Abstract
Purpose
To investigate the efficacy and toxicity of intravitreous melphalan for treatment of retinoblastoma, as a single agent or with concomitant topotecan.
Participants
130 eyes of 120 retinoblastoma patients receiving 630 intravitreous (melphalan, topotecan) and/or topotecan periocular injections. 83 (64%) of these eyes were treated with concomitant ophthalmic artery chemosurgery.
Design
Retrospective cohort study
Methods
Indirect ophthalmoscopy and clinical imaging were used to evaluate clinical response. Ocular survival and disease-free survival were estimated using Kaplan-Meier methods in 130 eyes. Ocular toxicity was evaluated by clinical findings and electroretinography (ERG) on 244 evaluable injections in 63 patients: 30 Hz flicker responses were recorded at baseline, immediately prior to each injection and at each follow-up visit. Analysis was performed using linear mixed effects models with a random intercept and slope for each patient and a fixed effect for number of injections, in addition to any other fixed effect of interest.
Main Outcome Measures
Ocular survival, disease-free survival, electroretinogram (ERG): Peak-to-peak ERG amplitudes in response to 30-Hz photopic flicker stimulation.
Results
There were no disease or treatment-related deaths and no patient developed externalization of tumor or metastatic disease. 2-year Kaplan-Meier estimates of ocular survival and disease-free survival were 94.2% (95% CI 89.2-99.4%) and 86.2% (95%CI 78.7-94.5%), respectively. There was a significant association between number of injections and diminished ERG responses, such that on average each intravitreous melphalan injection was associated with a 5.3 μV decrease in ERG amplitude (p-value <.001). Concomitant intra-arterial chemotherapy (p=0.01) and greater inherent ocular pigment was also significantly associated with a reduction in ERG (p = 0.045). Patient age, weight, new injection site location, addition of topotecan, concomitant focal treatment and time interval between injection were not significantly associated with toxicity.
Conclusion
Intravitreous melphalan is an effective treatment for vitreous seeding in retinoblastoma resulting in high rates of ocular survival and disease-free survival. However, in this study, each injection of melphalan was associated, on average, with a decrement in ERG response. The findings suggest increased toxicity 1) when ophthalmic artery chemosurgery is given within one week of the intravitreous injection and 2) in more deeply pigmented eyes.
Introduction
Intravitreous chemotherapy effectively treats retinoblastoma vitreous seeds and saves eyes that once would have been enucleated1. However, as we have previously published, this comes at the expense of ocular toxicity2. The posterior segment is involved and retinal damage occurs such that for every injection, we have reported a 5.8μV decrease in the ERG recording2. Furthermore, toxic effects may also occur in the anterior segment of the eye including iris recession, cataracts, iris depigmentation and iris thinning along with scleromalacia3.
The alternative management for vitreous seeds (or recalcitrant subretinal and retinal tumor) includes ophthalmic artery chemotherapy or enucleation. Intraarterial chemotherapy can be effective for vitreous disease and causes minimal retinal toxicity4,5. However, ophthalmic artery chemotherapy requires a team of specialists and resources that are not available to all retinoblastoma centers. For many centers that rely on intravitreous chemotherapy or centers that are deciding between intravitreous and intraarterial chemotherapy, the question becomes: how can we maintain the efficacy of intravitreous chemotherapy while limiting its toxicity? We have done more than 600 intravitreous injections for retinoblastoma since 2012 and also have an extensive database of electroretinogram recordings, so we undertook a retrospective analysis of 630 chemotherapy injections in an attempt to help answer this question. In addition to evaluating efficacy of the injections, we investigated a number of patient and treatment characteristics to determine if these influenced retinal toxicity.
Methods
This Institutional Review Board (IRB)-approved study included all eyes that received injections of melphalan and/or topotecan for the management of intraocular retinoblastoma at Memorial Sloan Kettering Cancer Center (MSKCC) between September 2012 and September 2016. Informed consent was obtained for each patient from their guardian, caregiver or parent. The study was Health Insurance Portability and Accountability Act (HIPAA) compliant. Research adhered to the tenets of the Declaration of Helsinki.
The intravitreous injections were performed as follows: after induction of anesthesia, the intraocular pressure was lowered by digital massage to a target pressue of less than 10mmHg. Intravitreous melphalan (25-30μg in 0.05 to 0.072mL) was injected through the conjunctiva, sclera and pars plana with a 33-gauge needle-usually 3mm from the limbus. 32 injections of 25μg melphalan were given to 8 eyes, and in 7 of these eyes, melphalan was administered with concomitant intravitreous topotecan; the remaining injections were 30μg melphalan. Prior to needle withdrawal, the injection site was sealed and sterilized with cryotherapy1. The ocular surface was submerged in irrigating sterile water for 3 minutes6. Periocular injections of 1mg topotecan were performed in a manner previously described7. Periocular or intravitreal topotecan was used to supplement intravitreal melphalan in cases where intravitreal melphalan was not resulting in the desired response and it was believed that additional treatment was warranted. Ophthalmic artery chemosurgery was given as concomitant treatment in 84 eyes in a manner previously described8. In brief, melphalan (2.5mg to 8mg), topotecan (0.3 to 2mg) and carboplatin (20 to 70mg) were used. The number of drugs and dose were determined by a number of factors including laterality of disease, age of patient, prior response to treatment etc.
The clinical status was evaluated under anesthesia with indirect ophthalmoscopy, RetCam fundus photography (Clarity, Pleasanton, CA, USA), B-scan ultrasonography (Ellex, Adelaide, Australia) and ultrasonic biomicroscopy (Ellex, Adelaide, Australia). At each subsequent exam, the burden of residual disease was reevaluated and additional injections were given on either a weekly or monthly schedule. Additional injections were given if the seeds were not in a state of regression by clinical exam. Seeds that enlarged in size without dismantling and dispersing into smaller pieces were deemed as active.
Patient data included sex, laterality, age and weight at start of injection course, degree of ocular pigmentation, (blue = blue iris with blonde fundus, light brown= brown iris with moderate fundus pigment, dark brown= brown iris with deep fundus pigment), eye status (salvaged or enucleated), indication for chemotherapy injection (vitreous seeds, subretinal seeds or retinal tumor), follow-up time from beginning of injection course. Treatment data included number of injections, number of clock hours where injections were administered, time interval between injections, clock hours of salt and pepper retinopathy, concomitant OAC or focal treatment (laser or cryotherapy) defined as occurring within one week of the injection but exclusive of the injection site cryotherapy, concomitant periocular/intravitreous topotecan injection at the time of melphalan injection. For ocular survival, an adverse event was defined as enucleation (no eyes received external beam radiation as salvage treatment). For disease free survival an event was defined as recurrence of seeds requiring enucleation or a subsequent course of injections. Tumor data included Reese-Ellsworth (RE) classification, Children's Oncology group (COG) version of International Classification (IC) and seed classification at presentation (class 1 = dust, class 2 = spheres +/- dust, or class 3 = clouds +/- spheres or dust).
Ocular toxicity
Electroretinogram (ERG) recordings were obtained during regularly scheduled examination under anesthesia, according to an International Society for Clinical Electrophysiology of Vision (ISCEV) standard protocol which had been modified to limit anesthesia time, as previously described2,9. Reported here are the response amplitudes to 30-Hz photopic flicker stimulation, which are representative of the full protocol10. Electroretinogram responses were performed at baseline, immediately prior to each injection and at each follow-up visit. ERG studies were deemed inevaluable if the baseline recording amplitudes were not sufficient enough to allow demonstration of ERG change over the injection course (for example, each injection has the potential to decrease the ERG by about 5μV2 – therefore an eye with an 8μV amplitude at baseline would not have sufficient baseline ERG signal strength to demonstrate change over 6 injections), or if there was no ERG testing performed (due to the absence of an electrophysiologist).
Statistical analysis
Ocular survival and disease-free survival were estimated using Kaplan-Meier methods in 130 eyes of 120 patients. Ocular toxicity was evaluated by clinical findings and electroretinography (ERG) on 244 evaluable injections in 63 patients. We explored trends in the data through a line plot of each individual patient's trajectory of ERG over injections, with a locally weighted scatterplot smoothing (LOWESS) line showing the overall trend in the data. Then, linear mixed effects models with a random intercept and slope for each patient and a fixed effect for number of injections, in addition to any other fixed effect of interest, were fit to the ERG data. All statistical analyses were conducted using R software version 3.2.5 (R Core Development Team, Vienna, Austria) and a p-value < 0.05 was considered statistically significant.
Results
56 patients had unilateral disease, while 64 patients had bilateral disease (10 patients had injections in both eyes). There were no disease- or treatment-related deaths and no patient developed externalization of tumor or metastatic disease. One patient died from trauma. The median follow-up among those eyes that were not enucleated was 14.3 months (0.3-47.4 months) and median age at initial treatment was 25.8 months (5.2-216.3 months). 630 injections in 130 eyes (Reese-Ellsworth Classification IA = 2 eyes, IB = 2 eyes, IIA = 3 eyes, IIB = 1 eye, IIIA = 4 eyes, IIIB = 4 eyes, IVA = 1 eye, VA = 16 eyes, VB = 97 eyes), International Classification A = 2 eyes, B = 8 eyes, C = 4 eyes, D = 83 eyes and E = 33 eyes) were included in this study. The median interval between injections was 12 days (6-44 days). Classification of vitreous seeds, delivery and drugs used in the injection and indication for the injection are all shown in Table 1. Treatment and disease characteristics are demonstrated in Table 2 and show that the majority of eyes received prior treatment (OAC, external beam radiation and/or intravenous chemotherapy before intravitreal injections and the majority of eyes received concomitant OAC. All eyes received intravitreous chemotherapy either following prior treatment and/or with concomitant OAC.
Table 1. Number, delivery and indication for 630 chemotherapy injections.
| Indication for injections | No. eyes | No. Intravit melphalan | No. Concomitant Intravit topotecan |
|---|---|---|---|
| Vitreous disease | 94 | 374 (4.0) | 26 (0.3) |
| 1 | 27 | 63 (2.3) | 0 (0) |
| 2 | 46 | 207 (4.5) | 16 (0.3) |
| 3 | 21 | 104 (5.0) | 10 (0.5) |
| Non-vitreous disease | 36 | 118 (3.3) | 28 (0.8) |
| Anterior Chamber | 1 | 3 (3.0) | 0 (0) |
| Subretinal Seeds | 17 | 52 (3.1) | 10 (0.6) |
| Retinal tumor | 18 | 63 (3.5) | 18 (1.0) |
| TOTAL | 130 | 492 (3.8) | 52 (0.4) |
Mean number of injections per eye is shown in parentheses.
Table 2. Treatment and disease characteristics for 130 eyes.
| Treatment and disease details | All (n=130) | Vitreous (n=94) | Non-Vitreous (n=36) |
|---|---|---|---|
| Disease status | |||
| Primary | 60 (45%) | 42 (45%) | 18 (50%) |
| Recurrent | 70 (55%) | 52 (55%) | 18 (50%) |
| Treatment status | |||
| Naïve | 22 (17%) | 19 (20%) | 3 (8%) |
| Prior treatment | 108 (83%) | 75 (80%) | 33 (92%) |
| OAC | 38 | 25 | 13 |
| IVC | 34 | 24 | 10 |
| OAC + IVC | 32 | 22 | 10 |
| EBR + IVC | 1 | 1 | |
| EBR + OAC | 1 | 1 | |
| EBR + IVC + OAC | 2 | 2 | |
| Concomittant OAC | |||
| Yes | 83 (64%) | 66 (70%) | 17 (47%) |
| No | 47 (36%) | 28 (30%) | 19 (53%) |
OAC = ophthalmic artery chemosurgery, IVC = intravenous chemotherapy, EBR = external beam radiation
Percentage shown in parentheses.
As shown in Figure 1, the overall two-year Kaplan-Meier estimate for ocular survival was 94.2% (95% confidence interval (CI) 89.2-99.4%). The two-year Kaplan-Meier estimate for disease-free survival was 86.2% (95%CI 78.7-94.5%).
Figure 1.
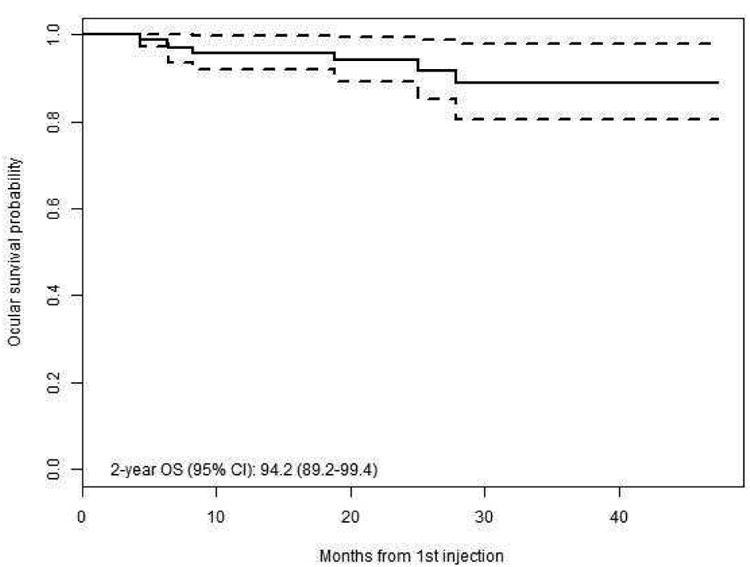

Kaplan Meier survival curves for (left) Ocular survival of all eyes, (right) Event free survival of all eyes.
The 63 patients evaluable for ERG each received between 2 and 9 injections for a total of 295 injection observations. The change in ERG for each injection of melphalan ranged from an increase of 43.4 μV to a decrease of 68.5μV as shown in Figure 2. Individual patient trajectories of ERG over time, as measured by number of injections, are plotted in Figure 3. The LOWESS smooth line shows that there is an overall downward trend in ERG over time. In a linear mixed with a random effect for patient and both a random and fixed effect for injection number, there was a significant association between number of injections and ERG decrement, such that on average each intravitreous melphalan injection was associated with a 5.3 μV degradation in ERG response (p-value <.001). When additional fixed effects variables were added to the model one at a time, we found that concomitant intra-arterial chemotherapy was associated with 8.0 μV decrease in ERG. Light brown or dark brown versus blue ocular pigment was also significantly associated with a reduction in ERG (p = 0.045) (Figure 4). Both concomitant OAC and ocular pigment were confirmed to be statistically significant on multivariate analysis. Age, weight, new injection site clock hour, addition of topotecan, concomitant focal treatment and time interval between injection were not significantly associated with toxicity.
Figure 2.
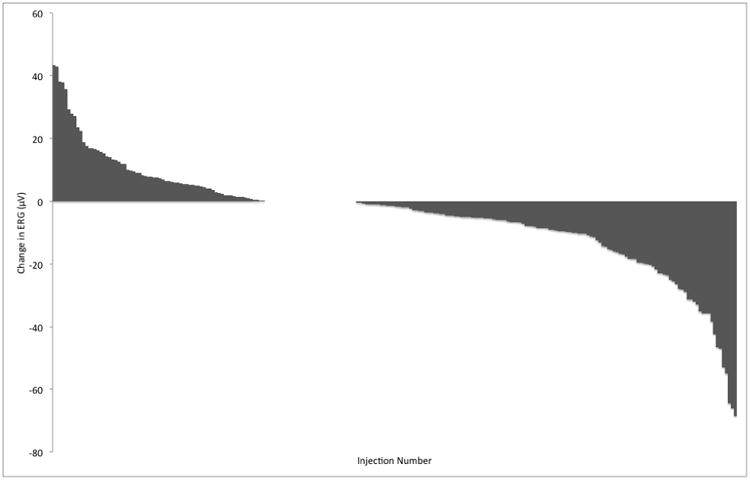
Waterfall plot demonstrating change in electroretinogram (ERG) response recorded after each intravitreal injection of melphalan
Figure 3.
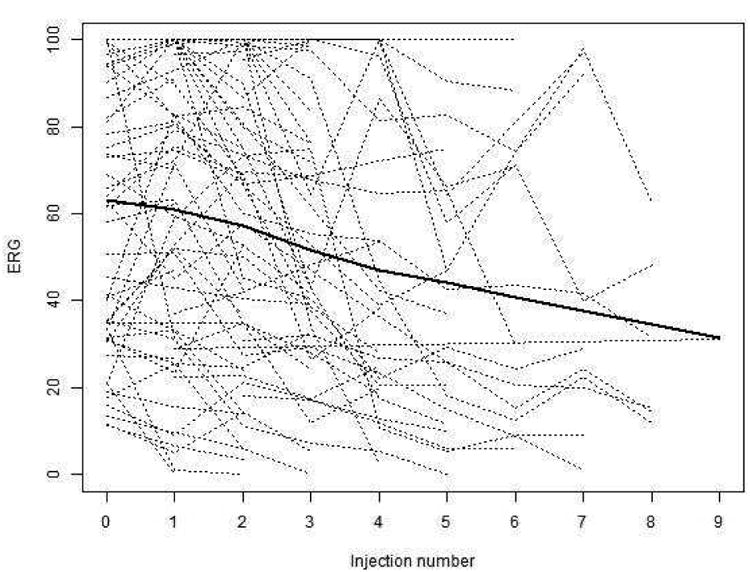
Individual patient trajectories of ERG over time, as measured by number of injections, (dotted lines) with a LOWESS smooth depicting the overall trend (solid line).
Figure 4.
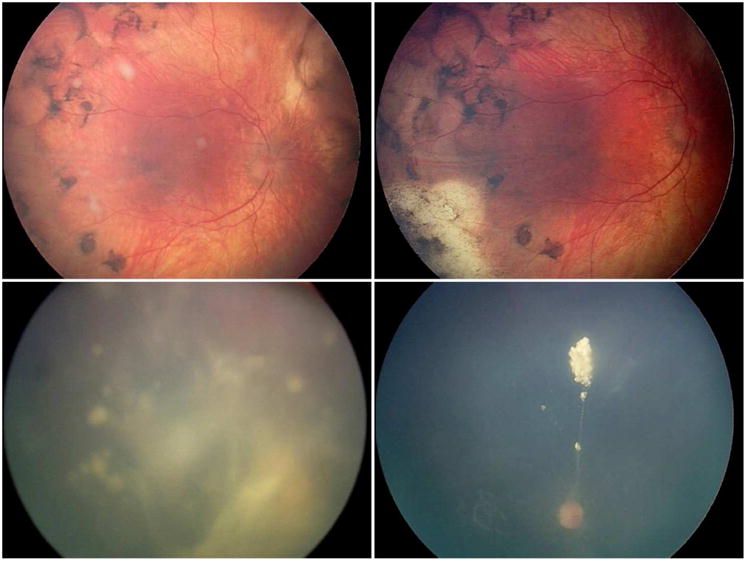
Representative cases of each seed classification (class 1, 2, and 3) and response to intravitreous melphalan. Representative eye with blue iris and class 2 (spheres) vitreous seeds (upper left) had a degradation of 3.6μV following 3 melphalan injections (upper right). Representative eye with dark brown iris and class 3 (cloud predominant) vitreous seeds (lower left) had comparatively more degradation (26.4μV) following 3 melphalan injections (lower right).
Discussion
Initial concerns regarding the use of intravitreous chemotherapy for retinoblastoma centered on questions of its safety and the risk of externalization of tumor. Thanks to a resurgence of interest stimulated by Munier et al. and a coordinated effort to adopt safety enhanced techniques, intravitreous chemotherapy injections have proven relatively safe in practice11,12. As the technique has been more widely implemented it has proven to be very effective for treating vitreous seeds1,13,14. However, even though intravitreous chemotherapy can save eyes, it is also toxic to the eye and to the retina2. In further refining the intravitreous chemotherapy technique, of the question now arises as to how can we make these injections less toxic and more amenable to saving not only the eye, but also to the potential of saving vision.
Patient characteristics potentially related to the size of the eye (age and weight) were not associated with increased toxicity. This may come as a surprise, since it might be that younger, smaller patients may have an increased concentration of drug (owing to a smaller volume of vitreous due to less axial elongation at a younger age15), or more viscous vitreous resulting in less drug diffusion and “pockets” of higher drug concentration in proximity to the retina, both of which might increase toxicity.
In a porcine model, it has been shown that a higher concentration of melphalan accumulated in the retinal pigment epithelium (RPE)-choroid than in the retina following intraarterial chemotherapy, suggesting melphalan may be preferentially taken up by pigmented tissues16. We have previously speculated that more deeply pigmented eyes may absorb increased levels of melphalan resulting in more RPE toxicity, and, by extension, retinal and choroidal toxicity2. Our current findings align with this theory: using iris and fundus pigment as a proxy for inherent ocular pigmentation demonstrated a statistical impact on retinal toxicity such that eyes with brown irides had more retinal toxicity compared to eyes with blue irides. This raises the question as to whether more deeply pigmented eyes may benefit from melphalan dose reduction. However, it is to be determined whether a lower dose of melphalan in more pigmented eyes would result in lower toxicity while, more importantly, still being efficacious.
It is commonly found that concomitant treatments are additive in their efficacy but also in their toxicity, and that toxicity may be worse with a shorter interval between modalities. Our results demonstrate no statistically significant impact on retinal toxicity when melphalan injections are given within a week of concomitant focal treatment or topotecan injections. However, even though ophthalmic artery chemosurgery alone has only a minimal impact on electroretinogram recordings5, it appears concomitant administration within a week of intravitreous melphalan heightens the retinal toxicity of intravitreous melphalan to a statistically significant extent. Presumably the melphalan delivered via ophthalmic artery chemosurgery is additive with drug administered intravitreally – and while this may result in more toxicity, it may also have enhanced efficacy. The question of the ideal interval between these two drug delivery modalities, such that efficacy is optimized and toxicity minimized, would benefit from further investigation.
Besides concomitant therapies, other treatment factors were evaluated for their influence on toxicity. Our current results confirmed our previous findings that more numerous intravitreous injections result in a statistically significant increase in toxicity. More specifically, for every melphalan injection, the electroretinogram recordings decrease by 5.3μV (which is close to our previous finding of a 5.8μV decrement for each injection in a smaller cohort2). We previously demonstrated that retinal toxicity was observed promptly (detectable at one week following the injection) and was stable without further decline after that initial one-week interval. This may explain our present finding that the interval between injections does not significantly influence toxicity. One could deduce that the toxicity is recordable and stable by at least one week post-injection and adding additional injections at one week, two weeks or one month would have little influence on the toxicity of that prior injection. It is still to be determined whether monthly injections afford more time for tumoricidal seed response, thereby resulting in fewer injections and therefore less toxicity.
Studies have suggested that there is an increased concentration of the drug at the site of the injection as clinically demonstrated by salt and pepper retinopathy (sometimes referred to as “melphalan pigment epitheliopathy”)2. There is a belief that repeated injections in the same clock hour may limit exposure of the drug to a single portion of the retina and thereby reduce toxicity. However our results do not support this, and in fact show no statistically significant relationship between number of clock hour injection sites and retinal toxicity. Perhaps each melphalan injection creates an area of vitreous liquefaction in which the drug concentrates and remains in proximity to the retina; and each subsequent injection into this same location expands this area of vitreous liquefaction while also expanding drug exposure and impact on the retina.
Our current study validates our prior, smaller case series which showed that each intravitreous melphalan (30mcg) injection results in approximately 5μV degradation in retinal response. This larger cohort demonstrates a patient characteristic (ocular pigment) and a treatment factor (concomitant OAC within 1 week), which influences toxicity and thereby provides a potential avenue for future modifications to limit toxicity.
Table 3. Details on 69 eyes of 63 patients whom had evaluable electroretinogram responses.
| Gender | Laterality | RE | ICRB | Prior treatment | Disease Status | Indication for inj* | Concmt OAC | No. of OAC drugs | No. of OAC cycles | No. of M injs | No. of intravit T injs | No. of PO T injs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | B | 5B | E | OAC, Sc | recurrent | 3 | yes | M, T, C | 3 | 8 | ||
| F | B | 5B | E | OAC, EBR | recurrent | 2 | yes | M, T, C | 2 | 6 | ||
| F | B | 5B | D | OAC | recurrent | 1 | no | 5 | ||||
| F | B | 1B | B | none | primary | R | no | 2 | ||||
| F | B | 1A | B | OAC, Sc | primary | R | no | 3 | ||||
| F | B | 5B | D | OAC, Sc, EBR | recurrent | 2 | yes | M, T, C | 2 | 3 | ||
| M | B | 5B | C | OAC, Sc | recurrent | 2 | no | 5 | 4 | |||
| F | U | 5B | D | OAC, Sc | recurrent | 2 | yes | M, T, C | 2 | 6 | ||
| F | B | 5B | C | Sc | recurrent | 2 | yes | M, T | 3 | 3 | ||
| M | B | 5B | D | OAC | recurrent | R | no | 2 | ||||
| M | B | 5B | D | Sc, EBR | recurrent | 2 | yes | M, T, C | 3 | 8 | ||
| M | B | 5B | E | OAC, Sc | recurrent | 2 | yes | M, T, C | 3 | 4 | 4 | 3 |
| F | U | 5D | E | none | primary | 3 | yes | M, T, C | 3 | 7 | ||
| M | B | 5B | D | Sc | recurrent | 2 | yes | M, T, C | 2 | 2 | ||
| M | U | 5B | D | OAC | recurrent | 1 | yes | M, T, C | 5 | 2 | ||
| F | U | 5B | D | OAC | primary | 3 | yes | M, T, C | 3 | 3 | ||
| M | B | 5B | D | OAC | primary | 2 | yes | M, T, C | 2 | 2 | 4 | |
| M | B | 5B | E | OAC | recurrent | 2 | no | 8 | ||||
| M | U | 5B | D | OAC | primary | 1 | yes | M, T, C | 3 | 2 | ||
| F | B | 5B | D | Sc | primary | 2 | yes | M, T, C | 7 | 3 | ||
| M | U | 5B | D | none | primary | 3 | yes | M, T | 2 | 7 | 2 | |
| F | U | 5B | D | Sc | primary | 3 | yes | M, T, C | 2 | 5 | ||
| M | B | 2A | B | OAC | recurrent | R | yes | M, C | 2 | 7 | ||
| F | B | 5B | E | OAC | primary | 1 | no | M, T, C | 3 | 3 | ||
| F | B | 5B | D | Sc | recurrent | 2 | no | 7 | 7 | |||
| F | B | 5B | D | OAC | recurrent | R | yes | M, T, C | 7 | 2 | 2 | |
| F | U | 5B | D | none | primary | 3 | yes | M, T, C | 2 | 4 | ||
| M | U | 5B | E | Sc | primary | 2 | yes | M, T, C | 3 | 6 | 8 | |
| F | U | 5B | D | OAC | recurrent | 2 | yes | M, T, C | 2 | 7 | ||
| M | B | 5B | D | Sc | primary | 2 | yes | 8 | ||||
| F | U | 5B | D | none | primary | 1 | yes | 1 | ||||
| M | B | 5B | E | none | primary | 1 | yes | 4 | ||||
| F | B | 5A | E | OAC, Sc | recurrent | 2 | no | M, T, C | 3 | 8 | ||
| M | U | 5B | D | OAC | primary | 1 | no | 1 | ||||
| F | U | 5B | D | Sc | primary | 3 | yes | M, T, C | 2 | 8 | ||
| M | B | 5B | D | OAC, Sc | recurrent | 2 | no | 3 | ||||
| M | B | 5B | D | OAC, Sc | recurrent | 2 | no | 1 | 1 | |||
| F | U | 5B | C | none | primary | 1 | yes | M | 3 | 5 | 2 | |
| M | U | 5B | D | Sc | recurrent | 2 | yes | M, T, C | 1 | 8 | ||
| M | B | 5B | D | OAC, Sc | recurrent | 2 | no | 6 | ||||
| F | B | 5B | E | Sc | primary | 1 | yes | M, C | 1 | 3 | ||
| M | U | 5B | D | none | primary | 3 | yes | M, T, C | 3 | 4 | ||
| F | B | 1B | B | OAC, Sc | recurrent | R | yes | M, T, C | 3 | 5 | 5 | |
| F | B | 2A | D | OAC | recurrent | R | no | 4 | 2 | |||
| M | B | 5B | D | OAC | recurrent | 2 | no | 5 | ||||
| M | B | 5B | D | OAC, Sc | recurrent | R | yes | M, T, C | 4 | 8 | 2 | 5 |
| F | B | 5B | E | OAC | recurrent | 2 | yes | M, T, C | 3 | 3 | ||
| M | U | 5B | E | OAC | primary | 1 | no | 3 | ||||
| M | U | 5B | E | Sc | primary | 3 | yes | M, T, C | 3 | 3 | ||
| M | U | 5B | D | none | primary | 3 | yes | M, T, C | 3 | 2 | ||
| M | U | 4A | D | OAC, Sc | recurrent | 2 | no | 4 | ||||
| M | U | 5B | D | Sc | primary | 2 | yes | M, C | 2 | 2 | 2 | |
| F | B | 3B | D | OAC, Sc | recurrent | SRS | no | 3 | ||||
| F | B | 3B | D | OAC, Sc | recurrent | SRS | no | 3 | ||||
| M | U | 3A | D | Sc | primary | SRS | yes | M, T, C | 4 | 3 | ||
| M | U | 3B | C | OAC | primary | R | no | 2 | ||||
| M | U | 5B | E | none | primary | 1 | yes | M, T, C | 2 | 1 | ||
| F | U | 5B | E | Sc | primary | 3 | yes | M, T, C | 3 | 6 | ||
| M | U | 5B | D | none | primary | 2 | yes | M, T, C | 3 | 1 | ||
| M | B | 5B | D | Sc | primary | 1 | yes | C, T | 2 | 1 | ||
| M | B | 5A | D | OAC | recurrent | SRS | no | 3 | ||||
| M | B | 5B | D | Sc | recurrent | 2 | yes | M, T, C | 3 | 6 | ||
| M | U | 5B | D | OAC, Sc | primary | 2 | no | 2 | ||||
| M | B | 3A | B | none | primary | R | yes | M, T, C | 1 | 3 | ||
| F | B | 5B | E | none | primary | 3 | yes | M, T, C | 4 | 3 | ||
| M | U | 5B | D | OAC | recurrent | R | no | 2 | ||||
| M | B | 5B | D | Sc | primary | R | yes | M, T, C | 2 | 2 | ||
| F | U | 5B | E | OAC, Sc | primary | SRS | no | 2 | ||||
| M | B | 2B | B | OAC | primary | R | no | 3 |
No. = number, RE = Reese-Ellsworth, ICRB = international classification of retinoblastoma, inj = injection, Concmt = concomitant, OAC = ophthalmic artery chemosurgery, M = melphalan, T = topotecan, C = carboplatin, PO = periocular, * = class of vitreous seed, F = female, M = male, U = unilateral, B = bilateral, Sc = systemic chemotherapy, EBR = external beam radiation, R = retinal tumor, SRS = subretinal seed.
Acknowledgments
This study was supported by The Fund for Ophthalmic Knowledge, The New York Community Trust, Research to Prevent Blindness and Cancer Center Support Grant (P30 CA008748). The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
This work was presented at the American Academy of Ophthalmology meeting 2016.
No conflicting relationship exists for any author, except Marr B who is a consultatnt for Aura Biosciences, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Munier FL, Gaillard MC, Balmer A, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012 doi: 10.1136/bjophthalmol-2011-301450. [DOI] [PubMed] [Google Scholar]
- 2.Francis JH, Schaiquevich P, Buitrago E, et al. Local and systemic toxicity of intravitreal melphalan for vitreous seeding in retinoblastoma: a preclinical and clinical study. Ophthalmology. 2014;121:1810–1817. doi: 10.1016/j.ophtha.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Francis JH, Marr BP, Brodie SE, Abramson DH. Anterior Ocular Toxicity of Intravitreous Melphalan for Retinoblastoma. JAMA Ophthalmol. 2015;133:1459–1463. doi: 10.1001/jamaophthalmol.2015.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abramson DH, Marr BP, Dunkel IJ, et al. Intra-arterial chemotherapy for retinoblastoma in eyes with vitreous and/or subretinal seeding: 2-year results. Br J Ophthalmol. 2012;96:499–502. doi: 10.1136/bjophthalmol-2011-300498. [DOI] [PubMed] [Google Scholar]
- 5.Francis JH, Abramson DH, Gobin YP, et al. Electroretinogram monitoring of dose-dependent toxicity after ophthalmic artery chemosurgery in retinoblastoma eyes: six year review. PLoS ONE. 2014;9:e84247. doi: 10.1371/journal.pone.0084247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis JH, Xu XL, Gobin YP, et al. Death by water: precautionary water submersion for intravitreal injection of retinoblastoma eyes. Open Ophthalmol J. 2014;8:7–11. doi: 10.2174/1874364101408010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunkel IJ, Lee TC, Shi W, et al. A phase II trial of carboplatin for intraocular retinoblastoma. Pediatr Blood Cancer. 2007;49:643–648. doi: 10.1002/pbc.21163. [DOI] [PubMed] [Google Scholar]
- 8.Gobin YP, Dunkel IJ, Marr BP, et al. Intra-arterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol. 2011;129:732–737. doi: 10.1001/archophthalmol.2011.5. [DOI] [PubMed] [Google Scholar]
- 9.Brodie SE, Paulus YM, Patel M, et al. ERG monitoring of retinal function during systemic chemotherapy for retinoblastoma. Br J Ophthalmol. 2012;96:877–880. doi: 10.1136/bjophthalmol-2011-301248. [DOI] [PubMed] [Google Scholar]
- 10.Liu CY, Jonna G, Francis JH, et al. Non-selectivity of ERG reductions in eyes treated for retinoblastoma. Doc Ophthalmol. 2013 doi: 10.1007/s10633-013-9416-8. e–pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Munier FL, Soliman S, Moulin AP, et al. Profiling safety of intravitreal injections for retinoblastoma using an anti-reflux procedure and sterilisation of the needle track. Br J Ophthalmol. 2012;96:1084–1087. doi: 10.1136/bjophthalmol-2011-301016. [DOI] [PubMed] [Google Scholar]
- 12.Smith SJ, Smith BD. Evaluating the risk of extraocular tumour spread following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol. 2013 doi: 10.1136/bjophthalmol-2013-303188. [DOI] [PubMed] [Google Scholar]
- 13.Shields CL, Manjandavida FP, Arepalli S, et al. Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: preliminary results. JAMA Ophthalmol. 2014;132:319–325. doi: 10.1001/jamaophthalmol.2013.7666. [DOI] [PubMed] [Google Scholar]
- 14.Ghassemi F, Shields CL, Ghadimi H, et al. Combined intravitreal melphalan and topotecan for refractory or recurrent vitreous seeding from retinoblastoma. JAMA Ophthalmol. 2014;132:936–941. doi: 10.1001/jamaophthalmol.2014.414. [DOI] [PubMed] [Google Scholar]
- 15.Zadnik K, Mutti DO, Mitchell GL, et al. Normal eye growth in emmetropic schoolchildren. Optom Vis Sci. 2004;81:819–828. doi: 10.1097/01.opx.0000145028.53923.67. [DOI] [PubMed] [Google Scholar]
- 16.Schaiquevich P, Buitrago E, Taich P, et al. Pharmacokinetic analysis of melphalan after superselective ophthalmic artery infusion in preclinical models and retinoblastoma patients. Invest Ophthalmol Vis Sci. 2012;53:4205–4212. doi: 10.1167/iovs.12-9501. [DOI] [PubMed] [Google Scholar]


