Abstract
Kallistatin was first identified in human plasma as a tissue kallikrein-binding protein and a serine proteinase inhibitor. Kallistatin via its two structural elements regulates differential signaling cascades, and thus a wide spectrum of biological functions. Kallistatin’s active site is essential for: inhibiting tissue kallikrein’s activity; stimulating endothelial nitric oxide synthase and sirtuin 1 expression and activation; and modulating the synthesis of the microRNAs, miR-34a, miR-21 and miR-203. Kallistatin’s heparin-binding site is crucial for antagonizing the signaling pathways of vascular endothelial growth factor, tumor necrosis factor-α, Wnt, transforming growth factor-β and epidermal growth factor. Circulating kallistatin levels are markedly reduced in patients with prostate and colon cancer. Kallistatin administration attenuates angiogenesis, inflammation, tumor growth and invasion in animal models and cultured cells. Therefore, tumor progression may be substantially suppressed by kallistatin’s pleiotropic activities. In this review, we will discuss the role and mechanisms of kallistatin in the regulation of cancer development.
Keywords: Kallistatin, Cancer, Vascular endothelial growth factor, Angiogenesis, Inflammation, Apoptosis, Hyperoxia
1. Introduction
Chronic inflammation and angiogenesis are critical components of cancer development, as inflammatory cells are actively recruited to the tumor microenvironment [1, 2]. Tumor necrosis factor-α (TNF-α) functions as an inflammatory cytokine, and induces vascular endothelial growth factor (VEGF) expression through paracrine mechanisms [3]. VEGF, in turn, is an essential contributor to the development of angiogenesis and tumor growth [4, 5]. Moreover, transforming growth factor-β (TGF-β) is a potent regulator of tumor metastasis by inducing endothelial-mesenchymal transition (EndMT) in endothelial cells and epithelial-mesenchymal transition (EMT) in epithelial cancer cells [6, 7]. In addition, Wnt signaling is critical for macrophage-induced cancer cell invasion by activating canonical Wnt/β-catenin signal transduction and Wnt-mediated transactivation of epidermal growth factor receptor (EGFR) [8–10]. Furthermore, tissue kallikrein (TK), which is present in endothelial and tumor cells, stimulates angiogenesis via VEGF synthesis and promotes cancer cell migration and invasion [11, 12]. Due to the multiple factors that regulate cancer development, it is a challenge to develop therapeutic regimens that target these diverse signaling pathways.
Kallistatin was discovered in human plasma as a tissue kallikrein-binding protein (KBP) and identified as a unique serine proteinase inhibitor (serpin) in our laboratory [13–17]. Serpins share a general frame of structure, but have diverse functions in the biological processes of apoptosis, inflammation and tumorigenesis [18, 19]. Serpins have been shown to either promote or inhibit tumor growth. For example, high levels of plasminogen activator inhibitor-1 are associated with poor prognosis and predicted adverse outcomes in patients with breast and ovarian cancer [20]. Moreover, α1-antichymotrypsin is up-regulated in multiple cancer types, and the elevated levels are positively correlated with worsening prognosis in patients with breast, lung and gastric cancers [21–23]. Conversely, maspin, an epithelial-specific member of the serpin superfamily, is associated with better prognosis and overall cancer patient survival [24]. Pigment epithelium-derived factor is widely reported to have anti-tumor effects in 17 different types of human cancers [25], and exhibits diverse and significant anti-tumor activities, including inhibition of tumor angiogenesis and metastasis, and enhanced apoptosis in cancer cells [26]. Likewise, circulating kallistatin levels are markedly reduced in patients with prostate and colon cancer, sepsis syndrome and liver disease [27, 28]. Kallistatin administration via local or systemic delivery in animal models retards tumor progression in breast, liver, stomach, colon and lung carcinomas by inhibiting angiogenesis, inflammation and metastasis, thereby implicating a role of kallistatin in tumor suppression [29–34].
2. Kallistatin via its structural domains exerts multiple anti-tumor actions
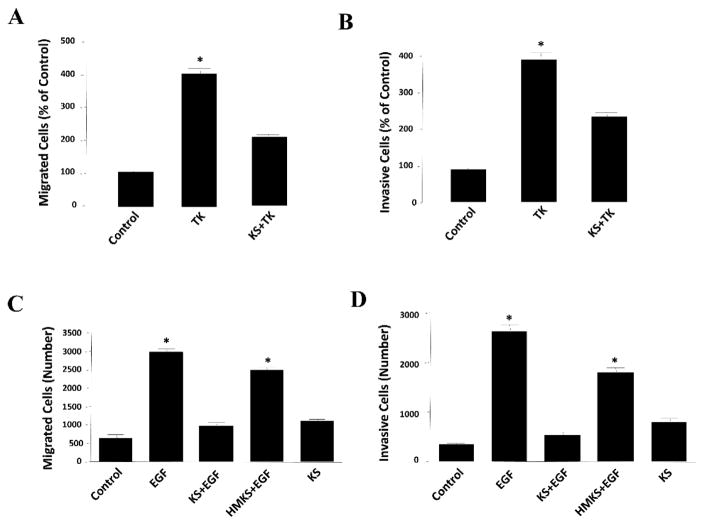
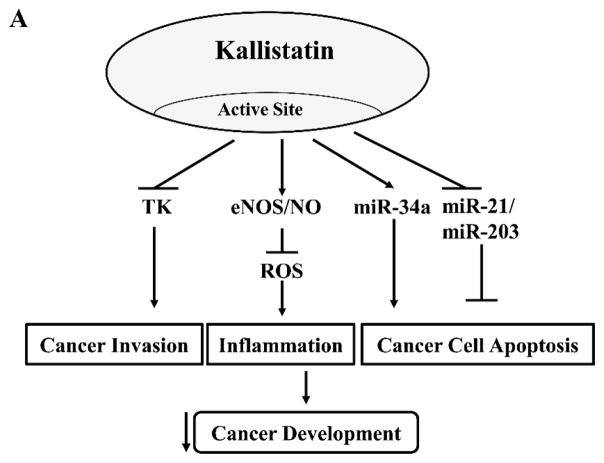
Kallistatin consists of two functional domains, an active site and a heparin-binding site [35, 36], that regulate differential signaling pathways and a wide spectrum of biological functions. Kallistatin is a unique serpin as its active site consists of Phe-Ser residues at P1-P1’ site, which can be cleaved by TK, thereby forming a covalent complex with TK [35]. Kallistatin via its active site inhibits TK’s enzymatic activity and bioavailability [37, 38], and suppresses TK-induced cancer cell migration and invasion (Fig. 1A & B). Kallistatin’s active site is also the key for stimulating endothelial nitric oxide synthase (eNOS) and sirtuin 1 (SIRT1) expression and activation by interaction with a tyrosine kinase [39]. Up-regulation of eNOS and SIRT1 by kallistatin leads to increased NO levels, a potent anti-inflammatory agent and anti-oxidant [40, 41]. Kallistatin through its active site induces cancer apoptosis by stimulating miR-34a and suppressing miR-21 and miR-203 synthesis in breast cancer cells [42]. Thus, kallistatin’s active site plays a significant role in inhibiting cancer development by modulating the effects mediated by TK, eNOS, miR-34a, miR-21 and miR-203. In addition, kallistatin via its heparin-binding domain binds to heparin sulfate proteoglycans to antagonize the following signaling pathways: VEGF-induced angiogenesis and vascular permeability [43, 44]; TNF-α- and high mobility group box 1 (HMGB1)-induced NF-κB activation and inflammatory gene expression [44, 45]; TGF-β-mediated oxidative stress and EndMT [39]; Wnt-induced cancer cell proliferation, migration and invasion [42, 46]; and epidermal growth factor (EGF)-induced cancer cell migration and invasion (Fig. 1C & D). Moreover, kallistatin induces cancer cell autophagy by preventing Wnt-mediated inhibition of peroxisome proliferator-activated receptor-γ (PPAR-γ) signaling [42]. Thus, kallistatin, through its functional domains – an active site and a heparin-binding site – regulates multiple signaling pathways to inhibit cancer development (Fig. 2A & B). These findings indicate that kallistatin via its two structural domains exerts multiple cellular functions to suppress cancer development.
Figure 1.
Kallistatin inhibits the migration and invasion of prostate cancer cells. Kallistatin (1 μM) prevented tissue kallikrein (TK, 0.1 μM)-induced (A) migration and (B) invasion of DU-145 prostate cancer cells. Wild-type kallistatin (KS, 1 μM), but not heparin-binding site mutant kallistatin (HMKS, 1 μM), antagonized epidermal growth factor (EGF, 1 ng/ml)-induced (C) migration and (D) invasion of DU-145 prostate cancer cells, indicating that kallistatin’s effects are mediated via its heparin-binding domain. n=3, *P<0.05 vs. other groups.
Figure 2.
Kallistatin’s active site and heparin-binding site play differential roles in blocking cancer development. (A). Kallistatin’s active site is key for modulating the effects induced by tissue kallikrein (TK), endothelial nitric oxide synthase (eNOS), nitric oxide (NO), miR-34a, miR-21 and miR-203. (B). Kallistatin’s heparin-binding site is essential for inhibiting the effects mediated by vascular endothelial growth factor (VEGF), tumor necrosis factor-α (TNF-α), high mobility group box 1 (HMGB1), transforming growth factor-β (TGF-β), epidermal growth factor (EGF) and Wnt.
3. Kallistatin retards tumor growth and metastasis in tumor-bearing animals
Kallistatin possesses anti-tumor activity as evidenced by inhibiting tumor growth and metastasis in animal models [29–34, 47]. For example, intramural injection of adenovirus carrying the human kallistatin gene into pre-established breast cancer xenografts in nude mice results in significant suppression of tumor growth and reduction of blood vessel numbers [29]. Recombinant kallistatin protein administration by intraperitoneal (IP) injection attenuates tumor growth and intramural neovascularization in either grafted hepatocarcinoma mice or xenografted hepatocarcinoma athymic mice, and reduces VEGF and hypoxia-inducible factor (HIF)-1α expression in hepatocellular carcinoma cells [30]. Likewise, exogenous kallistatin protein treatment by IP injection represses tumor growth and angiogenesis of gastric carcinoma xenografts in mice, and reduces VEGF and HIF-1α synthesis in gastric carcinoma cells [31]. Moreover, adeno-associated virus-mediated kallistatin gene transfer by intramuscular injection reduces angiogenesis and xenographic colon tumor growth in mice [32], and subcutaneous injection of plasmid DNA-mediated kallistatin gene delivery attenuates xenograft lung tumor growth in mice [33]. Furthermore, lentivirus-mediated kallistatin gene delivery by intravenous injection dramatically decreases tumor metastasis into lungs in association with reduced angiogenesis and inflammation, and enhances the survival of tumor-bearing mice [34]. In addition, a combination of local kallistatin gene therapy with meloxicam (a selective cyclooxygenase-2 inhibitor) was shown to have a better therapeutic effect in combating liver cancer in mice by inhibiting tumor growth and angiogenesis, and inducing apoptosis of human hepatocellular carcinoma cells [47]. Thus, addition of extrinsic kallistatin with various strategies by adenovirus, adeno-associated virus, lentivirus, plasmid DNA, or recombinant kallistatin protein, can retard tumor growth and progression in xenografted mice with breast, liver, stomach, colon and lung carcinomas. Importantly, systemic injection of lentivirus carrying human kallistatin cDNA reduces tumor metastasis into lungs and prolongs the survival of mice. Taken together, kallistatin therapy may serve as an effective and promising approach for controlling tumor growth and metastasis.
4. Kallistatin inhibits VEGF-induced angiogenesis
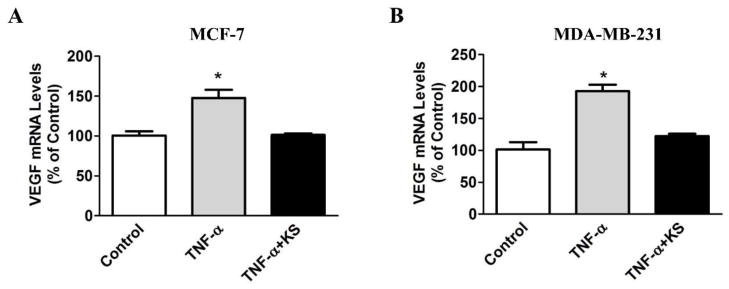
Angiogenesis is an important contributor to cancer progression, and VEGF is critical in the development of new blood vessels and tumor growth [48–51]. Since VEGF is a heparin-binding growth factor, kallistatin via its heparin-binding site competes with VEGF binding to cell surface heparan sulfate proteoglycans, thereby blocking VEGF-mediated signaling pathways and angiogenesis [43]. Moreover, kallistatin was shown to inhibit gastric carcinoma growth and angiogenesis in conjunction with reduced VEGF expression [31]. Likewise, intraperitoneal injection of kallistatin suppresses tumor growth and angiogenesis in xenografts of hepatocarcinoma in mice, and decreases VEGF synthesis in hepatocarcinoma cells [30]. TNF-α has pro-angiogenic activity via enhancing VEGF expression and secretion in macrophages and tumor cells [52]. However, kallistatin was shown to inhibit TNF-α induced VEGF expression in endothelial cells [53] and breast cancer cells (Fig. 3A & B). Therefore, kallistatin exerts anti-angiogenic actions not only by blocking VEGF-mediated signaling, but also by preventing TNF-α-induced VEGF synthesis.
Figure 3.
Kallistatin blocks tumor necrosis factor-α (TNF-α)-induced vascular endothelial growth factor (VEGF) expression in breast cancer cells. Cells were pre-treated with kallistatin (KS, 1 μM) for 30 min, followed by incubation with TNF-α (2 ng/ml) for 12 hr. Kallistatin significantly inhibited TNF-α induced VEGF expression in (A) MCF-7 and (B) MDA-MB-231 breast cancer cells. n=3, *P<0.05 vs. other groups.
5. Kallistatin inhibits cancer-related inflammation
TNF-α is produced in a wide variety of tumors and acts as a master switch in establishing an intricate link between inflammation and cancer [49]. Inflammation aids in the proliferation and survival of cancer cells and promotes angiogenesis and metastasis [54]. Activated macrophages are the major source of TNF-α, and it is well recognized that TNF-α functions as a key regulator of the tumor microenvironment [55]. Evidences indicate a critical role of TNF-α in cancer-related inflammation by promoting tumor angiogenesis, proliferation, migration and invasion [56]. Kallistatin levels are markedly reduced in patients and animal models with inflammatory disorders, sepsis syndrome and cancer [27, 28, 57, 58]. A recent study indicated that kallistatin levels in HIV-infected patients are negatively correlated with systemic inflammatory markers, such as IL-6 and high sensitivity C-reactive protein [59]. Kallistatin functions as an anti-inflammatory agent as shown by its ability to suppress: lipopolysaccharide-induced inflammation and lethality in kallistatin transgenic mice; joint swelling and inflammatory response in a rat arthritis model; vascular leakage in a mouse permeability model; inflammatory cell infiltration and TNF-α levels in rat models; and inflammatory responses in septic mice [44, 45, 58, 60–63]. Kallistatin protects against inflammation by antagonizing TNF-α- and HMGB1-mediated nuclear factor (NF)-κB activation and expression of pro-inflammatory genes in cultured endothelial cells [44, 45] and breast cancer cells [53]. Moreover, kallistatin stimulates eNOS expression by interacting with the transcription factor Kruppel-like factor 4, and also increases eNOS activity and NO generation by triggering a phosphoinositide 3-kinase (PI3K)-Akt signaling cascade in endothelial cells and endothelial progenitor cells (EPCs) [40, 41, 64]. Kallistatin not only stimulates eNOS expression [39], but also prevents TNF-α-mediated inhibition of eNOS synthesis in endothelial cells (unpublished results). NO production can inhibit the expression of cell adhesion molecules by preventing activation of the pro-inflammatory transcription factor NF-κB [65]. Therefore, kallistatin exerts anti-inflammatory actions via regulating differential signaling pathways, namely: 1) preventing TNF-α- and HMGB1-induced inflammatory gene expression; 2) stimulating eNOS synthesis and activation, and NO formation; 3) reversing TNF-α mediated suppression of eNOS synthesis; and 4) reducing VEGF-induced vascular permeability. Thus, kallistatin is a unique anti-inflammatory agent in protection against cancer development.
6. Kallistatin inhibits TGF-β-induced EndMT and EMT
As metastasis causes 90% of cancer patient mortality, understanding the initial step of metastasis is critical to the future development of novel strategies to prevent the spread of cancer. EndMT and EMT play pivotal roles in fibrosis and tumor metastasis [66–68]. TGF-β is the most potent inducer of EndMT and EMT, as TGF-β signaling is involved in controlling endothelial or epithelial plasticity by eliciting their transition to a mesenchymal state [68–70]. miR-21 is an important player in organ fibrosis and tumor invasion, and its expression levels rise to a significant extent during EndMT [70, 71]. TGF-β-induced EndMT is partly regulated by miR-21, as blockade of miR-21 was found to prevent EndMT [6, 39]. Moreover, reactive oxygen species (ROS) production leads to increased miR-21 synthesis [72]. Kallistatin treatment exerts beneficial effects on cardiac and renal fibrosis by suppressing TGF-β synthesis and oxidative stress in animal models, and antagonizing TGF-β-induced collagen synthesis in cardiac myofibroblasts [73, 74]. In endothelial cells, kallistatin via its heparin-binding site antagonizes TGF-β-induced miR-21 synthesis and ROS formation, while its active site is crucial for stimulating the synthesis of antioxidant genes, including eNOS, SIRT1 and forkhead box O1 (FoxO1) [39]. Furthermore, kallistatin suppresses TGF-β-induced EMT in epithelial MCF-7 breast cancer cells, as identified by increased E-cadherin and reduced snail-1, fibronectin and vimentin synthesis (unpublished results). In addition to TGF-β, EMT is also triggered by other secreted growth factors, such as Wnt, TNF-α and EGF [75–78]. Therefore, kallistatin could protect against cancer invasion, in part, by suppressing EndMT and EMT.
7. Kallistatin inhibits cancer cell proliferation, migration and invasion
Kallistatin is capable of inhibiting the proliferation, migration and invasion of cancer cells induced by Wnt, TGF-β and EGF [39, 43, 46]. Wnt signaling is critical for macrophage-induced cancer cell invasion by activating the canonical Wnt/β-catenin signaling pathway and Wnt-mediated transactivation of EGFR [8, 9]. Kallistatin via its heparin-binding site suppresses Wnt3a-induced proliferation, migration and invasion of breast cancer cells [42, 46]. Kallistatin antagonizes the Wnt3a signaling pathway by forming a complex with Wnt co-receptor low-density lipoprotein receptor-related protein 6 (LRP6) in breast cancer and retinal epithelial cells [46, 61]. As TNF-α has been shown to promote Wnt signalling in gastric tumor cells [79], kallistatin may also block TNF-α-mediated Wnt/β-catenin signaling. In hepatocellular carcinoma, decreased β-II spectrin (SPTBN1) is associated with reduced kallistatin levels, leading to elevated Wnt signal transduction [80]. Kallistatin administration inhibits colon tumor growth by inhibiting angiogenesis and tumor cell proliferation [32]. Furthermore, kallistatin could retard cancer cell invasion by blocking TGF-β-mediated EndMT [39] and EMT. In addition, kallistatin via its heparin-binding site antagonizes EGF-induced migration and invasion of prostate cancer cells (Fig. 1C & D). Thus, kallistatin through its heparin-binding site inhibits the growth, migration and invasion of cancer cells mediated by Wnt3a, TGF-β and EGF.
8. Kallistatin inhibits TK/kinin-induced cancer development
TK is a serine proteinase that cleaves low molecular weight kininogen substrate to release kinin peptides [81]. Kinins and their receptors are well-known inducers of the pro-inflammatory response [82, 83]. TK is present in many tumors, such as those of the breast, lung, stomach, pancreas, pituitary, prostate, and uterus [84]. The kallikrein-kinin system is involved in tumor development, as administration of icatibant (a kinin B2 receptor antagonist) suppressed angiogenesis, vascular permeability and tumor growth in mice [85]. Kinin B2 receptor signaling facilitates cancer progression, as tumor-associated angiogenesis and growth are markedly suppressed in rodent models genetically deficient in kininogen and kinin B2 receptor [86]. Moreover, a selective TK inhibitor or a kinin B2 receptor antagonist ameliorates vascular permeability and inflammatory cell infiltration in the intestine and lung following ischemia/reperfusion injury [87, 88]. TK gene transfer improves cardiac function and promotes neovascularization by increasing Akt and glycogen synthase kinase-3β (GSK-3β) phosphorylation, leading to increased VEGF and VEGF receptor-2 expression in ischemic myocardium and cultured endothelial cells [89]. Likewise, kinin stimulates endothelial cell proliferation and capillary tube formation through transactivation of VEGF receptor-2 by the kinin B2 receptor [90, 91]. Thus, TK and kinin promote neovascularization and restore blood flow through kinin B2 receptor-Akt-GSK-3β and VEGF signaling pathways [89]. As an endogenous TK inhibitor, kallistatin is capable of blocking TK-induced migration and invasion of prostate cancer cells (Fig. 1A & B). These findings indicate a potential role of kallistatin in controlling TK/kinin-mediated angiogenesis, tumor growth and invasion.
9. Kallistatin induces cancer cell apoptosis and autophagy
Numerous evidences indicate that microRNAs are key players in cancer biology, acting as oncogenes or tumor suppressor genes [92]. Among miRNAs, miR-34a is a critical tumor suppressor in many types of cancers [93–96]. In breast cancer, miR-34a expression is decreased [96], and overexpression of miR-34a induces apoptosis and suppresses cell proliferation and migration [96–98]. On the other hand, miR-21 is a well-recognized tumor inducer, as elevation of miR-21 in breast cancer patients is associated with poor survival [99]. An anti-miR-21 inhibitor has been shown to inhibit levels of the survival protein Bcl-2 in breast cancer [100]. miR-203 may also play a role in cancer progression as it is overexpressed in human breast cancer, while miR-203 knockdown sensitizes apoptotic cell death in MCF-7 breast cancer cells [101]. Moreover, recent studies have shown that autophagy can suppress tumor cell growth, which is a promising strategy for the treatment of breast cancer [102, 103].
Kallistatin was found to induce apoptosis in breast cancer cells by stimulating miR-34a and inhibiting miR-21 and miR-203 synthesis in MDA-MB-231 breast cancer cells [42]. In addition, kallistatin promotes breast cancer cell autophagy, which was mediated by preventing Wnt-mediated inhibition of PPAR-γ signaling [42]. Furthermore, kallistatin (SERPINA3K) induces apoptotic cell death in cultured colorectal cancer cells via activating the PPAR-γ-Fas-FasL signaling pathway [104]. Kallistatin’s active site plays a key role in modulating miR-34a, miR-21 and miR-203 synthesis, while its heparin-binding site is essential for blocking Wnt-PPAR-γ signaling in breast cancer cells [42]. Therefore, kallistatin can suppress tumor development by inducing cancer cell apoptosis and autophagy.
10. Role of kallistatin in hyperoxia-mediated cancer cell death
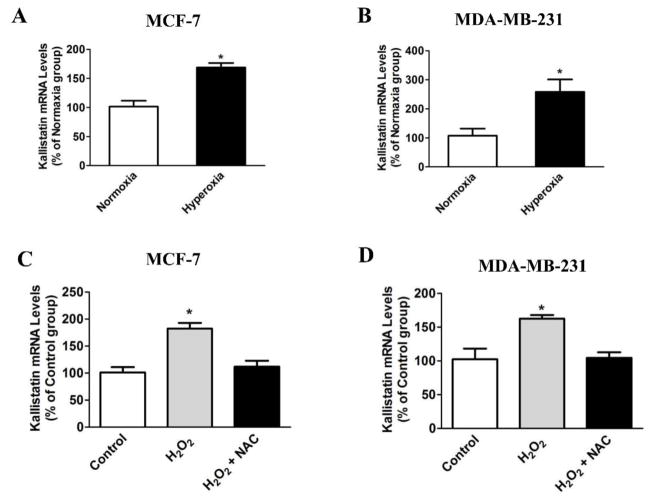
Hyperoxia can retard growth and induce apoptosis in rat mammary tumors [105]. Likewise, kallistatin inhibits tumor growth and metastasis in tumor-bearing mice [29, 34], and induces apoptosis and autophagy in breast cancer cells [42]. Hyperoxia treatment or H2O2 incubation markedly increases kallistatin expression in both MCF-7 and MDA-MB-231 breast cancer cells (Fig. 4A–D). Moreover, the antioxidant N-acetyl-L-cysteine (NAC) blocks H2O2-induced kallistatin expression (Fig. 4C & D). These findings indicate that hyperoxia and H2O2, through ROS formation, induce kallistatin expression in cancer cells. As with kallistatin [42], hyperoxia treatment also induces the expression of apoptosis and autophagic markers, such as BAX, ATG5 and Beclin-1, in breast cancer cells (unpublished results). These findings implicate a potential role of kallistatin in mediating the effect of hyperoxia on cancer cell death.
Figure 4.
Hyperoxia or H2O2 treatment increases kallistatin expression in breast cancer cells. Hyperoxia (95% O2 and 5% CO2) treatment for 18 hr significantly increased kallistatin synthesis in (A) MCF-7 and (B) MDA-MB-231 breast cancer cells. H2O2 incubation (30 μM) for 12 hr also increased kallistatin synthesis in (C) MCF-7 and (D) MDA-MB-231 breast cancer cells. Kallistatin expression was blocked by the antioxidant N-acetyl-L-cysteine (NAC, 2 mM). n=3, *P<0.05 vs. other groups.
11. Conclusion
Kallistatin exerts multi-factorial activities in retarding tumor progression. Cancer cells are able to switch a normal microenvironment to one that supports tumor growth and metastasis by inducing angiogenesis and inflammation. Kallistatin is an effective inhibitor of angiogenesis and inflammation. In murine models of tumor xenografts, kallistatin treatment inhibits the development of diverse cancers, including breast, liver, colon, lung and gastric carcinoma [29–34, 47]. Moreover, systemic delivery of the kallistatin gene dramatically inhibits experimental lung metastasis, inflammation and angiogenesis in tumor-bearing mice [34]. Kallistatin through its two functional domains regulates differential signaling pathways, thus protects against tumor growth and metastasis. Kallistatin’s active site is crucial for inhibiting TK-mediated cancer progression; suppressing inflammation by increasing eNOS and SIRT1 levels; and inducing cancer cell apoptosis by stimulating miR-34a and reducing miR-21 and miR-203 synthesis [42]. Kallistatin via its heparin-binding site antagonizes VEGF-induced angiogenesis; TNF-α- and HMGB1-induced inflammation; TGF-β-mediated EndMT and EMT; Wnt3a-, TGF-β- and EGF-induced cancer cell migration and invasion; and Wnt-PPARγ-mediated cancer cell autophagy [39, 42–46]. Therefore, kallistatin exerts pleiotropic activities to inhibit tumor progression. Plasma kallistatin levels are significantly reduced in patients with prostate and colon cancer [28]. Recent studies highlight the potential of kallistatin as a biomarker for the diagnosis of liver cirrhosis and an independent prognostic indicator for colorectal cancer [106, 107]. Therefore, it is possible that kallistatin’s role as a biomarker can also be applied to diverse human cancers. Moreover, kallistatin-based therapy may constitute a significant advancement in cancer treatment due to its multiple anti-tumor actions. The potential role of kallistatin therapy in humans requires further investigation to warrant clinical studies of kallistatin in cancer treatment.
Highlights.
Kallistatin treatment inhibits tumor growth and metastasis in animal models
Kallistatin inhibits tumor angiogenesis and cancer-related inflammation
Kallistatin inhibits EndMT and EMT
Kallistatin inhibits cancer cell proliferation, migration and invasion
Kallistatin inhibits tissue kallikrein-mediated cancer cell invasion
Kallistatin induces cancer cell apoptosis and autophagy
Acknowledgments
This work was supported by the National Institutes of Health grants HL118516.
Footnotes
Conflict of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Kimura YN, Watari K, Fotovati A, et al. Inflammatory stimuli from macrophages and cancer cells synergistically promote tumor growth and angiogenesis. Cancer Sci. 2007;98:2009–18. doi: 10.1111/j.1349-7006.2007.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crisostomo PR, Wang Y, Markel TA, et al. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–82. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 5.Milkiewicz M, Ispanovic E, Doyle JL, et al. Regulators of angiogenesis and strategies for their therapeutic manipulation. Int J Biochem Cell Biol. 2006;38:333–57. doi: 10.1016/j.biocel.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Kumarswamy R, Volkmann I, Jazbutyte V, et al. Transforming growth factor-beta-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler Thromb Vasc Biol. 2012;32:361–9. doi: 10.1161/ATVBAHA.111.234286. [DOI] [PubMed] [Google Scholar]
- 7.Yeh YH, Wang SW, Yeh YC, et al. Rhapontigenin inhibits TGF-beta-mediated epithelialmesenchymal transition via the PI3K/AKT/mTOR pathway and is not associated with HIF-1alpha degradation. Oncol Rep. 2016;35:2887–95. doi: 10.3892/or.2016.4664. [DOI] [PubMed] [Google Scholar]
- 8.Menck K, Klemm F, Gross JC, et al. Induction and transport of Wnt 5a during macrophage-induced malignant invasion is mediated by two types of extracellular vesicles. Oncotarget. 2013;4:2057–66. doi: 10.18632/oncotarget.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pukrop T, Klemm F, Hagemann T, et al. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci U S A. 2006;103:5454–9. doi: 10.1073/pnas.0509703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musgrove EA. Wnt signalling via the epidermal growth factor receptor: a role in breast cancer? Breast Cancer Res. 2004;6:65–8. doi: 10.1186/bcr737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao L, Smith RS, Chen LM, et al. Tissue kallikrein promotes prostate cancer cell migration and invasion via a protease-activated receptor-1-dependent signaling pathway. Biol Chem. 2010;391:803–12. doi: 10.1515/BC.2010.084. [DOI] [PubMed] [Google Scholar]
- 12.Kryza T, Achard C, Parent C, et al. Angiogenesis stimulated by human kallikrein-related peptidase 12 acting via a platelet-derived growth factor B-dependent paracrine pathway. Faseb J. 2014;28:740–51. doi: 10.1096/fj.13-237503. [DOI] [PubMed] [Google Scholar]
- 13.Chao J, Tillman DM, Wang MY, et al. Identification of a new tissue-kallikrein-binding protein. Biochem J. 1986;239:325–31. doi: 10.1042/bj2390325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou GX, Chao L, Chao J. Kallistatin: a novel human tissue kallikrein inhibitor. Purification, characterization, and reactive center sequence. J Biol Chem. 1992;267:25873–80. [PubMed] [Google Scholar]
- 15.Chen VC, Chao L, Chao J. Roles of the P1, P2, and P3 residues in determining inhibitory specificity of kallistatin toward human tissue kallikrein. J Biol Chem. 2000;275:38457–66. doi: 10.1074/jbc.M005605200. [DOI] [PubMed] [Google Scholar]
- 16.Chai KX, Chen LM, Chao J, et al. Kallistatin: a novel human serine proteinase inhibitor. Molecular cloning, tissue distribution, and expression in Escherichia coli. J Biol Chem. 1993;268:24498–505. [PubMed] [Google Scholar]
- 17.Chao J, Chao L. Biochemistry, regulation and potential function of kallistatin. Biol Chem Hoppe Seyler. 1995;376:705–13. [PubMed] [Google Scholar]
- 18.Law RH, Zhang Q, McGowan S, et al. An overview of the serpin superfamily. Genome Biol. 2006;7:216. doi: 10.1186/gb-2006-7-5-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzinic SH, Bernardo MM, Oliveira DS, et al. Tumor suppressor maspin as a modulator of host immune response to cancer. Bosn J Basic Med Sci. 2015;15:1–6. doi: 10.17305/bjbms.2015.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortenberry YM, Brandal SM, Carpentier G, et al. Intracellular Expression of PAI-1 Specific Aptamers Alters Breast Cancer Cell Migration, Invasion and Angiogenesis. PLoS One. 2016;11:e0164288. doi: 10.1371/journal.pone.0164288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamura J, Miyoshi Y, Tamaki Y, et al. mRNA expression level of estrogen-inducible gene, alpha 1-antichymotrypsin, is a predictor of early tumor recurrence in patients with invasive breast cancers. Cancer Sci. 2004;95:887–92. doi: 10.1111/j.1349-7006.2004.tb02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higashiyama M, Doi O, Yokouchi H, et al. Alpha-1-antichymotrypsin expression in lung adenocarcinoma and its possible association with tumor progression. Cancer. 1995;76:1368–76. doi: 10.1002/1097-0142(19951015)76:8<1368::aid-cncr2820760812>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 23.Tahara E, Ito H, Taniyama K, et al. Alpha 1-antitrypsin, alpha 1-antichymotrypsin, and alpha 2-macroglobulin in human gastric carcinomas: a retrospective immunohistochemical study. Hum Pathol. 1984;15:957–64. doi: 10.1016/s0046-8177(84)80125-2. [DOI] [PubMed] [Google Scholar]
- 24.Machowska M, Wachowicz K, Sopel M, et al. Nuclear location of tumor suppressor protein maspin inhibits proliferation of breast cancer cells without affecting proliferation of normal epithelial cells. BMC Cancer. 2014;14:142. doi: 10.1186/1471-2407-14-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becerra SP, Notario V. The effects of PEDF on cancer biology: mechanisms of action and therapeutic potential. Nat Rev Cancer. 2013;13:258–71. doi: 10.1038/nrc3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belkacemi L, Zhang SX. Anti-tumor effects of pigment epithelium-derived factor (PEDF): implication for cancer therapy. A mini-review. J Exp Clin Cancer Res. 2016;35:4. doi: 10.1186/s13046-015-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao J, Schmaier A, Chen LM, et al. Kallistatin, a novel human tissue kallikrein inhibitor: levels in body fluids, blood cells, and tissues in health and disease. J Lab Clin Med. 1996;127:612–20. doi: 10.1016/s0022-2143(96)90152-3. [DOI] [PubMed] [Google Scholar]
- 28.Chao J, Bledsoe G, Chao L. Protective Role of Kallistatin in Vascular and Organ Injury. Hypertension. 2016 doi: 10.1161/HYPERTENSIONAHA.116.07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miao RQ, Agata J, Chao L, et al. Kallistatin is a new inhibitor of angiogenesis and tumor growth. Blood. 2002;100:3245–52. doi: 10.1182/blood-2002-01-0185. [DOI] [PubMed] [Google Scholar]
- 30.Lu L, Yang Z, Zhu B, et al. Kallikrein-binding protein suppresses growth of hepatocellular carcinoma by anti-angiogenic activity. Cancer Lett. 2007;257:97–106. doi: 10.1016/j.canlet.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Zhu B, Lu L, Cai W, et al. Kallikrein-binding protein inhibits growth of gastric carcinoma by reducing vascular endothelial growth factor production and angiogenesis. Mol Cancer Ther. 2007;6:3297–306. doi: 10.1158/1535-7163.MCT-06-0798. [DOI] [PubMed] [Google Scholar]
- 32.Diao Y, Ma J, Xiao WD, et al. Inhibition of angiogenesis and HCT-116 xenograft tumor growth in mice by kallistatin. World J Gastroenterol. 2007;13:4615–9. doi: 10.3748/wjg.v13.i34.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia D, Zheng C, Feng J, et al. Plasmid mediated kallistain gene expression via intramuscular electroporation delivery in vivo for treatment of NCI-H446 subcutaneous xenograft tumor. Pak J Pharm Sci. 2014;27:633–6. [PubMed] [Google Scholar]
- 34.Shiau AL, Teo ML, Chen SY, et al. Inhibition of experimental lung metastasis by systemic lentiviral delivery of kallistatin. BMC Cancer. 2010;10:245. doi: 10.1186/1471-2407-10-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen VC, Chao L, Chao J. Reactive-site specificity of human kallistatin toward tissue kallikrein probed by site-directed mutagenesis. Biochim Biophys Acta. 2000;1479:237–46. doi: 10.1016/s0167-4838(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 36.Chen VC, Chao L, Pimenta DC, et al. Identification of a major heparin-binding site in kallistatin. J Biol Chem. 2001;276:1276–84. doi: 10.1074/jbc.M005791200. [DOI] [PubMed] [Google Scholar]
- 37.Chen VC, Chao L, Chao J. A positively charged loop on the surface of kallistatin functions to enhance tissue kallikrein inhibition by acting as a secondary binding site for kallikrein. J Biol Chem. 2000;275:40371–7. doi: 10.1074/jbc.M005691200. [DOI] [PubMed] [Google Scholar]
- 38.Xiong W, Tang CQ, Zhou GX, et al. In vivo catabolism of human kallikrein-binding protein and its complex with tissue kallikrein. J Lab Clin Med. 1992;119:514–21. [PubMed] [Google Scholar]
- 39.Guo Y, Li P, Bledsoe G, et al. Kallistatin inhibits TGF-beta-induced endothelial-mesenchymal transition by differential regulation of microRNA-21 and eNOS expression. Exp Cell Res. 2015;337:103–10. doi: 10.1016/j.yexcr.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen B, Gao L, Hsu YT, et al. Kallistatin attenuates endothelial apoptosis through inhibition of oxidative stress and activation of Akt-eNOS signaling. Am J Physiol Heart Circ Physiol. 2010;299:H1419–27. doi: 10.1152/ajpheart.00591.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen B, Smith RS, Jr, Hsu YT, et al. Kruppel-like factor 4 is a novel mediator of Kallistatin in inhibiting endothelial inflammation via increased endothelial nitric-oxide synthase expression. J Biol Chem. 2009;284:35471–8. doi: 10.1074/jbc.M109.046813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li P, Guo Y, Bledsoe G, et al. Kallistatin induces breast cancer cell apoptosis and autophagy by modulating Wnt signaling and microRNA synthesis. Exp Cell Res. 2016;340:305–14. doi: 10.1016/j.yexcr.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miao RQ, Chen V, Chao L, et al. Structural elements of kallistatin required for inhibition of angiogenesis. Am J Physiol Cell Physiol. 2003;284:C1604–13. doi: 10.1152/ajpcell.00524.2002. [DOI] [PubMed] [Google Scholar]
- 44.Yin H, Gao L, Shen B, et al. Kallistatin inhibits vascular inflammation by antagonizing tumor necrosis factor-alpha-induced nuclear factor kappaB activation. Hypertension. 2010;56:260–7. doi: 10.1161/HYPERTENSIONAHA.110.152330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li P, Bledsoe G, Yang ZR, et al. Human kallistatin administration reduces organ injury and improves survival in a mouse model of polymicrobial sepsis. Immunology. 2014;142:216–26. doi: 10.1111/imm.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Yang Z, Li P, et al. Kallistatin antagonizes Wnt/beta-catenin signaling and cancer cell motility via binding to low-density lipoprotein receptor-related protein 6. Mol Cell Biochem. 2013;379:295–301. doi: 10.1007/s11010-013-1654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang X, Li H, Qiao H, et al. Combining kallistatin gene therapy and meloxicam to treat hepatocellular carcinoma in mice. Cancer Sci. 2009;100:2226–33. doi: 10.1111/j.1349-7006.2009.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Byrne KJ, Dalgleish AG, Browning MJ, et al. The relationship between angiogenesis and the immune response in carcinogenesis and the progression of malignant disease. Eur J Cancer. 2000;36:151–69. doi: 10.1016/s0959-8049(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 49.Kundu JK, Surh YJ. Emerging avenues linking inflammation and cancer. Free Radic Biol Med. 2012;52:2013–37. doi: 10.1016/j.freeradbiomed.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 50.Reuter S, Gupta SC, Chaturvedi MM, et al. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeo EJ, Cassetta L, Qian BZ, et al. Myeloid WNT7b mediates the angiogenic switch and metastasis in breast cancer. Cancer Res. 2014;74:2962–73. doi: 10.1158/0008-5472.CAN-13-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao R, Ji H, Yang Y, et al. Collaborative effects between the TNFalpha-TNFR1-macrophage axis and the VEGF-C-VEGFR3 signaling in lymphangiogenesis and metastasis. Oncoimmunology. 2015;4:e989777. doi: 10.4161/2162402X.2014.989777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang KF, Huang XP, Xiao GQ, et al. Kallistatin, a novel anti-angiogenesis agent, inhibits angiogenesis via inhibition of the NF-kappaB signaling pathway. Biomed Pharmacother. 2014;68:455–61. doi: 10.1016/j.biopha.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 54.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 55.Sunderkotter C, Steinbrink K, Goebeler M, et al. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–22. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 56.Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010;102:639–44. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin WC, Lu SL, Lin CF, et al. Plasma kallistatin levels in patients with severe community-acquired pneumonia. Crit Care. 2013;17:R27. doi: 10.1186/cc12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen LM, Chao L, Chao J. Beneficial effects of kallikrein-binding protein in transgenic mice during endotoxic shock. Life Sci. 1997;60:1431–5. doi: 10.1016/s0024-3205(97)00094-5. [DOI] [PubMed] [Google Scholar]
- 59.Eckard AR, Cho S, O’Riordan MA, et al. Kallistatin Levels in HIV-infected Patients and Effects of Statin Therapy. Biomarkers. 2016:1–26. doi: 10.1080/1354750X.2016.1204002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hsieh JL, Shen PC, Shiau AL, et al. Adenovirus-mediated kallistatin gene transfer ameliorates disease progression in a rat model of osteoarthritis induced by anterior cruciate ligament transection. Hum Gene Ther. 2009;20:147–58. doi: 10.1089/hum.2008.096. [DOI] [PubMed] [Google Scholar]
- 61.Liu X, Zhang B, McBride JD, et al. Antiangiogenic and antineuroinflammatory effects of kallistatin through interactions with the canonical Wnt pathway. Diabetes. 2013;62:4228–38. doi: 10.2337/db12-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu SL, Tsai CY, Luo YH, et al. Kallistatin modulates immune cells and confers anti-inflammatory response to protect mice from group A streptococcal infection. Antimicrob Agents Chemother. 2013;57:5366–72. doi: 10.1128/AAC.00322-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li P, Guo Y, Bledsoe G, et al. Kallistatin treatment attenuates lethality and organ injury in mouse models of established sepsis. Crit Care. 2015;19:200. doi: 10.1186/s13054-015-0919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao L, Li P, Zhang J, et al. Novel role of kallistatin in vascular repair by promoting mobility, viability, and function of endothelial progenitor cells. J Am Heart Assoc. 2014;3:e001194. doi: 10.1161/JAHA.114.001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spiecker M, Darius H, Kaboth K, et al. Differential regulation of endothelial cell adhesion molecule expression by nitric oxide donors and antioxidants. J Leukoc Biol. 1998;63:732–9. [PubMed] [Google Scholar]
- 66.Lin F, Wang N, Zhang TC. The role of endothelial-mesenchymal transition in development and pathological process. IUBMB Life. 2012;64:717–23. doi: 10.1002/iub.1059. [DOI] [PubMed] [Google Scholar]
- 67.Diepenbruck M, Christofori G. Epithelial-mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol. 2016;43:7–13. doi: 10.1016/j.ceb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 68.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–84. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshimatsu Y, Watabe T. Roles of TGF-beta signals in endothelial-mesenchymal transition during cardiac fibrosis. Int J Inflam. 2011;2011:724080. doi: 10.4061/2011/724080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Meeteren LA, ten Dijke P. Regulation of endothelial cell plasticity by TGF-beta. Cell Tissue Res. 2012;347:177–86. doi: 10.1007/s00441-011-1222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo F, Ji J, Liu Y, et al. MicroRNA-21, up-regulated by arsenite, directs the epithelial-mesenchymal transition and enhances the invasive potential of transformed human bronchial epithelial cells by targeting PDCD4. Toxicol Lett. 2014;232:301–9. doi: 10.1016/j.toxlet.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Ling M, Li Y, Xu Y, et al. Regulation of miRNA-21 by reactive oxygen species-activated ERK/NF-kappaB in arsenite-induced cell transformation. Free Radic Biol Med. 2012;52:1508–18. doi: 10.1016/j.freeradbiomed.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 73.Chao J, Yin H, Yao YY, et al. Novel role of kallistatin in protection against myocardial ischemia-reperfusion injury by preventing apoptosis and inflammation. Hum Gene Ther. 2006;17:1201–13. doi: 10.1089/hum.2006.17.1201. [DOI] [PubMed] [Google Scholar]
- 74.Gao L, Yin HS, Smith RJ, et al. Role of kallistatin in prevention of cardiac remodeling after chronic myocardial infarction. Lab Invest. 2008;88:1157–66. doi: 10.1038/labinvest.2008.85. [DOI] [PubMed] [Google Scholar]
- 75.Koeck S, Amann A, Huber JM, et al. The impact of metformin and salinomycin on transforming growth factor beta-induced epithelial-to-mesenchymal transition in non-small cell lung cancer cell lines. Oncol Lett. 2016;11:2946–52. doi: 10.3892/ol.2016.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J, Tian XJ, Xing J. Signal Transduction Pathways of EMT Induced by TGF-beta, SHH, and WNT and Their Crosstalks. J Clin Med. 2016:5. doi: 10.3390/jcm5040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu Y, Cheng Y, Guo Y, et al. Protein kinase D2 contributes to TNF-alpha-induced epithelial mesenchymal transition and invasion via the PI3K/GSK-3beta/beta-catenin pathway in hepatocellular carcinoma. Oncotarget. 2016;7:5327–41. doi: 10.18632/oncotarget.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsai PC, Fu YS, Chang LS, et al. Taiwan cobra cardiotoxin III suppresses EGF/EGFR-mediated epithelial-to-mesenchymal transition and invasion of human breast cancer MDA-MB-231 cells. Toxicon. 2016;111:108–20. doi: 10.1016/j.toxicon.2016.01.051. [DOI] [PubMed] [Google Scholar]
- 79.Oguma K, Oshima H, Aoki M, et al. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. Embo J. 2008;27:1671–81. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhi X, Lin L, Yang S, et al. betaII-Spectrin (SPTBN1) suppresses progression of hepatocellular carcinoma and Wnt signaling by regulation of Wnt inhibitor kallistatin. Hepatology. 2015;61:598–612. doi: 10.1002/hep.27558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- 82.Calixto JB, Cabrini DA, Ferreira J, et al. Kinins in pain and inflammation. Pain. 2000;87:1–5. doi: 10.1016/S0304-3959(00)00335-3. [DOI] [PubMed] [Google Scholar]
- 83.Couture R, Harrisson M, Vianna RM, et al. Kinin receptors in pain and inflammation. Eur J Pharmacol. 2001;429:161–76. doi: 10.1016/s0014-2999(01)01318-8. [DOI] [PubMed] [Google Scholar]
- 84.Obiezu CV, Diamandis EP. Human tissue kallikrein gene family: applications in cancer. Cancer Lett. 2005;224:1–22. doi: 10.1016/j.canlet.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 85.Wu J, Akaike T, Maeda H. Modulation of enhanced vascular permeability in tumors by a bradykinin antagonist, a cyclooxygenase inhibitor, and a nitric oxide scavenger. Cancer Res. 1998;58:159–65. [PubMed] [Google Scholar]
- 86.Ikeda Y, Hayashi I, Kamoshita E, et al. Host stromal bradykinin B2 receptor signaling facilitates tumor-associated angiogenesis and tumor growth. Cancer Res. 2004;64:5178–85. doi: 10.1158/0008-5472.CAN-03-3589. [DOI] [PubMed] [Google Scholar]
- 87.Reshef A, Leibovich I, Goren A. Hereditary angioedema: new hopes for an orphan disease. Isr Med Assoc J. 2008;10:850–5. [PubMed] [Google Scholar]
- 88.Souza DG, Pinho V, Pesquero JL, et al. Role of the bradykinin B2 receptor for the local and systemic inflammatory response that follows severe reperfusion injury. Br J Pharmacol. 2003;139:129–39. doi: 10.1038/sj.bjp.0705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yao YY, Yin H, Shen B, et al. Tissue kallikrein promotes neovascularization and improves cardiac function by the Akt-glycogen synthase kinase-3beta pathway. Cardiovasc Res. 2008;80:354–64. doi: 10.1093/cvr/cvn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thuringer D, Maulon L, Frelin C. Rapid transactivation of the vascular endothelial growth factor receptor KDR/Flk-1 by the bradykinin B2 receptor contributes to endothelial nitric-oxide synthase activation in cardiac capillary endothelial cells. J Biol Chem. 2002;277:2028–32. doi: 10.1074/jbc.M109493200. [DOI] [PubMed] [Google Scholar]
- 91.Miura S, Matsuo Y, Saku K. Transactivation of KDR/Flk-1 by the B2 receptor induces tube formation in human coronary endothelial cells. Hypertension. 2003;41:1118–23. doi: 10.1161/01.HYP.0000064345.33807.57. [DOI] [PubMed] [Google Scholar]
- 92.Stahlhut C, Slack FJ. MicroRNAs and the cancer phenotype: profiling, signatures and clinical implications. Genome Med. 2013;5:111. doi: 10.1186/gm516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–52. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tazawa H, Tsuchiya N, Izumiya M, et al. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci U S A. 2007;104:15472–7. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wiggins JF, Ruffino L, Kelnar K, et al. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;70:5923–30. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang S, Li Y, Gao J, et al. MicroRNA-34 suppresses breast cancer invasion and metastasis by directly targeting Fra-1. Oncogene. 2013;32:4294–303. doi: 10.1038/onc.2012.432. [DOI] [PubMed] [Google Scholar]
- 97.Deng X, Cao M, Zhang J, et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials. 2014;35:4333–44. doi: 10.1016/j.biomaterials.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 98.Li L, Yuan L, Luo J, et al. MiR-34a inhibits proliferation and migration of breast cancer through down-regulation of Bcl-2 and SIRT1. Clin Exp Med. 2013;13:109–17. doi: 10.1007/s10238-012-0186-5. [DOI] [PubMed] [Google Scholar]
- 99.Yan LX, Huang XF, Shao Q, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. Rna. 2008;14:2348–60. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen L, Bourguignon LY. Hyaluronan-CD44 interaction promotes c-Jun signaling and miRNA21 expression leading to Bcl-2 expression and chemoresistance in breast cancer cells. Mol Cancer. 2014;13:52. doi: 10.1186/1476-4598-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ru P, Steele R, Hsueh EC, et al. Anti-miR-203 Upregulates SOCS3 Expression in Breast Cancer Cells and Enhances Cisplatin Chemosensitivity. Genes Cancer. 2011;2:720–7. doi: 10.1177/1947601911425832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zarzynska JM. The importance of autophagy regulation in breast cancer development and treatment. Biomed Res Int. 2014;2014:710345. doi: 10.1155/2014/710345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li JP, Yang YX, Liu QL, et al. The pan-inhibitor of Aurora kinases danusertib induces apoptosis and autophagy and suppresses epithelial-to-mesenchymal transition in human breast cancer cells. Drug Des Devel Ther. 2015;9:1027–62. doi: 10.2147/DDDT.S74412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yao Y, Li L, Huang X, et al. SERPINA3K induces apoptosis in human colorectal cancer cells via activating the Fas/FasL/caspase-8 signaling pathway. Febs J. 2013;280:3244–55. doi: 10.1111/febs.12303. [DOI] [PubMed] [Google Scholar]
- 105.Raa A, Stansberg C, Steen VM, et al. Hyperoxia retards growth and induces apoptosis and loss of glands and blood vessels in DMBA-induced rat mammary tumors. BMC Cancer. 2007;7:23. doi: 10.1186/1471-2407-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheng Z, Lv Y, Pang S, et al. Kallistatin, a new and reliable biomarker for the diagnosis of liver cirrhosis. Acta Pharm Sin B. 2015;5:194–200. doi: 10.1016/j.apsb.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun HM, Mi YS, Yu FD, et al. SERPINA4 is a novel independent prognostic indicator and a potential therapeutic target for colorectal cancer. Am J Cancer Res. 2016;6:1636–49. [PMC free article] [PubMed] [Google Scholar]