Abstract
Live poultry-associated salmonellosis is an emerging public health issue in the United States. Public and animal health officials collaborated to investigate one of the largest (356 cases, 39 states) of these outbreaks reported to date. A case was defined as illness in a person infected with the outbreak strain of Salmonella Typhimurium with illness onset between 1 March and 22 October 2013. The median patient age was seven years (range: < 1–87 years); 58% of ill persons were children ≤ 10 years, 51% were female, 25% were hospitalized; 189 (76%) of 250 patients reported live poultry exposure in the week before illness; and 149 (95%) of 157 reported purchasing live poultry from agricultural feed stores. Traceback investigations identified 18 live poultry sources, including 16 mail-order hatcheries. Environmental sampling was conducted at two mail-order hatcheries. One (2.5%) of 40 duplicate samples collected at one hatchery yielded the outbreak strain. Live poultry are an important source of human salmonellosis, particularly among children, highlighting the need for educational campaigns and comprehensive interventions at the mail-order hatchery and agricultural feed store levels. Prevention and control efforts depend on a One Health approach, involving cooperation between public and animal health officials, industry, health professionals, and consumers.
Keywords: Salmonellosis, Live poultry, Outbreaks, One health, Zoonoses, Mail-order hatcheries
Graphical abstract

Highlights
-
•
Live poultry-associated salmonellosis is an emerging public health issue.
-
•
We summarize one of the largest of these outbreaks reported to date (356 cases).
-
•
Prevention and control efforts depend on a One Health approach.
1. Introduction
Non-typhoidal salmonellosis is the most common enteric bacterial illness in the United States, resulting in an estimated 1.2 million illnesses, 19,000 hospitalizations, and 370 deaths annually [1]. Although the majority of Salmonella spp. infections are foodborne, an estimated 11% are attributed to animal contact, or are zoonotic [2]. Salmonella is naturally found in the intestinal tract of many animals. Based on reported outbreak investigations, reptiles, amphibians, live poultry, and small non-traditional pets present a high risk for zoonotic salmonellosis [3], [4], [5], [6]. Importantly, infected animals often appear healthy, but can intermittently shed bacteria [5]. Zoonotic Salmonella infections can occur through direct contact with infected animals; or through indirect contact with anything in the areas where animals live and roam, or consumption of food or drink prepared in contaminated environments [2], [6], [7].
Backyard flocks are increasing in popularity in the United States as a result of the local foods movement and the desire to raise live poultry for fun or hobby [8]; concurrently, live poultry-associated salmonellosis is an emerging public health issue [6]. The mail-order hatchery industry is the primary producer of baby poultry for sale to private individuals, with approximately 20 core mail-order hatcheries across the nation [6], [9], [10], [11]. Mail-order hatcheries produce and sell more than fifty million chicks annually, and may distribute a variety of live poultry nationwide. Baby poultry is typically distributed through the U.S. Postal Service to agricultural feed stores, and is also sold directly to customers through catalog and internet orders.
Mail-order hatcheries are voluntarily regulated through the United States Department of Agriculture National Poultry Improvement Plan (USDA-NPIP). USDA-NPIP is a voluntary partnership between industry and state and federal government, with the goal of eliminating or controlling certain poultry diseases in breeder flocks to prevent egg-transmitted and hatchery-disseminated diseases [12]. The NPIP's original purpose was to serve as an avenue for the commercial poultry industry to officially test birds and demonstrate degrees of freedom from economically devastating vertically-transmitted diseases of poultry, such as Pullorum and Typhoid. The program has since expanded to include important diseases that impact trade, by including surveillance programs for Low Pathogenic Avian Influenza. Through constant evolution, the NPIP has expanded with the impetus to add voluntary programs to the NPIP provisions through periodic amendments as a means to ultimately protect the U.S. poultry industry and continue trade through diagnostic testing. One of these amendments to the NPIP provisions includes the addition of a new certification program that was proposed and accepted in 2010 and officially adopted as a program within the NPIP on 8 August 2014 [13]. The US voluntary Salmonella Monitoring Program is a program in which mail-order hatcheries have the opportunity to voluntarily participate. Participation in this program will certify their flocks are monitored for Salmonella organisms that may cause illness in humans. By participating, mail-order hatcheries should be able to track trends in Salmonella over time through diagnostic testing. The intent of this program is to reduce the incidence of Salmonella in day-old poultry in the hatchery and give the poultry industry a better opportunity to reduce the incidence of Salmonella in their products.
PulseNet, the national molecular subtyping network for foodborne disease surveillance, routinely performs pulsed-field gel electrophoresis (PFGE) on isolates from clinical cases of Salmonella and other reportable enteric diseases to identify clusters of human infections that may represent outbreaks [14]. From 1990 to 2012, 45 live poultry-associated salmonellosis outbreaks were identified, resulting in 1581 illnesses, 221 hospitalizations, and 5 deaths [6]. Since this time, additional live poultry-associated salmonellosis outbreaks have been investigated [15], [16]. Additionally, a recent study of live poultry hatchling shipment boxes to multiple locations of a national farm store chain was published, further highlighting the link between mail-order hatcheries and human illness for particular non-typhoidal salmonella genotypes and antimicrobial susceptibility patterns [17]. Although many individuals recognize the risk of salmonellosis from handling raw poultry meat products, people are generally not aware that Salmonella can also be acquired through live poultry contact [6], [18].
The Centers for Disease Control and Prevention (CDC) collaborated with public health and agriculture officials in many states and the U.S. Department of Agriculture Animal and Plant Health Inspection Service (USDA-APHIS) to investigate a multistate outbreak of human Salmonella Typhimurium infections. The objectives of the investigation were to identify the source of the outbreak through epidemiologic, traceback, and laboratory investigations; implement strategies to control the outbreak and prevent future illnesses; and provide recommendations to appropriate stakeholders to prevent future outbreaks.
2. Material and methods
2.1. Outbreak detection, case definition, and case finding
On 8 April 2013, a cluster of Salmonella enterica serotype Typhimurium isolates with an identical PFGE pattern (i.e., outbreak strain) was detected by PulseNet. CDC contacted state and local health departments to further investigate this cluster. Cases were defined as ill persons with laboratory-confirmed Salmonella Typhimurium infections with a PFGE pattern indistinguishable from the outbreak strain and illness onset (or isolation date if illness onset date unknown) between 1 March and 22 October 2013. Throughout the investigation, PulseNet notified CDC epidemiologists of isolates matching the outbreak strain that were newly uploaded to the database. CDC contacted epidemiologists in states with matching isolates.
2.2. Hypothesis generation
Health department personnel conducted hypothesis-generating interviews with patients using state or local enteric disease questionnaires, which typically address shopping locations, the foods patients ate at home and outside the home, travel, and animal contact during the week before illness onset. When patients reported live poultry exposure, personnel were asked to interview patients with a live poultry supplemental questionnaire. This questionnaire addresses specific baby and adult live poultry exposures and collects purchase, behavior, and backyard flock information. Interview results were compared by binomial probability to the Foodborne Diseases Active Surveillance Network (FoodNet) Population Survey Atlas of Exposures, 2006–2007, a national survey of healthy people regarding foods consumed in the 7 days before interview [19].
2.3. Traceback investigation
When patients reported a live poultry purchase from an agricultural feed store, epidemiologists contacted the store to complete the feed store information section of the live poultry supplemental questionnaire. This section addresses species of live poultry sold, months and numbers sold, as well as the sources of live poultry sold at the store. When possible, CDC confirmed live poultry sources with agricultural feed store chain corporate headquarters. CDC also worked with state public health and agriculture partners to confirm feed store shipments with identified mail-order hatcheries.
2.4. Laboratory investigation
Live poultry and environmental samples were collected from ill persons' homes. Environmental sampling was conducted at two mail-order hatcheries, using drag swabs according to standard protocol [13]. Samples were cultured for Salmonella, serotyped, and subtyped by PFGE [20] using enzyme XbaI at state public health laboratories, the National Veterinary Services Laboratories, and the CDC laboratory. Multiple-Locus Variable-number tandem repeat Analysis (MLVA) pattern typing [21] was performed on multiple isolates matching the outbreak strain by certified state public health laboratories and the CDC laboratory. Select human and environmental isolates were tested for antimicrobial susceptibility [22] by the CDC National Antimicrobial Resistance Monitoring System laboratory.
3. Results
3.1. Case finding and patient characteristics
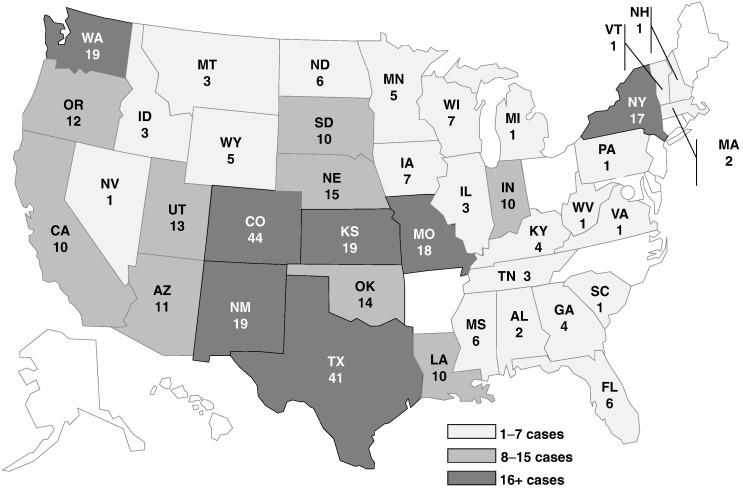
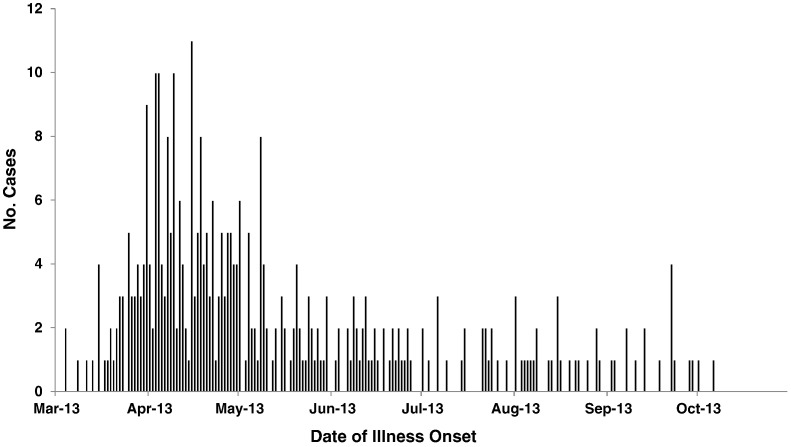
The outbreak strain (Salmonella Typhimurium, PFGE pattern XbaI JPXX01.0286) had been seen in PulseNet before, with an average of 33 (range = 20–51) uploads per year during 2008–2012. By 22 October 2013, 356 persons infected with the outbreak strain were reported from 39 states (Fig. 1). Estimated and reported illness onset dates were between 4 March and 6 October 2013 (Fig. 2). Ill persons ranged in age from < 1 to 87 years, with a median age of 7 years (Table 1). 58% of ill persons were children ≤ 10 years of age. 51% of ill persons were female. Among ill persons with available information, 25% were hospitalized. No deaths were reported.
Fig. 1.
Geographic distribution of persons infected with the outbreak strain of Salmonella Typhimurium, by state, n = 356.
See attached file: AndersonT_ManuscriptFig1_30Jun2015.
Fig. 2.
Date of illness onset of persons infected with the outbreak strain of Salmonella Typhimurium, n = 356.
Table 1.
Characteristics of persons infected with the outbreak strain of Salmonella Typhimurium.
| Demographics | # | n or range | % |
|---|---|---|---|
| Age, median (years) | 7 | (< 1–87) | n/a |
| Age, ≤ 10 years | 198 | 344 | 58 |
| Female | 172 | 338 | 51 |
| Outcomes | |||
| Hospitalizations | 64 | 261 | 25 |
| Deaths | 0 | 263 | 0 |
3.2. Hypothesis generation
Early epidemiologic information suggested an association between patient illness and baby poultry exposure. During interviews, 189 (76%) of 250 patients reported exposure to any live poultry in the 7 days before illness onset (Table 2). Of 177 patients with specific exposure information, 84% reported live poultry exposure including chicks or chickens and 56% reported exposure to only chicks or chickens. 42% of patients reported live poultry exposure including ducklings or ducks and 15% reported exposure to only ducklings or ducks. The 2006–2007 FoodNet population survey estimates that 1% of the U.S. population reports exposure to chicks and 3% reports exposure to chickens in a typical 7 day period. The exposures reported by patients during this investigation were much higher than these percentages, supporting the association of this outbreak with live poultry (P < 0.0001, binomial probability that observed live poultry exposures were greater than exposures in the U.S. population).
Table 2.
Reported live poultry exposures of persons infected with the outbreak strain of Salmonella Typhimurium.
| Exposures | # | n | % |
|---|---|---|---|
| Any live poultry | 189 | 250 | 76 |
| Any chick or chicken | 149 | 177 | 84 |
| Only chick or chicken | 100 | 177 | 56 |
| Any duckling or duck | 75 | 177 | 42 |
| Only duckling or duck | 26 | 177 | 15 |
| FoodNet Pop Survey, chick (19) | 82 | 8718 | 1 |
| FoodNet Pop Survey, chicken (19) | 222 | 8718 | 3 |
For patients with available purchase information, the median time from purchase to illness onset was 18 days (Range = 1–127 days). In interviews, 128 patients reported multiple reasons for purchasing live poultry, including one or more of the following: eggs (58%), pets (41%), fun/hobby (21%), meat (9%), Easter present (5%), fair/exhibition (3%), school project (2%), pest control (2%), and to release to a lake or pond (2%). Patients also reported high risk behaviors with their live poultry. Of 123 patients with available information, 50% reported keeping live poultry indoors, 49% reported holding/snuggling live poultry, and 16% reported kissing live poultry.
3.3. Traceback investigation
Live poultry were purchased from a variety of locations; however, 149 (95%) of 157 patients reported purchasing live poultry from agricultural feed stores, representing 33 companies in 116 locations nationwide. Ill persons also reported purchasing live poultry directly from mail-order hatcheries [4 (3%)], online [3 (2%)], another individual [2 (1%)], a farmers' market [1 (< 1%)], and a flea market [1 (< 1%)].
Sources of live poultry were reported through supplemental questionnaires for 114 (98%) of 116 identified agricultural feed store locations. Fifteen sources of live poultry, including 13 mail-order hatcheries, were initially identified. Based on supplemental questionnaires, these sources shipped live poultry to 1%–68% of the feed stores. Four mail-order hatcheries voluntarily provided shipment information for these stores, and drop-shipping (i.e., when a shipment of birds is sent directly to a customer from a second hatchery under the first hatchery's name [9]) was frequently reported when confirming mail-order hatchery shipments. Drop-shipping information identified three additional mail-order hatchery live poultry sources, as well as the previously unknown source for one of the feed stores; therefore, 18 sources of baby poultry located in 12 states were identified for 115 feed stores. Ultimately, Mail-order Hatchery A in the Western United States shipped birds to 104 (90%) of these stores.
3.4. Laboratory investigation
The outbreak strain was isolated from three live poultry and environmental samples collected at patient homes in New Mexico, New York, and Vermont. In addition to matching by PFGE XbaI pattern, 46 human and 23 non-human isolates tested by MLVA exhibited identical or closely related patterns. Of seven isolates (six human, one non-human) tested for antimicrobial susceptibility, two were pansusceptible to antimicrobials included on the test panel, one was resistant to streptomycin, and four (including the non-human isolate) were resistant to tetracycline.
Environmental sampling was conducted at Mail-order Hatchery A in May 2013 by state department of health and USDA-NPIP representatives. Thirty-six drag swabs were collected from the hatchery and two hatchery-owned breeder farms, representing thousands of birds and a variety of species. No Salmonella was detected. Further, no Salmonella was reportedly detected during routine monthly testing conducted by the hatchery during January–June 2013.
Additional environmental sampling was conducted at Mail-order Hatchery A in July 2013 by CDC, state department of health, and USDA-NPIP representatives. Forty duplicate environmental samples (i.e., 80 total samples) were collected throughout Mail-order Hatchery A, including in the hatchery and at two hatchery farms. One (2.5%) of 40 duplicate samples, collected from a duck pen on the hatchery's main farm, yielded the outbreak strain; several yielded other Salmonella serotypes that were not linked with human illnesses. The outbreak isolate matched the MLVA pattern observed for the majority of human and non-human isolates obtained during the investigation, and antimicrobial sensitivity testing indicated the isolate was pansusceptible to antimicrobials included on the test panel.
One other mail-order hatchery identified during the investigation was willing to have environmental sampling conducted specifically in response to this outbreak. Salmonella was detected in two samples; however, the outbreak strain was not identified. Other identified mail-order hatcheries did not voluntarily have sampling conducted in response to this outbreak, but three reported that the outbreak strain was not identified in routine drag swabs collected in 2013.
4. Discussion
We investigated one of the largest live poultry-associated salmonellosis outbreaks reported to date in the United States, with 356 confirmed human Salmonella Typhimurium infections in 39 states. Many more cases likely occurred than were reported [1]. Similar to previously reported live poultry-associated salmonellosis outbreaks [6], a large percentage of ill persons were children ≤ 10 years of age. 76% of patients with available information reported live poultry exposure in the seven days prior to illness onset, and the percentages of patients reporting duckling and duck exposures were higher than previously reported in other outbreaks [9], [10], [11]. 95% of patients with purchase information reported purchasing their live poultry from agricultural feed stores, and the traceback for this outbreak was the most complicated to date. Live poultry were purchased by consumers from > 30 agricultural feed store companies in > 100 locations, and agricultural feed stores obtained live poultry from 18 sources across the nation. Collaboration between public health and agriculture officials and the mail-order hatchery and agricultural feed store industries was critical to the success of the investigation, and highlights the importance of a One Health approach to zoonotic salmonellosis outbreak investigations [6].
This investigation had several limitations, including missing or incomplete exposure and purchase information (e.g., patients often could not specify breeds, some purchase dates were approximated), extensive drop-shipping complicated traceback efforts, and it was not possible to review records to determine drop-shipping practices or conduct environmental sampling at the majority of identified mail-order hatcheries. For these reasons, although the outbreak strain was isolated from an environmental sample collected at Mail-order Hatchery A, it is not possible to definitively rule out an additional source (or sources) of infection.
Concurrent with this outbreak investigation, a study was conducted of Salmonella strains in shipment boxes of hatchling poultry shipped to 36 locations of a large national farm store chain [17]. Due to confidentiality agreements, it is unknown if the specific store locations overlapped between the study and this outbreak investigation; however, consumers did report purchasing live poultry at the same national chain during this investigation. Salmonella strains were recovered from 59 (27%) of 219 shipment boxes, with 13 isolates collected at 10 different farm stores matching the outbreak strain (Salmonella Typhimurium, PFGE pattern XbaI JPXX01.0286) of this investigation. In addition, the antimicrobial susceptibility patterns of these isolates were comparable to those obtained during this investigation, with isolates being pansusceptible, resistant to tetracycline, or resistant to streptomycin and tetracycline. Shipment information was available for four of ten shipment boxes, with three originating from Mail-order Hatchery A (referred to as Hatchery B in the study publication) and one being drop-shipped from another source. It was noted that shipment boxes that were positive for the same human Salmonella outbreak strain were typically shipped from the same hatchery; however, since this outbreak strain was shipped from two different locations, it could have been more widespread. As previously noted, we established a strong link between Mail-order Hatchery A and this outbreak during the epidemiologic, traceback, and laboratory investigations; however the investigation's noted limitations and the findings of the hatchling shipment study highlight that an additional source (or sources) of infection cannot be ruled out.
Due to a previous multi-year outbreak of Salmonella Montevideo [9] linked to the hatchery, Mail-order Hatchery A has become an industry leader in Salmonella prevention and control. If the primary source of infection for this Salmonella Typhimurium outbreak was ducklings from Mail-order Hatchery A, then several questions relevant to the broader hatchery industry remain. Additional research is needed, and CDC is continuing to work with multiple stakeholders to better understand the larger implications of this and other outbreaks linked to live poultry.
The increasing popularity of backyard poultry, combined with poor public awareness and understanding of the risk of salmonellosis after contact with live poultry [6], [18], makes human Salmonella infections linked to live poultry from mail-order hatcheries a growing but preventable public health concern. From 1990 to 2012, 45 live poultry-associated salmonellosis outbreaks were reported to CDC. These outbreaks were linked to multiple mail-order hatcheries and resulted in > 1581 illnesses, 221 hospitalizations, and five deaths [6]. In 2013, four Salmonella outbreaks linked with live poultry from mail-order hatcheries were investigated, resulting in 579 cases and 94 hospitalizations [4], [23]. The hatchling shipment study results further supported the hatchery sources identified during these outbreak investigations [17]. The largest outbreak documented to date since occurred in 2014 with a total of 363 persons from 43 states and Puerto Rico infected with the outbreak strains of Salmonella Infantis, Newport, or Hadar [15]. The outbreak was traced to a single mail-order hatchery. CDC has identified multiple One Health partners to address live poultry-associated salmonellosis outbreaks including public health agencies (state and local health departments), agriculture agencies (USDA/APHIS, state departments of agriculture), industry (mail-order hatcheries, agricultural feed stores), and health professionals (especially pediatricians and veterinarians). Interventions implemented by mail-order hatcheries, agricultural feed stores, and consumers can reduce human Salmonella infections linked to live poultry contact. Specific recommendations are discussed, and summarized in Table 3.
Table 3.
Live poultry-associated salmonellosis prevention and control recommendations for identified stakeholders.
State and local health departments
|
The distribution of eggs and birds through multiple mail-order hatcheries emphasizes the importance of comprehensive Salmonella prevention and control programs being implemented and maintained at mail-order hatcheries and associated breeder farms. To reduce the chance of Salmonella, according to USDA, participating mail-order hatcheries and their source flocks should comply with management and sanitation practices outlined by the new NPIP certification program [13]. The USDA-NPIP recently published a best management practices handbook, which provides specific recommendations to mitigate Salmonella contamination at poultry hatcheries [24]. USDA-NPIP, state agriculture departments, and hatchery staff can collaborate to develop hatchery-level interventions. Mail-order hatcheries linked with previous outbreaks have implemented successful strategies to reduce Salmonella and prevent human illness such as: consulting with a poultry veterinarian to customize interventions to reduce Salmonella in the hatchery and breeder flocks; implementing strict biosecurity practices; using flock Salmonella vaccination and autogenous vaccines specific to outbreak strains identified in the hatchery environment; and collecting monthly environmental samples for regular Salmonella monitoring including testing for strains of Salmonella that cause human illness, but not poultry disease. To further reduce Salmonella risk, USDA recommends mail-order hatcheries should provide health-related information to owners and potential purchasers of these birds prior to the point of purchase to include information about the risk of acquiring a Salmonella infection from contact with live poultry [6]. Ideally, educational messages could be distributed with each box of shipped birds and displayed on hatchery websites. Free educational posters are available in English, French, and Spanish [25].
Because the majority of baby poultry are sold in agricultural feed stores, it is important for these venues to be involved with education and prevention. State and local agriculture and public health officials can remind feed stores annually about prevention of human Salmonella infections linked to live poultry contact. A feed store letter template, which includes current information and recommendations, is distributed annually by CDC for state and local use. Advice is also available in the National Association of State Public Health Veterinarians' Compendium of Measures to Prevent Diseases Associated with Animals in Public Settings [5].
Consumers and health professionals should be aware that healthy poultry can carry Salmonella and other germs that can make people sick. Specific recommendations regarding how to prevent human Salmonella infections are available (Table 3). Additional outreach efforts have included CDC features [26], [27], and educational materials in print and social media. The general public and health professionals can learn more about this issue on the CDC website [16], [28].
5. Conclusions
Live poultry are an important source of human salmonellosis, particularly among children, highlighting the need for educational campaigns and comprehensive interventions at the mail-order hatchery and agricultural feed store levels. Prevention and control efforts depend on a One Health approach, involving cooperation between public and animal health officials, industry, health professionals, and consumers.
Financial support
This work was supported by the Centers for Disease Control and Prevention, the United States Department of Agriculture, and state departments of health and agriculture. This investigation was conducted by employees of federal and state agencies, as part of their duties of employment. There was no other source of funding outside of employment.
Conflicts of interest
None.
Disclaimer
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.
Acknowledgements
We thank CDC staff in the Outbreak Response and Prevention Branch and the Enteric Diseases Laboratory Branch; USDA/APHIS staff in Veterinary Services, the National Veterinary Services Laboratories, and the National Poultry Improvement Plan; multiple state and local health departments and state departments of agriculture; and the mail-order hatchery and agricultural feed store industries for their assistance with this investigation and the overall issue of live poultry-associated salmonellosis.
References
- 1.Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.-A., Roy S. Foodborne illness acquired in the United States–major pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hale C.R., Scallan E., Cronquist A.B., Dunn J., Smith K., Robinson T. Estimates of enteric illness attributable to contact with animals and their environments in the United States. Clin. Infect. Dis. 2012;54:S472–S479. doi: 10.1093/cid/cis051. [DOI] [PubMed] [Google Scholar]
- 3.Harris J.R., Neil K., Barton Behravesh C., Sotir M., Angulo F. Recent multistate outbreaks of human Salmonella infections acquired from turtles: a continuing public health challenge. Clin. Infect. Dis. 2010;50:554–559. doi: 10.1086/649932. [DOI] [PubMed] [Google Scholar]
- 4.Basler C., Forshey T.M., Machesky K., Erdman C.M., Gomez T.M., Nguyen T.-A. Notes from the field: multistate outbreak of human Salmonella infections linked to live poultry from a mail-order hatchery in Ohio — March–September 2013. MMWR Morb. Mortal. Wkly Rep. 2014;63:222. [PMC free article] [PubMed] [Google Scholar]
- 5.National association of state public health veterinarians animal contact compendium committee 2013. Compendium of measures to prevent disease associated with animals in public settings, 2013J. Am. Vet. Med. Assoc. 2013;243:1270–1288. doi: 10.2460/javma.243.9.1270. [DOI] [PubMed] [Google Scholar]
- 6.Barton Behravesh C., Brinson D., Hopkins B.A., Gomez T.M. Backyard poultry flocks and salmonellosis: a recurring, yet preventable public health challenge. Clin. Infect. Dis. 2014;58:1432–1438. doi: 10.1093/cid/ciu067. [DOI] [PubMed] [Google Scholar]
- 7.Mettee Zareki S.L., Bennett S.D., Hall J., Yaeger J., Lujan K., Adams-Cameron M. US outbreak of human Salmonella infections associated with aquatic frogs, 2008–2011. Pediatrics. 2013;131:724–731. doi: 10.1542/peds.2012-2031. [DOI] [PubMed] [Google Scholar]
- 8.United States Department of Agriculture . U.S. Department of Agriculture, Animal and Plant Health Inspection Services, Veterinary Services, Center for Epidemiology and Animal Health, Fort Collins, CO. 2012. Poultry 2010, urban chicken ownership in four U.S. cities. [Google Scholar]
- 9.Gaffga N.H., Barton Behravesh C., Ettestad P.J., Smelser C.B., Rhorer A.R., Cronquist A.B. Outbreak of salmonellosis linked to live poultry from a mail-order hatchery. N. Engl. J. Med. 2012;366:2065–2073. doi: 10.1056/NEJMoa1111818. [DOI] [PubMed] [Google Scholar]
- 10.Loharikar A., Briere E., Schwensohn C., Weninger S., Wagendorf J., Scheftel J. Four multistate outbreaks of human Salmonella infections associated with live poultry contact, United States, 2009. Zoonoses Public Health. 2012;59:347–354. doi: 10.1111/j.1863-2378.2012.01461.x. [DOI] [PubMed] [Google Scholar]
- 11.Loharikar A., Vawter S., Warren K., Deasy M., III, Moll M., Sandt C. Outbreak of human Salmonella Typhimurium infections linked to contact with baby poultry from a single agricultural feed store chain and mail-order hatchery, 2009. Pediatr. Infect. Dis. J. 2013;32:8–12. doi: 10.1097/INF.0b013e3182755e28. [DOI] [PubMed] [Google Scholar]
- 12.National Poultry Improvement Plan [Internet] Georgia: U.S. Department of Agriculture, Animal and Plant Health Inspection Services, Veterinary Services, National Poultry Improvement Plan. Jun 30 2015. http://www.poultryimprovement.org/default.cfm [cited. Available from.
- 13.National Poultry Improvement Plan and Auxiliary Provisions, 9C.F.R. Parts 56, 145, 146, and 147. 2014. http://www.gpo.gov/fdsys/pkg/FR-2014-07-09/pdf/2014-16037.pdf [cited 2015 Jun 30]. Available from.
- 14.Swaminathan B., Barrett T.J., Hunter S.B., Tauxe R.V., PulseNet Task Force C.D.C. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg. Infect. Dis. 2001;7:382–389. doi: 10.3201/eid0703.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basler C., Forshey T.M., Machesky K., Erdman C.M., Gomez T.M., Brinson D.L. Notes from the field: multistate outbreak of human Salmonella infections linked to live poultry from a mail-order hatchery in Ohio — February–October 2014. MMWR Morb. Mortal. Wkly Rep. 2015;64:258. [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention [Internet] Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Jun 30 2015. Reports of selected Salmonella outbreak investigations.http://www.cdc.gov/salmonella/outbreaks.html [cited. Available from. [Google Scholar]
- 17.Habing G.G., Kessler S.E., Mollenkopf D.F., Wittum T.E., Anderson T.C., Barton Behravesh C. Distribution and diversity of Salmonella strains in shipments of hatchling poultry, United States, 2013. Zoonoses Public Health. 2015;62:375–380. doi: 10.1111/zph.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beam A., Garber L., Sakugawa J., Kopral C. Salmonella awareness and related management practices in U.S. urban backyard chicken flocks. Prev. Vet. Med. 2013;110:481–488. doi: 10.1016/j.prevetmed.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention . Atlanta (Georgia): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2006–2007. Foodborne active surveillance network (FoodNet) population survey atlas of exposures. [Google Scholar]
- 20.Ribot E.M., Fair M.A., Gautom R., Cameron D.N., Hunter S.B., Swaminathan B. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 2006;3:59–67. doi: 10.1089/fpd.2006.3.59. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention [Internet] Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Jun 30 2015. Salmonella MLVA protocols, 2013.http://www.cdc.gov/pulsenet/pathogens/mlva.html [cited. Available from. [Google Scholar]
- 22.Centers for Disease Control and Prevention . Human Isolates Final Report, 2012. Atlanta (Georgia): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2014. National antimicrobial resistance monitoring system for enteric bacteria (NARMS) [Google Scholar]
- 23.Centers for Disease Control and Prevention [Internet] Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Jun 23 2016. Multistate outbreak of human Salmonella Typhimurium infections linked to live poultry in backyard flocks (final update)http://www.cdc.gov/salmonella/typhimurium-live-poultry-04-13/index.html [cited. Available from. [Google Scholar]
- 24.United States Department of Agriculture . Conyers (Georgia): U.S. Department of Agriculture, Animal and Plant Health Inspection Services, Veterinary Services, National Poultry Improvement Plan. March 2014. Best management practices handbook: a guide to the mitigation of Salmonella contamination at poultry hatcheries. [Google Scholar]
- 25.Centers for Disease Control and Prevention [Internet] Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Jun 30 2015. Salmonella and baby poultry.http://www.cdc.gov/healthypets/publications/index.html [cited. Available from. [Google Scholar]
- 26.Centers for Disease Control and Prevention [Internet] Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Jun 30 2015. Spring and baby poultry are here.http://www.cdc.gov/features/salmonellababybirds/ [cited. Available from. [Google Scholar]
- 27.Centers for Disease Control and Prevention [Internet] Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Jun 30 2015. Keeping backyard poultry.http://www.cdc.gov/Features/SalmonellaPoultry/ [cited. Available from. [Google Scholar]
- 28.Centers for Disease Control and Prevention [Internet] Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Jun 30 2015. Gastrointestinal (enteric) diseases from animals.http://www.cdc.gov/zoonotic/gi/ [cited. Available from. [Google Scholar]