in the UK) and preterm births (∼38 weeks). Therefore, data from this period come largely from miscarriages where there are potential confounding factors with respect to fetal and/or placental normality. An unrepresentative age sample is a problem in studying the ontogeny of transporter expression as the transporter of interest may not be expressed during the timeframe leading to potentially erroneous conclusions.
Fetal sex
A review by Clifton and Murphy [80] has stated that disregarding the sex of the fetus whilst studying the placenta is improper practice. While some studies do follow this advice [81, 82], most studies disregard the sex of the fetus and pool placental samples (e.g. [17–19, 23]). Trophoblast cells are derived from the embryo, however, and so they are sex-specific and recent studies have shown a marked difference in placental transcript levels between male and female fetuses [83]. There is also considerable evidence from other studies to suggest functional differences in the placentas from different sexes. As discussed above, influences of the intrauterine environment shape fetal programming and may lead to disease onset later in life [84]. Pertinently, it has been reported that the time to onset and severity of the resulting disorder differ between sexes [85]. Discrepancies in growth ratios between males and females were recognized 50 years ago [86], and altered fetal growth is known to be intimately associated with trophic delivery via the placenta [72]. This raises the question whether some of these sex differences could be explained by preferential transporter expression.
Currently, there are no data that show transporter expression being influenced by fetal sex in the human placenta. However, the endocrine environment of the fetus is sexually dimorphic and placental transporters are known to be regulated by hormones. ABCG2 expression, for example, has been reported to be altered by progesterone and estradiol exposure in the BeWo cell line [87]. Similarly, recent evidence has suggested that the adverse pregnancy outcomes in polycystic ovary syndrome could be caused by hormonal influence on amino acid transport across the placenta due to much higher circulating levels of androgens [88]. As the endocrine environment is established with respect to fetal sex from as early as 8 weeks of gestation, there may be sex-specific differential expression levels of transporters in both the fetus and placenta during pregnancy. A further complication with respect to the sex of the fetus has arisen from a study [89], which reported that housekeeping genes commonly used to normalize human placental qPCR studies differ in expression levels depending on fetal sex. This could either serve to accentuate or reduce sex-specific differences, thereby invalidating the study and highlighting the need to choose normalization strategies carefully.
Medication in pregnancy
There is growing concern about the use of medication during pregnancy with evidence emerging for increasing use and prevalence of medication by pregnant women [90]. Medicating during pregnancy is often necessary to ameliorate acute or chronic illnesses of the mother or even to treat problems with the developing fetus, such as fetal arrhythmia or antiretroviral treatment if the mother is HIV positive. A major problem however is that, for good reasons, pregnant woman are normally excluded from drug trials, leading to a paucity of knowledge on correct dosage and usage of medications in expectant mothers [91]. There is a risk both of underdosing or overdosing and exposing the fetus to potential harm as many drugs are able to reach the fetal compartment [92]. There is also the potential for pregnancy-specific drug interactions as drugs crossing the syncytiotrophoblast could saturate transporters; indeed, certain antibiotics have been shown to block transporters [93]. Exogenous glucocorticoids are the preferred treatment for woman at risk of adverse pregnancy outcomes such as preterm birth and recurrent miscarriages. Glucocorticoids have been shown, however, to reduce amino acid transporter expression in vitro [94], and this altered transporter expression in the placenta could be the reason glucocorticoid use in pregnancy is linked to intrauterine growth restriction [95].
Understanding the developmental expression of the transporters may provide insights into both maternal and fetal medicating, especially with respect to when it is or is not safe to do so. ABC expression, for instance, changes throughout gestation and any decrease in protective drug efflux would aid in drug delivery to the fetal compartment. Nitrofurantoin, for example, is widely used to treat urinary tract infections and continued treatment is necessary, even in pregnancy, since a change in the bacterial environment heightens risk of preterm delivery. Nitrofurantoin is eliminated by ABCG2 [96] so any decrease in ABCG2 expression may harm the fetus, and knowing this time frame (currently a controversy in the literature) could inform medical professionals when to take precaution when prescribing. Analgesics are extensively used during pregnancy (e.g., to ease maternal discomfort) and are considered safe but recent data challenge this. A shortened anogenital distance has been observed, for example, in male fetuses exposed to paracetamol during weeks 8–14 of gestation [97]. The mechanism of this effect is not clear but paracetamol can decrease ABCG2 expression [98] suggesting that it may induce nonphysiological changes to transporter expression with downstream effects on placental function and fetal health—particularly as ABCG2 is one of the key players at the maternal–fetal interface.
In conclusion, there is a need to perform placental transporter studies using human tissue with consideration of fetal sex and with the largest age spectrum achievable. This would uncover more sex and/or time differences in transporter expression, data which the field is either lacking or unable to agree upon. It also may be of interest to study transporter expression in the fetal liver from the same pregnancy as it is the next major gate keeper to prevent fetal exposure, receiving 70% of the blood from the placenta. A better understanding of transporter regulation, both physiologically and pathologically, could shed light on molecular disposition within the placental and fetal compartments. Future studies following these criteria may demonstrate to what extent, and why, transporter expression is up/downregulated over the trimesters. It is also important to relate mRNA and protein levels to transporter activity within the human placenta, an area of research that is far from conclusive. Furthermore, convincing establishment of membrane localization of all identified transporters is required so that an accurate “map” of transport directions can be established.
References
- 1. Winder NR, Krishnaveni GV, Hill JC, Karat CL, Fall CH, Veena SR, Barker DJ. Placental programming of blood pressure in Indian children. Acta Paediatr 2011; 100:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yinon Y, Nevo O, Xu J, Many A, Rolfo A, Todros T, Post M, Caniggia I. Severe intrauterine growth restriction pregnancies have increased placental endoglin levels. Am J Pathol 2008; 172(1):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Redman CWG. Current topics: pre-eclampsia and the placenta. Placenta 1991; 12:301–308. [DOI] [PubMed] [Google Scholar]
- 4. Barker DJP. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition 1997; 13:807. [DOI] [PubMed] [Google Scholar]
- 5. Fox H. Aging of the placenta. Arch Dis Child Fetal Neonatal Ed 1997; 77(3):F165–F170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mikheev AM, Nabekura T, Kaddoumi A, Bammler TK, Govindarajan R, Hebert MF, Unadkat JD. Profiling gene expression in human placentae of different gestational age: an OPRV network and UW SCOR study. Reprod Sci 2008; 15:866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Konkel L. Lasting impact of an ephemeral organ: the role of the placenta in fetal programming. Environ Health Perspect 2016; 124(7):A124–A129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res 2001; 42:1007–1017. [PubMed] [Google Scholar]
- 9. Gaccioli F, Lager S, Powell T, Jansson T. Placental transport in response to altered maternal nutrition. J Dev Orig Health Dis 2013; 4(2):101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pettitt DJ, Nelso RG, Mf Saad, Bennett PH, Knowler WC. Diabetes and obesity in the offspring of pima indian woman with diabetes during pregnancy. Diabetes Care 1993; 16:310–314. [DOI] [PubMed] [Google Scholar]
- 11. Prouillac C, Lecoeur S. The role of the placenta in fetal exposure to xenobiotics: importance of membrane transporters and human models for transfer studies. Drug Metab Dispos 2010; 38:1623–1635. [DOI] [PubMed] [Google Scholar]
- 12. Barker DJP, Godfrey KM, Gluckman PD, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993; 341:938–941. [DOI] [PubMed] [Google Scholar]
- 13. Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol 2007; 165(8):849–857. [DOI] [PubMed] [Google Scholar]
- 14. Mao Q. BCRP/ABCG2 in the placenta: expression, function and regulation. Pharm Res 2008; 25(6):1244–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Staud F, Cerveny L, Ceckova M. Pharmacotherapy in pregnancy; effect of ABC and SLC transporters on drug transport across the placenta and fetal drug exposure. J Drug Target 2012; 20:736–763. [DOI] [PubMed] [Google Scholar]
- 16. Ceckova-Novotna M, Pavek P, Staud F. P-glycoprotein in the placenta: expression, localization, regulation and function. Reprod Toxicol 2006; 22:400–410. [DOI] [PubMed] [Google Scholar]
- 17. Malek A, Obrist C, Wenzinger S, von Mandach U. The impact of cocaine and heroin on the placental transfer of methadone. Reprod Biol Endocrinol 2009; 7(61):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mathias AA, Hitti J, Unadkat JD. P-glycoprotein and breast cancer resistance protein expression in human placentae of various gestational ages. Am J Physiol Regul Integr Comp Physiol 2005; 289:R963–R969. [DOI] [PubMed] [Google Scholar]
- 19. Gil S, Saura R, Forestier F, Farinotti R. P-glycoprotein expression of the human placenta during pregnancy. Placenta 2005; 26:268–270. [DOI] [PubMed] [Google Scholar]
- 20. Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. Expression of the multidrug resistance P-glycoprotein, (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta 2006; 27:602–609. [DOI] [PubMed] [Google Scholar]
- 21. Lye P, Bloise E, Dunk C, Javam M, Gibb W, Lye SJ, Matthews SG. Effect of oxygen on multidrug resistance in the first trimester human placenta. Placenta 2013; 34:817–823. [DOI] [PubMed] [Google Scholar]
- 22. Kimura Y, Morita SY, Matsuo M, Ueda K. Mechanism of multidrug recognition by MDR1/ABCB1. Cancer Sci 2007; 98:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Evseenko DA, Paxton JW, Keelan JA. ABC drug transporter expression and functional activity in trophoblast-like cell lines and differentiating primary trophoblast. Am J Physiol 2006; 290(5):R1357–R1365. [DOI] [PubMed] [Google Scholar]
- 24. Patel P, Weerasekera N, Hitchins M, Boyd CAR, Johnston DG, Williamson C. Semi quantitative expression analysis of MDR3, FIC1, BSEP, OATP-A, OATP-C, OATP-D, OATP-E and NCTP gene transcripts in 1st and 3rd trimester human placenta. Placenta 2003; 24:39–44. [DOI] [PubMed] [Google Scholar]
- 25. Jacquemin E, Cresteil D, Manouvrier S, Boute O, Hadchouel M. Heterozygous non-sense mutation of the MDR3 gene in familial intrahepatic cholestasis of pregnancy. Lancet 1999; 353:210–211. [DOI] [PubMed] [Google Scholar]
- 26. Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst 2000; 92:1295–1302. [DOI] [PubMed] [Google Scholar]
- 27. Serrano MA, Macias RIR, Briz O, Monte MJ, Blazquez AG, Williamson C, Kubitz R, Marin JJG. Expression in human trophoblast and choriocarcinoma cell lines, BeWo, Jeg-3 and JAr of genes involved in the hepatobiliary-like excretory function of the placenta. Placenta 2007; 28:107–117. [DOI] [PubMed] [Google Scholar]
- 28. St-Pierre MV, Serrano MA, Macias RI, Dubs U, Hoechli M, Lauper U, Meier PJ, Marin JJ. Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol Regul Integr Comp Physiol 2000; 279:R1495–R1503. [DOI] [PubMed] [Google Scholar]
- 29. Macias RI, Marin JJ, Serrano MA. Excretion of biliary compounds during intrauterine life. World J Gastroenterol 2009; 15:817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reider MJ. Prevention of neural tube defects with periconceptional folic acid. Clin Perinatol 1994; 21:483–503. [PubMed] [Google Scholar]
- 31. Pascolo L, Fernetti C, Pirulli D, Crovella S, Amoroso A, Tiribelli C. Effects of maturation on RNA transcription and protein expression on four MRP genes in human placenta and in BeWo cells. Biochem Biophys Res Commun 2003; 303:259–265. [DOI] [PubMed] [Google Scholar]
- 32. Williams PJ, Mistry HD, Morgan L. Folate transporter expression decreases in the human placenta throughout pregnancy and in pre-eclampsia. Pregnancy Hypertens 2012; 2:123–131. [DOI] [PubMed] [Google Scholar]
- 33. Sasaki K, Duan J, Murohara T, Ikeda H, Shintani S, Shimada T, Akita T, Egami K, Imaizumi T. Rescue of hypercholesterolemia-related impairment of angiogenesis by oral folate supplementation. J Am Coll Cardiol 2003; 42(2):364–372. [DOI] [PubMed] [Google Scholar]
- 34. Meyer zu Schwabedissen HE, Jedlitschky G, Gratz M, Haenisch S, Linnemann K, Fusch C, Cascorbi I, Kroemer HK. Variable expression of MRP2 (ABCC2) in human placenta: influence of gestational age and cellular differentiation. Drug Metab Dispos 2005; 33(7):896–904. [DOI] [PubMed] [Google Scholar]
- 35. Meyer zu Schwabedissen HE, Grube M, Heydrich B, Linnemann K, Fusch C, Kroemer HK, Jedlitschky G. Expression, localization and function of MRP5 (ABCC5), a transporter for cyclic nucleotides, in human placenta and cultured human trophoblasts: effects of gestational age and cellular differentiation. Am J Pathol 2005; 166:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res 1998; 58:5337–5339. [PubMed] [Google Scholar]
- 37. Morrissey KM, Wen CC, Johns SJ, Zhang L, Huang SM, Giacomini KM. The UCSF-FDA TransPortal: a public drug transporter database. Clin Pharmacol Ther 2012; 92(5):545–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Evseenko DA, Murthi P, Paxton JW, Reid G, Emerald BS, Mohankumar KM, Lobie PE, Brennecke SP, Kalionis B, Keelan JA. The ABC transporter BCRP/ABCG2 is a placental survival factor, and its expression is reduced in idiopathic human fetal growth restriction. Faseb J 2007; 21:3592–3605. [DOI] [PubMed] [Google Scholar]
- 39. Sieppi E, Vahakangas K, Rautio A, Letta F, Paulesu L, Myllynen P. The xenoestrogens, bisphenol A and para-nonylphenol, decrease the expression of the ABCG2 transporter protein in human term placental explant cultures. Mol Cell Endocrinol 2016; 429:41–49. [DOI] [PubMed] [Google Scholar]
- 40. Yeboah D, Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. Expression of breast cancer resistance protein (BCRP/ABCG2) in human placenta throughout gestation and at term before and after labor. Can J Physiol Pharmacol 2006; 84:1251–1258. [DOI] [PubMed] [Google Scholar]
- 41. Petrovic V, Kojovic D, Cressman A, Piquette-Miller M. Maternal bacterial infections impact expression of drug transporters in human placenta. Int Immunopharmacol 2015; 26(2):349–356. [DOI] [PubMed] [Google Scholar]
- 42. Meyer zu Schwabedissen HE, Grube M, Dreisbach A, Jedlitschky G, Meissner K, Linnemann K, Fusch C, Ritter CA, Volker U, Kroemer HK. Epidermal growth factor-mediated activation of the map-kinase cascade results in altered expression and function of ABCG2 (BCRP). Drug Metab Dispos 2006; 34:524–533. [DOI] [PubMed] [Google Scholar]
- 43. Sitras V, Fenton C, Paulssen R, Vartun A, Acharya G. Differences in gene expression between first and third trimester human placenta: a microarray study. PLoS One 2012; 7:e33294 doi: 10.1371/journal.pone.0033294. pmid:22442682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. He L, Vasiliou K, Nebert DW. Analysis and update of the human solute carrier (SLC) gene superfamily. Hum Genomics 2009; 3:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol 2012; 165:1260–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Obaidat A, Roth M, Hagenbuch B. The expression and function of organic anion transporting polypeptides in normal tissues and in cancer. Annu Rev Pharmacol Toxicol 2012; 52:135–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang H, Yan Z, Dong M, Zhu X, Wang H, Wang Z. Alteration in placental expression of bile acids transporters OATP1A2, OATP1B1, OATP1B3 in intrahepatic cholestasis of pregnancy. Arch Gynecol Obstet 2012; 285(6):1535–1540. [DOI] [PubMed] [Google Scholar]
- 48. Phillips RJ, Fortier MA, Bernal AL. Prostaglandin pathway gene expression in human placenta, amnion and choriodecidua is differentially affected by preterm and term labour and by uterine inflammation. BMC Pregnancy Childbirth 2014; 14:241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. St-Pierre MV, Hagenbuch B, Ugele B, Meier PJ, Stallmach T. Characterization of an organic anion-transporting polypeptide (OATP-B) in human placenta. J Clin Endocrinol Metab 2002; 87(4):1856–1863. [DOI] [PubMed] [Google Scholar]
- 50. Lofthouse EM, Brooks S, Cleal JK, Hanson MA, Poore KR, O’Kelly IM, Lewis RM. Glutamate recycling may drive organic anion transport on the basal membrane of human placental syncytiotrophoblast. J Physiol 2015; 593(20):4549–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sato K, Sugawara J, Sato T, Mizutamari H, Suzuki T, Ito A, Mikkaichi T, Onogawa T, Tanemoto M, Unno M, Abe T, Okamura K. Expression of organic anion transporting polypeptide E (OATP-E) in human placenta. Placenta 2003; 24(2-3):144–148. [DOI] [PubMed] [Google Scholar]
- 52. Sata R, Ohtani H, Tsujimoto M, Murakami H, Koyabu N, Nakamura T, Uchiumi T, Kuwano M, Nagata H, Tsukimori K, Nakano H, Sawada Y. Functional analysis of organic cation transporter 3 expressed in human placenta. J Pharm Exp Ther 2005; 315(2):888–895. [DOI] [PubMed] [Google Scholar]
- 53. Grube M, Schwabedissen HM, Draber K, Prager D, Moritz KU, Linnemann K, Fusch C, Jedlitschky G, Kroemer HK. Expression, localization, and function of the carnitine transporter OCTN2 (SLC22A5) in a human placenta. Drug Metab Dispos 2005; 33:31–37. [DOI] [PubMed] [Google Scholar]
- 54. Settle P, Mynett K, Speake P, Champion E, Doughty IM, Sibley CP, D’Souzs SW, Glazier J. Polarized lactate transporter activity and expression in the syncytiotrophoblast of the term human placenta. Placenta 2004; 25(6):469–504. [DOI] [PubMed] [Google Scholar]
- 55. Ganapathy V, Prasad PD. Role of transporters in placental transfer of drugs. Toxicol Appl Pharmacol 2005; 207(2):381–387. [DOI] [PubMed] [Google Scholar]
- 56. Hosoyamada M, Sekine T, Kanai Y, Endou H. Molecular cloning and functional expression of a multispecfic organic anion transporter from human kidney. Am J Physiol 1999; 276:F122–F128. [DOI] [PubMed] [Google Scholar]
- 57. Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterisation of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem 2000; 275:4507–4512. [DOI] [PubMed] [Google Scholar]
- 58. Ugele B, St-Pierre MV, Pihusch M, Bahn A, Hantschmann P. Characterisation and identification of steroid sulfate transporters of human placenta. Am J Physiol Endocrinol Metab 2003; 284:E390–E398. [DOI] [PubMed] [Google Scholar]
- 59. Yasuda S, Hasui S, Kobayashi M, Itagaki S, Hirano T, Iseki K. The mechanism of carrier-mediated transport of folates in BeWo cells: the involvement of heme carrier protein 1 in placental folate transport. Biosci Biotechnol Biochem 2008; 72(2):329–334. [DOI] [PubMed] [Google Scholar]
- 60. Rask-Andersen M, Marusam S, Fredriksson R, Schioth HB. Solute carriers as drug targets: current use, clinical trials and perspective. Mol Cell Ther 2014; 2(15):702–710. [DOI] [PubMed] [Google Scholar]
- 61. Nigam SK. What do drug transporters really do? Nat Rev Drug Discov 2015; 14:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tomi M, Eguchi H, Ozaki M, Tawara T, Nishimura S, Higuchi K, Maruyama T, Nishimura T, Nakashima E. Role of OAT4 in uptake of estriol precursor 16α-hydroxydehydroepiandrosterone sulfate into human placental syncytiotrophoblasts from fetus. Endocrinology 2015; 156(7):2704–2712. [DOI] [PubMed] [Google Scholar]
- 63. Ahmadimoghaddam D, Hofman J, Zemankova L, Nachtigal P, Dolezelova E, Cerveny L, Ceckova M, Micuda S, Staud F. Synchronized activity of organic cation transporter 3 (oct3/slc22a3) and multidrug and toxin extrusion 1 (mate1/slc47a1) transporter in transplacental passage of MPP+ in rat. Toxicol Sci 2012; 128(2):471–481. [DOI] [PubMed] [Google Scholar]
- 64. Ahmadimoghaddam D, Zemankova L, Nachtigal P, Dolezelova E, Neumanova Z, Cerveny L, Ceckova M, Kacerovsky M, Micuda S, Staud F. Organic cation transporter 3 (oct3/slc22a3) and multidrug and toxin extrusion 1 (mate1/slc47a1) transporter in the placental and fetal tissues: expression profile and fetus protective role at different stages of gestation. Biol Reprod 2013; 88(3):55. [DOI] [PubMed] [Google Scholar]
- 65. Loubiere LS, Vasilopoulou E, Bulmer JN, Taylor PM, Stieger b, Verrey F, McCabe CJ, Franklyn JA, Kilby MD, Chan SY. Expression of thyroid hormone transporters in the human placenta and changes associated with intrauterine growth restriction. Placenta 2010; 31:295–304. [DOI] [PubMed] [Google Scholar]
- 66. Alzamil HA, Pawade J, Fortier MA, Bernal AL. Expression of the prostaglandin F synthase AKR1B1 and the prostaglandin transporter SLCO2A1 in human fetal membranes in relation to spontaneous term and preterm labor. Front Physiol 2014; 5:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schmidt A, Morales-Prieto DM, Pastuschek J, Frohlich K, Markert UR. Only humans have human placentas: molecular differences between mice and humans. J Reprod Immunol 2015; 108:65–71. [DOI] [PubMed] [Google Scholar]
- 68. Beckman DA, Koszalka TR, Jensen M, Brent RL. Experimental manipulation of the rodent visceral yolk sac. Teratology 1990; 41:395–404. [DOI] [PubMed] [Google Scholar]
- 69. Chu X, Bleasby K, Evers R. Species differences in drug transporters and implications for translating preclinical findings to humans. Expert Opin Drug Metab Toxicol 2013; 9(3):237–252. [DOI] [PubMed] [Google Scholar]
- 70. Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet 2001; 2:538–548. [DOI] [PubMed] [Google Scholar]
- 71. Mayhew TM. Allometric studies on growth and development of the human placenta: growth of tissue compartments and diffusive conductances in relation to placental volume and fetal mass. J Anat 2006; 208(6):785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jansson T, Powell TL. Human placental transport in altered fetal growth: does the placenta function as a nutrient sensor? Placenta 2006; 27:S91–S97. [DOI] [PubMed] [Google Scholar]
- 73. Rodrigues RF, Carter AM, Ambrosio CES, dos Santos TC, Miglino MA. The subplacenta of the red-rumped agouti (dasyprocta leporina L). Reprod Biol Endocrinol 2006; 4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schatten H. Animal Models and Human Reproduction: Cell and Molecular Approaches With Reference to Human Reproduction. New Jersey: John Wiley and Sons; 2017:79. [Google Scholar]
- 75. Barry JS, Anthony RV. The pregnant sheep as a model for human pregnancy. Theriogenology 2008; 69(1):55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev 2016; 96:1509–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mathiesen L, Morck TA, Zuri G, Anderson MH, Pehrson C, Frederiksen M, Mose T, Rytting E, Poulsen MS, Nielsen JKS, Knudsen LE. Modelling of human transplacental transport as performed in Copenhagen Denmark. Basic Clin Pharmacol Toxicol 2014; 115:93–100. [DOI] [PubMed] [Google Scholar]
- 78. Nanovskaya T, Nekhayeva I, Karunaratne N, Audus K, Hankins GDV, Ahmed MS. Role of P-glycoprotein in transplacental transfer of methadone. Biochem Pharmacol 2005; 69(12):1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pattillo RA, Gey GO. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res 1968; 28:1231–1236. [PubMed] [Google Scholar]
- 80. Clifton VL, Murphy VE. Maternal asthma as a model for examining fetal sex-specific effects on maternal physiology and placental mechanisms that regulate human fetal growth. Placenta 2004; 25:S45–S52. [DOI] [PubMed] [Google Scholar]
- 81. Vyhlidal CA, Riffel AK, Haley KJ, Sharma S, Dai H, Tantisira KG, Weiss ST, Leeder JS. Cotinine in human placenta predicts induction of gene expression in fetal tissues. Drug Metab Dispos 2013; 41(2):305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Niu Z, Xie C, Wen X, Tian F, Ding P, He Y, Lin J, Yuan S, Guo X, Jia, D, Chen WQ. Placenta mediates the association between maternal second hand smoke exposure during pregnancy and small for gestational age. Placenta 2015; 36:876–880. [DOI] [PubMed] [Google Scholar]
- 83. Buckberry S, Bianco-Miotto T, Bent SJ, Dekker GA, Roberts CT. Integrative transcriptome meta-analysis reveals wide-spread sex-biased gene expression at the human fetal-maternal interface. Mol Hum Reprod 2014; 20(8):810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Barker DJ. The origins of the developmental origins theory. J Intern Med 2007; 261(5):412–417. [DOI] [PubMed] [Google Scholar]
- 85. Van Abeelen AF, de Rooij SR, Osmond C, Painter RC, Veenendaal MV, Bossuyt PM, Elias SG, Grobbee DE, van der Schouw YT, Barker DJ, Roseboom TJ. The sex-specific effects of famine on the association between placental size and later hypertension. Placenta 2011; 32:694–698. [DOI] [PubMed] [Google Scholar]
- 86. Lubchencho LO, Hansman C, Dressler M, Boyd E. Intrauterine growth as estimated from live born birth-weight data at 24 to 42 weeks of gestation. Pediatrics 1963; 32:793–800. [PubMed] [Google Scholar]
- 87. Vore M, Leggas M. Progesterone acts via progesterone receptors A and B to regulate breast cancer resistance protein expression. Mol Pharmacol 2008; 73(3):613–615. [DOI] [PubMed] [Google Scholar]
- 88. Fornes R, Hu M, Maliqueo M, Kokosar M, Benrick A, Carr D, Billig H, Jansson T, Manni L, Stener-Victorin E. Maternal testosterone and placental function: effect of electroacupuncture on placental expression of angiogenic markers and fetal growth. Mol Cell Endocrinol 2016; 433:1–11. [DOI] [PubMed] [Google Scholar]
- 89. Cleal JK, Day PL, Hanson MA, Lewis RM. Sex differences in the mRNA levels of housekeeping genes in human placenta. Placenta 2010; 31(6):556–557. [DOI] [PubMed] [Google Scholar]
- 90. Parisi MA, Spong CY, Zajicek A, Guttmacher AE. We don't know what we don't study: the case for research on medication effects in pregnancy. Am J Med Genet C Semin Med Genet 2011; 157(3):247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shields KE, Lyerly AD. Exclusion of pregnant woman from industry-sponsored clinical trials. Obstet Gynecol 2013; 122:1077–1081. [DOI] [PubMed] [Google Scholar]
- 92. Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet 2004; 43(8):487–514. [DOI] [PubMed] [Google Scholar]
- 93. Munic V, Kelneric Z, Mikac L, Erakovic Haber V. Differences in assessment of macrolide interaction with human MDR1 (ABCB1, P-gp) using rhodamine-123 efflux, ATPase activity and cellular accumulation assays. Eur J Pharm Sci 2010; 41:86–95. [DOI] [PubMed] [Google Scholar]
- 94. Audette MC, Challis JRG, Jones RL, Sibley CP, Matthews SG. Synthetic glucocorticoid reduces human placental system a transport in woman treated with antenatal therapy. J Clin Endocrinol Metab 2014; 99(11):E2226–E2233. [DOI] [PubMed] [Google Scholar]
- 95. Murphy KE, Hannah ME, Willian AR, Hewson SA, Ohlsson A, Kelly EN, Matthews SG, Saigal S, Asztalos E, Ross S, Delisle MF, Amankwah K et al. . Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet 2008; 372:2143–2151. [DOI] [PubMed] [Google Scholar]
- 96. Merino G, Jonker JW, Wagenaar E, van Herwaarden AE, Schinkel AH. The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol Pharmacol 2005; 67:1758–1764. [DOI] [PubMed] [Google Scholar]
- 97. Fisher BG, Thankamony A, Hughes IA, Ong KK, Dunger DB, Acerini CL. Prenatal paracetamol exposure is associated with shorter anogenital distance in male infants. Hum Reprod 2016; (in press). Published online ahead of print 8 Sept 2016; DOI 10.1093/humrep/dew196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Blazquez AG, Briz O, Gonzalez-Sanchez E, Perez MJ, Ghanem CI, Marin JJ. The effect of acetaminophen on the expression of BCRP in trophoblast cells impairs the placental barrier to bile acids during maternal cholestasis. Toxicol Appl Pharmacol 2014; 277(1):77–85. [DOI] [PubMed] [Google Scholar]
- 99. Albrecht C, Soumain S, Tetlow N, Patel P, Sullivan MHF, Lakasing L, Nicolaides K, Williamson C. Placental ABCA1 expression is reduced in primary antiphospholipid syndrome compared to pre-eclampsia and controls. Placenta 2007; 28:701–708. [DOI] [PubMed] [Google Scholar]
- 100. Aye IL, Waddell BJ, Mark PJ, Keelan JA. Placental ABCA1 and ABCG1 transporters efflux cholesterol and protect trophoblasts from oxysterol induced toxicity. Biochim Biophys Acta 2010; 1801(9):1013–1024. [DOI] [PubMed] [Google Scholar]
- 101. Bloise E, Ortiga-Carvalho TM, Reis FM, Lye SJ, Gibb W, Matthews SG. ATP-binding cassette transporters in reproduction: a new frontier. Hum Reprod Update 2016; 22(2):164–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Azzaroli F, Mennone A, Feletti V, Simoni P, Baglivo E, Montagnani M, Rizzo N, Pelusi G, DE Aloysio D, Lodato F, Festi D, Colecchia A et al. . Clinical trial: modulation of human placental multidrug resistance proteins in cholestasis of pregnancy by ursodeoxycholic acid. Aliment Pharmacol Ther 2007; 26:1139–1146. [DOI] [PubMed] [Google Scholar]
- 103. Marin JJG, Macias RIR, Briz O, Perez MJ, Blazquez AG, Arrese M, Serrano MA. Molecular bases of the fetal-placenta-maternal liver excretory pathway for cholephilic compounds. Liver Int 2008; 28(4):435–454. [DOI] [PubMed] [Google Scholar]
- 104. Faller DP, Egan DA, Ryan MP. Evidence for location of the CFTR in human placental apical membrane vesicles. Am J Physiol 1995; 269(1):C148–C155. [DOI] [PubMed] [Google Scholar]
- 105. Litman T, Jensen U, Hansen A, Covitz KM, Zhan Z, Fetsch P, Abati Am Hansen PR, Horn T, Skovsgaard T, Bates SE. Use of peptide antibodies to probe for the mitoxantrone resistance-associated protein MXR/BCRP/ABCP/ABCG2. Biochim Biophys Acta 2002; 1565:6–16. [DOI] [PubMed] [Google Scholar]