Abstract
Background
Clinical efficacy of balloon pulmonary angioplasty (BPA) to the patients with non-operable chronic thromboembolic pulmonary hypertension (CTEPH) for improving pulmonary hemodynamics and exercise tolerance has been reported in these several years. However, reperfusion pulmonary injury (RPI) remains to be a major complication of BPA to overcome. This study elucidated the local predictor of RPI.
Methods
Twenty-eight consecutive patients with non-operable CTEPH underwent BPA for lesions in the segmental or sub-segmental vessels. Pre- and post-BPA pulmonary arterial pressures at proximal (Pp) and distal (Pd) to the stenosis were measured by a 0.014-in. pressure wire. Positive or negative RPI was evaluated by chest computed tomography in each re-perfused segment separately 4 h after BPA.
Results
Pressure measurements pre- and post-BPA were obtained from 110 lesions, where Pd and pressure ratio (Pd/Pp) increased after BPA in all lesions. Among them, RPI was observed in 49 lesions (44.5%). In the RPI-positive lesions, post-BPA Pd and post-BPA Pd/Pp were higher compared with the RPI-negative lesions. Multivariate logistic analysis revealed that the post-BPA Pd was independently associated with RPI incidence. Receiver operating characteristic curve analysis demonstrated the best cut-off value of 19.5 mm Hg for post-BPA Pd to predict RPI.
Conclusions
High reperfusion pressure after BPA could be a predictor of RPI. Monitoring local pressure during BPA procedure may have a potential to reduce the incidence of RPI.
Abbreviations: BPA, balloon pulmonary angioplasty; CI, cardiac index; CO, cardiac output; CT, computed tomography; CTEPH, chronic thromboembolic pulmonary hypertension; IVUS, intravascular ultrasound; MLD, minimal lumen diameter; NIPPV, non-invasive positive pressure ventilation; PAG, pulmonary angiography; PAP, pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; PEA, pulmonary endarterectomy; Pd, mean pulmonary arterial pressure distal to the stenosis; Pp, mean pulmonary arterial pressure proximal to the stenosis; PVR, pulmonary vascular resistance; ROC, receiver-operating characteristic; RPI, reperfusion pulmonary injury; 95% CI, 95% confidence interval
Keywords: Chronic thromboembolic pulmonary hypertension, Balloon pulmonary angioplasty, Reperfusion pulmonary injury, Complication, Predictor
1. Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by the obstruction of the pulmonary arteries with organized thrombus and intraluminal fibrous tissue, which leads to elevated pulmonary vascular resistance (PVR), severe pulmonary hypertension, and right heart failure [1], [2], [3], [4], [5]. In addition, if appropriate medical intervention is not provided, the prognosis of patients with CTEPH is extremely poor, with a reported only 10% five-year survival rate in patients with a mean pulmonary arterial pressure (PAP) of > 50 mm Hg [6]. In this context, pulmonary endarterectomy (PEA) is a gold standard of treatment that can dramatically reduce PAP and improve the prognosis of patients with CTEPH [7], [8], [9]. However, PEA for CTEPH with peripherally located organized thrombi has been reported to be less effective for the improvement in pulmonary hemodynamics and a higher perioperative mortality rate compared with that for CTEPH with proximal thrombi [10], [11]. Thus, it has been reported that up to 40% of patients with CTEPH were considered non-operable, due to peripherally located organized thrombus or other comorbidities [7]. Therefore, a safer and more effective treatment for non-operable CTEPH is urgently needed.
The first report of balloon pulmonary angioplasty (BPA) for a patient with CTEPH was published in 1988 [12]. In 2001, Feinstein et al. reported marked improvements in the pulmonary hemodynamics and exercise tolerance in the series of BPA to 18 patients with CTEPH [13]. Recent several studies reported the refined BPA procedure and agitated the interest of many interventionists due to its clinical efficacy [14], [15], [16], [17], [18]. In addition, we have reported that BPA could achieve comparable reduction in mean PAP with PEA [15]. At the same time, reperfusion pulmonary injury (RPI), a major complication of BPA is yet to be resolved. Previous reports have shown that RPI after BPA occurred in 53–64% of cases [13], [14], [15], [16]. RPI includes hemosputum, worsening of hypoxemia, or increased density of chest computed tomography (CT) in the dilated segment regardless of symptoms [14]. RPI is thought to be caused by pulmonary edema and/or pulmonary bleeding. Because of the difficulty to differentiate between these etiologies by only chest X-ray or CT, it is called reperfusion pulmonary “injury”. Although it has been hypothesized that exposure to high reperfusion pressure after balloon dilation may lead to RPI [14], no published reports have monitored the local pressure around the stenosis before and after BPA. In the present study, we analyzed the effect of BPA on the local pressure hemodynamics, and investigated whether the local pressure might be a predictor of RPI.
2. Methods
2.1. Patient enrollment
Between October 2012 and April 2015, 48 patients were diagnosed as CTEPH at Kobe University Hospital in Japan. The diagnosis of CTEPH was established according to the published clinical guidelines [19]. All patients had a more than 25 mm Hg of mean PAP and less than 15 mm Hg of pulmonary capillary wedge pressure (PCWP), and were definitively diagnosed as CTEPH after identification of multiple stenosis and obstruction of the bilateral pulmonary arteries via pulmonary angiography (PAG). The distribution of the thromboembolic lesions and intimal thickening of the pulmonary artery was evaluated using contrast-enhanced chest CT and PAG, and the results were reviewed by experienced cardiologists, cardiovascular surgeons, and radiologists. The assessment of operability was conducted by cardiologists and cardiovascular surgeons according to the criteria for PEA [20], which consider the distribution of stenosis, obstruction, and intimal thickening, age, and comorbidities. Based on these assessments, 19 patients were judged to be operable and underwent PEA, while 29 patients were judged to be non-operable and therefore suitable for BPA. Only data from BPA were included in this analysis.
The design of the present study was approved by the Ethics Committee of Kobe University, and conformed to the tenets of the Declaration of Helsinki. All enrolled patients provided their informed written consent.
2.2. Hemodynamic and exercise tolerance analysis
We performed right heart catheterization and measured the PAP, right atrial pressure, and PCWP using a flow-directed thermodilution catheter immediately before the first BPA procedure in each patient. PVR and cardiac output (CO) were calculated using the Fick method. In addition, 6-min walk distance and the World Health Organization functional class were obtained 1 or 2 days before the first BPA procedure.
2.3. Balloon pulmonary angioplasty and pulmonary arterial pressure analysis
In each BPA session, we placed a 9-Fr sheath (XEMEX Introducer Set, Zeon Medical, Tokyo, Japan) into the vein (typically into the femoral vein or the internal jugular), and then brought a 70 cm or 90 cm 6-Fr Britetip sheath introducer (BRITE TIP® Interventional Sheath Introducer; Cordis/Johnson & Johnson, Bridgewater, NJ, USA) to the main pulmonary artery via the 9-Fr sheath, using a Radifocus 0.035-in. guide wire (Terumo, Tokyo, Japan) and a 5-Fr pigtail catheter (Goodtec catheter, Goodman, Aichi, Japan). When the sheath was inserted, an initial dose of heparin (2000–5000 U) was administered, and 1000 U of heparin was administered every hour to maintain 200–300 s of intra-operative activated clotting time. We then selected a segmental or sub-segmental pulmonary artery using a 6-Fr guide catheter (Autobahn; Multipurpose, or Judkins right 4.0; NIPRO Corporation, Osaka, Japan) via the long sheath, and performed angiography to identify any stenoses or occlusions. A 0.014-in. guide wire (Cruise; Asahi Intecc, Aichi, Japan or Chevalier 14 Floppy, FMD, Saitama, Japan) was passed through the target lesions under the support of a microcatheter (Prominent Raptor, Tokai Medical Products, Aichi, Japan). Using intravascular ultrasound (IVUS) (Eagle Eye® Platinum; Volcano Corp, Rancho Cordova, CA, USA), we measured the vessel diameter at the site of the thrombi [14], and the lesions were dilated to an appropriate size using balloon catheters (2.0–9.0 mm, depending on the diameter of the vessel). To prevent pulmonary arterial injury, the maximum balloon size was slightly smaller than the actual vessel diameter, and did not exceed 90% of the actual vessel diameter [14]. The procedures were repeated on other segments after one week interval during the same admission until the mean PAP had normalized. RPI symptom comprises hemosputum, desaturation, intratracheal intubation, hemodynamic compromise which needs percutaneous cardiopulmonary support and peri-procedural mortality. Procedure time for one BPA session was limited up to 2 h for safety assurance. If necessary, we administrated oxygen or non-invasive positive pressure ventilation (NIPPV) to the patient.
2.4. Angiographic analysis
Pulmonary angiographic data before and immediately after BPA were analyzed using edge-detection techniques (QCA-CMS5.1, Medis Imaging Systems, Leiden, The Netherlands). The quantitative angiographic parameters included: (1) the minimal lumen diameter (MLD), (2) the reference vessel diameter, which was measured at an apparently normal area proximal to the target stenosis, (3) the vessel expansion ratio, which was defined as the MLD after balloon expansion divided by the MLD before the wire passed through the lesion, and (4) the balloon-vessel ratio, which was defined as the balloon diameter divided by reference vessel diameter before the BPA.
2.5. Pulmonary arterial pressure assessment
The local PAP was measured at proximal and distal to the stenosis. Exclusion criteria for pressure assessment included severe bending lesions, uncrossable lesions, lesions without pressure gradient, and lesions with short peripheral margin. After we passed a 0.014-in. guide wire through the target lesion, the guide wire was changed to a pressure wire (Aeris, Saint Jude Medical Systems, Uppsala, Sweden). After dilation of the stenosis by balloon catheter, we performed additional assessments of the mean proximal and distal PAPs. Pp was defined as the mean PAP proximal to the stenosis measured at the tip of guiding catheter and Pd as the mean PAP distal to the stenosis measured using the pressure wire sensor. The pressure ratio was calculated as Pd divided by Pp, and the absolute increase in Pd as the post-BPA Pd was subtracted by the pre-BPA Pd.
2.6. Computed tomography analysis
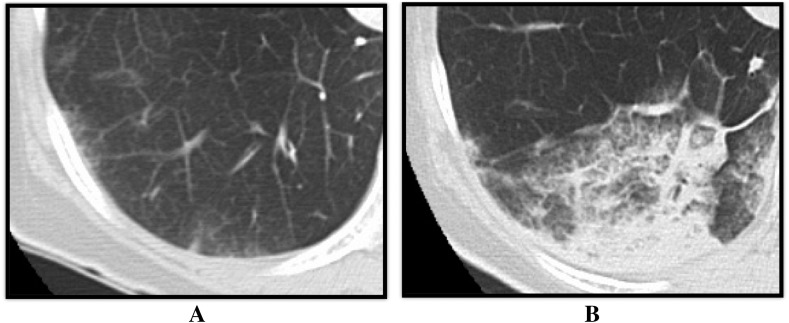
4 h after each BPA session, we took chest CT to evaluate incidence and location of RPI. RPI was defined when there was any increase in density (regardless of the size) observed on CT image in the corresponding pulmonary segment of the dilated pulmonary artery (Fig. 1). All CT images were analyzed by two independent observers who were blinded to all clinical information. When the two observers' decisions were inconsistent, a third observer analyzed the images for making the final decision.
Fig. 1.
Representative case of computed tomography imaging of the reperfusion pulmonary injury. (A) Chest CT image before the BPA. (B) Chest CT image 4 h after the BPA. During the BPA, the right pulmonary artery A10 was dilated, and the density of S10 increased during chest CT imaging as the reperfusion pulmonary injury occurred in S10. BPA: balloon pulmonary angioplasty, CT: computed tomography.
2.7. Statistics analysis
All data were presented as mean ± standard deviation or odds ratios and 95% confidence intervals (95% CI), unless otherwise stated. Analyses were performed using SPSS statistical software (v. 17.0, SPSS, Chicago, Illinois, USA), and two-sided p value of < 0.05 was considered statistically significant. Student's t test was used for all normally distributed variables, and the Mann–Whitney U test or Wilcoxon matched-pairs signed-rank test were used for non-normally distributed variables, as appropriate. Comparisons of proportions were performed using the chi-square test or Fisher's exact test. Inter- and intra-observer reliability was assessed using the kappa test for chest CT diagnosis of RPI. Univariate logistic regression analysis was used to examine the relationship between the incidence of RPI and the predictive variables. Multivariate logistic regression analysis was used to examine the independent effect of each variable on the incidence of RPI. The optimum predictive threshold for RPI was evaluated using a receiver-operating characteristic (ROC) curve, and the Youden index was used to define the optimum cut-off value on the ROC curve.
3. Results
3.1. Baseline patient characteristics
Among the 29 patients who were deemed unsuitable for PEA and were enrolled for BPA, one in-hospital death resulted from infection via the central venous catheter that was used to administer epoprostenol before the BPA. Therefore, 28 patients underwent BPA, and their baseline characteristics are shown in Table 1.
Table 1.
Baseline patient characteristics.
| Variables | n = 28 |
|---|---|
| Female, n (%) | 19 (67.9%) |
| Age, years | 64.8 ± 12.1 |
| WHO functional class, I/II/III/IV | 0/8/16/4 |
| 6MWD, m | 303.0 ± 91.9 |
| Heart rate, beat/min | 72.0 ± 8.4 |
| Systolic PAP, mm Hg | 61.1 ± 18.8 |
| Diastolic PAP, mm Hg | 19.7 ± 5.8 |
| Mean PAP, mm Hg | 34.2 ± 10.4 |
| PCWP, mm Hg | 7.9 ± 2.4 |
| RAP, mm Hg | 4.8 ± 2.3 |
| CO, L/min | 4.1 ± 1.3 |
| PVR, dyne/s/cm5 | 574.4 ± 316.7 |
| BNP, pg/ml | 160.4 ± 233.4 |
| Warfarin, n (%) | 28 (100) |
| Administration of pulmonary vasodilators, n (%) | 20 (71.4) |
| PGI2, n (%) | 5 (17.9) |
| ERAs, n (%) | 16 (57.1) |
| PDE-5 inhibitors, n (%) | 6 (21.4) |
| sGC | 1 (3.6%) |
Values are mean ± SD or percent. BNP, brain natriuretic peptide; CO, cardiac output; ERA, endothelin receptor antagonist; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PDE-5, phosphodiesterase type 5; PGI2, prostaglandin analog; PVR, pulmonary vascular resistance; RAP, right atrial pressure; sGC, soluble guanylate cyclase activator; WHO, World Health Organization; 6MWT, 6 min walk test.
3.2. BPA results
A total of 84 BPA sessions were performed for the 28 patients (average, 3.0 ± 1.4 sessions per patient), and their hemodynamics and exercise tolerance significantly improved after the BPA procedures (Table 2). Regarding periprocedural complications, one or more RPI symptoms occurred in 56 sessions (66.7%). Hemosputum was observed in 14 sessions (16.7%), and desaturation requiring NIPPV was observed in 13 sessions (15.4%). None of the patients required intratracheal intubation or percutaneous cardiopulmonary support, and no one died (Table 3).
Table 2.
Hemodynamics and exercise tolerance before and after BPA.
| Variables | Before BPA | After BPA | p value |
|---|---|---|---|
| WHO Fc | 0/8/16/4 | 16/11/1/0 | < 0.01 |
| 6MWD, m | 303.0 ± 91.9 | 394.7 ± 124.2 | < 0.01 |
| HR, beat/min | 72.0 ± 8.4 | 69.1 ± 10.3 | 0.10 |
| sPAP, mm Hg | 61.1 ± 18.8 | 33.1 ± 8.7 | < 0.01 |
| dPAP, mm Hg | 19.7 ± 5.8 | 10.5 ± 4.3 | < 0.01 |
| mPAP, mm Hg | 34.2 ± 10.4 | 19.2 ± 5.7 | < 0.01 |
| PCWP, mm Hg | 7.9 ± 2.4 | 6.4 ± 2.3 | 0.02 |
| RAP, mm Hg | 4.8 ± 2.3 | 2.9 ± 2.1 | < 0.01 |
| CO, L/min | 4.1 ± 1.3 | 4.5 ± 1.6 | 0.14 |
| PVR, dyne/s/cm5 | 574.4 ± 316.7 | 258.3 ± 170.7 | < 0.01 |
| BNP, pg/ml | 160.4 ± 233.3 | 26.1 ± 30.5 | < 0.01 |
Values are mean ± SD. BNP, brain natriuretic peptide; CO, cardiac output; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; WHO, World Health Organization; 6MWT, 6 min walk test.
Table 3.
Complications related to BPA.
| Variables | n = 84 |
|---|---|
| Wire perforation, n (%) | 5 (6.0) |
| RPI, n (%) | 56 (66.7) |
| Right heart failure required for PCPS, n (%) | 0 (0) |
| Desaturation required for intratracheal intubation, n (%) | 0 (0) |
| Desaturation required for NIPPV, n (%) | 13 (15.4) |
| Hemosputum, n (%) | 14 (16.7) |
| Only CT scan, n (%) | 39 (46.4) |
| Death, n (%) | 0 (0) |
| None, n (%) | 28 (33.3) |
Data indicates the number of sessions. The number of RPI indicates the number of session one or more RPI symptoms occurred. NIPPV, non-invasive positive pressure ventilation; only CT scan, increased density of the dilated segment detected by a chest CT scan without any symptoms; PCPS, percutaneous cardiopulmonary support; RPI, reperfusion pulmonary injury.
3.3. Lesions, procedural characteristics, and angiographic analysis
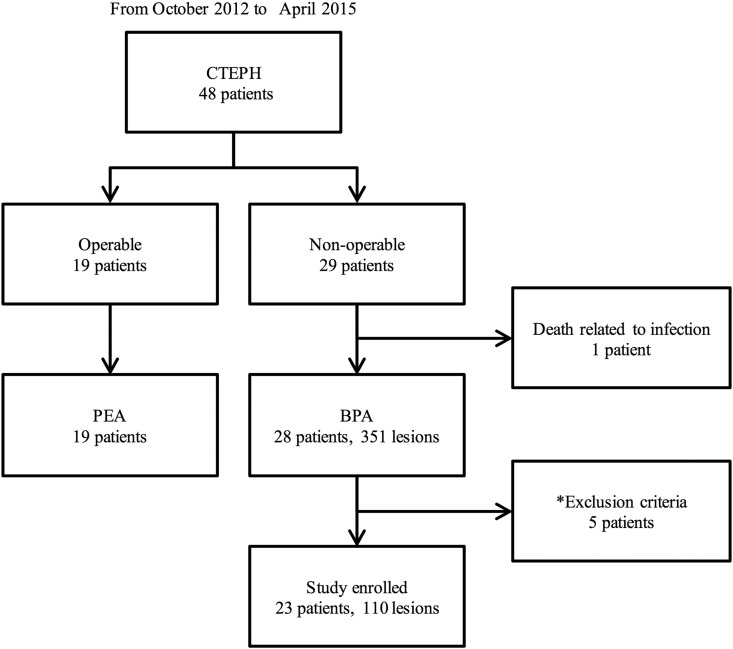
Based on the exclusion criteria, we excluded 5 patients from the local pulmonary artery pressure measurements. Therefore, Pp and Pd data (with chest CT data) were obtained from 110 lesions in 23 patients (Fig. 2). The results of the angiographic analysis are shown in Table 4. MLD (from 2.22 ± 1.18 to 3.39 ± 1.30 mm, p < 0.01) and reference vessel diameter (from 5.50 ± 1.86 mm to 6.10 ± 1.75, p < 0.01) were significantly increased after balloon dilatation as showing 1.94 ± 1.28 of a vessel expansion ratio.
Fig. 2.
Study flow chart. BPA, balloon pulmonary angioplasty; CTEPH, chronic thromboembolic pulmonary hypertension; PEA, pulmonary endarterectomy. *: Exclusion criteria are mentioned in the main text.
Table 4.
Lesion and angiographic analysis.
| Variables | n = 77 |
|---|---|
| Side, right/left, n (%) | 72 (64.9)/38 (34.2) |
| Lobe, superior/middle/inferior, n (%) | 22 (19.8)/2 (1.8)/86 (77.5) |
| Pre-BPA reference vessel diameter, mm | 5.50 ± 1.86 |
| Post-BPA reference vessel diameter, mm | 6.10 ± 1.75⁎⁎ |
| Pre-BPA MLD, mm | 2.22 ± 1.18 |
| Post-BPA MLD, mm | 3.39 ± 1.30⁎⁎ |
| Vessel expansion ratio | 1.94 ± 1.28 |
| Final balloon diameter, mm | 5.26 ± 1.44 |
| Balloon-vessel ratio | 0.89 ± 0.21 |
Values are mean ± SD or percent. BPA, balloon pulmonary angioplasty; MLD, minimal lumen diameter.
p value was < 0.01 compared with before BPA.
3.4. Pulmonary arterial pressure analysis
Before the BPA, a pressure gradient was observed across the pulmonary artery stenosis (mean Pp: 26.5 ± 7.5 mm Hg; mean Pd: 11.3 ± 7.2 mm Hg; p = 0.027, mean pressure gradient: 15.4 ± 8.8 mm Hg). After the BPA, the Pp slightly decreased (from 26.5 ± 7.5 mm Hg to 25.7 ± 7.5 mm Hg, p < 0.01), the post-BPA Pd (from 11.3 ± 7.2 mm Hg to 19.9 ± 7.9 mm Hg, p < 0.01) and the post-BPA pressure ratio significantly increased (from 0.44 ± 0.25 to 0.76 ± 0.19, p < 0.01) (Table 5).
Table 5.
Local pulmonary artery pressure analysis.
| Variables (n = 77) | Pre-BPA | Post-BPA | p value |
|---|---|---|---|
| Pp, mm Hg | 26.5 ± 7.5 | 25.7 ± 7.5 | 0.008 |
| Pd, mm Hg | 11.3 ± 7.2 | 19.9 ± 7.9 | < 0.01 |
| Pressure ratio | 0.44 ± 0.25 | 0.76 ± 0.19 | < 0.01 |
Values are mean ± SD. BPA, balloon pulmonary angioplasty; Pd, mean pulmonary arterial pressure distal to the stenosis measured by the pressure wire sensor; Pp, mean pulmonary arterial pressure proximal to the stenosis measured by the guiding catheter.
3.5. Computed tomography analysis
Based on the CT analysis, RPI under the dilated lesion were observed in 49 (44.5%) lesions. Inter- and intra-observer reliability testing (tested at > 30 days apart) regarding the diagnosis of RPI exhibited good agreement (kappa = 0.947 and 0.947, respectively).
3.6. RPI analysis
In the analysis based on the dilated lesions, a comparison of the RPI-positive and RPI-negative groups is shown in Table 6, although no significant differences were observed in location of targeted pulmonary artery, pre-BPA Pd, pre-BPA pressure ratio, the vessel expansion ratio, or balloon-vessel ratio between the two groups. However, the RPI-positive group had significantly higher pre-BPA Pp (30.3 ± 6.1 vs. 23.4 ± 7.4 mm Hg, p < 0.01), post-BPA Pp (29.5 ± 6.2 vs. 22.7 ± 7.2 mm Hg, p < 0.01), post-BPA Pd (24.3 ± 6.4 vs. 16.4 ± 7.2 mm Hg, p < 0.01), post-BPA pressure ratio (0.83 ± 0.14 vs. 0.71 ± 0.21, p < 0.01), and absolute Pd increase (12.5 ± 8.4 vs. 5.5 ± 5.0 mm Hg, p < 0.01), compared with the RPI-negative group. Multivariate analysis revealed that post-BPA Pd was strongly related to the incidence of RPI (odds ratio: 1.139, 95% CI: 1.053–1.231, p = 0.001) (Table 7). The area under ROC curve of post-BPA Pd for predicting RPI was 0.814, with an optimum cut-off value of 19.5 mm Hg (sensitivity: 79.6%, specificity: 75.4%) (Fig. 3).
Table 6.
Comparison between RPI-positive group and RPI-negative group.
| Variables | RPI-positive group (n = 49) |
RPI-negative group (n = 61) |
p value |
|---|---|---|---|
| Side, right/left, n (%) | 28 (57.1)/21 (42.9) | 44 (72.1)/17 (27.9) | 0.10 |
| Lobe, superior/middle/inferior, n (%) | 11 (22.4)/0 (0)/38 (77.6) | 11 (18.0)/2 (3.3)/48 (78.7) | 0.51 |
| Vessel expansion ratio | 1.64 ± 0.45 | 2.45 ± 6.21 | 0.25 |
| Balloon-vessel ratio | 0.89 ± 0.19 | 0.89 ± 0.22 | 0.99 |
| Pre-BPA Pp, mm Hg | 30.3 ± 6.1 | 23.4 ± 7.4 | < 0.01 |
| Pre-BPA Pd, mm Hg | 11.8 ± 7.6 | 10.9 ± 6.8 | 0.53 |
| Pre-BPA pressure ratio | 0.40 ± 0 .27 | 0.46 ± 0.23 | 0.23 |
| Post-BPA Pp, mm Hg | 29.5 ± 6.2 | 22.7 ± 7.2 | < 0.01 |
| Post-BPA Pd, mm Hg | 24.3 ± 6.4 | 16.4 ± 7.2 | < 0.01 |
| Post-BPA pressure ratio | 0.83 ± 0.14 | 0.71 ± 0.21 | < 0.01 |
| Pd increase, mm Hg | 12.5 ± 8.4 | 5.5 ± 5.0 | < 0.01 |
Values are mean ± SD or percent. BPA, balloon pulmonary angioplasty; Pd, mean pulmonary arterial pressure distal to the stenosis measured by the pressure wire sensor; Pp, mean pulmonary arterial pressure proximal to the stenosis measured by the guiding catheter.
Table 7.
Multivariate analysis to examine the independent effect of each variable on the occurrence of RPI.
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | |
| Pre-BPA Pp | 1.159 | 1.083–1.2040 | < 0.001 | |||
| Post-BPA Pp | 1.156 | 1.082–1.235 | < 0.001 | |||
| Post-BPA Pd | 1.191 | 1.106–1.284 | < 0.001 | 1.139 | 1.053–1.231 | 0.001 |
| Pd increase | 1.185 | 1.097–1.280 | < 0.001 | 1.126 | 1.033–1.229 | 0.007 |
| Pre-BPA MLD | 0.689 | 0.483–0.982 | 0.040 | |||
BPA, balloon pulmonary angioplasty; MLA, minimal lumen diameter; Pd, mean pulmonary arterial pressure distal to the stenosis measured by the pressure wire sensor; Pp, mean pulmonary arterial pressure proximal to the stenosis measured by the guiding catheter.
Fig. 3.
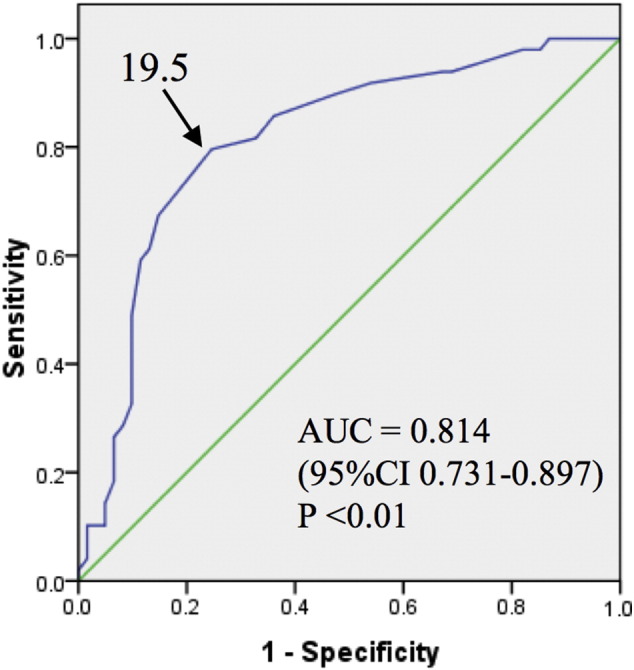
Receiver operating characteristic curve of post-BPA Pd for predicting RPI.
The arrow indicates the optimal cut-off value for predicting reperfusion pulmonary injury after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. AUC: area under the curve, CI: confidence interval.
4. Discussion
As recently reported, BPA has the potential to improve hemodynamics and exercise tolerance in patients with inoperable CTEPH. However, no consensus has been established in technical aspects of BPA procedure, so that various clinical efficacies on hemodynamics and prognosis derived from individual optimization have been reported [13], [14], [15], [16], [17], [18]. So, there is an urgent need for establishing the index which guides us to safer and more effective BPA. For example, although Feinstein et al. achieved a 21% decrease in mean PAP, final PAP was still so far above the normal range that it would raise relatively high incidence of intratracheal intubation (17%), and periprocedural mortality (5.6%) [13]. In contrast, Mizoguchi et al. achieved a 47% decrease in the mean PAP through routine use of IVUS, with lower complications of severe RPI (6%) and periprocedural mortality (1.5%) [14]. Therefore, IVUS can potentially reduce operative complications by facilitating to select optimal balloon size. In the present study, we also used IVUS in a similar way used by Mizoguchi et al., and we achieved a 44% decrease in the mean PAP without any severe complications like intratracheal intubation or resulted in periprocedural death.
At the same time, despite the use of IVUS, Mizoguchi et al. reported RPI in 60% of their procedures and we also observed RPI in 66.7% of our procedures. Now therefore, the high incidence of RPI still remains as an issue for BPA. These findings indicate that routine use of IVUS does not sufficiently reduce the incidence of RPI. Several groups recommend the limitation of the number of treatment lesions for the prevention of severe desaturation caused by RPI, whereas this strategy would likely reduce the overall efficacy of BPA. Therefore other monitoring methodologies are desired for further improvement of safety and efficacy.
It has been hypothesized that exposure of high reperfusion pressure to the reperfused area after balloon dilation may lead to RPI [14], [18], and Feinstein et al. have reported that RPI was frequently in the patients with > 35 mm Hg of mean PAP before BPA [13]. However, no previous study has assessed the relation between the local pressure and safety before and after BPA, and this study is the first documentation which clarifies the association between distal reperfusion pressure and the incidence of RPI (Fig. 4). In addition, we identified an optimal cut-off value (19.5 mm Hg) as a novel monitoring parameter to assure stable BPA result. We would like to suggest that combined strategy with IVUS for selecting an appropriate balloon size and a pressure wire for monitoring distal perfusion pressure would facilitate safe and effective BPA procedures, which will allow more aggressive BPA by increasing the target number of dilations in one session.
Fig. 4.
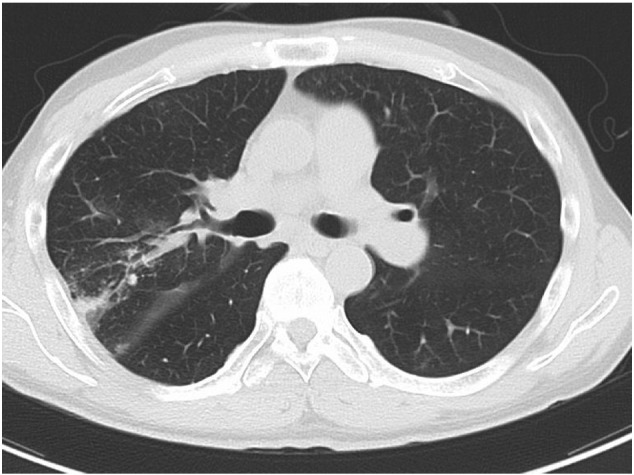
Both pulmonary artery right A2 and A3 were dilated by BPA. RPI was observed in pulmonary segment 2 where Pd changed from 10 to 28 mm Hg, but not observed in pulmonary segment 3 where Pd changed from 8 to 19 mmHg. BPA, balloon pulmonary angioplasty; Pd, mean pulmonary arterial distal to the stenosis; Pp, mean pulmonary arterial proximal to the stenosis; RPI, reperfusion pulmonary injury.
4.1. Study limitations
There were several limitations in the present study. First, female patients were noticeably more likely to undergo BPA (67.9% in this study). Although it has been reported that there is no sex-related bias for the incidence of CTEPH, it has also been reported that 72.2% of Japanese patients with CTEPH are women (per the Respiratory Failure Research Group from the Ministry of Health, Labor and Welfare, Japan [21]). Therefore, we believe that the predominance of women was not related to any selection bias in the present study. Second, this was a single-center study with a small sample size, therefore a multi-center trial with a larger number of patients is needed to confirm our findings. Third, although post-BPA RPI was observed in 66.7% of the procedures, modest desaturation requiring NIPPV occurred in 15.4% of the sessions even if there is no need of intratracheal intubation or extracorporeal lung assistance. The relationship between CT-diagnosed RPI and the clinical symptoms of hypoxia was not analyzed, and further studies are needed to clarify the degree of RPI that can cause hypoxia. Finally, the long-term efficacy of BPA was not evaluated in this study, and it needs to be further investigated.
5. Conclusion
Overloaded high reperfusion pressure after BPA would be an independent predictor for RPI. Monitoring the local pressure changes during BPA may help reduce the incidence of RPI.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgment
There is no grant support for this manuscript.
References
- 1.Piazza G., Goldhaber S.Z. Chronic thromboembolic pulmonary hypertension. N. Engl. J. Med. 2011;364:351–360. doi: 10.1056/NEJMra0910203. [DOI] [PubMed] [Google Scholar]
- 2.Fedullo P., Kerr K.M., Kim N.H., Auger W.R. Chronic thromboembolic pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2011;183:1605–1613. doi: 10.1164/rccm.201011-1854CI. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M. Pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: pathophysiology. Eur. Respir. Rev. 2010;19:59–63. doi: 10.1183/09059180.00007309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonderman D., Skoro-Sajer N., Jakowitsch J., Adlbrecht C., Dunkler D., Taghavi S. Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation. 2007;115:2153–2158. doi: 10.1161/CIRCULATIONAHA.106.661041. [DOI] [PubMed] [Google Scholar]
- 5.Hoeper M.M., Mayer E., Simonneau G., Rubin L.J. Chronic thromboembolic pulmonary hypertension. Circulation. 2006;113:2011–2020. doi: 10.1161/CIRCULATIONAHA.105.602565. [DOI] [PubMed] [Google Scholar]
- 6.Riedel M., Stanek V., Widimsky J., Prerovsky I. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest. 1982;81:151–158. doi: 10.1378/chest.81.2.151. [DOI] [PubMed] [Google Scholar]
- 7.Pepke-Zaba J., Delcroix M., Lang I., Mayer E., Jansa P., Ambroz D. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124:1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 8.Mayer E., Jenkins D., Lindner J., D'Armini A., Kloek J., Meyns B. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J. Thorac. Cardiovasc. Surg. 2011;141:702–710. doi: 10.1016/j.jtcvs.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Jamieson S.W., Kapelanski D.P., Sakakibara N., Manecke G.R., Thistlethwaite P.A., Kerr K.M. Pulmonary endarterectomy: experience and lessons learned in 1500 cases. Ann. Thorac. Surg. 2003;76:1457–1462. doi: 10.1016/s0003-4975(03)00828-2. (discussion 62-4) [DOI] [PubMed] [Google Scholar]
- 10.Thistlethwaite P.A., Madani M., Jamieson S.W. Outcomes of pulmonary endarterectomy surgery. Semin. Thorac. Cardiovasc. Surg. 2006;18:257–264. doi: 10.1053/j.semtcvs.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Jais X., D'Armini A.M., Jansa P., Torbicki A., Delcroix M., Ghofrani H.A. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J. Am. Coll. Cardiol. 2008;52:2127–2134. doi: 10.1016/j.jacc.2008.08.059. [DOI] [PubMed] [Google Scholar]
- 12.Voorburg J.A., Cats V.M., Buis B., Bruschke A.V. Balloon angioplasty in the treatment of pulmonary hypertension caused by pulmonary embolism. Chest. 1988;94:1249–1253. doi: 10.1378/chest.94.6.1249. [DOI] [PubMed] [Google Scholar]
- 13.Feinstein J.A., Goldhaber S.Z., Lock J.E., Ferndandes S.M., Landzberg M.J. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001;103:10–13. doi: 10.1161/01.cir.103.1.10. [DOI] [PubMed] [Google Scholar]
- 14.Mizoguchi H., Ogawa A., Munemasa M., Mikouchi H., Ito H., Matsubara H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ. Cardiovasc. Interv. 2012;5:748–755. doi: 10.1161/CIRCINTERVENTIONS.112.971077. [DOI] [PubMed] [Google Scholar]
- 15.Taniguchi Y., Miyagawa K., Nakayama K., Kinutani H., Shinke T., Okada K. Balloon pulmonary angioplasty: an additional treatment option to improve the prognosis of patients with chronic thromboembolic pulmonary hypertension. EuroIntervention. 2014;10:518–525. doi: 10.4244/EIJV10I4A89. [DOI] [PubMed] [Google Scholar]
- 16.Kataoka M., Inami T., Hayashida K., Shimura N., Ishiguro H., Abe T. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ. Cardiovasc. Interv. 2012;5:756–762. doi: 10.1161/CIRCINTERVENTIONS.112.971390. [DOI] [PubMed] [Google Scholar]
- 17.Sugimura K., Fukumoto Y., Satoh K., Nochioka K., Miura Y., Aoki T. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ. J. 2012;76:485–488. doi: 10.1253/circj.cj-11-1217. [DOI] [PubMed] [Google Scholar]
- 18.Andreassen A.K., Ragnarsson A., Gude E., Geiran O., Andersen R. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart. 2013;99:1415–1420. doi: 10.1136/heartjnl-2012-303549. [DOI] [PubMed] [Google Scholar]
- 19.Galie N., Brundage B.H., Ghofrani H.A., Oudiz R.J., Simonneau G., Safdar Z. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 20.Klepetko W., Mayer E., Sandoval J., Trulock E.P., Vachiery J.L., Dartevelle P. Interventional and surgical modalities of treatment for pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004;43:73s–80s. doi: 10.1016/j.jacc.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 21.The Respiratory Failure Research Group from the Ministry of Health LaW, Japan Kokyufuzen ni kansuru Rinshochosa (Clinical study of respiratory failure). 2011; 2012:249–252. (http://mklwgrants.niph.go.jp/niph/search/Download.do?nendo=2012&jigyoId=123151&bunkenNo=201231040A&pdf=201231040A0012.pdf).