Abstract
T regulatory cells (Tregs) are critical in shaping the latent HIV/SIV reservoir, as they are preferentially infected, reverse CD4+ T cell activation status and suppress CTL responses. To reactivate latent virus and boost cell-mediated immune responses, we performed in vivo Treg depletion with Ontak (Denileukin diftitox) in two SIVsab-infected controller macaques. Ontak induced significant (>75%) Treg depletion, major CD4+ T cell activation and only minimally depleted CD8+ T cells. The overall ability of Tregs to control immune responses was significantly impaired in spite of their incomplete depletion, resulting in both reactivation of latent virus (virus rebound to 103 vRNA copies/ml of plasma in the absence of antiretroviral therapy) and a significant boost of SIV-specific CD8+ T cell frequency, with rapid clearance of reactivated virus. As none of the latency reversing agents in development have such dual activity, our strategy holds great promise for cure research.
Keywords: Simian immunodeficiency virus, rhesus macaques, viral replication, single copy assay, T regulatory cells, Ontak, SIVsab
Introduction
The burden of the HIV epidemic, which spreads unabated such that for every HIV-infected person that starts antiretroviral therapy (ART) two new people become infected, calls for a cure (1). The “Berlin patient” (2) demonstrated that a cure for HIV infection is feasible, but the mechanisms responsible for achieving success in this case are complex and poorly understood. Further, virus relapse in the “Mississippi baby” (3, 4) and nonhuman primate (NHP) studies documenting seeding of the viral reservoir prior to detectable viremia (5) suggest that virus eradication strategies should involve therapeutic approaches that go beyond ART.
Multiple strategies for an HIV cure are currently being investigated. One of the most advanced strategies is the “shock and kill” approach, consisting of induction of viral expression in latently infected cells that could trigger immune-mediated clearance of the infected cells through cytotoxic T-lymphocytes (CTLs), natural killer (NK) cells or immunotoxins (6, 7). The most critical limitation of this strategy is the lack of effective latency reversing agents (LRAs). Available LRAs activate only a minor fraction of resting cells, the most critical component of the reservoirs (1, 8). On the other hand, even if such effective compounds become available, reactivated virus cannot be effectively cleared due to HIV/SIV-specific CTL impairment and exhaustion (4). Therefore, there is intense research aimed at both improving LRA efficacy, as well as restoring the impairment of SIV-specific T cells.
There is strong evidence that Tregs can become latently infected with HIV and may represent a potentially important HIV reservoir: (i) Tregs expand in blood and tissues in chronically HIV-infected patients and SIV-infected macaques (9); (ii) the fraction of Tregs containing HIV/SIV DNA is higher than that of non-Tregs in HIV-infected patients on ART (10) and in SIV-infected rhesus macaques (RMs) (11); (iii) Tregs are less susceptible to cell death than conventional T cells (9). As such, therapeutic interventions aiming at Treg depletion may directly contribute to the reduction of the size of virus reservoir.
Furthermore, through their regulatory function, Tregs can indirectly shape the reservoir. During the acute HIV/SIV infection, Tregs may decisively contribute to the rapid establishment of the HIV reservoir by reversing the immune activation status of CD4+ T cells (9). During chronic HIV/SIV infection, Tregs contribute to the impairment of CTL responses, as suggested by the following observations: Treg expansion correlates with loss of CTL function (12–14); ex vivo Treg depletion from blood and lymph nodes (LNs) enhances T cell responses to HIV or SIV antigens (9); HIV nonprogressors have a high perforin/Fox-P3 ratio; and HLA B27+ and B57+ HIV-specific CD8+ T cells from elite controllers are able to evade Treg suppression (15, 16). Inhibition of cellular immune responses by Tregs was also reported in other infectious and noninfectious conditions, such as hepatitis C and several types of cancer (9). Furthermore, Treg depletion resulted in a significant improvement of cellular immune responses and prolonged survival in cancer patients with T lymphomas (9). Altogether, these observations support a major involvement of Tregs in suppressing the protective effector immune responses against HIV. It is thus conceivable that Treg depletion in HIV/SIV-subjects may improve cell-mediated immunity and increase HIV/SIV-infected cell clearance. This effect of the Treg depletion may be all the more critical for the “shock and kill” strategies, which require increased killing of the reactivated virus.
Here, we assessed Treg contribution to shaping the SIV reservoir by depleting Tregs in vivo in two spontaneous-controller RMs infected with SIVsab (17, 18). RM treatment with Ontak (recombinant IL-2 coupled with diphtheria toxin) resulted in significant Treg depletion, induced T cell activation and virus reactivation. The combined effect of Treg depletion and antigenic stimulation by the reactivated virus boosted SIV-specific T lymphocytes. As such, Treg depletion, alone or in combination with other LRAs, appears to be a promising approach for virus eradication.
Materials and Methods
Animals, infection, treatments, and samples
Two RMs (Macacca mulatta) received plasma equivalent to 300 50% tissue culture-infective doses (TCID50) of the SIVsab92018 (18). Animals were housed and handled in accordance with guidelines for the care and use of laboratory animals from the U.S. Public Health Service, the American Association for Accreditation of Laboratory Animal Care, and the Animal Welfare Act (19). The University of Pittsburgh Institutional Animal Care and Use Committee approved all protocols and procedures.
SIVsab infection follow-up was performed as described (17, 18). After one and a half year postinfection, when the animals had completely controlled the virus, they were treated twice with Ontak (Ligand Pharmaceutical, La Jolla CA) intravenously (15 μg/kg) for 5 consecutive days at 21 days interval, as reported (20). Blood samples were collected prior to Ontak administration and then at 1, 3, 7, 10, 14, 17, 21 days posttreatment (dpt) initiation. Plasma and mononuclear cells were isolated as described (17, 18) for viral load (VL) quantification and flow cytometry.
Viral quantification
Plasma VLs were quantified with an ultrasensitive qPCR assay, as described (17). This assay has a sensitivity of 1 vRNA copies/ml; however, due to frequent sampling which limited the volumes of plasma, assay sensitivity was 5 copies/ml.
Antibodies and flow cytometry
Whole blood was stained for flow cytometry with multiple combinations of the following mAbs: CD3-FITC (SP34) ; CD20-PE (3G8); CD8-PerCP (SK1); CD4-APC (L200); CD25-FITC (2A3); HLA-DR-PerCP (l243); Ki-67-FITC (B56) (all from BD Biosciences) and FoxP3-APC (PCH101) (EBiosciences), as described (17, 18). Data were acquired with a FACSCalibur flow cytometer (BD Immunocytometry Systems) and analyzed with CellQuest software (BD Biosciences). CD4+ and CD8+ T cell percentages were obtained by first gating on lymphocytes and then on CD3+ T cells. Immune activation, and proliferation markers were determined by gating on lymphocytes, then on CD3+ T cells, and finally on CD4+CD3+ or CD8+CD3+ T cells.
Intracellular staining for SIV-specific T cells
Env and Gag SIVsab-specific CD4 and CD8 responses (IFNγ, TNFα, IL-2, MIP-1β and CD107a) were measured by intracellular staining (ICS) and assessed by flow cytometry, as described (8), using SIVsab-specific peptide pools: Env (52 peptides) and Gag (pool 1: 1–68, pool 2: 69–136 peptides). SIV-specific cells were acquired the same day on a custom four-laser BD LSR-II instrument (BD Bioscience). Only singlet events were gated and a minimum of 250,000 live CD3 cells were acquired with FACSDIVA 8.0. Populations were analyzed using FlowJo software version 7.6.5 (Tree Star Inc. Ashland, OR) and the graphs were generated with GraphPad Prism 6.04.
Granzyme and perforin measurements
The measurements were performed using anti-Granzyme B PE (eBioscience, San Diego, CA) and anti-human perforin antibodies from BD Biosciences (δG9). Standard intracellular cytokine stimulation and staining procedures were performed as described (21). Monensin (1 μg/mL) and concanamycin A (CMA; 0.2 μM; Sigma-Aldrich) were used for inhibition of cytotoxic granule acidification. For each tube, between 500,000 and 1,000,000 total events were acquired with an LSRII flow cytometer (BD Immunocytometry Systems, San Jose, CA). Antibody capture beads (BD Biosciences) were used to prepare individual compensation tubes for each antibody. Data analysis was performed using FlowJo (version 8.5.2; TreeStar, Ashland, OR). Reported data have been corrected for background, when appropriate.
Statistical analyses
The limited number of included RMs did not permit us to perform statistical calculations. However, the various parameters were compared within the same animal prior and after reactivation with Ontak, to assess the biological effects of this treatment. For the increases in perforin expression by CD4+ and CD8+ T cells, results of both treatments were grouped together and paired t test was performed using Prism Graph Pad.
Results and Discussion
Treg depletion in SIVsab-infected RMs superelite controllers
Two RMs were experimentally infected with SIVsab and followed for 500 days. Similar to our previous results (17, 18), after very active virus replication during the acute infection (Supplemental Fig. 1a), with significant depletion of CD4+ T cells (Supplemental Fig. 1b), SIVsab was completely controlled in RMs during chronic infection (Supplemental Fig. 1a). This allowed for control of chronic immune activation (Supplemental Fig. 1 c and d) and restoration of the CD4+ T cells in the gut (Supplemental Fig. 1b). As suggested by previous results, control was likely due to effective cellular immune responses (18).
Effective Treg depletion with Ontak
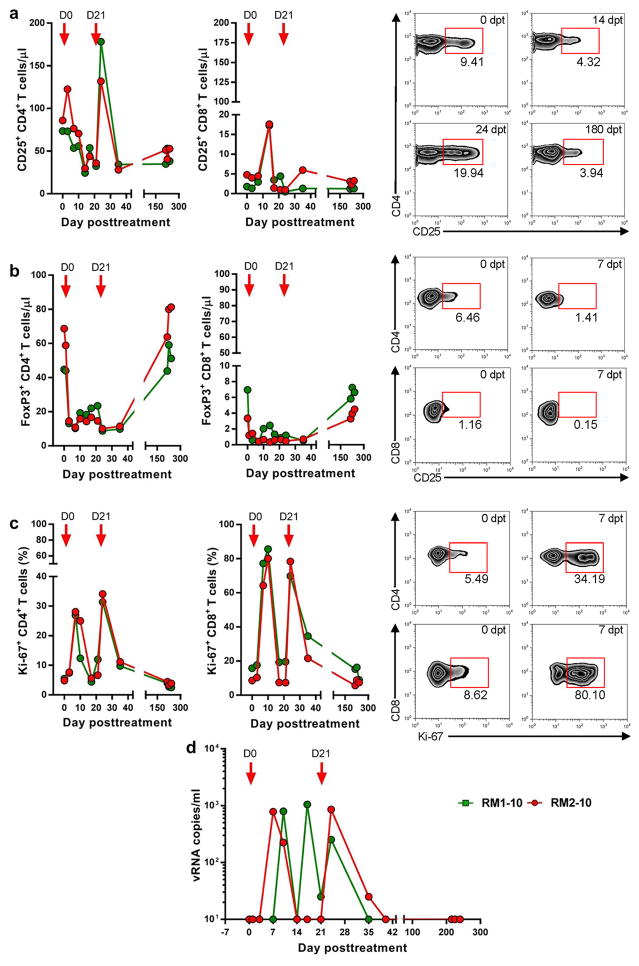
Ontak administration to SIVsab-infected controller RMs after 500 days of complete control of viral replication resulted in significant depletion of the CD25+ CD4+ T cells (Figure 1a). Depletion was rapid and massive, with up to >50% of the CD25+ CD4+ T cells being depleted by 14 dpt. However, after the second administration of Ontak, there was a very robust increase in the number of CD25+ CD4+ T cells (Figure 1a). The number of CD8+ T cells expressing CD25 showed a similar pattern of initial depletion followed by a robust rebound (Figure 1a).
Figure 1. Treg depletion with Ontak results in increased T cell proliferation and virus reactivation in SIVsab-infected RM controllers.
(a) Depletion of T cells expressing CD25 is transient and is followed by robust increases coincidental with increases in the levels of T cell activation; (b) Efficient depletion of Tregs (FoxP3+ CD4+ T cells) and minimal changes in the levels of FoxP3+ CD8+ T cells; (c) Increases in the immune activation/proliferation levels (Ki-67) of CD4+ and CD8+ T cells. Representative flow cytometry plots illustrate CD25-expressing CD4+ T cell and Treg depletion and the increases in immune activation; (d) Plasma virus rebounds after Ontak administration. Times of Ontak administration are depicted as red arrows. dpt-day posttreatment.
We further monitored the dynamics of Fox-P3+ CD25+ CD4+ T cells and report that Ontak administration induces a rapid and massive (75–85%) depletion of the Fox-P3+ CD25+ CD4+ T cells (Figure 2b). A trend of Fox-P3+ CD25+ CD4+ T cell rebound was observed prior to the second administration of Ontak (Figure 1a). The impact on the CD8+ T cells was only moderate, as the frequency of CD8+ T cells expressing Fox-P3 is very low (Figure 1b).
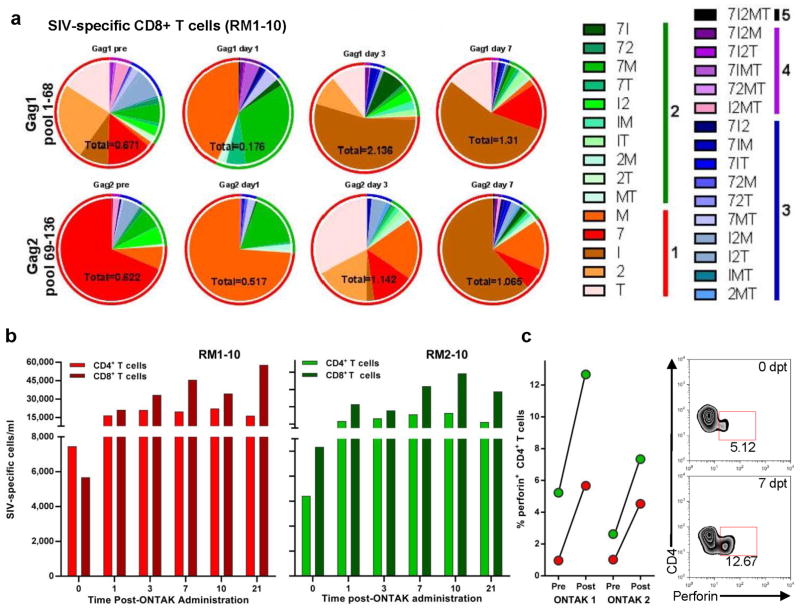
Figure 2. Ontak administration boosted the overall CTL responses in SIVsab-infected controller RMs.
(a) Serial monitoring of SIV-specific T cell polyfunctionality after the first round of Ontak administration was achieved by stimulating PBMCs with Gag SIVsab92018 peptide pools followed by intracellular cytokine staining. Cytokines tested for include: TNF-α (T); IL-2 (2); IFN-γ (I); CD107α (7); and MIP-1β (M). Data are representative of both RMs. Absolute numbers of CD8+ T cells/ml for each time point are marked beneath each pie graph. The pie charts depict functionality based on the combination of cytokines expressed. The color scheme represents the number of cytokines produced by the SIV-specific T cells and their proportion is illustrated as color-coded ring surrounding the pie charts. (b) Absolute numbers (per ml) of the aggregated Gag- and Env-specific CD4+ and CD8+ T cells at different time points following the first round of Ontak administration. The x-axis numbers represent days post-Ontak administration. (c) Significant boosting of the frequency of CD4+ T cells expressing perforin after Ontak administration. Representative flow cytometry plots illustrate increase in perforin expression by the CD4+ T cells after Treg depletion.
Experimental Treg depletion with Ontak in spontaneous controller RMs boosts T cell immune activation
A major increase of CD4+ T cell activation was observed after administration of Ontak. The frequency of the circulating CD4+ and CD8+ T cells expressing Ki-67 dramatically increased (on an average of 8–10 fold) after each Ontak administration (Figure 1c). These robust increases of CD4+ and CD8+ T cell activation persisted beyond the five-day course of each Ontak administration.
One may argue that these Ontak-induced increases in the levels of immune activation may have inadvertently boosted the size of the CD25+ CD4+ T cell fraction, which is associated with cell activation. This is not necessary a weakness of our approach. Ontak targets the cells that express CD25 and depletes them through the immunotoxin component of the drug. As such, increases in the pool of CD25-expressing CD4+ T cells will only result in an increase in the pool of the Ontak-depleted CD4+ cells, thus improving the drug ability to directly target the reservoir.
Experimental Treg depletion with Ontak reactivated the latent SIVsab in controller RMs
Prior to Ontak administration, the levels of SIVsab in plasma were below 5 vRNA copies/ml (Figure S1). After treatment, SIVsab rebounded up to 103 copies/ml (Figure 1d). This virus rebound, albeit relatively low, is not negligible and strongly suggests that Ontak could be used either alone or in combination with other conventional LRAs for virus reactivation. Unlike LRAs currently tested, which require the presence of effective CTLs to clear the cells expressing the reactivated virus, Ontak has the advantage that the cells targeted by this drug are destroyed by the diphtheria toxin that is coupled to the IL-2. Furthermore, through Treg depletion, Ontak has also the ability to boost the cell mediated immune responses necessary for effective clearance of the reactivated virus.
Experimental Treg depletion with Ontak boosted SIV-specific T cells in SIVsab-infected controller RMs
One of the most notable effects of Treg depletion with Ontak was a significant boost of SIV-specific CD8+ T cells (Figure 2a). This increase occurred in every test performed, with every tested peptide pool, and thus the absolute numbers (/ml) of the aggregated SIV-specific CD4+ and CD8+ T cells at different time points following different rounds of Ontak administration, as determined by intracellular cytokine staining and flow cytometry, were significantly boosted (p=0.0001 and p=0.0029 for CD4+ and CD8+ T cell, respectively) in Ontak-treated RM (Figure 2b). SIV-specific CD4+ T cells increased in RM1-10 from 6,430/ml prior to Ontak administration to 23,437/ml at 7 dpt, while in RM2-10 they increased from 7,456/ml prior to Ontak administration to 19,864/ml at 7 dpt. Similarly, SIV-specific CD8+ T cells increased in RM1-10 from 10,346/ml prior to Ontak administration to 47,899/ml at 7 dpt, while in RM2-10 they increased from 5,678/ml prior to Ontak administration to 45,673/ml at 7 dpt. Furthermore, measurements of granzyme and perforin as surrogate markers of cytotoxic responses revealed a massive boost of their expression on both CD4+ and CD8+ T cells. Thus, perforin expression increased 4–8 folds on CD4+ T cells (p=0.0342) and 2–7 folds on the CD8+ T cells (p=0.0313) at 7 dpt (Figure 2c).
Altogether, our results demonstrate the feasibility of Treg depletion with Ontak in chronically SIV-infected RMs, also pointing to the efficacy of the proposed approach on both reactivating the latent virus and boosting CTL responses.
Other approaches targeting Tregs have also been reported to have potential impact for cure research. Treg blockade with the anti-CTLA-4 drug ipilimumab in an HIV-infected patient on ART had significant effects on the total number and phenotype of CD4+ T cells. Furthermore, the drug induced a profound increase in cell-associated unspliced HIV RNA, resulting in a subsequent decline in plasma HIV RNA. (22). Similarly, Treg blockade with the anti-CTLA-4 human antibody MDX-010 in SIVmac-infected RMs receiving ART (23) resulted in decreased expression of IDO and TGF-β in tissues and was associated with decreased viral RNA levels in the lymph nodes and increased effector function of both SIV-specific CD4+ and CD8+ T cells. Dampening Treg function in SIV-infected RMs did not have detrimental virologic effects and was shown to provide a valuable approach to complement ART and therapeutic vaccination during treatment of HIV-1 infection (23). These results further validate our approach as a promising cure strategy.
As such, our results provide strong proof of concept data that supports approaches aimed at altering the number and function of Tregs as a strategy for cure research. Treg depletion has a dual effect, leading to both reactivation of the latent virus and improved clearing of the reactivated virus. As none of the LRAs in development has been reported to have such a dual activity, Treg depletion, alone or in combination with other LRAs, holds great promises for cure research, and has a real potential to provide both improved “shock (through reactivation by LRA AND Treg-depleting agent) and kill (through CTL boosting after Treg depletion)” effects.
Supplementary Material
Acknowledgments
We would like to thank Drs. Claire Chougnet, Nicholas Chomont and Ruy M. Ribeiro for very helpful discussion.
References
- 1.Deeks SG, Lewin SR, Ross AL, Ananworanich J, Benkirane M, Cannon P, Chomont N, Douek D, Lifson JD, Lo YR, Kuritzkes D, Margolis D, Mellors J, Persaud D, Tucker JD, Barre-Sinoussi F International AIDS Society Towards a Cure Working Group. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nature medicine. 2016;22:839–850. doi: 10.1038/nm.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allers K, Hutter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, Schneider T. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood. 2011;117:2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 3.Luzuriaga K, Gay H, Ziemniak C, Sanborn KB, Somasundaran M, Rainwater-Lovett K, Mellors JW, Rosenbloom D, Persaud D. Viremic relapse after HIV-1 remission in a perinatally infected child. The New England journal of medicine. 2015;372:786–788. doi: 10.1056/NEJMc1413931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siliciano JD, Siliciano RF. AIDS/HIV. Rekindled HIV infection. Science. 2014;345:1005–1006. doi: 10.1126/science.1259452. [DOI] [PubMed] [Google Scholar]
- 5.Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, Smith JY, Brinkman AL, Peter LE, Mathew SI, Smith KM, Borducchi EN, Rosenbloom DI, Lewis MG, Hattersley J, Li B, Hesselgesser J, Geleziunas R, Robb ML, Kim JH, Michael NL, Barouch DH. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512:74–77. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margolis DM. Eradication therapies for HIV Infection: time to begin again. AIDS research and human retroviruses. 2011;27:347–353. doi: 10.1089/aid.2011.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Policicchio BB, Pandrea I, Apetrei C. Animal Models for HIV Cure Research. Frontiers in immunology. 2016;7:12. doi: 10.3389/fimmu.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Policicchio BB, Xu C, Brocca-Cofano E, Raehtz KD, He T, Ma D, Li H, Haret-Richter GS, Dunsmore T, Trichel A, Hahn BH, Shaw GM, Mellors JW, Ribeiro RM, Pandrea I, Apetrei C. Multi-dose romidepsin reactivates latent, replication competent SIV in postantiretroviral rhesus macaque controllers. PLoS pathogens. 2016;12:e1005879. doi: 10.1371/journal.ppat.1005879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno-Fernandez ME, Presicce P, Chougnet CA. Homeostasis and function of regulatory T cells in HIV/SIV infection. Journal of virology. 2012;86:10262–10269. doi: 10.1128/JVI.00993-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran TA, de Goer de Herve MG, Hendel-Chavez H, Dembele B, Le Nevot E, Abbed K, Pallier C, Goujard C, Gasnault J, Delfraissy JF, Balazuc AM, Taoufik Y. Resting regulatory CD4 T cells: a site of HIV persistence in patients on long-term effective antiretroviral therapy. PloS one. 2008;3:e3305. doi: 10.1371/journal.pone.0003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allers K, Loddenkemper C, Hofmann J, Unbehaun A, Kunkel D, Moos V, Kaup FJ, Stahl-Hennig C, Sauermann U, Epple HJ, Schneider T. Gut mucosal FOXP3+ regulatory CD4+ T cells and Nonregulatory CD4+ T cells are differentially affected by simian immunodeficiency virus infection in rhesus macaques. Journal of virology. 2010;84:3259–3269. doi: 10.1128/JVI.01715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bi X, Suzuki Y, Gatanaga H, Oka S. High frequency and proliferation of CD4+ FOXP3+ Treg in HIV-1-infected patients with low CD4 counts. European journal of immunology. 2009;39:301–309. doi: 10.1002/eji.200838667. [DOI] [PubMed] [Google Scholar]
- 13.Nikolova M, Lelievre JD, Carriere M, Bensussan A, Levy Y. Regulatory T cells differentially modulate the maturation and apoptosis of human CD8+ T-cell subsets. Blood. 2009;113:4556–4565. doi: 10.1182/blood-2008-04-151407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss L, Donkova-Petrini V, Caccavelli L, Balbo M, Carbonneil C, Levy Y. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104:3249–3256. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 15.Elahi S, Dinges WL, Lejarcegui N, Laing KJ, Collier AC, Koelle DM, McElrath MJ, Horton H. Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nature medicine. 2011;17:989–995. doi: 10.1038/nm.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsson J, Boasso A, Velilla PA, Zhang R, Vaccari M, Franchini G, Shearer GM, Andersson J, Chougnet C. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma D, Xu C, Cillo AR, Policicchio B, Kristoff J, Haret-Richter G, Mellors JW, Pandrea I, Apetrei C. Simian immunodeficiency virus SIVsab infection of rhesus macaques as a model of complete immunological suppression with persistent reservoirs of replication-competent virus: Implications for cure research. Journal of virology. 2015;89:6155–6160. doi: 10.1128/JVI.00256-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandrea I, Gaufin T, Gautam R, Kristoff J, Mandell D, Montefiori D, Keele BF, Ribeiro RM, Veazey RS, Apetrei C. Functional cure of SIVagm infection in rhesus macaques results in complete recovery of CD4+ T cells and is reverted by CD8+ cell depletion. PLoS pathogens. 2011;7:e1002170. doi: 10.1371/journal.ppat.1002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Research Council. Guide for the care and use of laboratory animals. National Academy Press; Washington, D.C: 1996. [Google Scholar]
- 20.Pandrea I, Gaufin T, Brenchley JM, Gautam R, Monjure C, Gautam A, Coleman C, Lackner AA, Ribeiro RM, Douek DC, Apetrei C. Cutting edge: Experimentally induced immune activation in natural hosts of simian immunodeficiency virus induces significant increases in viral replication and CD4+ T cell depletion. Journal of immunology. 2008;181:6687–6691. doi: 10.4049/jimmunol.181.10.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chattopadhyay PK, Betts MR, Price DA, Gostick E, Horton H, Roederer M, De Rosa SC. The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. Journal of leukocyte biology. 2009;85:88–97. doi: 10.1189/jlb.0208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wightman F, Solomon A, Kumar SS, Urriola N, Gallagher K, Hiener B, Palmer S, McNeil C, Garsia R, Lewin SR. Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. Aids. 2015;29:504–506. doi: 10.1097/QAD.0000000000000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hryniewicz A, Boasso A, Edghill-Smith Y, Vaccari M, Fuchs D, Venzon D, Nacsa J, Betts MR, Tsai WP, Heraud JM, Beer B, Blanset D, Chougnet C, Lowy I, Shearer GM, Franchini G. CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood. 2006;108:3834–3842. doi: 10.1182/blood-2006-04-010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.