Abstract
Objective
Red-cell distribution width (RDW) has been identified as a novel prognostic marker in a heterogeneous group of heart failure patients. In this group, diastolic dysfunction is associated with worse outcome. As the evidence is limited, the aim of the present study was to assess the relationship of RDW to diastolic markers in patients with left ventricular dysfunction (LVD) diagnosed during cardiac catheterization.
Methods
Clinical and angiographic data were collected retrospectively on a total of 291 stable patients (mean age 62 years, 199 males) with systolic dysfunction documented during cardiac catheterization in a regional medical center between January 2006 and December 2010.
Results
Positive association was seen between RDW and Left ventricular end diastolic pressure (LVEDP), estimated systolic pulmonary arterial pressure(sPAP), and left atrial dimension (LAD) (r: 0.18, 0.24, 0.28, respectively; p:<0.05).Three year retrospective survival analysis for 108 patients admitted in the first 2 years showed a statistically significant decrease in survival patients with high RDW(> 14.5) vs. normal RDW (73%vs.88%;log rank p:0.03). This was seen even in the asymptomatic subgroup (71% vs. 94%; log rank p: 0.01).
Conclusion
RDW correlates with markers of diastolic dysfunction in patients with LVD. Additionally, in patients asymptomatic LVD, high RDW is still associated with lower survival.
Keywords: Diastolic dysfunction, RDW, Left ventricular dysfunction
1. Introduction
Red cell distribution width (RDW) is a readily available test from a standard full blood count, and is a measure of variation in red blood cell size. It has been shown in many studies to be a powerful predictor of outcome in heart failure patients [1], [2], [3]. Most of these studies enrolled patients with congestive symptoms and some included patients with preserved systolic function. Among other predictors of worse outcome in heart failure population is the presence of diastolic dysfunction [4], [5]. In this work, we sought to study the correlation between RDW and markers of diastolic dysfunction in patients with symptomatic and asymptomatic LVD.
2. Methods
Medical and angiographic records of patients who underwent elective cardiac catheterization in a 500-bed teaching community hospital in urban New Jersey from January 2006 to December 2010 were retrospectively reviewed. Patients with normal EF on left ventriculogram, iron deficiency anemia, positive hemoccult blood, Hemoglobin < 10 g/dL, cancer, AIDS, elevated tropinins, or stage III-V chronic kidney disease were excluded. Data regarding estimated ejection fraction (EF), number of diseased vessels, and Left ventricular end diastolic pressure (LVEDP) were retrieved from cardiac catheterization reports. If right heart catheterization was not performed, systolic pulmonary artery pressure was estimated using tricuspid regurgitation velocity, in addition to estimated right atrial pressure from transthoracic echocardiography in the same admission. Echocardiography reports were also used to assess chamber dimension and size. Other demographic and clinical data were collected from the electronic medical records of those patients. All-cause mortality data were obtained from social security death index for patients in the first 2 years of the study to calculate the 3 year retrospective survival analysis. The study was conducted in Trinitas Regional Medical Center, where it was approved by the Institutional Review Board.
Highest RDW and brain natriuretic peptide (BNP) results during the index admission were recorded. Patients were considered to have symptomatic LVD if they were known to have symptoms and signs of heart failure at, or before the time of catheterization. Non-ischemic cardiomyopathy (NICD) was defined as the presence of LVD with no obstructive lesion (stenosis > 50%) in any of the three coronary arteries or their major branches.
3. Statistical analysis
Interval variables were tested for normality using the D'Agostino-Pearson omnibus normality test. Groupwise comparisons for normally distributed variables were made using Student's t test; variables that were not normally distributed were subjected to a nonparametric (Mann–Whitney) test. For non-normally distributed data, medians and interquartile ranges (IRs) are provided; for normally distributed variables, data are expressed as means and standard deviations (SDs). Categorical data were compared using Fisher's exact test. Odds Ratios (OR) and 95% confidence intervals (CIs) are provided. Spearman's rank correlation coefficient was used to measure the statistical association between two non-normally distributed variables. Logistic regression was used to adjust ORs for differences in baseline characteristics if the P value for the difference between the groups was less than 0.05. For the present study, α was set at 0.05; thus, P < 0.05 (two-sided) was considered to be statistically significant. Data were analyzed using Prism software (GraphPad Corp, USA), except for logistic regression, which was performed using an online routine (available at www.stat-pages.org/logistic.html; accessed on July, 14, 2011). Retrospective survival analysis was done using Kaplan–Meier curve with log rank test.
4. Results
We reviewed the angiographic reports of 2150 patients, of them 291 patients were included (199 men, mean age 61.9 ± 13.5 yr). This cohort was divided into 2 groups, Group 1 (patients with high RDW) included 117 patients, and Group 2 (normal RDW group) included 174 patients (Table 1). Ischemic cardiomyopathy (ICD) was found in 169 patients, and non-ischemic cardiomyopathy (NICD) was found in the remaining 122 patients. Symptomatic LVD was found in 41% of the group. Patients with high RDW were more likely to be symptomatic (OR 4.5 (2.7–7.4; p: < 0.0001)), more likely to have non- ischemic cardiomyopathy (OR: 2.9 (1.8–4.7; p: < 0.0001)).They were more likely to have lower EF (median EF 35% (IR: 25–40%) vs. normal RDW median EF 40% (IR 30–45%); p:0.0007), higher BNP (median BNP: 907 pg/ml (IR: 253–1923) vs.347 pg/ml (IR: 127–676); p: < 0.0001), larger LAD (median LAD 4.17 cm (3.65–4.80) vs. normal RDW median LAD 3.78 cm (IR: 3.38–4.2); p: < 0.0001).There was also a trend toward having more atrial fibrillation in group 1 vs. group 2 (OR: 1.9 (1.01–3.06); p: 0.08), and larger left ventricular end diastolic volume index(LVEDVI) (68 ml/m2 in group 1 vs. 61 ml/m2 in group 2; p: 0.05). Differences in LVEDP did not reach statistical significance (group 1 LVEDP 18 vs. 16 mm Hg in group 2, p: 0.09) (Table 2). In the symptomatic group, patients who had high RDW were more likely to have abnormal maximum left ventricular dP/dt (OR: 6 (1.3–28); p: 0.04). NICD patients were more likely to have abnormal RDW in comparison to ICD patients (OR: 2.9 (1.783–4.715); p: < 0.001). This was independent of the presence of symptoms, or severe LVD (EF < 30%) (p value for interaction > 0.05).In this study, Hypertension was the presumed etiology in 67% of NICD patients. The latter group were relatively younger than ICD patients (median age 57 yr. (47–68) vs ICD 65 yr. (54–75)),they were less likely to be on statins(OR: 0.4 (0.24–0.68); p: 0.005), and they were found to have larger ventricles(median LVEDVI 74 ml/m2 vs. 64 ml/m2; p: 0.006).
Table 1.
Abbreviations: ACEi/ABR blockers: angiotensin converting enzyme inhibitors/angiotensin receptor blockers; BMI: body mass index; BNP: brain natriuretic peptide; BUN: blood urea nitrogen; Cr: creatinine; Hb: hemoglobin, Na: serum sodium.
| Group 1 (RDW > 14.5%) 117 patients | Group 2 (RDW ≤ 14.5%) 174 patients | P value | |
|---|---|---|---|
| Age (mean) | 61 ± 13 years | 62 ± 14 years | 0.6 |
| Male gender | 79 | 120 | 0.8 |
| Race | < 0.0001 | ||
| Caucasians | 38 (33%) | 75(43%) | |
| Hispanics | 23 (20%) | 60 (35%) | |
| Blacks | 56 (47%) | 39(22%) | |
| Diabetes | 49 | 71 | 0.9 |
| Hypertension | 94 | 122 | 0.06 |
| Dyslipidemia | 55 | 80 | 0.9 |
| Smoking | 57 | 70 | 0.16 |
| BMI (kg/m2) | 28 (25–32) | 28 (25–32) | 0.4 |
| Symptoms of CHF | 73 | 47 | < 0.0001 |
| Atrial fibrillation | 25 | 22 | 0.08 |
| Cr (mg/dl) | 0.9 (0.8–1.1) mg/dl | 1.1 (0.9–1.3) mg/dl | < 0.0001 |
| BUN (mg/dl) | 14 (11–18) | 16 (12–24) | 0.005 |
| Na (meq/dl) | 137 (136–140) meq/dl | 137(136–140) | 0.38 |
| BNP (pg/ml) | 907 (252–1923) | 348 (128–676) | < 0.0001 |
| Hb (gm/dl) | 13.3 (12.3–14.2) | 14 (12.7–14.9) | 0.003 |
| Medications | |||
| Beta blockers | 52 | 76 | 0.9 |
| ACEi./ARB Blockers | 70 | 75 | 0.006 |
| Statins | 41 | 65 | 0.7 |
| Diuretics | 41 | 48 | 0.07 |
| Digoxin | 17 | 9 | 0.005 |
| warfarin | 11 | 11 | 0.3 |
Table 2.
Abbreviations: L. A.: left atrium; LVEDP: left ventricular end diastolic volume; LVEDVI: left ventricular end diastolic volume index; LVIDd: left ventricular internal dimension in diastole, LVIDs: left ventricular internal dimension in sytole; sPAP: systolic pulmonary artery pressure.
| Group1 | Group 2 | P value | |
|---|---|---|---|
| Ejection fraction | 35%(25–40) | 40% (30–45) | 0.0007 |
| Diseased vessels | < 0.0001 | ||
| None | 67(57%) | 55 (31%) | |
| Single vessel | 15 (13%) | 39 (22%) | |
| Two vessels | 20 (17%) | 31 (18%) | |
| Three vessels | 15 (13%) | 49 (29%) | |
| LVEDP (mm Hg) | 18 (12–25) | 16 (11–21) | 0.09 |
| Maximum LV dP/dt (mm Hg/s) |
1171 (965–1471) | 1340 (1109–1564) | 0.2 |
| L.A. Dimension(cm) | 3.8 (3.4–4.2) | 4.2 (3.7–4.8) | < 0.0001 |
| LVEDVI (ml/m2) | 68 (53–86) | 62 (50–77) | 0.05 |
| LVIDd (cm) | 5.4 (4.9–6.3) | 5.6 (4.9–6.3) | 0.7 |
| LVIDs(cm) | 4.1(3.6–5) | 3.9(3.5–4.8) | 0.4 |
| Estimated sPAP (mm Hg) | 27 (16–46) | 21(15–29) | 0.009 |
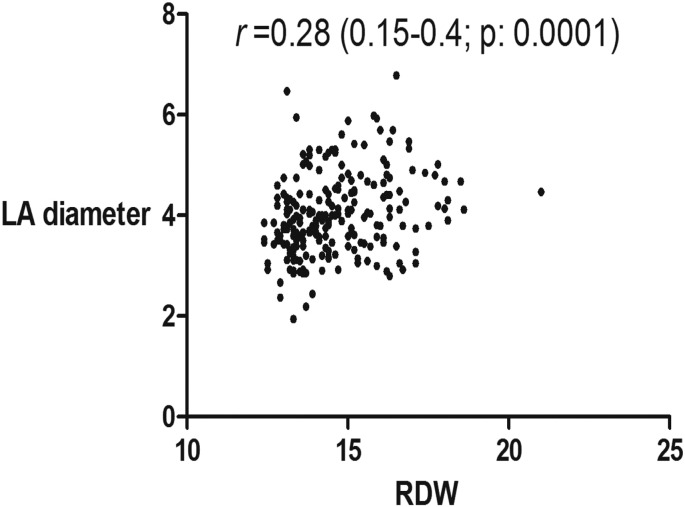
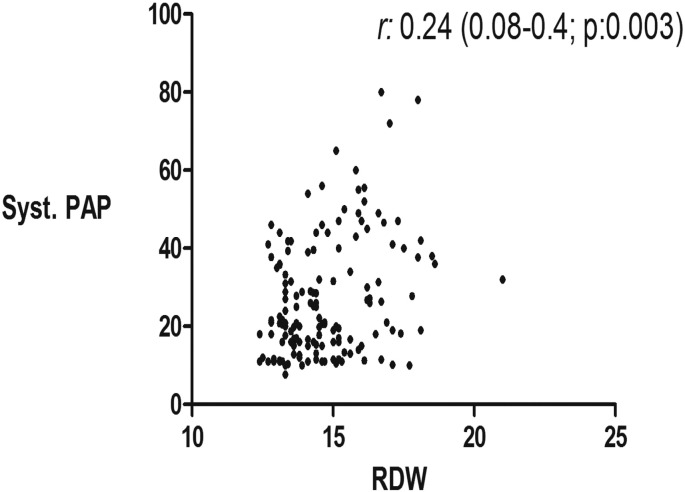
In addition to the well-established correlation between RDW and MCV, creatinine, BUN, EF, we found a modest but statistically significant correlations between RDW and LAD (r: 0.28; p: < 0.0001), sPAP(r: 0.24; p: 0.0028), LVEDP (r: 0.18; p: 0.039), and BNP (r: 0.3; p: < 0.0001) (Table 3, Fig. 1, Fig. 2, Fig. 3).
Table 3.
Abbreviations: LVEDP: left ventricular end diastolic pressure; sPAP: systolic pulmonary artery pressure.
| The biomarker | Correlation factor (r) | P value |
|---|---|---|
| Blood urea nitrogen | 0.2 | 0.0006 |
| Serum creatinine | 0.25 | < 0.0001 |
| BNP | 0.3 | < 0.0001 |
| Hemoglobin | − 0.17 | 0.003 |
| Mean corpuscular volume | − 0.2 | 0.0002 |
| Ejection fraction | − 0.25 | < 0.0001 |
| Left atrial dimension | 0.28 | < 0.0001 |
| Estimated sPAP | 0.24 | < 0.003 |
| LVEDP | 0.13 | 0.04 |
Fig. 1.
The correlation between RDW and left atrial dimension (L.A.).
Fig. 2.
The correlation between RDW and estimated systolic pulmonary artery pressure (sPAP).
Fig. 3.
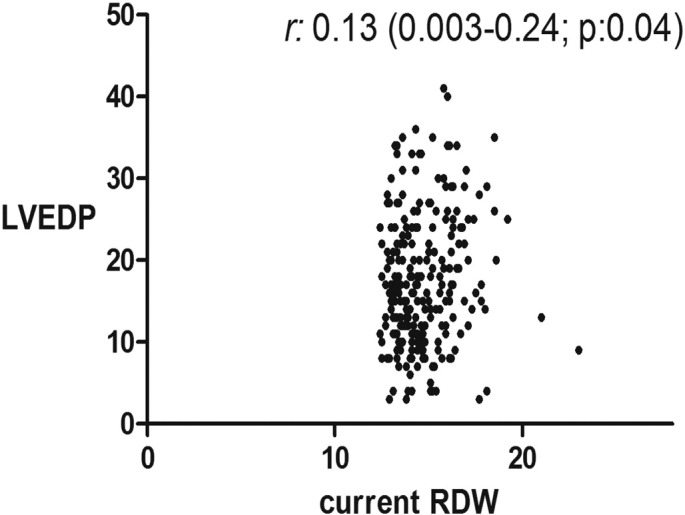
Correlation between RDW and left ventricular end diastolic pressure (LVEDP).
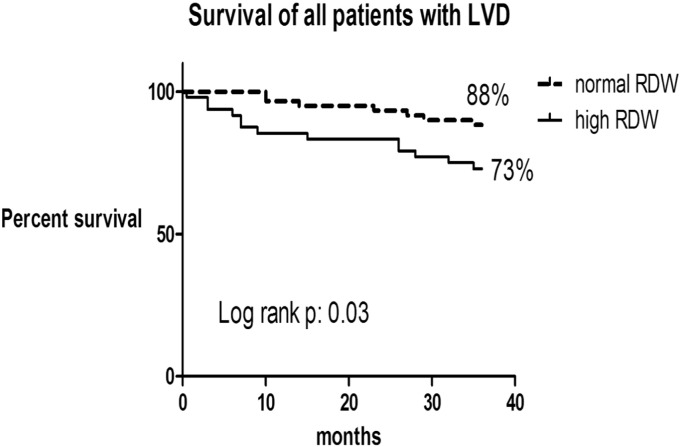
Data for 108 patients (52 patients asymptomatic) from the period (Jan 2006 to Dec. 2007) were included in 3-year retrospective survival analysis, and they were divided according to RDW into 2 groups. Three year survival was higher in the patients with LVD and normal RDW vs. high RDW (88% vs. 73%; log rank p: 0.03) (Fig. 4).This was also seen even in the asymptomatic patients (normal RDW group 94% vs. high RDW group 71%; log rank p: 0.01) (Fig. 5).Survival curves were not different in the symptomatic group between high and normal RDW group (log rank p: > 0.05).
Fig. 4.
Kaplan–Meier curves for all-cause mortality in all patients with left ventricular dysfunction (LVD) based on their RDW.
Fig. 5.
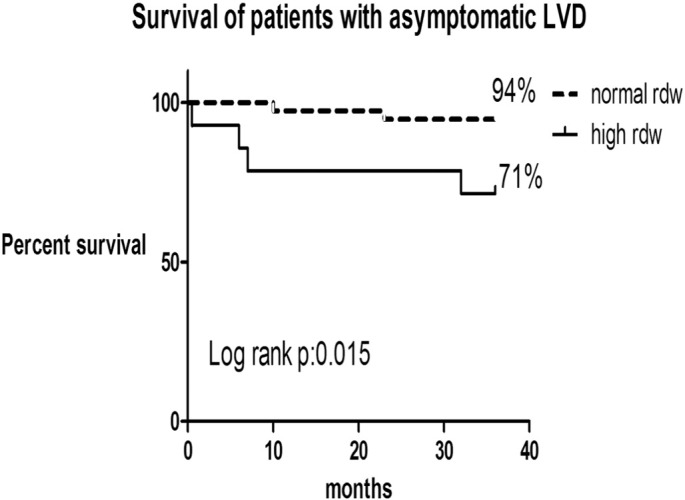
Kaplan–Meier curves for all-cause mortality in patients with asymptomatic left ventricular dysfunction (LVD) based on their RDW.
5. Discussion
High RDW values have been shown to be independently related to increased mortality and cardiovascular events in people with congestive heart failure [1], [2], [3], acute coronary syndrome [6], [7], and in patients referred for coronary angiography [8]. This study extends the prognostic value of RDW to all patients with systolic LVD even the asymptomatic group, a group that is less presented in other studies.
As this correlation was independent of hemoglobin level in most of the studies [1], [2], [3], [4], [9], RDW is presumed to reflect the degree of neurohormonal activation in HF patients [10], [11]. Not surprising was our finding that RDW correlates with BUN and BNP, two other markers of neurohormonal activation [12].
Elevated LVEDP is a hemodymanic indicator of diastolic dysfunction. Chronic longstanding diastolic dysfunction leads to left atrial dilation and increase in pulmonary circulation pressure. Brucks et al. proved that in patients who had systolic HF, the degree of diastolic dysfunction influenced survival rate (OR 1.64, p < 0.05), whereas EF and LVEDVI did not. Diastolic dysfunction was a better predictor of B-type natriuretic peptide levels and mortality than ejection fraction or LVEDVI [4]. In the aforementioned study, BNP correlates with diastolic dysfunction but not with EF. Here in this work, RDW correlates with both systolic (EF) and diastolic measures of left ventricular function. This result comes in agreement with a previous study of acute heart failure patients from South Korea where a value of RDW > 13.45% was predictive for early mitral inflow velocity to early diastolic mitral annular velocity (E/E′) > 15 in echocardiography, suggesting elevated LVEDP [13]. The association between RDW and LAD helps explain the trend toward more atrial fibrillation in patients with high RDW in our cohort and in previous studies in heart failure patients [1], [14].
In our group we found a positive correlation between sPAP and RDW. Although we attribute this to diastolic dysfunction, other mechanisms may play a role as recent studies have shown that RDW is a prognostic factor in patients with primary pulmonary hypertension [15].
NICD patients were more likely to have high RDW than ICD in our cohort despite being younger patients. This was independent of symptoms, and severe LVD (EF < 30%). A closer look at this group reveals that most of them have LVD due to hypertension. Studies on left ventricle systolic function in hypertensive patients indicate that only about 1/6 of hypertensive patients with left ventricular hypertrophy (LVH) present with systolic dysfunction. If hypertension is not controlled, the modifications of left ventricle will progressively increase, leading to LV dilation which seems to herald the transition to heart failure [16]. Because NICD group had larger ventricles (expressed as LEDVI) than ICD, we think that they represented a more advanced stage of LVD in poorly treated and probably noncompliant patients. In recent papers the transition from LVH to CHF has been related to a marked increase in myocardial expression of growth factor TGF beta 1, which influences interstitial fibrosis, and stimulation of apoptosis by myocardial expression of tumor necrosis factor alpha and the subsequent increase in inducible NO-synthase and oxidative stress [16]. We speculate that these humeral factors affect the bone marrow adversely, and are suppressed by statins which were used more often in our ICD patients, and which were found in other studies to be associated with lower RDW [2], [8].
Knowing that BNP was used in previous studies to screen hypertensive patients for systolic and diastolic dysfunction [17], [18], [19], it would be prudent to consider looking for these complications in non-anemic patients with poorly controlled hypertension and high RDW.
Our study suffers from the limitations of retrospective design, lack of detailed echocardiographc data like left atrial volume, and diastolic dysfunction grading by echocardiography. Also, the small number of patients available in the first 2 years for retrospective survival analysis has limited our capability to draw any valid conclusion from the negative results.
6. Conclusion
RDW correlates with markers of diastolic dysfunction in patients with LVD, and high values were associated with lower survival even in asymptomatic patients.
Acknowledgments
None of the authors has any conflict of interest, financial, or other disclosures to acknowledge.
References
- 1.Najjar Y., Goode K.M., Zhang J., Cleland J.G., Clark A.L. Red cell distribution width: an inexpensive and powerful prognostic marker in heart failure. Eur. J. Heart Fail. 2009;11:1155–1162. doi: 10.1093/eurjhf/hfp147. [DOI] [PubMed] [Google Scholar]
- 2.Zalawadiya S.K., Zmily H., Farah J., Daifallah S., Ali O., Ghali J.K. Red cell distribution width and mortality in predominantly African-American population with decompensated heart failure. J. Card. Fail. 2011;17:292–298. doi: 10.1016/j.cardfail.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Van Kimmenade R.R., Mohammed A.A., Uthamalingam S., van der Meer P., Felker G.M., Januzzi J.L., Jr. Red blood cell distribution width and 1-year mortality in acute heart failure. Eur. J. Heart Fail. 2010;12:129–136. doi: 10.1093/eurjhf/hfp179. [DOI] [PubMed] [Google Scholar]
- 4.Brucks S., Little W.C., Chao T., Kitzman D.W., Wesley-Farrington D., Gandhi S., Shihabi Z.K. Contribution of left ventricular diastolic dysfunction to heart failure regardless of ejection fraction. Am. J. Cardiol. 2005;95:603–606. doi: 10.1016/j.amjcard.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Rihal C.S., Nishimura R.A., Hatle L.K., Bailey K.R., Tajik A.J. Systolic and diastolic dysfunction in patients with clinical diagnosis of dilated cardiomyopathy. Relation to symptoms and prognosis. Circulation. 1994;90:2772–2779. doi: 10.1161/01.cir.90.6.2772. [DOI] [PubMed] [Google Scholar]
- 6.Yaman H., Celik T., Akgul E.O., Cayci T., Kurt Y. Red cell distribution width and acute coronary syndromes. Int. J. Cardiol. 2010;145:353. doi: 10.1016/j.ijcard.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Dabbah S., Hammerman H., Markiewicz W., Aronson D. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am. J. Cardiol. 2010;105:312–317. doi: 10.1016/j.amjcard.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Cavusoglu E., Chopra V., Gupta A., Battala V., Poludasu S., Eng C., Marmur J.D. Relation between red blood cell distribution width (RDW) and all-cause mortality at two years in an unselected population referred for coronary angiography. Int. J. Cardiol. 2010;141:146. doi: 10.1016/j.ijcard.2008.11.187. [DOI] [PubMed] [Google Scholar]
- 9.Pascual-Figal D.A., Bonaque J.C., Redondo B., Caro C., Manzano-Fernandez S., Sánchez-Mas J., Garrido I.P., Valdes M. Red blood cell distribution width predicts long-term outcome regardless of anemia status in acute heart failure patients. Eur. J. Heart Fail. 2009;11:840–846. doi: 10.1093/eurjhf/hfp109. [DOI] [PubMed] [Google Scholar]
- 10.Allen L.A., Felker G.M., Mehra M.R., Chiong J.R., Dunlap S.H., Ghali J.K., Lenihan D.J., Oren R.M., Wagoner L.E., Schwartz T.A., Adams K.F., Jr. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J. Card. Fail. 2010;16:230–238. doi: 10.1016/j.cardfail.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Förhécz Z., Gombos T., Borgulya G., Pozsonyi Z., Prohászka Z., Jánoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am. Heart J. 2009;158:659–666. doi: 10.1016/j.ahj.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Kazory A: emergence of blood urea nitrogen as a biomarker of neurohormonal activation in heart failure. Am. J. Cardiol. 2010;106:694–700. doi: 10.1016/j.amjcard.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Oh J., Kang S.M., Hong N., Choi J.W., Lee S.H., Park S., Shin M.J., Jang Y., Chung N. Relation between red cell distribution width with echocardiographic parameters in patients with acute heart failure. J. Card. Fail. 2009;15:517–522. doi: 10.1016/j.cardfail.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Zalawadiya S.K., Zmily H., Farah J., Daifallah S., Ali O., Ghali J.K. Red cell distribution width is independently associated with atrial fibrillation in hospitalized patients with heart failure. Circulation. 2010;122:A18773. [Google Scholar]
- 15.Rhodes C.J., Wharton J., Howard L.S., Gibbs J.S., Wilkins M.R. Red cell distribution width outperforms other potential circulating biomarkers in predicting survival in idiopathic pulmonary arterial hypertension. Heart. 2011;97:1054–1060. doi: 10.1136/hrt.2011.224857. [DOI] [PubMed] [Google Scholar]
- 16.Nogueira J.B. Hypertensive cardiomyopathy: from arterial hypertension to congestive heart failure. Rev. Port. Cardiol. 1999;18:635–646. [PubMed] [Google Scholar]
- 17.Vasan R.S., Benjamin E.J., Larson M.G., Leip E.P., Wang T.J., Wilson P.W., Levy D. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. J. Am. Med. Assoc. 2002;288:1252–1259. doi: 10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 18.Wang T.J., Evans J.C., Benjamin E.J., Levy D., LeRoy E.C., Vasan R.S. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 19.Wang T.J., Levy D., Benjamin E.J., Vasan R.S. The epidemiology of “asymptomatic” left ventricular systolic dysfunction: implications for screening. Ann. Intern. Med. 2003;138:907–916. doi: 10.7326/0003-4819-138-11-200306030-00012. [DOI] [PubMed] [Google Scholar]