Abstract
Livestock represent a fundamental economic and nutritional resource for many households in the developing world; however, a high burden of infectious disease limits their production potential. Here we present an ecological framework for estimating the burden of poultry disease based on coupled models of infectious disease and economics. The framework is novel, as it values humans and livestock as co-contributors to household wellbeing, incorporating feedbacks between poultry production and human capital in disease burden estimates. We parameterize this coupled ecological–economic model with household-level data to provide an estimate of the overall burden of poultry disease for the Ifanadiana District in Madagascar, where over 72% of households rely on poultry for economic and food security. Our models indicate that households may lose 10–25% of their monthly income under current disease conditions. Results suggest that advancements in poultry health may serve to support income generation through improvements in both human and animal health.
Keywords: Poultry, Poverty, Economics, Infectious disease modeling, Livestock
Highlights
-
•
We describe a new framework that couples models of infectious disease ecology and economics.
-
•
Using this framework, we estimate the burden of poultry disease on rural households in Madagascar.
-
•
We find that monthly household income is reduced by 10–25% in the majority of model simulations.
-
•
Feedbacks between human and poultry productivity amplify the economic impact of poultry disease.
Introduction
Globally, there are 2.2 billion people who live on less than 2 USD a day [1], and approximately half rely on livestock for their livelihoods [2]. For the rural poor who rely on agricultural production for subsistence [3], production outputs are often generated from physical labor, and may be sold, consumed, or traded for goods and services. Livestock have been shown to contribute to the production process through multiple pathways. They provide financial capital, as a liquid asset; physical capital, when used for transportation or traction; natural capital, when manure is used to improve soil fertility; and human capital, through the effects of improved nutrition on physiological growth, cognitive development, and education attainment [4].
The majority of poor livestock keepers live in South Asia (600 million) and Sub-Saharan Africa (300 million) [2], regions of the world where infectious and parasitic diseases continue to undermine the health and productivity of both humans and livestock [5], [6]. Good health is essential for the development of human capital, as it influences an individual's capacity to grow, learn, and actively engage in productive labor [7]. Like their human counterparts, livestock are complex biological organisms whose reproduction, growth, and economic value are necessarily influenced by their health status [8]. Therefore, diseases that afflict livestock threaten the subsistence of poor households in ways that are directly comparable to the effects of human disease; not only through direct loss of income, but also by undermining immediate and long term human capital accumulation through poor nutrition and health. Although there is a growing body of literature that supports the role of improving livestock health in poverty reduction [9], [10], [11], we lack frameworks for measuring disease burden that capture the cumulative impacts of livestock disease on the livelihoods and health of the rural poor.
Madagascar, a country with high levels of material deprivation, malnutrition, and reliance on agriculture, provides an exemplar setting for exploring these relationships. As one of the poorest countries in the world, Madagascar consistently ranks within the bottom 20% of all countries for indicators of development [12] and food security [13]. Eighty-seven percent of Malagasy survive on less than 1.25 USD per day [1], and approximately 15 million people, or two-thirds of the total population, live in rural areas where agriculture and livestock production are the primary forms of employment [14]. Madagascar has over 27 million chickens and 9.2 million other varied poultry species [14]; and as in many other low income countries, poultry are important livestock species for the poor. They produce meat and eggs for household consumption, and are easily transported to local markets for sale [15], [16].
Many of the major pathogens with the potential to impact poultry production have been reported in the country, including: avian influenza virus, Newcastle Disease virus, infectious bursal disease virus, fowl cholera (Pasteurella multocida), and intestinal parasites [17], [18], [19]. These pathogens decrease production as a result of high morbidity and mortality, with sequela including transient immune suppression, poor weight gain, and reduced egg production [20]. In order to quantify the effects of poultry disease on economic productivity, we present an ecological model framework that accounts for long-term interactions between poverty and infectious disease. The model is based on the premise that human and poultry capital are complementary inputs for generating household income, and that human capital (in the form of nutrition) is also supported by poultry production. The dynamic relationship between poultry disease and economic outcomes is modeled by coupling well-established susceptible–infectious–susceptible (SIS) disease-type models [21], [22], [23] with a simple economic growth model [24].
The framework builds on a theory of infectious diseases as ecological drivers of poverty [25], [26], [27], and allows one to measure the impact of poultry disease dynamics on household income directly, and on household capital accumulation. In this study, we estimate the burden of poultry disease in the Ifanadiana District of Madagascar recognizing that feedbacks between loss of production and human capital can exacerbate the economic impact that infectious diseases of poultry can have on poor households. We calibrate our model using data from a 1520-household human health and demographic survey and an 80-household pilot study of livestock health.
Materials and methods
Model framework
The epidemiological model for poultry disease
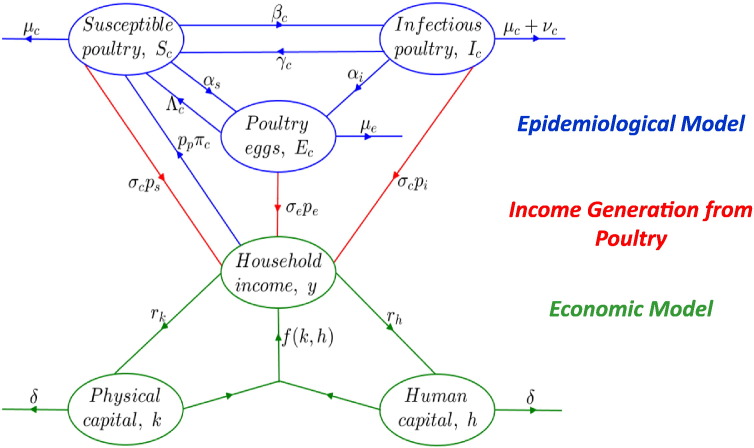
The poultry disease model is based on standard models in epidemiology and disease ecology [21], [22], [23], where the poultry population is apportioned into two compartmental classes, representing disease status. The susceptible or healthy class denoted by Sc consists of uninfected poultry with the potential of being infected, while the infectious or unhealthy class denoted by Ic, consists of infected poultry that can transmit disease. To account for differences in egg production between healthy and unhealthy poultry, we introduce a third class for eggs, denoted by Ec, and use two distinct egg production rates: αs for egg production by healthy poultry and αi for egg production by unhealthy poultry. A schematic of the model is presented in Fig. 1.
Fig. 1.
Schematic of the coupled model. Blue ellipses denote poultry compartments, while blue lines denote transition rates between poultry compartments such as disease transmission rate, βc, recovery rate, γc, natural mortality rate, μc, disease-induced mortality rate, νc, birth rate, Λc, egg-laying rates by healthy and unhealthy poultry, αs and αi, egg loss at rate, μe; and the use of income to purchase poultry, ppπc. Green ellipses denote economic model classes, while green lines denote the generation of income from physical and human capital, fk, h, and the reinvestment of a portion of income into these forms of capital at rates rk and rh. Red lines denote the generation of income through the sales and consumption of susceptible poultry, σcps, infectious poultry, σcpi, and eggs, σepe.
We present the following system of differential equations for the dynamics of the healthy and unhealthy poultry and egg populations:
| (1) |
| (2) |
| (3) |
We assume new births and purchases occur at respective rates, Λc and πc. The susceptible poultry population also increases when previously sick poultry recover from the infection at rate γc, and is reduced as a result of infection occurring at rate βc, natural mortality or loss at rate μc, or through sale or consumption at rate σc. The infectious poultry population grows as a result of new infections and is reduced as a result of both natural and disease-induced mortalities occurring at respective rates μc and νc. The infectious poultry population is also reduced when infected poultry recover to join the susceptible class or when they are sold or consumed at rate σc. Eggs, Ec, are laid by susceptible and infectious poultry at respective rates, αs and αi, and become consumed or sold at rate σe, or lost or hatched at rate μe.
Poultry-based income, M, is defined as:
| (4) |
where pe is the sales price of one unit of egg, ps is the unit sales price of a healthy bird, pi is the unit sales price of an unhealthy bird, and pp is the unit purchase cost of healthy poultry and πc is the purchase rate.
The economic model
In the economics literature, income generation is typically modeled with production functions [24], [28], [29], where income (i.e. the monetary value of total production) is generated from inputs, such as physical capital (i.e. infrastructure, equipment, land), human capital (i.e. education, human health, nutrition), and labor. We accordingly model household income with the following production function:
| (5) |
where represents household income from sources other than poultry. The state variables k and h represent household physical and human capital, respectively. The exponents αk and αh denote production elasticities. Typically, it is assumed that αk + αh < 1, so that the system experiences diminishing returns to all capital [24]. Through this production function, capital is transformed into income, a portion of which is reinvested into future capital, while the other proportion is consumed. As in [24] we model the dynamics of physical and human capital through the following system of differential equations:
| (6) |
| (7) |
where rk and rh are the rates of physical and human capital accumulation and δ is the rate at which capital depreciates.
The coupled epidemiological–economic model
The coupled model is obtained by combining Eqs. (1), (2), (3), (4), (5), (6), (7). The coupling occurs through income, M, which is generated from the poultry disease models and fed into the equation for total household income (Eq. (5)), and ultimately into the equations for physical and human capital (Eqs. (6), (7)). See [25], [26] for details on using such integrative approaches for modeling the dynamics of coupled economic-epidemiological systems.
Estimating the economic burden of poultry disease
The framework described by Eqs. (1), (2), (3), (4), (5), (6), (7) can be used to estimate the economic burden of poultry disease as the difference between household income with current poultry disease prevalence, , and the household income in the absence of any poultry disease, , where Ic⁎ is the infectious poultry population at equilibrium:
| (9) |
Data
Study site
The Ifanadiana District, centered at 21°18′S 47°38′E, has an average elevation of 466 m, average annual rainfall of 1700 mm, and average temperature of 24 °C [30]. The district is home to an estimated 185,000 people living in 11 Communes [31]. The western region of the district includes a large portion of the 41,600 hectare Ranomafana National Park (RNP), established in 1991 and since declared a UNESCO World Heritage site [32]. The vast majority of the peripheral zone around the RNP is agricultural land consisting largely of rice, cassava, and banana subsistence farms [30].
Survey data collection
Data used in this study were acquired from two sources (Appendix A.1). From April 1st to May 31st, 2014, the Madagascar Institute of Statistics (INSTAT) performed a two-step randomized survey of 1520 households of the Ifanadiana District [31]. Questionnaires were adapted from the 2008 Madagascar Demographic and Health Survey (DHS) [33] and the Multiple Indicator Clusters Survey (MICS) [34]. A smaller pilot survey of 80 households was conducted by INSTAT from June 9–20th, 2014 within four geographical clusters comprising the Ranomafana Commune, located within the Ifanadiana District. Questionnaires included questions taken from the Livestock Module for Multi-topic Household Surveys [35], and were used to measure production indicators among poultry-owning households (N = 48). Both studies were carried out in accordance with approved guidelines (Appendix A.2).
Analysis
Indicators of income, physical capital, and human capital
Indicators of income, physical capital and human capital were estimated at the household level using data obtained from the Ifanadiana District survey. Household income was measured as the combined annual income of all adult (≥ 15 years) household members. Physical capital was measured by the household wealth index, which was constructed for the Ifanadiana District following standard methods used in the DHS [36] (Appendix A.3). Human capital was determined at the household level using combined measures of the average nutrition and education of household members (Appendix A.4).
Statistical analysis
A descriptive analysis of household and individual-level characteristics for the population of the Ifanadiana District was performed. Multiple linear regression was used to establish the association between poultry ownership and: 1) the indicator variables of income, physical capital and human capital within district households; and 2) individual-level variables used to create the human capital index, i.e. stunting in children < 5 years, underweight in adults, and the average years of adult education (Appendix A.5). Descriptive statistics and linear regression analyses were performed using the survey procedures (SURVEYMEANS, SURVEYFREQ, SURVEYREG) available in SAS 9.3 (Cary, NC), which account for cluster sample design using Taylor linear approximation for variance estimation. Household weights were applied to provide estimates at the district and commune level.
Coupled model analysis
Due to a lack of specific disease data, we used general measures of poultry production and mortality collected from the pilot study in the Ranomafana Commune to parameterize the model where possible (Appendix A.6 and Table B.1). A sensitivity analysis using Latin Hyper-cube Sampling (LHS) and Partial Rank Correlation Coefficient (PRCC) approaches [37] was performed to evaluate how uncertainty in parameter estimates affected estimates of the economic burden of disease and to identify the parameters with the greatest impact on these disease burden estimates (Appendix A.6).
Results and discussion
Table 1 provides a general summary of household and individual-level characteristics of the Ifanadiana District. The average annual household income is 398 USD (± 24 USD), and 85% (± 0.9%) of working adults are employed in the agricultural sector. Seventy-two percent (± 2.2%) of households own poultry, with an average of twelve birds (± 0.5) per household. The majority of households own arable land (85% ± 2.4%), however ownership of other physical assets is generally low: less than half (47% ± 3.0%) of households own some form of latrine or toilet, 20% (± 3.1%) use improved roofing or flooring material in their homes, and only 15% (± 3.3%) have access to a protected water source. Indicators of nutritional status and education within the population are also low. The prevalence of underweight in adults is 29% (± 1.0%); 52% (± 1.7%) of children < 5 years are stunted; and over 80% (± 1.0%) of adults have received fewer than 5 years of formal education.
Table 1.
Household and individual-level characteristics for the population of the Ifanadiana District, Madagascar. Results are based on a 2014 population level health and economic survey of 1522 households [31].
| Variable | Weighted % (n) or mean | w SE |
|---|---|---|
| Household characteristics (N = 1522) | ||
| Annual household income | $398 USD | 24 USD |
| Physical capital index | 0.081 | 0.014 |
| Human capital indexa | 0.187 | 0.004 |
| Household members | 5.4 | 0.10 |
| Livestock ownership | ||
| Any livestock (poultry, pigs, cattle) | 75.3% (1188) | 2.2% |
| Poultry | 72.4% (1145) | 2.2% |
| Pigs | 23.2% (367) | 1.7% |
| Cattle | 13.9% (200) | 1.1% |
| Herd/flock size | ||
| Poultry | 11.80 | 0.52 |
| Pigs | 1.75 | 0.12 |
| Cattle | 3.62 | 0.20 |
| Own latrine/toilet | 47.4% (765) | 3.0% |
| Own arable land | 85.2% (1351) | 2.4% |
| Electricity | 9.3% (101) | 2.5% |
| Protected water source | 14.9% (179) | 3.3% |
| Improved housing materials | 20.3% (254) | 3.1% |
| Individual characteristics | ||
| Agriculture-related Job (N = 3698) | 84.8% (3221) | 0.9% |
| Adult education (N = 4053) | ||
| No formal school (0 years of education) | 24.3% (1041) | 0.9% |
| Some primary school (1–4 years of education) | 56.5% (2352) | 1.0% |
| Completed primary school or higher (≥ 5 years of education) | 19.2% (660) | 1.0% |
| Nutritional indicators | ||
| Underweightb adults ≥ 15 years (N = 3312) | 28.5% (679) | 1.0% |
| Stuntingc in children < 5 years (N = 1261) | 52.1% (642) | 1.7% |
For human capital index, N = 1313.
Defined as BMI < 18.5 for ages ≥ 18 years, and based on BMI-for-age cutoffs by gender for those aged 15–17, as defined by WHO [48].
Defined as a z-score < − 2 for the height-for-age ratio.
A significant positive linear relationship was found between the number of poultry owned and all three economic indicators, controlling for the number of household members and agricultural-related employment (Table 2). Based on model results, for every bird a household owns we expect the annual household income to increase by 2.50 USD (p < 0.001), the physical capital index to increase by 5.2% (p < 0.001), and the human capital index to increase by 1.0% (p = 0.009). Indicators for annual household income, physical capital, and human capital were positively correlated, demonstrating the potential impact of any one of these indicators on other key components of household economics (Figure B.1). The existence of these relationships within the population supports our premise that there are dynamical relationships between poultry production and household economic outcomes that cannot be directly measured as a loss of income alone.
Table 2.
Results of multiple linear regression models used to determine the association between the number of poultry owned with the indicator variables of annual household income (N = 1425), physical capital (N = 1502), and human capital (N = 1297), controlling for the number of household (HH) members and employment in an agricultural job (Ag Job) by at least one HH member.
| Parameters | Annual household income (log income in USD) |
Physical capital (log wealth index) |
Human capital (log human capital index) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Pr > |t| | R2 | Estimate | Pr > |t| | R2 | Estimate | Pr > |t| | R2 | |
| Intercept | 5.416 | < 0.001 | 0.072 | 1.820 | < 0.001 | 0.160 | 3.384 | < 0.001 | 0.098 |
| HH members | 0.058 | < 0.001 | 0.135 | < 0.001 | − 0.011 | 0.455 | |||
| Ag job | − 0.487 | < 0.001 | − 2.867 | < 0.001 | − 0.092 | < 0.001 | |||
| Number poultry | 0.011 | < 0.001 | 0.028 | < 0.001 | 0.010 | 0.009 | |||
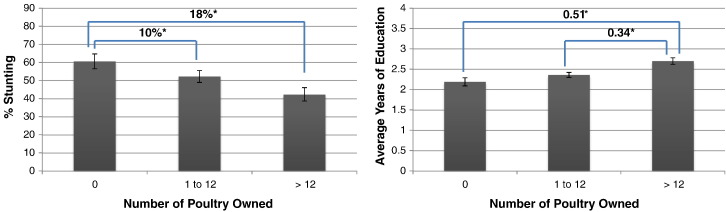
Using health and education as indicators of human capital is not unique to this study [7], [38], [39], [40], and is supported by our statistical results. Individual-level measures of stunting in children < 5 years and adult education in the Ifanadiana District were significantly associated with increasing numbers of poultry (Table B.2). Specifically, we found a lower prevalence of stunting in children < 5 years and higher education levels among adults with increasing flock size, controlling for important covariates (Fig. 3). We used nutritional indicators as our proxy for human health status, as malnutrition increases the risk of being affected by disease and may reflect an underlying chronic disease condition [41], [42]. By measuring human capital in this way, our estimate of the rate of human capital accumulation explicitly models the rate at which income is invested in education and nutrition at the household level.
Fig. 3.
Estimated prevalence of stunting in children < 5 years (N = 1261) and average years of schooling in adults ≥ 15 years (N = 4053) by number of household-owned poultry. Estimates are controlled for average values of annual household (HH) income, HH wealth index, number of HH members and employment in an agricultural job by at least one HH member. Compared to individuals living in HH without poultry, the prevalence of stunting in children < 5 years was 10% (95% CI: 1.3–18.5) lower for those living in HH that owned 1–12 birds, and 18% (95% CI: 8.3–28.1) lower for those living in HH with > 12 birds; adults ≥ 15 years living in HH with > 12 birds had 0.34 (95%: 0.13–0.55) more years of education compared to those living in HH with 1–12 birds, and 0.51 (95% CI: 0.24–0.77) more years than those living in HH without poultry. *p-value < 0.05.
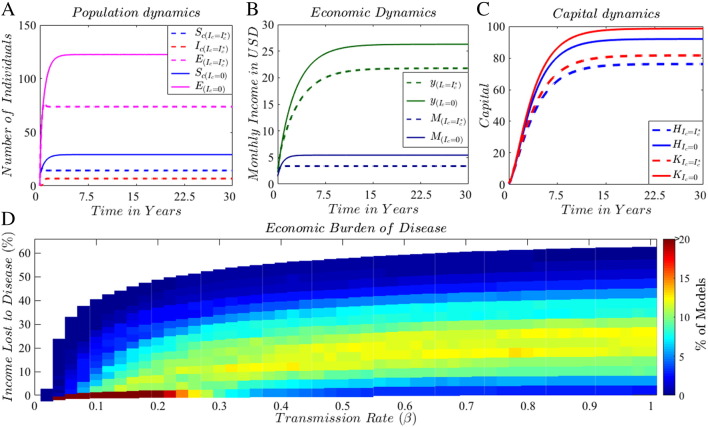
The epidemiological component of our model framework captures the monetary value of poultry and eggs sold and consumed by households, allowing it to vary based on production parameters influenced by general disease dynamics. Fig. 2A–C illustrates the epidemiological dynamics of poultry and egg production systems, and the dynamics of household economics for a period of 30 years with and without poultry disease. Each state variable relaxes to its equilibrium value signaling stability in the long term. The value provided by poultry (from sales and consumption) is incorporated into the income production function separately from other forms of income, as well as through its incorporation into the differential equations for human and physical capital. The presence of disease in the system reduces the number of live birds and eggs available for each household as a result of higher mortality rates and lower production rates of ill poultry (Fig. 2A). This reduced production results in a loss of approximately 4.5 USD per month or almost 17% of monthly household income at equilibrium (y Fig. 2B). The income loss is a result of the reduction of available income from poultry (M, Fig. 2B) and is exacerbated through feedbacks with human capital and physical capital (Fig. 2C).
Fig. 2.
Estimation of the economic burden of poultry diseases. Graphs A–C show the dynamics of the epidemiological and economic systems in the presence of disease (dashed lines) and in the absence of disease (continuous lines). Graph D presents the results of the sensitivity analysis of the economic burdens of poultry disease for a range of potential transmission rates. At each transmission rate, 1000 simulations were run, each with a different a combination of randomly selected parameters. The color code represents the % of models with a specific economic burden, where red indicates the most frequent equilibrium burden, and dark blue indicates the least frequent burden.
Equilibrium monthly household income was used to estimate the mean burden of disease as the percent of income lost to disease for a range of potential transmission rates, βc (Fig. 2D). Results from the sensitivity analysis were included in our burden estimation to account for the high uncertainty in model parameter values (Table B.1). Based on the Latin Hyper-cube sampling method, we ran 1000 simulations, each with a different combination of parameters, at transmission rates from 0 to 1. An exponential increase in the economic burden is observed at transmission rates below 0.4 while the burden approaches a fixed value at higher transmission rates. The majority of simulations with a transmission rate > 0.3 predicted a 10–25% loss of monthly income.
PRCC results suggest that in the presence of poultry disease, both economic and epidemiological parameters highly influence the outcomes of the coupled system, and hence the overall burden of poultry disease (Figure B.2). Uncertainty or variability in the production elasticities αk and αh, the physical and human capital investment rates rk and rh, the price of healthy poultry ps, the poultry harvest rate σc, the poultry fertility rate Λc, and the recovery rate γc, contributed most in generating uncertainty or variability in household income (Table B.3). Furthermore, seasonal or temporal fluctuations in disease transmission and other relevant parameters could impact the dynamics of the disease and economic systems. Indeed, diseases in many agriculture systems go through cycles of disease outbreaks, or periods of elevated incidence in the case of endemic diseases. These are not considered in our study due to the lack of longitudinal data on disease dynamics. Instead, we run the disease system with constant transmission rates for 30 years to analyze results at equilibrium for simplicity. Future iterations of this model would benefit from longitudinal data collection, including information on specific disease incidence and associated epidemiological disease parameters (i.e. rates of transmission and recovery); temporal changes in poultry purchases, sales, and consumption; and accurate ranges for capital accumulation and depreciation rates.
Other economic tools have been combined with epidemiological methods to assess the impact of livestock disease at various scales [43], [44], however, ours is the first to provide an analysis at the household level, targeting the livestock-owning population, and incorporating feedbacks of livestock productivity on both income generation and human capital accumulation. These are important model characteristics when estimating livestock disease burden on the rural poor. For example, in Ghana, an economy-wide impact of highly pathogenic avian influenza (HPAI) was assessed as minimal, with a loss of 70% of poultry production causing only a 0.47% drop in agricultural GDP [45]. This result suggests that HPAI control is not essential to the Ghanaian economy, but does not consider the potential devastating effect on the individual poultry farmer. In contrast, evaluations performed at the micro-level have found large impacts on household income and nutritional indicators. In Nigeria, where poultry production is responsible for up to 25% of household income, a loss of 75% of a flock from HPAI was estimated to reduce total household income by 8%, with income measured directly as sales of poultry and poultry products [46]. In Indonesia, assessment of the impact of HPAI, modeled as a reduction in protein intake, found increases in the prevalence of underweight, stunting, and anemia in children [47]. These studies highlight the importance of the level at which the economic analysis is performed and the measures used to calculate or quantify the burden of poultry disease.
Conclusion
By evaluating poultry disease within a framework that allows income and human capital to vary with disease dynamics, we gain new insight into the value of poultry as biological forms of capital whose health can play a role in poverty reduction though income generation and capital accumulation. Developing methods to measure the burden of livestock disease on the rural poor is important for governments, policy makers, and others in the global health and development communities who seek to include livestock disease prevention strategies as components of broader human health and development strategies. Future iterations of this model could be altered to address specific diseases in other important livestock species, and could include additional impacts on human health (beyond nutrition) through zoonotic transmission pathways [49].
Author contributions
CLR, CEB, PCW, TRG, and MHB designed the study. CLR, RHR, PCW, ACM, and RM oversaw field data collection. CLR, AG, and CNN, analyzed data. All authors wrote the paper and approved the final submission.
Acknowledgments
This study was funded in part by the Bill and Melinda Gates Foundation Phase I Grand Challenges Explorations Grant (#OPP1098855), the James and Robin Herrnstein Foundation, and the Emory Global Health Institute. MHB was funded by the James McDonnell Foundation (#220020322) and NIH grant (#K01TW008773) from the Fogarty International Center. Funders were not responsible for study design; the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the article for publication. We would like to thank the research support staff at Center ValBio, PIVOT, and the entire INSTAT team for their generous support in the field.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.onehlt.2015.10.002.
Appendix A. Supplementary data
Appendix A: Details of Study Materials and Methods.
Appendix B: Tables and Figures.
References
- 1.World Bank PovcalNet: An Online Analysis Tool for Global Poverty Monitoring. 2011. http://iresearch.worldbank.org/PovcalNet/index.htm [Online]. Available: (Accessed: 19-May-2015)
- 2.Staal S. International Livestock Research Institute; 2009. Targeting Strategic Investment in Livestock Development as a Vehicle for Rural Livelihoods. [Google Scholar]
- 3.International Fund for Agricultural Development . Rural Poverty Report 2011. IFAD; 2010. [Google Scholar]
- 4.Perry B.D. Investing in Animal Health Research to Alleviate Poverty. International Livestock Research Institute; 2002. Animal diseases and their impact on the poor; pp. 49–61. [Google Scholar]
- 5.Murray C.J.L. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 6.Perry B.D. Investing in Animal Health Research to Alleviate Poverty. International Livestock Research Institute; 2002. Animal disease impact on the poor: study results; pp. 67–77. [Google Scholar]
- 7.Bleakley H. Health, human capital, and development. Annu. Rev. Econ. 2010;2:283–310. doi: 10.1146/annurev.economics.102308.124436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamy E. Factors influencing livestock productivity. In: Sejian V., editor. Environmental Stress and Amelioration in Livestock Production. Springer-Verlag; 2012. pp. 19–45. [Google Scholar]
- 9.Perry B., Grace D. The impacts of livestock diseases and their control on growth and development processes that are pro-poor. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:2643–2655. doi: 10.1098/rstb.2009.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry B., Sones K. Poverty reduction through animal health. Science. 2007;315:333–334. doi: 10.1126/science.1138614. [DOI] [PubMed] [Google Scholar]
- 11.Bruckner G. Food and Agriculture Oragnization of the United Nations; 2002. Improved Animal Health for Poverty Reduction and Sustainable Livelihoods. [Google Scholar]
- 12.United Nations Development Programme . Madagascar Human Development Report 2014. UNDP; 2014. [Google Scholar]
- 13.Von Grebmer K. International Food Policy Research Institute; 2014. 2014 Global Hunger Index. [Google Scholar]
- 14.FAO FAOSTAT. 2015. http://faostat3.fao.org/faostat-gateway/go/to/home/E [Online]. Available: (Accessed: 19-May-2015)
- 15.Sonaiya E., Swan S. Food and Agriculture Organization of the United Nations; 2004. Small Scale Poultry Production: Technical Guide. [Google Scholar]
- 16.Randolph T.F. Invited review: role of livestock in human nutrition and health for poverty reduction in developing countries. J. Anim. Sci. 2007;85:2788–2800. doi: 10.2527/jas.2007-0467. [DOI] [PubMed] [Google Scholar]
- 17.Maminiaina O.F. Epidemiology of Newcastle disease in village poultry farming in Madagascar. Rev. Sci. Tech. 2007;26:691–700. [PubMed] [Google Scholar]
- 18.Rasamoelina Andriamanivo H. Risk factors for avian influenza and Newcastle disease in smallholder farming systems, Madagascar highlands. Prev. Vet. Med. 2012;104:114–124. doi: 10.1016/j.prevetmed.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 19.FAO, International Atomic Energy Agency . Proceedings of the joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture. 2006. Improving farmyard poultry production in Africa: interventions and their economic assessment; p. 283. [Google Scholar]
- 20.The Merck Veterinary Manual 2014. http://www.merckvetmanual.com/mvm/index.html [Online]. Available: (Accessed: 19-May-2015)
- 21.Kermack W.O., McKendrick A.G. A Contribution to the mathematical theory of epidemics. Proc. R. Soc. A. 1927;115:700–721. [Google Scholar]
- 22.Bailey N.T.J. 2nd edn. Griffin; 1975. The Mathematical Theory of Infectious Diseases and its Applications. [Google Scholar]
- 23.Keeling M.J., Rohani P. Princeton University Press; 2007. Modeling Infectious Diseases. [Google Scholar]
- 24.Mankiw N.G. A contribution to the empirics of economic-growth. Q. J. Econ. 1992;107:407–437. [Google Scholar]
- 25.Ngonghala C.N. Poverty, disease, and the ecology of complex systems. PLoS Biol. 2014;12 doi: 10.1371/journal.pbio.1001827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pluciński M.M. Clusters of poverty and disease emerge from feedbacks on an epidemiological network. J. R. Soc. Interface. 2013;10 doi: 10.1098/rsif.2012.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonds M.H. Poverty trap formed by the ecology of infectious diseases. Proc. Biol. Sci. 2010;277:1185–1192. doi: 10.1098/rspb.2009.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cobb C.W., Douglas P.H. A theory of production. Am. Econ. Rev. 1928;18:139–165. [Google Scholar]
- 29.Solow R.M. A contribution to the theory of economic growth. Q. J. Econ. 1956;70:65–94. [Google Scholar]
- 30.Bublitz D.C. Epidemiology of pathogenic enterobacteria in humans, livestock, and peridomestic rodents in rural Madagascar. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Direction de la Démographie et des Statistiques Sociales . Internal Report: Enquete de Base PIVOT, District Ifanadiana. INSTAT; 2014. [Google Scholar]
- 32.UNESCO Rainforests of the Atsinanana, World Heritage List. 2015. http://whc.unesco.org/en/list/1257/multiple=1&unique_number=1434 [Online]. Available:
- 33.Institut Nacional de la Statistique (INSTAT), ICF Macro . Enquête démographique et de santé: Madagascar 2008–2009. INSTAT; 2010. [Google Scholar]
- 34.UNICEF Multiple Indicator Cluster Surveys. 2014. http://mics.unicef.org/about [Online]. Available:
- 35.Pica-ciamarra U. World Bank; FAO, AU-IBAR, ILRI: 2011. Measuring the Contribution of Livestock to Household Livelihoods: A Livestock Module for Multi-Topic Household Surveys. [Google Scholar]
- 36.Rutstein S.O., Johnson K. ORC Macro; 2004. The DHS Wealth Index. [Google Scholar]
- 37.Marino S. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 2008;254:178–196. doi: 10.1016/j.jtbi.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grossman M. Handbook of, Health Economics. 2000. The human capital model; pp. 347–408. (1 pp.) [Google Scholar]
- 39.Lutz W., KC S. Global human capital: integrating education and population. Science. 2011;333:587–592. doi: 10.1126/science.1206964. [DOI] [PubMed] [Google Scholar]
- 40.Lanzi D. Capabilities, human capital and education. J. Socio. Econ. 2007;36:424–435. [Google Scholar]
- 41.Checkley W. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int. J. Epidemiol. 2008;37:816–830. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dewey K.G., Begum K. Long-term consequences of stunting in early life. Matern. Child Nutr. 2011;7:5–18. doi: 10.1111/j.1740-8709.2011.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rich K.M. Enhancing economic models for the analysis of animal disease. Rev. Sci. Tech. 2005;24:847–856. [PubMed] [Google Scholar]
- 44.Moll H. a J. Costs and benefits of livestock systems and the role of market and nonmarket relationships. Agric. Econ. 2005;32:181–193. [Google Scholar]
- 45.Diao X. Economywide impact of avian influenza in Ghana — a dynamic computable general equilibrium (DCGE) model analysis. Int. J. Livest. Prod. 2011;2:145–158. [Google Scholar]
- 46.Mensah-bonsu A. IFPRI/ILRI; 2009. Investigating the Role of Poultry and the Impact of HPAI on Livelihoods in Nigeria. [Google Scholar]
- 47.Iannotti L. IFPRI/ILRI; 2008. Animal Source Foods and Nutrition of Young Children: An Ex Ante Analysis of Impact of HPAI on Nutrition in Indonesia. [Google Scholar]
- 48.World Health Organization The WHO Child Growth Standards. http://www.who.int/childgrowth/standards/en/ [Online]. Available: (Accessed: 05-Jan-2015)
- 49.Rist C.L. The burden of livestock parasites on the poor. Trends Parasitol. 2015;31:527–530. doi: 10.1016/j.pt.2015.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A: Details of Study Materials and Methods.
Appendix B: Tables and Figures.