Abstract
There is an increasing interest in determining the impact of vaccine technologies developed using public funding targeted at international development, and understanding the factors and ingredients which contribute to the success and impacts of such vaccines. This paper chronicles the development of a live vaccine against East Coast fever, a tick-borne disease of cattle caused by Theileria parva. The paper describes the technological innovation, commonly known as infection-and-treatment, which was developed some 40 years ago, explores the institutional settings in which the vaccine was developed and refined, and discusses the political dynamics of both during the decades from first development to field deployment and impacts. The paper also analyses the direct and indirect indicators of success of ITM and the many qualifiers of these, the impacts that the emerging technology has had, both in positive and negative terms, and maps the key contributors and milestones on the research-to-impact pathway.
1. Introduction
Cattle are highly valued in Africa, and in the eastern, central and southern regions of the continent, they play diverse roles in the livelihoods and economies of peoples and countries. So when a highly fatal disease of cattle appears to be interfering with the exploitation of this diverse livestock resource, the call for a sustainable solution is loud. So it has been with the call for a vaccine to protect cattle against East Coast fever (ECF).
ECF is tick-borne and indigenous to the region, probably originally a parasite of the Cape buffalo (Syncerus caffer). It was first described in eastern Africa as Amakebe by Bruce et al. (1910) [1], where it had been endemic and apparently recognised for centuries as a relatively mild disease of calves. Surprisingly, it was only when tick-infested cattle were exported by boat from eastern to southern Africa in 1901 and 1902, and the disease appeared in what is now Zimbabwe, that the disease became widely recognised [2]. Rinderpest had earlier swept down through southern Africa, wiping out over 2.5 million cattle in South Africa alone, before it was eradicated from that country in 1899. As a result of rinderpest and of the effects of the Boer war (1899–1902), the cattle populations of southern Africa had become depleted, and were inadequate to meet the multiple needs of the region [2]. As a result, cattle were imported from many sources, including Kenya and Tanzania, where ECF, in its milder form in the resistant indigenous animals of the region, had been existing almost unnoticed for generations. It was only when the early European settlers started to arrive in eastern Africa, importing exotic cattle breeds, that the disease was recognised there, and it was not until 1911 that the endemic disease in eastern Africa and the epidemic highly fatal disease in southern Africa were found to be one and the same [3].
This narrative describes the story behind the development of a live vaccine against ECF, probing the technological achievements which laid the groundwork for the innovation, the institutional settings in which this occurred, and the political dynamic of both over the last 50 years. It also analyses the indicators of success and the many qualifiers of these, the impacts that the emerging technology has had, both in positive and negative terms, and maps the key contributors and milestones on the research to impact pathway.
2. Livestock disease priorities in Eastern Africa in the evolving social and political landscape
2.1. The legacy of colonial livestock systems and accompanying research imperatives
Somewhat surprisingly in the context of 2016, the fundamental and widespread belief in Kenya in the late 1960s and early 1970s was that the priority disease of cattle in eastern Africa was ECF. While this disease undoubtedly continues to feature in any livestock disease-ranking in Kenya and indeed in other countries of the eastern and southern African region, in today's context we would likely ask the questions: “priorities to whom?”…and…. “on what evidence?” The evidence that was available at the time to answer these unasked questions was derived almost entirely from the more commercially orientated livestock enterprises of the European settler community and the priorities of the former colonial government and its veterinary services and diagnostic laboratories. The changes and development in livestock systems, particularly in Kenya, have been phenomenal over the past 35 years, and especially over the past 15 years or so; the trend in the highland regions has been a progressive intensification1 of smallholder mixed farming systems in which livestock are central (see for example Van de Steeg et al., 2005) [4]. In these systems tick-borne disease control has moved from a broad public sector responsibility, administered through community cattle dips, to farmer operated backpack application, spray race systems and private dips, depending on the scale of the enterprise. This devolution of responsibility has provided much more effective control of ECF, even in the absence of a vaccine.
But it is not only the livestock production systems that have changed; the animal disease research environment has had its own dynamic. Looking back at the veterinary research landscape in Kenya during the late 1960s and early 1970s, the period immediately following independence, the already existing and competent disease research infrastructure which had existed was replaced, at least for a decade or so, by a wave of new technical assistance attempting to provide continued veterinary service and research support to post-colonial livestock enterprises, and to train the new generation of African scientists. But the story was not that simple.
2.2. The changing institutional framework
During the latter part of the colonial era in eastern Africa, the main centre of research on ECF and other tick-borne diseases (TBDs) was at the East African Veterinary Research Organization (EAVRO). EAVRO was one of five research organizations responsible to the East African Agricultural and Fisheries Research Council, under the East African High Commission (EAHC). The EAHC operated from 1948 to 1961, then became the East African Common Services Organization (EACSO) from 1961 to 1967, and finally the East African Community (EAC) from 1967 to 1977. The EAC collapsed in 1977, (but was revived in 2000). The 1956/57 Annual Report of EAVRO stated that their laboratories at Muguga North were completed towards the end of 1954 and opened by the Governor of Kenya Sir Evelyn Baring on 21st February 1957. The staff listing contained some notable names, such as Walter Plowright, who was to go on to receive the World Food Prize for his work on rinderpest tissue culture vaccine development,2 which contributed significantly to the ultimate global eradication of rinderpest in 2011.
The tick-borne disease research group at the time was led by Steve Barnett, with colleagues David Brocklesby and Peter Bailey, among several others in the team. ECF was a major focus of their work, principally studying the transmission and chemotherapy of the infection; some research on immunisation of cattle had started which involved a 28-day therapy regimen of the antibiotic aureomycin following their infestation with ECF-infected ticks, which was perhaps the first successful exploitation of an ITM approach undertaken at EAVRO.
In December 1963, just five years after the opening of EAVRO at Muguga, Kenya gained its independence from the UK, and the UK government continued its support to livestock disease research. In 1967 the United Nations Development Programme (UNDP) initiated the funding of a 10-year research programme on tick-borne disease research under the auspices of the Food and Agriculture Organisation (FAO), based at EAVRO, Muguga.3 It was this FAO-administered research programme which was ultimately responsible for developing the ITM live vaccination against ECF.
While having as its objective the study of controlling ticks and tick-borne diseases in general, the project placed special emphasis on immunological work designed to control ECF by means of a vaccine. The project started operations in May 1967 at the laboratories of EAVRO, originally a project of three years duration but as a result of two extensions it continued until the end of 1976, just prior to the collapse of the EAC. With the collapse of the EAC in 1977, EAVRO was absorbed into the Ministry of Agriculture and renamed the Veterinary Research Department (VRD) and in 1986 it was brought under the Kenya Agricultural Research Institute (KARI) as the National Veterinary Research Centre (NVRC). Research on ECF continued at Muguga at the renamed NVRC. Importantly, despite the end of FAO's important contributions to EAVRO, the organisation continued to be heavily committed to supporting research on ECF and other TBDs in eastern and southern Africa for several decades to come, and many of the subsequent regional discussion forums held were co-financed by FAO.
2.3. The birth of the CGIAR and ILRAD
There was substantial international interest in providing technical support to the newly independent countries of eastern Africa and elsewhere during the years immediately following Kenya's independence in 1963. The Ford and Rockefeller Foundations had helped launch the International Rice Research Institute (IRRI) in the Philippines, and two years later the two Foundations began discussing the possibilities of a centre concerned with improving the yield and quality of tropical food crops other than rice; the International Institute of Tropical Agriculture (IITA) was opened in 1967 near Ibadan, Nigeria [5]. This process gained momentum and in 1970 the Rockefeller Foundation proposed a worldwide network of agricultural research centres under a permanent secretariat. Notably, this was coincident with the early years of the FAO programme at EAVRO in Muguga. The concept was further supported and developed by the World Bank, FAO and UNDP, and the Consultative Group for International Agricultural Research (CGIAR) was established on May 19, 1971 to coordinate international agricultural research efforts aimed at reducing poverty and achieving food security in developing countries.
A study had been commissioned by the Rockefeller Foundation to explore the creation of a livestock disease research centre (McKelvey and Pino, 1971) [6]. The CGIAR created an “African Livestock Subcommittee”, which asked the Rockefeller Foundation to act as executing agency in negotiations with the EAC for the establishment of an animal disease research laboratory, to be known as the International Laboratory for Research on Animal Diseases (ILRAD) and to be located at EAVRO, Muguga. At a meeting of the EAC Council on July 20th 1972 a decision was taken that the EAC could not host an autonomous institution as had been proposed, and the invitation to establish ILRAD within the EAC was withdrawn. The subcommittee then agreed to pursue an alternative option of establishing ILRAD at Kabete outside Nairobi. The first step was to reopen negotiations with the Kenya Government, through a letter from the then President of the World Bank, Robert McNamara, to President Jomo Kenyatta. In the letter, reference was made to earlier correspondence in which President Kenyatta had expressed his interest in research on animal diseases in eastern Africa, and had offered facilities and support of the Kenya Government. The letter mentioned that if and when a comprehensive animal production and health centre should be established for tropical Africa (referring to parallel negotiations going on at the time with the Imperial Ethiopian government on the establishment of the International Livestock Centre for Africa -ILCA), there would be the possibility of a link between or even the integration of these two centres. Ironically in January 1995 the two CGIAR institutes of ILRAD, based in Nairobi, Kenya, and ILCA, based in Addis Ababa, Ethiopia, were united as the International Livestock Research Institute (ILRI).
ILRAD was duly established in 1973, four years before the end of the FAO research project at EAVRO, Muguga.
3. The technology/innovation
3.1. East Coast fever, the tick-borne malaria of cattle
ECF is a tick-transmitted disease of cattle which causes major economic losses throughout eastern, central and southern Africa, where it has been regarded as one of the most serious constraints to improving the livestock industries of the region. The disease can cause high mortality in cattle, particularly affecting improved dairy and beef cattle, as well as in zebu cattle in pastoralist areas and ranches. There is an extensive literature on the disease (see for example Norval et al., 1992 [7]; Perry and Young, 1993 [8]; Lawrence et al., 2004 [9]; and Gachohi et al., 2012 [10]). An economic impact assessment of the disease was undertaken in 1989 by Mukhebi et al., (1992) [11], which estimated the total regional losses due to the disease to be US$ 168 million, which included an estimated mortality of 1.1 million cattle. Surprisingly, there have been few follow-up studies to validate or update these estimates, and as a result this figure is still regularly cited as the cost of the disease.
3.2. The innovation behind the live vaccine against ECF
Few theory-based strategies in human and veterinary medicine are sounder than the use of vaccines to artificially induce population immunity, and vaccine development and deployment has an outstanding success record in the control (and eradication, in the case of smallpox and rinderpest) of major infectious diseases.
On the specifics of applying this theory to immunization of cattle to protect against the causal agent of ECF, there were several milestone technical innovations which contributed to the development and success of the Infection and Treatment (ITM) approach. These were:
-
•
The ability to harvest Theileria parva sporozoites from batches of infected Rhipicephalus appendiculatus ticks.
-
•
The ability to preserve T. parva stabilates (cryopreserved sporozoite preparations) in liquid nitrogen for extended periods of time without affecting their infectivity. This allowed the administration of a uniform and reproducible dose of infective material to cattle.
-
•
The ability to induce sustained protective immunity in cattle through the injection of T. parva stabilates in combination with long-acting oxytetracycline treatment
-
•
The identification and combination into a single stabilate of three parasite stocks (T. parva Muguga, T. parva Kiambu 5 and buffalo derived T. parva Serengeti transformed), which became known as the Muguga Cocktail (MC), which has been shown to afford very good protection against T. parva, both in the laboratory and the field in many parts of eastern and central Africa, despite the wide diversity of T. parva immunological strains found in the field
-
•
The adjustment of the dose of long acting oxytetracycline from 20% to 30% dramatically reduced the proportion of clinical reactors to ITM, allowing the live vaccine to be more widely applied in countries of the region
-
•
The preparation at ILRI in 1996 of a commercial scale batch of the MC at the request of FAO and funded by FAO through a Technical Cooperation Programme – TCP.4 These stabilates were therefore designated FAO 1 and FAO 2. A second commercial scale batch was produced again some 10 years later in 2007 at the request of the Inter-African Bureau of Animal Resources of the African Union (AU/IBAR5). This stabilate was designated ILRI 08.
These technical innovations were the result of numerous experiments and trials, and for the specific details of the different studies undertaken the reader is referred to the following reviews: Cunningham (1977) [12]; Radley (1981) [13]; Norval et al. (1992) [7]; Uilenberg (1999) [14]; Di Giulio et al., (2009) [15]; Yrjö-Koskinen et al., 2010 [16]; ILRI, 2014 [17].
The major features of these technical innovations are summarised below.
Fundamental to the concept of immunization, it had been observed that cattle which survive infection naturally develop long lasting immunity, and this was demonstrated experimentally by Burridge at al. (1972) [18], who showed that solid protection against homologous challenge lasted for up to three and a half years in the absence of reinfection.
Neitz (1953) [19] in Onderstepoort, South Africa, had earlier found that when Theileria parva-infected ticks were applied to cattle that were simultaneously treated with chlortetracycline (under the trade name Aureomycin), the cattle underwent mild reactions and were immune on challenge. The drug was administered intravenously at a dosage rate of 10 mg/kg, starting 24 h after tick infestation and continuing on approximately alternate days for 2–3 weeks.
Subsequently, Brockelsby and Bailey (1962; 1965) [20], [21] at EAVRO in Kenya applied T. parva-infected ticks to cattle which then received oral tetracycline at a dosage of 15 mg/kg, and they recommended the use of this immunization technique in valuable exotic breeds of cattle. This method was applied further by Jezierski et al. (1959) [22] in Rwanda and Jarrett et al. (1969) [23] in Kenya.
Under the leadership of Matt Cunningham, the FAO project made a breakthrough in the technique of ITM by the production of sporozoite stabilates, the infectious stage of T. parva that is found in ticks, known at the time as ground-up tick stabilates (GUTS). This allowed cattle to be infected with a specific predetermined dose (Cunningham et al., 1973) [24].
During the six-year period from 1971 to 1977, a series of experiments was undertaken to reduce the amount of drug required to control infection and yet permit a protective immune response to develop. It was found that two doses (10 mg/kg) of short acting oxytetracycline given on days 0 and 4 would permit good protection with this immunization regimen.
A major breakthrough came with the introduction by the pharmaceutical company Pfizer of a long-acting oxytetracycline product (Terramycin LA, Pfizer, UK). The long-acting formulation, when given at a single dose of 20 mg/kg at the time of infection with the sporozoite stabilate, resulted in the development of immunity to homologous T. parva stabilate challenge with minimal clinical response.
A severe limitation to the effective immunization of cattle against T. parva using ITM has been the recognition of different strains of T. parva in the field; this is particularly important in infections derived from buffalo. It was quickly established that different immunogenic stocks existed, and this led to the search for a “master” stock or stocks which would induce broad protection in immunized animals (Mutugi et al., 1990a) [25]. In a series of experiments, a combination of three stocks (T. parva Muguga, T. parva Kiambu 5 and buffalo derived T. parva Serengeti transformed), which were collectively given the name the “Muguga Cocktail (MC)” [26], and was shown to afford very good protection against T. parva, both in the laboratory and the field in many parts of eastern and central Africa [27], [13], [28].
During the ten years of the FAO project's life the following agencies established cooperative support projects with EAVRO: Ministry of Overseas Development, United Kingdom (latterly Overseas Development Administration ODA); United States Department of Agriculture (USDA); Pfizer International Incorporated; International Centre for Insect Physiology and Ecology (ICIPE); International Development Research Centre (IDRC) Canada; International Atomic Energy Agency (IAEA); Australian Volunteer Service; Nuffield Institute of Comparative Medicine, and the Rockefeller Foundation.
The first group to use the ITM method in a truly commercial setting, charging producers for each dose administered, were Lieve Lynen and colleagues at VetAgro in Tanzania. The technique was introduced there in 1993, initially restricted to the smallholder dairy sector, but from 1998 it was made available to pastoralist communities in the Arusha region of northern Tanzania [29], [30], [15]). Some initial problems with severe clinical reactions to the immunization were solved by raising the dose of simultaneously administered long-acting oxytetracycline formulation from 20% to 30% [30].
4. The pathway from the development of the live vaccine technology to its adoption, use and impact
4.1. The long and winding road, the scientific reticence, and the inevitable politics
The feasibility of immunisation using the ITM method was established by Willi Neitz in South Africa in the 1950s, a theoretical approach using oxytetracycline was established by Brocklesby and Bailey at EAVRO in the 1960s, and a pragmatic and practical application of this method using Pfizer's then newly emergent long-acting formulation of oxytetracycline was developed by the FAO project at EAVRO in the 1970s. This happened about 40 years ago, but it is only relatively recently that vaccine production capacity has been established outside ILRI, and that vaccine has been distributed in the different ECF-affected countries. What happened in the interim? What were the other factors which helped or hindered the application of this apparently simple technology? The trail from innovation to impact is reviewed below.
4.2. The void following the end of the UNDP project
The very productive UNDP-sponsored research project at Muguga finished in late 1976, winding up a most productive decade of intense research, and also bringing to an end the impetus for translating the live vaccine experimental successes into a product which could be delivered. In addition, the EAC collapsed shortly afterwards in 1977, bringing other institutional challenges to the research at Muguga. At the time, ILRAD, initiated in late 1973 and with facilities finally inaugurated in 1978, was going through its early establishment and development years, and it too was having a set of teething problems. These related firstly to its new role as an international research centre, housed in a country desperate to further develop and sustain its own veterinary research capacity on a par with the previous decade of FAO contributions and before. Secondly there were problems of institutional leadership; when Ross Gray became Director General (DG) in 1982 he was the fourth DG in seven years. The first, E. Sadun, died before assuming office. Sadun's successor, James Henson, resigned during his second term. The third, Anthony Allison, was asked to resign after less than two years (Lewin, 1982). Not a healthy start. Notably, Ross Gray went on to lead ILRAD for almost 14 years until the institute was merged with ILCA in January 1995 and ILRI was born.
ECF research continued at the NVRC in Muguga, but with much less in the way of funding and human resources. Funding support came from various sources, in particular the Overseas Development Administration (ODA) of the UK government, now known as the Department for International Development (DFID), and also from USAID, among others. There were a few ODA scientists at NVRC still working on ECF and ITM, notably Alan Young and Tom Dolan. But there was a degree of scepticism, and perhaps scientific arrogance, that was associated with ILRAD during its early years of existence. There was an impression among some scientists at ILRAD that furthering ITM immunisation was not science, and this view continued to be widely prevalent until the merger with ILCA and the birth of ILRI in 1995. There was also widespread belief among ILRAD scientists that a subunit ECF vaccine was just around the corner, and this influenced the Directors of Veterinary Services of the region (especially in Kenya), which in turn gave rise to the lukewarm support of the ITM. Nevertheless there was a small group of scientists at ILRAD who did venture into the field, and undertook parasite isolation and ITM immunisation studies at three government ranches located in the coastal region of Kenya (Morzaria, 1989) [31].
Then came the new institute ILRI in January 1995, with a new Director General, and a revived discomfort of the past unfulfilled promises of vaccines against trypanosomiasis and ECF vaccines “within five years”. These changes certainly turned the tide a little for ITM, resulting in several new engagements within the region, and also paved the way for a renewed link with FAO and the ensuing request in 1996 for ILRI to prepare a commercial scale MC stabilate.
4.3. The concern about live vaccines and spread of vaccine strains to new areas
Several concerns had been raised about the wider use of the ITM MC vaccine. These mostly centred on the awareness that the use of a live vaccine meant that vaccinated animals become “carriers” of the T. parva strains being used in the vaccine, and that they then provide a source of these strains to ticks in the field, and as a result have the potential to infect co-grazing non-vaccinated animals with these strains. These concerns, and their implications in the light of subsequent understanding, have been extensively reviewed by Declan McKeever [32], [33]. Over many years became a central obstacle to the acceptance of the MC as an option to be deployed in different countries of the region. This led to extreme caution on the part of Directors of Veterinary Services and other government officials unfamiliar with the science behind the vaccine, who did not want to be seen to be condoning the introduction of “foreign” parasites into a new area. Part of this was because many new stabilates isolated were given a name, usually referring to the geographical area or region in which it had first been identified. Interestingly, even Kenya was opposed to the use of the MC as it contained a buffalo stock! The confusion was further deepened by the scientists themselves, inadvertently fuelling the debate in publications and meetings.
The NVRC in Kenya searched for a master stock without buffalo origin parasites. The isolation of T. parva Marikebuni was believed to be such a strain [25]. For the next 20 years Kenya invested heavily in the development of Marikebuni as the vaccine of choice. ODA support was intended to lead to the commercialisation of Marikebuni and a MoU was signed with Cooper Kenya, but this partnership was not successful.
It is now generally believed that the risk of adverse reactions from the use of MC and the resultant introduction of its strains is of no significant consequence, as there is a substantial degree of sexual recombination among T. parva strains in the field, resulting in huge parasite diversity (Oura et al., 2007 [34]; Patel et al., 2007 [35] However, there are still people who ask the question whether strain variation is important and whether use of the MC as a standard regional approach can lead to the introduction of strains into countries where they do not currently exist. The evidence has never been good enough to silence the doubters. As a result, current initiatives coordinated by GALVmed in Rwanda and the Democratic Republic of Congo (DRC), for example, are taking a cautious and pragmatic approach by testing MC under controlled trials in new markets prior to requesting the right to import. Importantly, it is known that MC has been used in certain of these countries in the past, seemingly without adverse effects.
4.4. The reorientation of the ECF vaccine research agenda
The success of the UNDP-sponsored FAO project at Muguga had a significant impact on the confidence and hope of parasite immunologists, demonstrating proof of principle that cattle could be exposed to Theileria strains and develop a strong and long-lasting immunity, boosted by new challenge from infected ticks in the field. Although the mechanism of immunity was still unknown in 1977, and the role of cytotoxic t-cells was not yet discovered, an air of confidence had emerged that paved the way for a new pathway for ECF vaccines. This wave of confidence coincided with the establishment of ILRAD, with its highly sophisticated facilities, its staff of highly qualified scientists recruited from different corners of the world, and its specific mandate to develop vaccines. The result was that the technicalities and mechanisms for further deployment of ITM was in effect side-lined for the next 20 years. These became the wilderness years of ITM and the MC. It certainly was not forgotten, as the ensuing 20 years saw much discussion on the differing epidemiology of ECF under different production systems, in different breeds and at different latitudes, but virtually nothing was done to move ITM to the next level and have it deployed as a commercial product in the field. ILRAD, and its successor ILRI (as it became from 1995), moved into the field of developing new recombinant vaccines, and of following the trend at the time of mapping the Theileria genome [35], [36], [37].
As well as looking to new generation vaccines, ILRAD and ILRI used these years to develop a wide variety of molecular tools to better understand and characterise the different T. parva parasites being investigated as potential vaccine candidates. As far as ITM was concerned, these molecular techniques brought extremely valuable tools to its characterisation and use. These included: the ability to establish quality control of the ITM vaccines such as the MC; the ability to monitor for possible vaccine breakthroughs and breakdowns; and the capacity to assess the impact of introducing new parasite strains into the vaccine on its long-term efficacy. The work focused on two main categories of tools; serological (such as schizont - specific monoclonal antibodies [38]) and DNA-based (various genome-wide polymorphic markers, southern blotting, polymerase chain reaction (PCR) and single strand conformation polymorphism) [39].
4.5. The years of regional dialogues
The successes in Kenya, combined with a continued interest from FAO, a new field epidemiology and impact assessment initiative by ILRAD, and an increasing engagement of AU-IBAR, triggered a set of valuable regional dialogues on ECF and its control over a period of 15 years.
The first workshop in this series was held in Nairobi in October 1984, when participants provided updates on theileriosis in the countries of the region, reports on immunization trials, and papers expanding on elements of immunization [40]. The second workshop, held in Nairobi in September 1985, emphasised data collection and handling as well as analyses of productivity in relation to immunization [41]. This workshop introduced participants to practical data handling and computer use. The third workshop was originally planned for November 1987 but was postponed due to uncertainty about the funding of some of the FAO tick and tick-borne disease control projects. The workshop was eventually held in Lilongwe, Malawi, in September 1988, with considerable support from the Malawi Government.
The Lilongwe meeting had an important spin-off. It provided an opportunity for a sharing of information and understanding on theileriosis throughout the eastern and southern African region, and it became apparent that there were significant differences in the disease epidemiology between the eastern and southern regions. Whether this was real or due to the diversity of studies that had been undertaken or interpretations thereof was not entirely clear. Whatever the source, it stimulated a discussion, and the Lilongwe meeting was where the only text book on the epidemiology and control of ECF was conceived. The preface to “The Epidemiology of Theileriosis in Africa” [7] comments: “during discussions after the day's session, Alan Young suggested that the three of us (Andy Norval, Brian Perry and Alan) attempt to prepare a review article on the epidemiology of theileriosis throughout its distribution in Africa, with the objective of rationalising the differences in patterns of the disease, particularly those between eastern and southern Africa. During early gestation, it became clear that a review article would not accommodate this objective, and the project quickly grew into a book”. The book was published by Academic Press, and became a key reference point for all working on the control of ECF.
Then in September 1989 FAO called an Expert Consultation at FAO headquarters in Rome on the revision of strategies for the control of ticks and tick-borne diseases, the proceedings of which were published in Parassitologia (a journal of the Institute of Parasitology of the University of Rome; Parassitologia, 1990). Next it was the turn of Uganda to host a regional meeting in September 1991 (Dolan, 1993 [42]), which was again organised jointly by OAU/IBAR), FAO and ILRAD. The workshop was timed to coincide with the annual review meeting of the FAO national and regional projects, and the Government of Uganda convened an additional supplementary meeting to address the specific tick and tick-borne disease problems in Uganda.
There was then a gap in the regional meetings until March 1996, when a meeting focussing on the epidemiology of tick-borne diseases across the region was held in Harare, organised by FAO in association with the new emerging institute ILRI [43]. This was followed a year later in March 1997 by a workshop at ILRI which was again jointly organised by OAU/IBAR, FAO and ILRI [45]. The workshop focussed on the deployment of live ECF vaccines and the problems associated with their delivery.
These meetings played both positive and negative roles. On the positive side, they provided a very valuable opportunity to share studies and research in what became a “club” atmosphere, and provide the opportunity for younger scientists to both present their results and to discuss informally with their peers from other countries and institutions. On the negative side, they were at times seen as a hub for conspiracy theories, and were termed “disruptive” by some due to the tabling of sometimes damaging suggestions and concerns on the role and application of ITM.
4.6. The devolution of field studies
In part as a result of the earlier successes in Kenya, and in part from the regional dialogues, there was a growing interest among scientists and other donor organisations to explore ECF immunisation in other countries of the region. Until the collapse of the EAC in 1977, a joint FAO/UNDP project was operating in Tanzania, and following a study of the use of the MC in experimental cattle held at Pugu near Dar es Salaam, recommended its routine use on a large scale in the country [27]. However the collapse of the EAC brought the UNDP funding to an end. The Danish International Development Agency (DANIDA) then came in 1980 with support for a series of projects on ECF control in Malawi, Zambia and Zimbabwe, and eventually, in 1990, brought Tanzania into this programme.
In Zambia, a Belgian animal disease control project started using the ITM method of immunisation against ECF, but using a T. parva stock isolated from Zambia (the Katete isolate), rather than using the MC (Lynen et al., 1993) [46]. This was extremely successful, and Zambia has continued to use this stock, with the vaccine being prepared at the Centre for Ticks and Tick-borne Diseases (CTTBD) in Malawi. Interestingly, Lieve Lynen then moved to Tanzania, where she continued to pioneer the use of ITM, but using the MC, initially supplied to her project by the VPC in Malawi (which later became the CTTBD), but after 1996 using the FAO 1 batch produced at ILRI.
Malawi started using the MC in 1984 following extensive field trials showing its efficacy under Malawi conditions. This continues, with vaccine being sourced from CTTBD.
Various trials were also carried out at different intensities in other countries, notably Rwanda, Uganda and Zimbabwe, reported in Morzaria and Williamson, 1999) [45].
4.7. The production of commercial scale batches of MC vaccine by ILRI
At the birth of ILRI in 1995 there was a strong awareness that on the one hand the new long-promised recombinant vaccines were still a long way off, and on the other that the much discussed ITM vaccine was still far from a practical reality in the field. In 1996 FAO requested ILRI to prepare a commercial scale batch of the MC. Julio de Castro was the incoming Senior Animal Health Officer at FAO headquarters with particular responsibility for tick-borne disease control, and was reportedly concerned that although FAO had invested so much over many years in ITM, no workable product had emerged. While he realised that some of the southern Africa countries, and the donors supporting their work, wanted their own stocks and strains for immunisation, which had become a substantial political force at the time, he felt that it was high time for the MC to be more widely available; countries could make their own decisions on its use. He made available a Technical Cooperation Programme – TCP project for about $250,000. There was a shortage of immunising stabilates in the region due to increasing demand in the different countries of the region, and because the Vaccine Production Centre (VPC) in Malawi (see section 4.10 below) was not functioning at full capacity, ILRI agreed to prepare the vaccine stabilate and send it to Malawi. At the time of the request, it was considered that there was no other institution in the region with the capacity to produce the MC.
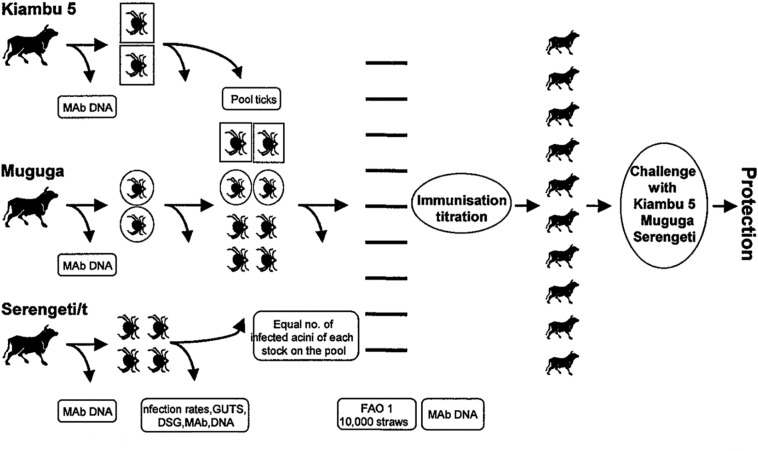
The key players at ILRI responsible for preparing the stabilate were Paul Spooner, supported by Subhash Morzaria, Tony Musoke and Stephen Mwaura. Certain modifications from the original protocol for MC preparation were introduced, some of which were adopted as part of the standard ILRI procedure for stabilate preparation. Other amendments were also introduced to fulfil the requirements of the Office International des Epizooties (OlE) recommended standards for biological products (OlE 1996) [47], and the proposed new standards that had been set by the Standards Committee, representing the OAU, FAO and ILRI. A protocol for the production of MC was published in a meeting proceedings (Morzaria et al., 1997) [44] (see Fig. 1), and this protocol has recently been brought up to date by Patel et al. (2016) [48].
Fig. 1.
The complexities of preparing a Muguga cocktail (MC) stabilate. From Morzaria et al. (1997) [44].
The first batch produced by ILRI was designated FAO 1, comprising about 250,000 doses, and it was sent to VPC in Malawi (VPC changed its name in 2001 to Centre for Tick and Tick borne Diseases (CTTBD)). The intention was that funds from the sale of vaccine would be reinvested in new production capacity at VPC in order to create a sustainable vaccine production centre for the region, but it appears that did not happen.
Then in 2007 ILRI was called on yet again to produce another large batch of MC. This time the request came from the AU/IBAR, and again driven by the lack of availability of MC, the decline in effectiveness of the VPC, and a continuing demand from the countries of the region. The preparation of the batch was undertaken by Paul Spooner, Steve Mwaura and Phil Toye, and the batch was designated as ILRI 08. Perhaps surprisingly, it was kept at ILRI in Nairobi and distributed from there to Tanzania (the majority), Kenya, Uganda and Malawi, among other distributor demands.
4.8. The DFID drive towards a poverty reduction agenda
In 1997, the incoming Labour Government of the UK established a new Department for International Development (DFID), with responsibility for the £4 billion aid budget and other aspects of UK development policy, led by Clare Short. In the subsequent eight years, the new Department established a reputation for itself, and for the UK Government, as a leader in development thinking and practice. DFID was described by The Economist6 as “a model for other rich countries.” What has this got to do with ECF vaccine development?
The new minister Clare Short changed the approach to international development assistance in the White Paper presented to parliament in the UK.7 In her introductory comments, she said: “The White Paper is first and most importantly about the greatest single challenge which the world faces – eliminating poverty. It is about ensuring the poorest people in the world benefit as we move towards a new global society”. Of particular relevance to the role of livestock and livestock disease control was the section on sustainable livelihoods, where six elements were highlighted: pro-poor economic growth; efficient and well-regulated markets; access of poor people to land, resources and markets; good governance and the realisation of human rights; prevention and resolution of conflict; and the removal of gender discrimination.
Working for DFID at the time with the Livestock & Wildlife Advisory Group in the Rural Livelihoods Department, and with responsibility for animal disease control investments, was Sarah Holden. Sarah picked up on the wind of change in DFID, and applied the new principles to animal disease research. On 22nd November 2000 Sarah wrote to Brian Perry at ILRI saying: “DFID is re-thinking the way in which we provide support to animal health research. This will involve a re-orientation of the research programme to focus diseases that are important to poor people, rather than diseases of production (the two may or may not be the same). To kick start the process we need to commission a study to identify and prioritise those disease research issues that are likely to be important to poor livestock keepers. Would you be interested/able/willing to do the study for DFID?”
The answer was of course yes, and this initiated a process which brought together scientists and opinion leaders in Africa, Asia, Europe and North America, and which was to deliver one of the highest impact products of ILRI's epidemiology group (Perry et al., 2002) [49]. The Inter-Agency Development Group (IADG) of international donors to livestock research and development, of which DFID was a leading member, had just been formed to bring greater coordination between its membership in the funding of livestock research.8 As part of this process, it sought to better define the animal health research priorities, and DFID proposed that these be placed in the context of poverty reduction.9 This required first defining poverty and the association with livestock, and then quantifying the association, a process which continues to be refined [50].
This study has had a lasting impact on animal health research priorities for development, and is still the most cited reference in this context. In addition, it set the stage for measuring the association of poverty and livestock, and for applying greater emphasis to the impacts that research in animal health has on processes of poverty reduction, rather than simply on national agricultural development. Other research and development sponsors were also influenced.
There were several important implications for ECF and the ITM immunisation. Firstly, even in the region in which the disease occurs, ECF was ranked as 11th on the priority scale, while at the global level it ranked as 35th [49]. There are many factors behind this. The low ranking was due to the fact that it occurs in just one species, cattle, and although that species ranks highly in eastern, central and southern Africa as important to the poor, it is usually the indigenous zebu and sanga breeds that play the most important role in poor households in mixed agro-pastoral systems, rather than the more susceptible European breeds, in which ECF has a high impact. The importance of ECF was thus characterised as constraining the processes of intensification by smallholders, rather than affecting the fundamental assets of poor livestock keepers. Importantly, however, the opportunity for delivery of ITM was identified as one of the “low hanging apples”, attainable within a short time frame and at a low to medium cost.
A follow-up meeting of the IADG was held in Paris in April 2002, at which it was decided to explore how the private sector might be encouraged to tackle some of the so-called low hanging fruit technologies. A month later, DFID received the ‘go-ahead’ from Claire Short for the design phase of a would-be Private Sector Initiative (called the Rural Enterprise Technology Facility, RETF). DFID commissioned the Cambridge Economic Policy Group, who developed what they initially called a draft Global Animal Health Alliance, but which evolved, in April 2003, to become the Public Private Partnerships on Livestock Vaccines for the Poor. This recognised that there was a global failure in markets to generate and deliver animal health technologies in these countries, and particularly for targeting the poor. The study discussed the concept with various actors in the private sector and reported that major pharmaceutical companies such as Merial, Intervet and Pfizer would be willing to participate in an alliance and to contribute expertise and IPR to the development of specific products, subject to appropriate funding and management structures being put in place. The onus was put on the donors to reach agreement on the concept, and to identify appropriate funding and management arrangements. This prompted DFID to explore various options, and it went on to facilitate the establishment of the Global Alliance for Livestock Vaccines (GALV) in 2004 with an appropriate legal and institutional framework and its first business plan. It was envisaged that GALV would work in a similar way to the GAVI Alliance,10 an organisation with a human vaccine agenda.
4.9. The emergence of GALVmed
DFID provided a £300,000 grant in an inception phase for GALV as it picked up the public-private partnership mantle on animal health technology refinement and delivery, including the ITM vaccine for ECF.11 Then GALV became GALVmed12 (Global Alliance for Livestock Veterinary Medicines), the change of name reportedly reflected the recognition by the management and board of GALVmed that although vaccines were important, the development of other types of veterinary medicines, including drugs, would also be relevant to their agenda. As seed funding from October 2004 to October 2008, DFID injected some £2.6 million to get the organisation off the ground. During this period, considerable time and effort was invested in fund raising, and the organisation took on Steve Sloan for this task, under former Merial scientist Xavier Fargetton as CEO.
This fundraising initiative was very successful, and led to a new Phase 1 of GALVmed from September 2009 to September 2012 of £14.8 million, of which the Bill and Melinda Gates Foundation (BMGF) contributed 80%, and DFID 20%.
Following this, DFID and BMGF decided to jointly fund a second phase of GALVmed from July 2012 to June 2017, to which they jointly committed to provide over £31.2 million (DFID contributing 20% and BMGF 80%). GALVmed has also been able to raise some £25 million from BMGF, DFID, the EU and BBSRC for a variety of other (non-ECF) programmes.
Since 2008 GALVmed has helped facilitate the improved functionality of the CTTBD in Lilongwe, Malawi, the successor of the VPC, through training, re-equipping and technical support (GALVmed 2015) [51]. CTTBD won the tender to produce the MC following a tendering process by AU-IBAR-chaired ECF regional task force, comprising government representatives of the four countries currently using the ECF MC vaccine (Malawi, Kenya, Tanzania and Uganda), GALVmed, ILRI and Pan-African Veterinary Vaccine Centre (PANVAC).
From the ECF live vaccine point of view, GALVmed has stepped into the breech and developed a most impressive public private partnership. It has been helped by the solid and continuing support of DFID, which contributes some 15% of the funding of GALVmed's wide range of activities (and which has recently indicated its intent to continue its support), and by the very successful fund raising, in particular with the BMGF, who have also placed both vaccine deployment and ECF high on its priorities. GALVmed currently plays the role of facilitator and broker for sustainable ITM production and deployment (using the term ECF-ITM to describe the product), seeking to end the wilderness years of ITM and MC. While it is carefully backing the opening up of new ECF vaccine delivery initiatives in Rwanda, Burundi, Democratic Republic of Congo (DRC), Uganda and South Sudan, it sees itself being replaced as broker of ITM production within the next two to five years, with the technical support being provided by a private enterprise.
4.10. The changing roles and fortunes of the Centre for Tick and Tick borne Diseases (CTTBD), Malawi
Towards the end of the 1970s, construction of a facility for ECF vaccine production capacity was set up outside Lilongwe in Malawi under the auspices of the African Union. The Tick-borne Diseases Vaccine Production Centre (VPC), managed by Fred Musisi, included the capacity to produce other TBD vaccines for babesiosis and anaplasmosis. The government of Malawi set up the VPC with technical assistance of FAO and financial co-operation of various donors, principally the governments of Denmark and the Netherlands, and UNDP. The objectives were to look into the available technologies for immunisation against TBDs and to subsequently evolve production systems that would meet the minimum standards of registerable vaccines under Good Manufacturing Practices (GMP). In his report of 1997, Chizonda (1999) [52] wrote that shortly after its inauguration, the VPC encountered several problems, including the loss of four of its five external technical assistance staff, and nearly half of its national technical support staff. Vaccine production was then suspended indefinitely.
In 1998 there was an initiative to resuscitate the vaccine facility in Malawi with support for five years by two donors (Netherlands and Belgium), renaming the facility in 2001 as the Centre for Tick and Tick borne Diseases. The main objective of the Centre was to produce live vaccines against ECF, provide training in epidemiology and other areas of TBD control, backstop field projects involved in the integrated control of TBDs, and undertake research. It was decided that the Centre would not produce vaccines against Babesia and Anaplasma, as it had previously.
There has been substantial debate on how and where to best provide the countries of the region with the ITM technology for ECF. The only places where the MC had been produced, until recently, was at Muguga in the 1970s, and at ILRI in 1996 and 2007. Kenya was, from many points of view, the logical place to produce MC, but this argument was complicated by the fact that not all countries of the region wanted to use MC. In fact many were quite adamantly opposed, during the era of a lack of understanding of the nature of introducing these Kenya-origin stocks into other countries.
Research at ILRAD, ILRI and other institutions in the countries of the region had successfully identified a variety of T. parva stocks, some of which were the products of research projects, and some of which became considered as potential candidate vaccine strains, particularly in countries whose authorities would not accept the importation of the MC. Examples were T. parva Katete and T. parva Chitongo in Zambia, and T. parva Boleni in Zimbabwe.
After the 2007 production of the MC (ILRI 08) at ILRI in Nairobi, there was an extended debate as to how sustainability in both production and distribution of the ITM technology could be established. The main political force at the time was the group of Directors of Veterinary Services of the countries with the strongest demand for the vaccine, namely Kenya, Tanzania, Malawi and Zambia. Through the leadership and facilitation of AU/IBAR, a committee involving this group was established, which also included GALVmed and the Pan-African Veterinary Vaccine Centre PANVAC. This committee opened a tender for both the manufacture and distribution of the vaccine. The views at the time were many. Some believed that vaccine should continue to be produced at ILRI, where the track record and all the facilities existed for the complexities of stabilate production. There were also strong feelings against ILRI producing the vaccine, both from some of the countries involved, and from ILRI itself, which is a research institute and not Good Manufacturing Practices (GMP) compatible for vaccine production.
In the end the tender for manufacture went to CTTBD. GALVmed had already committed to prioritising ECF control in its portfolio, and stepped in with significant investment to re-equip the laboratories during the period 2008–2014. Despite CTTBD winning the tender for ITM manufacture, there was to be a wait of six years before the revamped centre was launched (in December 201413). This was reportedly carefully planned to avoid yet another launch of the facility before all equipment and personnel were in place, and the first batch of vaccine produced and available.
GALVmed continues to provide support to CTTBD in various ways, including assistance to management, to its cash flow challenges associated with unpredictable vaccine sales to different players in the region, and to vaccine production and sales. Importantly it also provides consultancy services to CTTBD; ironically Tony Musoke, veteran of EAVRO at Muguga, is helping with the further refinement of facilities in Lilongwe, and is back at the front line involved in supporting new ITM initiatives in Rwanda, Burundi, Democratic Republic of Congo, Uganda, Mozambique and South Sudan.
CTTBD has a steering committee which meets regularly and oversees vaccine batch release. It also had a board, made up of the Directors of Veterinary Services from the purchasing countries meeting annually, until recently under the chairmanship of Musa Fanikiso, a former Director in Botswana. A new board has now been appointed, chaired by Tumusiime Rhoda Peace, Commissioner for Rural Economy and Agriculture at the African Union.
The challenge remains of moving a technology for which there appears to be a strong but variable demand in the region, and for which livestock producers are generally prepared to pay, into a sustainable private enterprise. GALVmed has just signed a contract with a private sector company to explore this option further, with the company bringing in management and technical contributions to quality assurance, eventually moving towards profit sharing.
CTTBD's main product is currently the MC ECF vaccine for the countries of eastern Africa (Tanzania, Uganda, and Kenya) and Malawi.14 In addition, for the past decade or so, CTTBD has been producing two subtypes of monovalent ECF vaccines for southern (Chitongo strain) and eastern Zambia (Katete strain), with the latest batches having been delivered in April 2016. All these vaccines are produced with the accompanying diluent as a package for the end use. ITM vaccine sales are now in excess of 1.5 million doses, with uptake (in order of size of market) in Tanzania, Zambia, Kenya and Uganda. These figures reflect sales from ILRI 08, MCL 01 and Zambia batches only, and exclude sales from earlier batches.
ECF-ITM vaccines are currently packaged in 0.5 ml straws containing 40 doses which may not be cost effective to smallholder farmers with 1–5 cattle. The use of a complete straw's doses is sometimes difficult to achieve, and vaccinators are left with a logistical challenge of organising a total of 40 animals.
5. The Muguga Cocktail; is it a success, and if so, what has been the impact?
5.1. The indicators and views of success
The original ITM live vaccine technology was developed about 40 years ago. Has it been a success? Clearly many people think it has, and it is a technology that is now out in the field and being used, and its wider distribution is being supported by governments, international donors and institutions. A few indicators are presented and discussed below.
-
•
There is no other vaccine to prevent ECF despite over 40 years of heavy investment and research. While this is hardly an indicator of success, the reality is that no other vaccine has yet been developed to protect against ECF in the field. At the time that the ability to protect cattle with this live vaccine was first demonstrated, work started at ILRAD, then continued at ILRI, to develop a new recombinant vaccine. Some partial success was achieved with a sporozoite vaccine, based on the surface protein p67 (Musoke et al., 2005) [53], giving a reduction in the severity of disease in immunised cattle, but there was inadequate protection to justify further development of this approach. Research continues at ILRI on a novel recombinant vaccine with support from the Bill and Melinda Gates Foundation (BMGF) and others at ILRI15,16
-
•
The MC immunisation provides long-term, some consider life-long, immunity in the field.
-
•
Over 1.5 million doses of MC have reportedly been administered in approximately eleven countries of eastern and southern Africa. The breakdown of this figure by country, production system and cattle type is not available.
-
•
The greatest impact appears to have been in Tanzania, in part due to the commitment of the group championing its use in and around Arusha, and in part due to the unique demand in the pastoralist communities in northern Tanzania and southern Kenya. These have been documented extensively by Di Giulio et al. (2009) [31].
-
•
The MC vaccine is only registered in Malawi. The MC vaccine has been registered in Kenya and Tanzania, but these registrations have lapsed. Nevertheless it is used in both of those countries, and in Uganda, through importation under special permit. The MC is also being used in DRC, South Sudan, Rwanda, and Burundi. The technology of ITM is also used in Zambia, using the Katete and Chitongo stocks of T. parva, not the MC. ITM technology has also been deployed on a commercial basis in Zimbabwe, using the Boleni stock of T. parva and without the use of tetracycline.
-
•
The impact on the establishment of ILRAD and the ECF vaccine research programme. What momentum did the successes of the FAO project at EAVRO, Muguga, have on the establishment of ILRAD? There was certainly an overlap in the timing, with the CGIAR launched in 1971, the livestock subcommittee a year later, concurrent with the first demonstration of protection with the MC, and the initial attempt to establish ILRAD at EAVRO. One can speculate that the EAVRO success might have inspired the creation of ILRAD. Former Director General of ILRAD Ross Gray argues that trypansomiasis became the primary objective behind ILRAD very early in the CGIAR's project, not tick borne diseases, and in the review carried out five years after ILRAD's establishment, equal weight appears to be given to both diseases, with the common theme of immunological approaches to control them (TAC, 1981) [54].
-
•
The impact on the understanding of the bovine immune system. The successes of the FAO team at EAVRO, and the establishment of ILRI with its mandate of vaccine development stimulated a new and intense scrutiny of the bovine immune responses to different parasites, and is perhaps well illustrated by Ivan Morrison's book [55].
-
•
The establishment of a functional international Public-Private Partnership to address the market failure of animal health technologies for the livestock enterprises of the poor. This must be one of the most significant indirect products of the ITM technology, and while this narrative cannot comment on the overall performance of GALVmed, there is no doubt that it is now firmly set on the path of ensuring that ECF-ITM is produced sustainably, and is effectively delivered. There can be few public private partnerships targeted at animal health technologies which combine a global funding agency at the scale of BMGF, and an international development office of a major industrialised country (DFID), and it appears that GALVmed is starting to achieve the kinds of impacts these partners were seeking. GALVmed itself does not rank ECF ITM as its greatest success in vaccine delivery, a position which it currently gives to its vaccine programme for Newcastle Disease of poultry, in which the programme GALVmed has engaged several private enterprise manufacturers and deliverers.
-
•
The capacity building of African scientists. There is no doubt that the funding and training opportunities presented by the work of NVRC, ILRAD and ILRI, as well as research institutions in Tanzania, Uganda, Zambia, Malawi and Zimbabwe played a significant role of capacity building of individual scientists across the region. It is less clear that it had a substantial impact on institutional capacity, with the clear exception of ILRAD and ILRI which attracted the cream of aspiring scientists, arguably at the expense of their national institutions.
5.2. The qualifiers of success
In addition to several clear indicators of success, and of both direct and indirect impacts of the development and deployment of the MC, there are certain qualifiers of these successes. These apply to the technology itself, and to the adoption process. These are presented below.
-
•
The manufacture of a commercial scale batch of the MC stabilate is a complex, costly and time consuming process. The facilities and technical support of ILRI in producing FAO 1, FAO 2 and ILRI 08 absorbed the prohibitively high cost associated with the production of these stabilates. Since then, in the production of the MCL 01 stabilate at CTTBD, financial and technical consultancy support has been essential, and even with that various technical challenges affecting product quality have been experienced. The sourcing of disease-free cattle in the relatively small country of Malawi is but one of those challenges.
-
•
To produce one million doses of vaccine requires 130 cattle that have not previously been exposed to the disease, 500 rabbits and at least 600,000 ticks. The entire process of making the batch takes up to 18 months. This raises some ethical issues. The vaccine release has now been amended to reduce the number of cattle used and to shorten the release. It was recognised that three refinements of the dilutions were not needed to quantify the effective dose, so two serial dilution steps were taken out. This shortens the production time and uses fewer cattle (with a beneficial ethical impact as well as a reduction in production costs).
-
•
There remains no good correlate of the potency of emerging stabilates, which need to be evaluated empirically through assessment of the number of infected acini in each infected tick batch. This is both inadequate in reflecting potency, and is extremely time consuming and labour intensive.
-
•
The registration enigma. It has been extremely difficult to secure and maintain registration of the MC. The MC has been registered in both Kenya and Tanzania, but these registrations have lapsed. GALVmed have contributed to the process of development of registration dossiers, but the major challenge is an insistence by the authorities on GMP capacity for vaccines. There are guidelines on GMP for veterinary vaccines, but it seems that these are interpreted by each country. Currently neither ILRI nor CTTBD meet GMP standards for vaccines. It is considered by some that this compliance failure is in part a reflection on the misalignments between the different Directorates of Veterinary Services and the relevant local regulatory body in the countries concerned.
-
•
The complexity of strains and stocks, and the failure to silence the doubters. There is no doubt that the 1996 initiative by FAO to request and fund the first full commercial scale batch of the MC helped substantially in gaining acceptance of the wide use of these stabilates across country borders. This acceptance was deepened by the apparent successes of MC in reducing ECF mortality in northern Tanzania. However, the reluctance by Directors of Veterinary Services and other decision makers to approve the manufacture and deployment of the MC severely hampered its impact, and continues to do so to a degree.
-
•
The necessity of a rigorous cold chain. The product requires a well-managed cold chain and careful handling to deliver and administer carefully and correctly so that it is has the maximum chance of full efficacy. This has generally required coordination by trained veterinarians on farms and ranches. This is not a straight forward process, and requires infrastructure, training and coordination. Responding to the need for greater attention to MC delivery, there is a USAID-supported project in Tanzania managed by ILRI which aims to widen the distributor network and provide training to different private enterprises. However, Tanzania does not have a liquid nitrogen distribution network that adequately meets the demand of the MC distribution aspirations. The artificial insemination (AI) programme provides a basis for this distribution in Kenya, but it is far less developed in Tanzania.
-
•
The MC successes in northern Tanzania have not always been straight forward. Prior to 1998 there was an unacceptably high level of clinical reactors in the Kilimanjaro region, and this was addressed by increasing the concentration of oxytetracycline, as described above.
-
•
Beyond the technical challenges encountered in the early stages of MC delivery in Tanzania, the mostly positive impacts were not straight forward in development terms. Homewood et al. (2006) [56] showed that although the ITM vaccine has an overwhelming impact on survival through protecting against fatal ECF, reducing calf mortality from over 20% to around 2%, the distribution of those impacts on livelihoods of cattle owners and their families were not uniform. They showed that when the vaccine was provided on a commercial basis, poorer livestock keeping households vaccinated a smaller proportion of their calves and immature animals (30–34%) compared to the wealthier households (up to 90%). Part of this was due to the cost of the vaccine (US$ 6–10), and in part due to the vaccine pack-size issue (referred to earlier). With the vaccine kept in straws in liquid nitrogen, and diluted in the field for immediate use into about 40 doses, only larger scale operators could gather the necessary numbers of calves for vaccination at one place and time. This discriminated against the smaller scale producers, who also were more prone to logistical problems of assembling their animals in the right place and at the right time.
-
•
Public or private good? In all disease control interventions, particularly those targeting specific diseases in low and middle income countries, and particularly in poor rural areas of such countries, the question of financial responsibility emerges (see Kirsten et al., 2009) [57]. As an endemic disease and one that is not affecting national or international trade, ECF control and prevention is generally considered a private good, and this is the position adopted by CTTBD and GALVmed. There are many component costs of the MC, and it emerges at something between $6–10 per dose. This is substantially more expensive than say the vaccine against foot-and-mouth disease. Nevertheless, in Kenya there is, at least in theory, a substantial market for the MC throughout the country to serve the growing livestock enterprises in different production systems. However, in reality there appears to be a dramatic difference in uptake by production system and agro-ecology. In some parts of Kenya, particularly the highland regions in which smallholder dairy is widespread, supplying the large part of Kenya's milk demand, there has been a conflict of interest with the business enterprises of private agro-pharma suppliers. Many such private distributors feel that the long term immunity associated with a vaccine will interfere with their established markets for acaricides and anti-theilerial drugs (such as parvaquone and buparvaquone), and reduce their profit margins on these products. There were reports of companies which put in bids to take over and monopolise the distribution of MC, but in reality were not planning to distribute any vaccine as they feared it would reduce the lucrative market for acaricides. There have been several reports of companies and veterinarians actively discouraging farmers from using the MC. As a result, a code of practice has been set up by the Directors of Veterinary Services; the Directorate of Veterinary Services has authorised only certain accredited distributors to deliver the MC. But the low uptake of MC is not just based on the business aspirations of the agro-pharma companies. As Kenya's smallholder dairy system is characterised by 1–3 cows kept in zero grazing stalls, not only is their risk of acquiring ECF lower with very little pasture grazing, but also the use of knap-sack spray application of acaricides regularly by the farmer is both easy and cost effective. In the dryer pastoralist regions of Kenya there is also a substantial demand for MC, as there is in northern Tanzania, and it is in these areas, particularly Kajiado and Narok where the greatest demand for the MC lies. This appears to be for various reasons. From a logistical point of view, it is easier to deliver MC to larger herds, given the 40 doses emerging from any one straw of stabilate. And secondly it would appear that the larger agro-pharma distributors are less concerned about their acaricide markets in these areas, likely linked to the logistical challenges of administering acaricides regularly to large herd without dip tank facilities. Indeed a complex matrix of different business interests.
-
•
The pack-size enigma. The irony is that the current format of the technology biases against a major market segment, not only in Kenya but throughout the region. The practicalities of delivery to small groups of cattle must surely be a research priority. It is understood that GALVmed is working with CTTBD on this, and in addition ILRI is reportedly exploring this dilemma. Issues to be addressed include the need to ensure that straws flush properly, that stability is not affected by the changed formulation, and it requires an amendment to the registration dossiers.
6. A synthesis of the MC research to delivery impact continuum
6.1. A review of the chronology of events and the institutions and players involved
-
•
1950s to early 1960s: EAVRO under the East African Community before Kenya's independence. A strong research capacity including names of Steve Barnett, Peter Bailey, David Brockelsby and others;
-
•
Mid 1960s to mid-1970s: EAVRO post-independence, the UNDP-supported FAO project led by Matt Cunningham, with a cast of many including Duncan Brown, Tony Musoke, Roger Purnell, Subhash Morzaria, Mike Burridge, David Radley, etc. The golden years of ITM research, and the birth of the MC.
-
•
Late 1970s to early 1990s: The end of the FAO project at EAVRO Muguga, coincides with the inauguration of ILRAD; the start of the reorientation of the ECF vaccine agenda to sporozoite and eventually schizont vaccines. An exit of resources and scientists from former EAVRO; a few (such as Alan Young, Tom Dolan) remain at the renamed NVRI Muguga, and new scientists emerge such as Dadson Kariuki, Jamlak Mutugi, Alice Maritim, Priscilla Ngumi, Sammy Ndungu, Sam Mbogo. Period focuses on refining the ITM technique, studying the parasite dynamics and strain variation, and developing molecular tools to refine the ITM procedure. The start of the wilderness years with respect to field application of ITM
-
•
1980s to late 1990s: Multiple initiatives in other countries of the region. Inputs in funding and projects from the Netherlands, Belgium, DANIDA, UK. Studies in Tanzania, Uganda, Zambia, Zimbabwe. A series of valuable regional workshops on ECF epidemiology initiated, coordinated by ILRAD, FAO and conducted under the auspices of AU/IBAR. ILRAD and ILCA become ILRI, under new leadership. The first commercial scale MC batch produced at ILRI. The wilderness years continue.
-
•
The Noughties: The DFID study of animal health research priorities for poverty reduction leads to the birth of a Public Private Partnership of what was to become GALVmed. The wilderness years continue, as ILRI produces a new batch of MC at the request of AU/IBAR. AU/IBAR chaired an ECF regional task force, comprising government representatives of the four countries currently using the ECF MC vaccine (Malawi, Kenya, Tanzania and Uganda), GALVmed, ILRI and Pan-African Veterinary Vaccine Centre (PANVAC); opened tenders for MC manufacture and distribution. Manufacture won by CTTBD. The wilderness years continue.
-
•
The Twenty-tens: GALVmed moves into a coordinating and facilitating role both for the rehabilitation of CTTBD and the extension of new MC initiatives into other countries in the region with renewed financial support from DFID and BMGF. GALVmed also pursues registration of MC in different countries. New delivery initiative launched in Tanzania with USAID support. Private sector delivery strengthened in Kenya with variable results based on highly differentiated business interests of pastoralist and dairy sectors. Still no recombinant vaccine alternative emerging.
6.2. The innovation to impact pathway: some lessons learned
The ITM innovation, in particular the MC, has indeed had many direct and indirect impacts, which have been presented and discussed above. The direct impacts on cattle have been survival of animals, and have thus contributed to the livelihoods of many who have had their cattle immunised. The indirect impacts have been many, in some cases of greater long term contribution to science and capacity development.
However, importantly, the direct impacts have not been on the scale that they could have been, nor in the production systems in which there is arguably the greatest need, nor over the time scale they could have been anticipated. The following overarching issues are considered to have been the principle contributors to these inadequacies.
-
•
A supply-driven, not demand-driven, approach to vaccine deployment has characterised ITM. The technology has been promoted by the handful of scientists engaged with ECF control. This has left a fundamental weakness in the understanding of the different livestock production systems in the countries of the region, and most importantly of the business aspirations of producers, farmers, pastoralists and health service providers embedded in the ECF-endemic environment. This aspect of development science was almost totally absent in the early days of independence, concurrent with the early ITM technology successes, and a current vision of the evolving and intensifying livestock production systems, as well as their complex idiosyncrasies in terms of technology adoption, remains grossly inadequate.
-
•
The void in leadership and responsibility to ensure sustainable MC stabilate production. It is argued by some that inadequate advantage was taken of the 1996 milestone of MC production at a commercial scale by ILRI, and particularly ten years later with production of the ILRI 08 batch in 2007. ILRI was generally considered to be (and some argue remains) the only institution in the region with the human, financial and technical resources necessary for the complex MC production process. Some have argued that ILRI even had an international responsibility to take a leadership role and continue producing stabilates. Opposing this was ILRI's management at the time, which felt that moving into vaccine production was a risk to ILRI's role and credibility as an international research institute. Furthermore ILRI is not a registered vaccine producer and does not have the structures to monitor and oversee vaccine production required to meet GMP guidelines. In addition, certain of the regional government authorities supported what they saw to be a more politically correct pathway of MC production under the auspices of AU/IBAR, which ILRI readily bought into. With hindsight, these two forces arguably conspired to circumvent the pragmatic decision in the public interest for ILRI to pick up the responsibility for ITM production, which some consider belonged to ILRI.
-
•
The merits of the new public private partnership, combined with the inadequacies and politics of the constituents it was given to work with. The ITM was in part responsible for the birth of GALVmed, by being one of the technologies identified in the DFID-sponsored analysis of animal health research priorities for poverty reduction. GALVmed has built up a portfolio of priority technologies for it to promote, and has successfully raised considerable funding to help move these to the field. GALVmed entered the stage concurrent with ILRI's production of MC batch ILRI 08. With ILRI not wishing to continue with ITM production, GALVmed welcomed the opportunity to take on a leadership role in this, and to start developing its own position in African livestock technology development and deployment. While the relationship with ILRI is now generally seen to be functional on several fronts (with ILRI collaborating closely in assistance to CTTBD, and GALVmed working with ILRI in the ECF consortium, for example), this relationship was strained in the early twenty-tens. GALVmed leadership at the time was seeking to broaden its funding base through partnerships with major donors, but some observers considered that it did not engage adequately with ILRI, and was seen as a new competitor for the scarce global funds for animal health technologies in developing countries. In addition, there was a corresponding reluctance from ILRI, particularly at the scientist level, to explore dialogue with the “new kid on the block”. An independent study of ILRI's biosciences research opportunities and comparative advantage commissioned in 2012 by the incoming Director General concluded “there is overall weakness in some areas in establishing partnerships with downstream partners and international organisations, and indeed in understanding the need for such engagement. This is particularly the case with GALVmed, where very little functional partnership seems to exist”. Fortunately, as emphasised above, these inadequacies of communication on both sides now appear to be in the past, and the two institutions have defined much more clearly their differential roles, ILRI in research and GALVmed in development and market creation.
-
•
In this environment, and with AU/IBAR advocating its role as an umbrella for future ITM deployment, GALVmed then helped oversee a tender process under AU/IBAR for regional ITM production, in which CTTBD emerged the winner. While GALVmed undoubtedly welcomed its new role as a key player, it then had to deal with the reality of resuscitating a twice-collapsed institution, and do this in a politically charged environment. At the beginning of this process there was sufficient of the ILRI 08 batch to satisfy the needs of the region, but this was followed by a shortage of ECF-ITM in 2014. On top of this the re-equipping of CTTBD took about three years. It is fully understandable that GALVmed took its time with the resuscitation process, and ensured that its first vaccine batch (MCL 01) was produced before opening the doors to the world's media in December 2014.
-
•
Were the wilderness years wasted? Yes they were in terms of the direct impact of ITM, but with hindsight they played an important role in broadening the understanding of ECF epidemiology and control at a regional level, bring all countries and their scientists together, and in raising the awareness, standard and profile of many key African scientists who now hold important positions in many public and private institutions.
Acknowledgements
I wish to thank the following people for several fascinating and informing discussions on the ECF ITM story:
Ross Gray, Tony Irvin, Peter Jeffries, Henry Kiara, Subhash Morzaria, Tony Musoke, Vish Nene and Phil Toye.
I thank Jeroen Dijkman for comments on an earlier draft of this manuscript, and I am most grateful for the support of the Independent Science and Partnership Council (ISPC) of the CGIAR who invited me to prepare this narrative.
Footnotes
FAO (2004) defined intensification as an increase in agricultural production per unit of inputs (which may be labour, land, time, fertilizer, seed, feed or cash). For practical purposes, intensification occurs when there is an increase in the total volume of agricultural production that results from a higher productivity of inputs, or agricultural production is maintained while certain inputs are decreased (such as by more effective delivery of smaller amounts of fertilizer, better targeting of plant or animal protection, and mixed or relay cropping on smaller fields). The ethics of sustainable agricultural intensification, FAO, Rome. http://www.fao.org/docrep/007/j0902e/j0902e00.htm#Contents
What was originally termed the Organisation for African Unity (OAU) was renamed the African Union in 2002. Within the organisation sits the Inter-African Bureau of Animal Resources (IBAR), established in 1951 as the Inter-African Bureau of Epizootic Diseases (IBED.
The Economist, Aid Policy, October 31st 2002.
Eliminating World Poverty: A Challenge for the 21st Century.
References
- 1.Bruce D.B., Hamerton A.E., Baterman H.R., Mackie F.P. Amakebe: a disease of calves in Uganda. Proceedings of the Royal Society: B. 1910;82:256–272. [Google Scholar]
- 2.Lawrence J.A. The History of Bovine Theileriosis in Southern Africa. In: Norval R.A.I., Perry B.D., Young A.S., editors. The Epidemiology of Theileriosis in Africa. Academic Press; London: 1992. 1999. [Google Scholar]
- 3.Anon . Government Printers; Nairobi: 1911. Annual Report of the Department of Agriculture, British East Africa. [Google Scholar]
- 4.de Steeg V., Jeannette P.V., Baltenweck I., Herrero M., Makokhac S., Staal S. Future of crop-livestock systems in the densely populated highlands of Kenya. 2005. http://www.tropentag.de/2005/abstracts/full/545.pdf
- 5.Eicher C.K., Ruhuni M. World Bank; Washington, D.C.: 2003. The CGIAR at 31: An Independent Meta Evaluation of the Consultative Group on International Agricultural Research. ( http://lnweb90.worldbank.org/oed/oeddoclib.nsf/DocUNIDViewForJavaSearch/FF995495091C533C85256D5600551683/$file/cgiar_wp_eich_ruki.pdf) [Google Scholar]
- 6.McKelvey J., Jr., Pino J.A. 1970. An International Laboratory for Animal Disease Research in Africa. [Google Scholar]
- 7.Norval R.A.I., Perry B.D., Young A.S. Academic Press; London: 1992. The Epidemiology of Theileriosis in Africa. (481 pp.) [Google Scholar]
- 8.Perry B.D., Young A.S. The naming game: the changing fortunes of East Coast fever and Theileria parva. Vet. Rec. 1993;133:613–616. [PubMed] [Google Scholar]
- 9.Lawrence J.A., Perry B.D., Williamson S. East Coast Fever. In: Coetzer J.A.W., Tustin R.C., editors. Infectious Diseases of Livestock. Vol. 1. Oxford University Press; Cape Town: 2004. pp. 448–467. [Google Scholar]
- 10.Gachohi J., Skilton R., Hansen F., Ngumi P., Kitala P. Epidemiology of East Coast fever (Theileria parva infection) in Kenya: past, present and the future. Parasit. Vectors. 2012;5:194. doi: 10.1186/1756-3305-5-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukhebi A.W., Perry B.D., Kruska R.L. Estimated economics of theileriosis control in Africa. Prev. Vet. Med. 1992;12:73–85. [Google Scholar]
- 12.Cunningham M.P. Immunization of Cattle against Theileria parva. In: Henson J.B., Campbell M., editors. Theileriosis: Report of a Workshop Held in Nairobi, 7–9 December 1976. International Development Research Centre; Ottawa: 1977. (1 12 pp.) [Google Scholar]
- 13.Radley D.E. Infection and treatment immunization against theileriosis. In: Irvin A.D., Cunningham M.P., Young A.S., editors. Advances in the Control of Theileriosis: Proceedings of an International Conference Held at ILRAD, Nairobi, 9–13 February 1981. Martinus Nijhoff Publishers; The Hague: 1981. pp. 227–236. [Google Scholar]
- 14.Uilenberg G. Immunization against diseases caused by Theileria parva: a review. Tropical Med. Int. Health. 1999;9:A12–A20. doi: 10.1046/j.1365-3156.1999.00446.x. ( http://www.ncbi.nlm.nih.gov/pubmed/10540307) [DOI] [PubMed] [Google Scholar]
- 15.Di Giulio G., Lynen G., Morzaria S., Oura C., Bishop R. Live immunization against East Coast fever–current status. Trends Parasitol. Feb 2009;25(2):85–92. doi: 10.1016/j.pt.2008.11.007. (Epub 2009 Jan 8) [DOI] [PubMed] [Google Scholar]
- 16.Yrjö-Koskinen, A. E., McKeever, D., Peters, A., Rushton, J. (2010). Considerations for the use of the ITM method for East Coast Fever immunization. Royal Veterinary College and GALVmed (Unpublished report).
- 17.ILRI A short history of the ‘live’ vaccine protecting Africa's cattle against East Coast fever. 2014. https://ilvac.net/2014/08/19/a-short-history-of-the-live-vaccine-protecting-africas-cattle-against-east-coast-fever/
- 18.Burridge M.J., Morzaria S.P., Cunningham M.P., Brown C.G.D. Duration of immunity to East Coast fever (Theileria parva) infection of cattle. Parasitology. 1972;64:511–515. doi: 10.1017/s0031182000045571. [DOI] [PubMed] [Google Scholar]
- 19.Neitz W.O. Aureomycin in Theileria parva infection. Nature. 1953;171:34–35. doi: 10.1038/171034a0. [DOI] [PubMed] [Google Scholar]
- 20.Brocklesby D.W., Bailey K.P. Oxytetracyline hydrochloride in East Coast fever (Theileria parva infection) Br. Vet. J. 1962;118:81–85. doi: 10.1016/s0007-1935(17)39352-1. [DOI] [PubMed] [Google Scholar]
- 21.Brocklesby D.W., Bailey K.P. The immunization of cattle against East Coast fever (Theileria parva infection) using Tetracyclines: a review of the literature and a reappraisal of the method. Bull. Epizoot. Dis. Afr. 1965;13:161–168. [PubMed] [Google Scholar]
- 22.Jerierski A., Lambelin G., Lateur L. Vol. 8. 1959. Immunisation Des Bovides Contre, (“East Coast Fever” [ECF]. (Theileriose a Theileria parva) pp. 1–21. (Bulletin a" Information d INEAC). [Google Scholar]
- 23.Jarrett W.F.H., Pirie H.M., Sharp N.C.C. Immunization agai1nst East Coast fever using tick infections and chlorotetracycline. Exp. Parasitol. 1969;24:147–151. doi: 10.1016/0014-4894(69)90151-9. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham M.P., Brown C.G.D., Burridge M.J., Purnell R.E. Cryopreservation of infective particles of Theileria parva. Int. J. Parasitol. 1973;3:583–587. doi: 10.1016/0020-7519(73)90082-9. [DOI] [PubMed] [Google Scholar]
- 25.Mutugi J.J., Young A.S., Maritim A.C., Ndungu S.G., Mining S.K., Linyonyi A., Ngumi P.N., Leitch B.L., Morzaria S.P., Dolan T.T. Immunisation of cattle against theileriosis in Coast Province, Kenya: laboratory evaluation of a Theileria parva parva stabilate for use in ‘infection and treatment’ immunisation in the field. Res. Vet. Sci. 1989;47(2):170–177. [PubMed] [Google Scholar]
- 26.Radley D.E., Brown C.G.D., Cunningham M.P., Kimber C.D., Musisi F.L., Payne R.C., Purnell R.E., Stagg D.A., Young A.S. East Coast fever. 3. Chemoprophylactic immunization of cattle using oxytetracycline and a combination of theilerial strains. Vet. Parasitol. 1975;1:51–60. [Google Scholar]
- 27.Uilenberg G., Silayo R.A., Mpangala C., Tondleur W., Tatchell. R.J., Sanga H.J.N. Studies on Theileriidae (Sporozoa) in Tanzania. X. A large-scale field trial on immunization of cattle against theileriosis. Tropenmed. Parasitol. 1977;28:499–506. [PubMed] [Google Scholar]
- 28.Musisi F.L. Methods currently used for the control of East Coast fever: their validity and proposals for future control strategies. Parassitologia. 1990;32(1):5–22. [PubMed] [Google Scholar]
- 29.Lynen G., Melewas J., Majaliwa K., Bakuname C., di Giulio G. The Use of a 30% Formulation of Oxytetracycline Long-Acting in East Coast Fever Immunizations in Tanzania. In: Mukaratirwa S., Obwolo M.J., editors. Animal Health and Production for Development. Proceedings of IX International Conference of Associations of Institutions of Tropical Veterinary Medicine, Harare, Zimbabwe, 14–18 September 1998. vol. 1. 1999. pp. 367–375. [Google Scholar]
- 30.Di Giulio G. Use of two Different Dose Rates of Oxytetracycline in East Coast Fever Immunisation in Tanzania. In: Kazimirova M., Labuda M., Nuttall P.A., editors. Proceedings of the 3rd International Conference on Tick and Tick-Borne Pathogens. Institute of Zoology; Bratislava: 2000. pp. 121–123. [Google Scholar]
- 31.Morzaria S.P. THEILERIOSIS in Eastern, Central and Southern Africa, Proceedings of a Workshop on East Coast Fever Immunization Held in Lilongwe, Malawi 20–22 September 1988. ILRAD; Nairobi, Kenya: 1989. A Systematic Approach to East Coast Fever Immunization in the Kilifi District of the Kenya Coast.http://www.fao.org/wairdocs/ilri/x5549e/x5549e0f.htm#TopOfPage [Google Scholar]
- 32.McKeever D.J. Live immunisation against Theileria parva: containing or spreading the disease? Trends Parasitol. 2007;23(12):565–568. doi: 10.1016/j.pt.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKeever D.J. Bovine immunity - a driver of diversity in Theileria parasites? Trends Parasitol. 2009;25(6):269–276. doi: 10.1016/j.pt.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Oura C.A., Bishop R., Asiimwe B.B., Spooner P., Lubega G.W., Tait A. Theileria parva live vaccination: parasite transmission, persistence and heterologous challenge in the field. Parasitology. 2007;134:1205–1213. doi: 10.1017/S0031182007002557. [DOI] [PubMed] [Google Scholar]
- 35.Patel E.H., Lubembe D.M., Gachanja J., Mwaura S., Spooner P., Toye P. Molecular characterization of live Theileria parva sporozoite vaccine stabilates reveals extensive genotypic diversity. Vet. Parasitol. 2011;179:62–68. doi: 10.1016/j.vetpar.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 36.Morzaria S.P., Young J.R. Restriction mapping of the genome of the protozoan parasite Theileria parva. Proc. Natl. Acad. Sci. 1992;89:5241–5245. doi: 10.1073/pnas.89.12.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nene V., Bishop R., Morzaria S., Gardner M.J., Sugimoto C., ole-MoiYoi O.K., Fraser C.M., Irvin A. Theileria parva genomics reveals an atypical apicomplexan genome. Int. J. Parasitol. 2000;10(30(4)):465–474. doi: 10.1016/s0020-7519(00)00016-3. [DOI] [PubMed] [Google Scholar]
- 38.Minami T., Spooner P.R., Irvin A.D., Ocama J.G.R., Dobbelaere D.A.E., Fujinaga T. Characterization of stocks of Theileria parva by monoclonal antibody profiles. Res. Vet. Sci. 1983;35:334–340. [PubMed] [Google Scholar]
- 39.Bishop R., Nene V., Spooner P., Mbogo S., Kariuki D., Payne R., Morzana S. Application of Molecular Tools in Support of Deployment of Theileria parva Live Vaccines. In: Morzaria S., Williamson S., editors. Live Vaccines for Theileria parva: Deployment in Eastern, Central and Southern Africa: Proceedings of an FAO/OAU-IBAR/ILRI Workshop Held at ILRI, Nairobi, Kenya, 10–12 March 1997. 1999. [Google Scholar]
- 40.Irvin A.D. ILRAD; Nairobi: 1985. Immunization against Theileriosis in Africa: Proceedings of a Workshop Sponsored by the International Laboratory for Research on Animal Diseases and the Food and Agriculture Organization of the United Nations, Held in Nairobi, Kenya, 1–5 October 1984. [Google Scholar]
- 41.Irvin A.D. ILRAD; Nairobi: 1986. Immunization against East Coast Fever: Report of a Workshop on Collection, Handling and Analysis of Performance and Productivity Data, Held in Nairobi, Kenya, 23–25 September 1985. [Google Scholar]
- 42.Dolan T.T. The International Laboratory for Research on Animal Diseases; Nairobi: 1993. Ticks and Tick-Borne Disease Control: Proceedings of a Joint OAU, FAO and ILRAD Workshop Held in Kampala, Uganda, 12–14 September 1991. [Google Scholar]
- 43.Irvin A.D., McDermott J.J. and Perry, B.D. Epidemiology of Ticks and Tick-borne Diseases in Eastern, Central and Southern Africa. Proceedings of a Workshop Held in Harare, 12–13 March 1996. ILRI (International Livestock Research Institute), Nairobi, Kenya.
- 44.Morzaria S.P., Spooner P., Bishop R., Mwaura S. The Preparation of a Composite Stabliate for Immunization against East Coast Fever. In: Morzaria S., Williamson S., editors. Live Vaccines for Theileria parva: Deployment in Eastern, Central and Southern Africa. ILRI (International Livestock Research Institute); Nairobi, Kenya: 1997. (Proceedings of an FAO, OAU-IBAR and ILRI Workshop Held at ILRI, Nairobi, Kenya 10–12 March 1997). (1999, 166 pp.) [Google Scholar]
- 45.Morzaria S., Williamson S., editors. Proceedings of an FAO, OAU-IBAR and ILRI Workshop Held at ILRI, Nairobi, Kenya 10–12 March 1997. ILRI (International Livestock Research Institute); Nairobi, Kenya: 1999. Live Vaccines for Theileria parva: Deployment in Eastern, Central and Southern Africa. (166 pp) [Google Scholar]
- 46.Lynen G.M., Makala L.H.C., Pas W.M. East Coast Fever Immunization in Eastern Province, Zambia. In: Dolan T.T., editor. Ticks and Tick-Borne Disease Control: Proceedings of a Joint OAU, FAO and ILRAD Workshop Held in Kampala, Uganda, 12–14 September 1991. The International Laboratory for Research on Animal Diseases; Nairobi: 1993. pp. 16–21. (1993) [Google Scholar]
- 47.OlE (Office International des Epizooties) Manual of Standards for Diagnostic Test and Vaccines. OlE; Paris, France: 1996. Theileriosis; pp. 321–330. [Google Scholar]
- 48.Patel E., Mwaura S., Kiara H., Morzaria S., Peters A., Toye P. Production and dose determination of the ITM method (ITM) MC vaccine used to control East Coast fever in cattle. Ticks Tick Borne Dis. Mar 2016;7(2):306–314. doi: 10.1016/j.ttbdis.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Perry B.D., Randolph T.F., McDermott J.J., Sones K.R., Thornton P.K. International Livestock Research Institute (ILRI); Nairobi, Kenya: 2002. Investing in Animal Health Research to Alleviate Poverty. (140 pp plus CD-ROM https://ilri.org/InfoServ/Webpub/fulldocs/investinginAnimal/Book1/media/) [Google Scholar]
- 50.Robinson T.P., Wint G.R.W., Conchedda G., Van Boeckel T.P., Ercoli V. Mapping the global distribution of livestock. PLoS One. 2014;9(5):e96084. doi: 10.1371/journal.pone.0096084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.GALVmed . GALVmed Position Paper 1. 2015. East Coast Fever Infection & Treatment Method. ( http://www.galvmed.org/wp-content/uploads/2015/02/GALVmed-Position-Paper-on-ECF-Feb-2015.pdf) [Google Scholar]
- 52.Chizonda C. ECF Immunization in Malawi. In: Morzaria S., Williamson S., editors. Live Vaccines for Theileria parva: Deployment in Eastem, Central and Southern Africa. Proceedings of an FAO, OAU-IBAR and ILRI Workshop held at ILRI, Nairobi, Kenya 10–12 March 1997. ILRI (International Livestock Research Institute); Nairobi, Kenya: 1999. (1999, 166 pp.) [Google Scholar]
- 53.Musoke A., Rowlands J., Nene V., Nyanjui J., Katende J., Spooner P., Mwaura S., Odongo D., Nkonge C., Mbogo S., Bishop R., Morzaria S. Subunit vaccine based on the p67 major surface protein of Theileria parva sporozoites reduces severity of infection derived from field tick challenge. Vaccine. 2005;23(23):3084–3095. doi: 10.1016/j.vaccine.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 54.TAC . CGIAR Technical Advisory Group Secretariat; Rome: 1981. Report of the TAC Quinquennial Review of the Ilnernational Laboratory for Research on Animal Disease (ILRAD) [Google Scholar]
- 55.Morrison W.I. Cambridge University Press; 1986. The Ruminant Immune System in Health and Disease. (570 pp.) [Google Scholar]
- 56.Homewood K., Trench P., Randall S., Lynen G., Bishop B. Livestock health and socio-economic impacts of veterinary interventions in Masailand: infection and treatment vaccine against East Coast fever. Agric. Syst. 2006;89:248–271. [Google Scholar]
- 57.Kirsten J.F., Dorward A., Poulton C., Vink N. International Food Policy Research Institute (IFPRI); Washington D.C.: 2009. Institutional Economics Perspectives on African Agricultural Development. [Google Scholar]