Abstract
Cross-species transmission can often lead to deleterious effects in incidental hosts. Parvoviruses have a wide host range and primarily infect members of the order Carnivora. Here we describe juvenile common palm civet cats (Paradoxurus musangus) that were brought to the Singapore zoo and fell ill while quarantined. The tissues of two individual civets that died tested PCR-positive for parvovirus infection. Phylogenetic analysis revealed this parvovirus strain falls in a basal position to a clade of CPV that have infected dogs in China and Uruguay, suggesting cross-species transmission from domestic to wild animals. Our analysis further identified these viruses as genotype CPV-2a that is enzootic in carnivores. The ubiquity of virus infection in multiple tissues suggests this virus is pathogenic to civet cats. Here we document the cross-species transmission from domestic dogs and cats to wild civet populations, highlighting the vulnerability of wildlife to infectious agents in companion animals.
Viruses in the subfamily Parvovirinae infect vertebrates and have a wide host range, infecting humans, pigs, bats, rodents, and several species in the mammalian order Carnivora [1]. There are main three parvoviruses that infect these animals; canine parvovirus (CPV), feline panleukopenia virus (FPV), and mink enteritis virus (MEV). Parvoviruses are primarily transmitted fecal-orally, though it may also be transmitted via predation, scavenging carcasses, or oronasally [2]. Virus is shed at high titers for 4–12 days post-infection [3], [4], typically in young animals, but it is exceptionally durable and can persist in the environment for weeks under ideal conditions [5]. CPV is thought to have originated from FPV from a cat or another carnivore [6]. Although cats can be infected with CPV, dogs are the natural reservoir.
Parvoviruses are non-enveloped single-stranded DNA viruses with a genome approximately 5 kb in length that consists of two open reading frames (ORF) flanked by the 5′ and 3′ untranslated regions [7]. The first ORF encodes the non-structural proteins NS1 and NS2, while the second ORF encodes the structural VP1 and VP2 proteins [8]. Host machinery transcribes their genomes into mRNAs and replication occurs in the nucleus. The capsid region determines host range [9] and is prone to immunological pressure [10].
Singapore is a highly urbanized country that has lost nearly all of its original habitats and suffered a consequent loss of wildlife diversity [11]. Wild animals have limited sites to inhabit within this complex urban island biogeography. This loss of habitat also increases interactions between forest species and peridomestic species, potentially leading to cross-species transmission between these two groups [12]. There are four confirmed species of civet cats in Singapore, one of which adapts readily to human habitation (Paradoxurus musangus) [13]. Their use of human domiciles may lead them into contact with feral dogs and cats.
In this study, we sequenced and characterized a parvovirus isolated from a juvenile civet cat to understand its relatedness to known isolates. Apparently healthy juvenile civet cats (Paradoxurus musangus) were brought to Wildlife Reserves Singapore (WRS) from central and eastern Singapore. Within 27 days after arrival one individual developed diarrhea and died, and shortly after another individual housed with the first, developed diarrhea and also died. To ensure other species at the zoo would not be exposed, the two remaining civets housed in the same area were euthanized as a viral infection was suspected.
Tissue samples were collected from two civet cats and delivered to Duke-NUS Medical School for screening. Tissues were placed into vials with virus transport media and silicate beads, then homogenized in a Fast-Prep-24 (MP Biomedicals, Santa Ana, CA). DNA was extracted from the homogenized tissues using Qiagen DNeasy Blood and Tissue Kit (Qiagen, Germantown, MD) following the manufacturer's instructions. Samples were initially tested for both canine parvovirus (CPV) and feline parvovirus (FPV) using a nested PCR to amplify a 428 bp region in the non-structural protein (NS1) gene following published protocols [14], [15] (supplementary data 1). A second PCR was run to amplify 583 bp of the VP2 capsid gene using a previously established protocol [16]. The resulting PCR products were purified with a QIAquick Gel Purification kit (Qiagen, Germantown, MD) and sent for sequencing to Axil Scientific (Singapore).
The brain, heart, liver, spleen, and small intestine from one P. musangus and the brain and heart samples from the second individual tested positive for canine parvovirus using the nested CPV NS1 PCR. The liver sample from the second individual tested negative with the CPV NS1 primers and all samples tested negative with the FPV primers. Extracted DNA from the tissues was pooled by individual and screened for CPV VP2, both of which were positive. Preliminary genetic analysis of the NS1 and VP2 segments sequenced from the diagnostic PCR demonstrated that they belonged to canine parvoviruses.
To generate a full parvovirus genome, we used samples from the first civet cat to display symptoms and designed primers for whole genome amplification using Primer3 in Geneious v7.1.6 [17] (Suppl. Table 1). When specific PCR products produced poor direct sequencing results, these amplicons were purified using a QIAquick PCR purification kit (Qiagen, Germantown, MD) and cloned with a p-GEM-T Easy Vector System (Promega, Madison, WI). Amplification of untranslated regions was performed using 3′ RACE and 5′ RACE Systems for Rapid Amplification of cDNA ends (Life Technologies, Carlsbad, CA) according to the manufacturer's instructions followed by a hemi-nested PCR, however, amplification of the 5′ and 3′ untranslated regions was unsuccessful.
To generate a full parvovirus genome, we used samples from the first civet cat to display symptoms and designed primers for whole genome amplification using Primer3 in Geneious v7.1.6 [17] (Suppl. Table 1). When specific PCR products produced poor direct sequencing results, these amplicons were purified using a QIAquick PCR purification kit (Qiagen, Germantown, MD) and cloned with a p-GEM-T Easy Vector System (Promega, Madison, WI). Amplification of untranslated regions was performed using 3′ RACE and 5′ RACE Systems for Rapid Amplification of cDNA ends (Life Technologies, Carlsbad, CA) according to the manufacturer's instructions followed by a hemi-nested PCR, however, amplification of the 5′ and 3′ untranslated regions was unsuccessful.
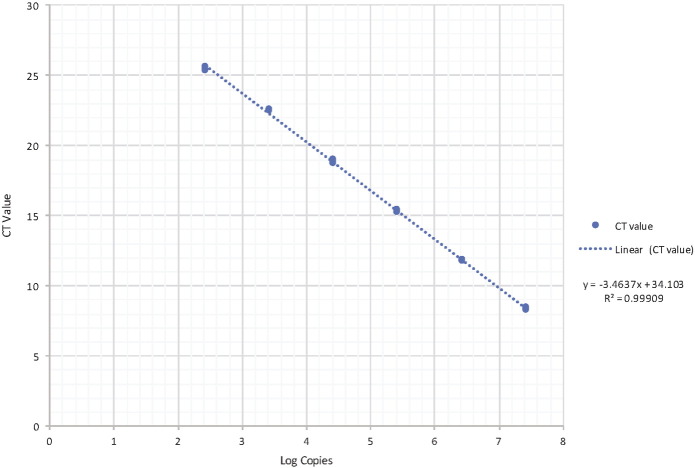
To quantify the viral DNA copy load per tissue, 1 g of each tissue type from each animal was homogenized in 1000 μl of viral transport media. A total of 200 μl of supernatant was used for the DNA extraction (as above) and eluted in 200 μl of Qiagen elution buffer. The qPCR reaction mixture was 10 μl of 2 × SensiFAST SYBR No-ROX (BIOLINE, London, UK), 10 μM of the forward (CPV-NS1 456F 5′- AGCTTCCAGGAGACTTTGGT-3′) and reverse primers (CPV-NS2 599R 5′- CCGCCCAGTTTTCATCCCAT-3′), 4 μl of water and 4 μl of DNA template. The PCR conditions consisted of an initial denaturation period at 95 °C for 5 min, followed by 40 cycles of 95 °C for 20 s and 55 °C for 30 s with a melt curve analysis from 45 °C–95 °C increasing 0.5 °C every 5 s and were run on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad). A standard curve was generated by amplifying a 461 bp fragment of the NS1 gene containing the qPCR target using the primers Parvo271F (5′-TTTTAGAATGCACGGATGGA-3′) and Parvo477R (5′- CTTTGCAACCTGGTGAATTT-3′). Serial 10-fold dilutions were quantified with the qPCR to generate an absolute standard curve (Suppl. Fig. S1).
Fig. S1.
A standard curve for detection of DNA copies of the NS1 gene. 4 μl of serial DNA dilutions from 0.271 to 2.7 × 107 DNA viral copies were run in triplicate and the standard curve was generated from 6 log10 concentrations of purified PCR products. The linear standard curve equation is y = − 3.4637x + 34.103 with an R2 value of 0.99909.
To quantify the viral DNA copy load per tissue, 1 g of each tissue type from each animal was homogenized in 1000 μl of viral transport media. A total of 200 μl of supernatant was used for the DNA extraction (as above) and eluted in 200 μl of Qiagen elution buffer. The qPCR reaction mixture was 10 μl of 2 × SensiFAST SYBR No-ROX (BIOLINE, London, UK), 10 μM of the forward (CPV-NS1 456F 5′- AGCTTCCAGGAGACTTTGGT-3′) and reverse primers (CPV-NS2 599R 5′- CCGCCCAGTTTTCATCCCAT-3′), 4 μl of water and 4 μl of DNA template. The PCR conditions consisted of an initial denaturation period at 95 °C for 5 min, followed by 40 cycles of 95 °C for 20 s and 55 °C for 30 s with a melt curve analysis from 45 °C–95 °C increasing 0.5 °C every 5 s and were run on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad). A standard curve was generated by amplifying a 461 bp fragment of the NS1 gene containing the qPCR target using the primers Parvo271F (5′-TTTTAGAATGCACGGATGGA-3′) and Parvo477R (5′- CTTTGCAACCTGGTGAATTT-3′). Serial 10-fold dilutions were quantified with the qPCR to generate an absolute standard curve (Suppl. Fig. S1).
Recombination detection analysis, using the publicly available CPV full genomes, implemented using GARD [18], revealed a recombination breakpoint that corresponds to the junction between the two ORFs, consistent with previous reports [19]. All subsequent phylogenetic analyses were therefore conducted on separate alignments of the NS1 and VP2 genes. The full-length VP2 dataset comprised a total of 417 parvovirus sequences, including 411 CPV sequences from different hosts such as bobcat (Lynx rufus), coyote (Canis latrans), dog (Canis lupus), fisher (Martes pennanti), gray wolf (Canis lupus), raccoon (Procyon lotor), red fox (Vulpes vulpes) stone marten (Martes foina); four MEV sequences from Mink species (Mustela lutreola); and one sequence from a feline species as an outgroup. For the full-length NS1 dataset, 104 sequences were included in the analysis. Phylogenetic relationships were inferred using maximum likelihood in RAxML v.8.0.14 [20], under the general time reversible model of nucleotide substitution with gamma distribution of rate variation among sites.
A total of 4363 bases were sequenced with a G + C content of 36.6% (Accession number: XXXXXX). Overlapping regions from directly sequence PCR products and cloned amplicons showed 8 nucleotide sites with variants, four of these with non-synonymous mutations (Suppl. Table 2). Where there was overlap, the civet cat parvovirus was 99.6% identical to canine parvovirus isolate SC02/2011 (JX660690) from China. The consensus parvovirus sequence generated lacked stop codons in the coding genes indicating it was a viable virus and that a non-viable genome was not sequenced. The first civet cat was much more heavily infected than the second civet cat. The comparative number of viral DNA copies was 20 × higher in the brain, 80 × higher in the heart and 4700 × higher in the liver in the first animal, indicating this animal was likely recently infected (Table 1). The spleen was the organ with the highest number of viral DNA copies, followed by the liver and small intestine.
Table 1.
Viral DNA copy numbers per 1 g of tissue per animal.
| Animal | Brain | Heart | Liver | Spleen | Small Intestine |
|---|---|---|---|---|---|
| Civet cat 1 (074F-0644) | 9.11 × 104 | 2.07 × 105 | 1.37 × 107 | 9.26 × 107 | 8.44 × 106 |
| Civet cat 2 (0728-87A6) | 4.43 × 103 | 2.61 × 103 | 2.88 × 103 |
A total of 4363 bases were sequenced with a G + C content of 36.6% (Accession number: KX618915). Overlapping regions from directly sequence PCR products and cloned amplicons showed 8 nucleotide sites with variants, four of these with non-synonymous mutations (Suppl. Table 2). Where there was overlap, the civet cat parvovirus was 99.6% identical to canine parvovirus isolate SC02/2011 (JX660690) from China. The consensus parvovirus sequence generated lacked stop codons in the coding genes indicating it was a viable virus and that a non-viable genome was not sequenced. The first civet cat was much more heavily infected than the second civet cat. The comparative number of viral DNA copies was 20 × higher in the brain, 80 × higher in the heart and 4700 × higher in the liver in the first animal, indicating this animal was likely recently infected (Table 1). The spleen was the organ with the highest number of viral DNA copies, followed by the liver and small intestine.
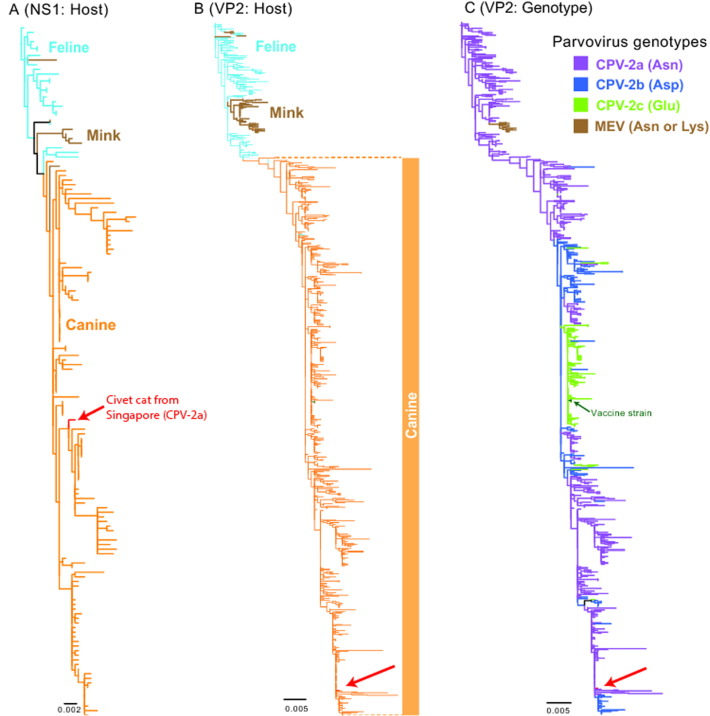
Phylogenetic analysis of the NS1 region revealed this parvovirus strain falls in a basal position to a clade of CPV that have infected dogs in China [21] and Uruguay [22] (Fig. 1A and Suppl. Fig. S2). The phylogeny of the VP2 gene showed a similar relationship, but with no statistical support (Fig. 1B and Suppl. Fig. S3). Our phylogenetic analysis therefore indicates the virus from civets is a canine parvovirus. As CPV populations show a strong spatial structure, with limited gene flow between populations [23], we hypothesize that these civet cats acquired the infection locally from an infected dog or dog feces. Furthermore, genotyping of the CPV from civet cats based on amino acid position 426 of the VP2 protein [24], [25] showed that it was a CPV-2a virus (Fig. 1C).
Fig. 1.
Phylogenetic trees of parvovirus based on (A) non-structural (NS1) and (B) capsid (VP2) nucleotide sequences from various hosts: canine parvovirus (CPV), feline parvovirus (FPV) and mink enteritis virus (MEV). (A) Maximum likelihood (ML) phylogeny of 150 full-length NS1 sequences. Colored branches denote host species. (B) ML phylogeny of 804 full-length VP2 sequences. Color branches denote host species. Red arrow indicates CPV sequences of a civet cat from Singapore. (C). Colored branches of VP2 phylogeny represent different genotypes at an amino acid position 426: CPV-2a (Asn), CPV-2b (Asp) and CPV-2c (Glu). Green arrow indicates live attenuated CPV vaccine. Abbreviations: Asn, asparagine; Asp, aspartic acid; Glu, glutamic acid; Lys, lysine.
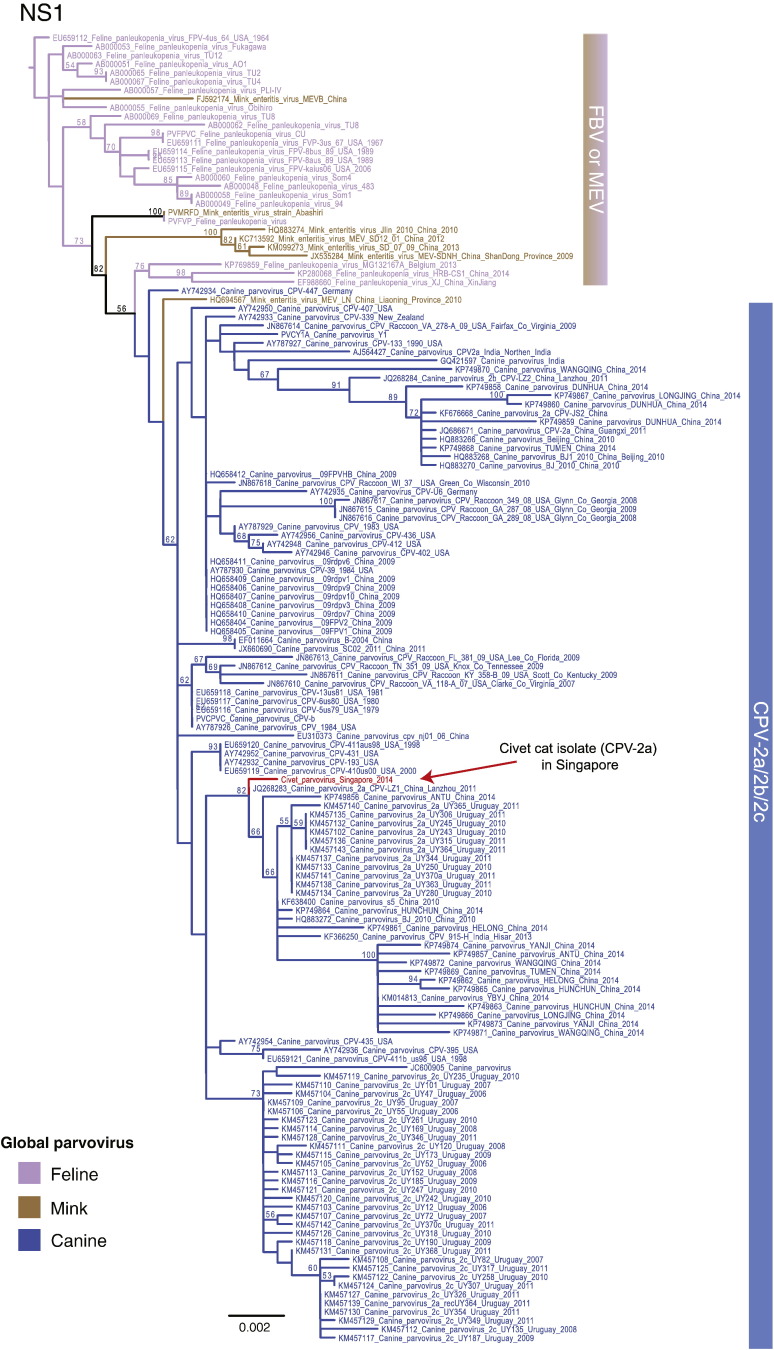
Fig. S2.
Phylogenetic tree of full-length NS1 gene of 150 global parvovirus sequences inferred using maximum likelihood method. Bootstrap values greater than 50% are indicated above branches. Different colored branches denote different hosts. A red arrow indicates the novel sequence from Singapore. The scale bar represents the number of substitutions per site.
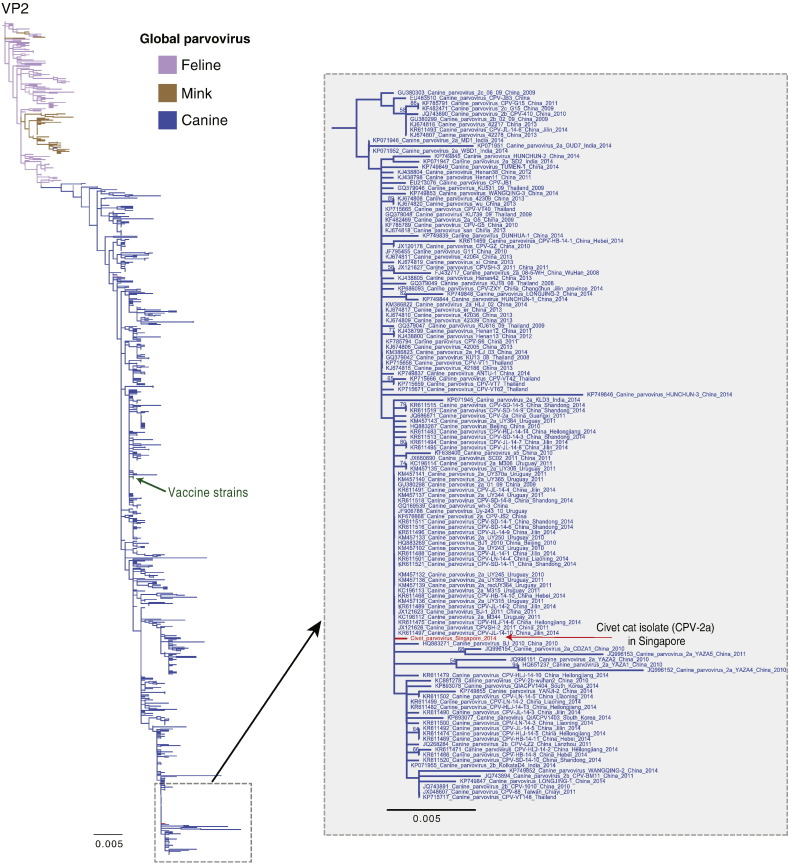
Fig. S3.
Phylogenetic tree of full-length VP2 gene of 804 global parvovirus sequences inferred using maximum likelihood method. Bootstrap values greater than 50% are indicated above branches. Different colored branches denote different hosts. A red arrow indicates the novel sequence from Singapore. The scale bar represents the number of substitutions per site. Green arrow indicates two vaccine strains (GenBank accession numbers: HW351002 and JA737999). The inset displays full sequence names and their corresponding accession numbers are shown.
Phylogenetic analysis of the NS1 region revealed this parvovirus strain falls in a basal position to a clade of CPV that have infected dogs in China [21] and Uruguay [22] (Fig. 1A and Suppl. Fig. S2). The phylogeny of the VP2 gene showed a similar relationship, but with no statistical support (Fig. 1B and Suppl. Fig. S3). Our phylogenetic analysis therefore indicates the virus from civets is a canine parvovirus. As CPV populations show a strong spatial structure, with limited gene flow between populations [23], we hypothesize that these civet cats acquired the infection locally from an infected dog or dog feces. Furthermore, genotyping of the CPV from civet cats based on amino acid position 426 of the VP2 protein [24], [25] showed that it was a CPV-2a virus (Fig. 1C).
Host range and host switching for parvoviruses appear to be correlated to specific mutations in the VP2 capsid protein [26]. The progenitor CPV genotype (CPV-2) affected dogs, but was unable to infect other carnivores. Subsequent mutations in VP2 residues produced two pandemic variants, CPV-2a and CPV-2b, with the ability to infect multiple species in Carnivora. In the VP2, there are a number of polymorphic sites that differ between hosts and mutations in these sites may modify antibody binding or change the structure of the capsid [27]. The CPV described here had the same amino acid profile in the VP2 as the most genetically closely related strain (GenBank accession number JX660690), but there was one key difference at amino acid residue 300 where the civet cat had an aspartic acid, while canine parvoviruses typically have a glycine (Table 2). This site has been shown to influence transferrin receptor binding of host cells and may indicate a virus adaptation as aspartic acid at the VP2 aa residue is often witnessed in raccoons [28]. Interestingly, there were several amino acid residue differences between this strain and a palm civet parvovirus detected in China. Additionally, canine parvoviruses are able to use feline receptors to enter cells and infect members of the Suborder Feliformia [29]. The absence of host adaptation related mutations in the capsid of this virus sequence, suggests that the civet cat infection was a direct or recent transfer from a dog, consistent with the phylogenetic analysis.
Table 2.
Amino acid residues in the VP2 of the civet cat parvovirus associated with the capacity of canine parvoviruses to infect other carnivores.
Cross-species transmission events are governed by several factors. Incidental hosts need to share space and time with infected reservoirs or encounter infectious material. These individuals need to be susceptible and the pathogen must possess the capacity to utilize host cell receptors and replication machinery [30], [31]. Infections are often ephemeral, resulting in acute host morbidity and mortality or failure of the parasite to thrive. In both cases, the parasite is infrequently sustainably transmitted [32], [33], but these barriers to cross-species transmission may be overcome by the etiological agent's plasticity. RNA viruses lack polymerase fidelity and mutate rapidly, however, smaller DNA viruses such as parvoviruses may have comparable rates of nucleotide substitution [34]. These viruses readily adapt to new carnivore hosts by mutations that allow them to bind to different host cell apical domains [27].
Parvoviruses demonstrate these characteristics as evidenced by their wide host range. The rapid decline of these juvenile civet cats limited the opportunity for the virus to mutate and escape into other hosts. Canine parvoviruses are important viruses in wild and domestic carnivores, especially regarding their deleterious effects on juveniles [2]. Though these infections are usually short-lived in the natural reservoirs, their environmental durability provides opportunities for incidental hosts to come in contact with fomites. These infections reveal the impact of cross-species transmission events even when the disease is vaccine preventable.
The following are the supplementary data related to this article.
Supplementary material.
Primer list for CPV genome sequencing.
Synonymous and non-synonymous mutations detected in the civet cat parvovirus genome.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.onehlt.2016.07.003.
Conflict of interest
The authors declare no conflict of interest in this study.
Acknowledgements
This study was supported by the Duke-NUS Signature Research Program funded by the Agency of Science, Technology and Research, Singapore, and the Ministry of Health, Singapore. We would also like to thank the Wildlife Reserves Singapore veterinary team who cared for the civets and collected samples.
Contributor Information
Ian H. Mendenhall, Email: ian.mendenhall@duke-nus.edu.sg.
Gavin J.D. Smith, Email: gavin.smith@duke-nus.edu.sg.
References
- 1.Allison A.B., Kohler D.J., Fox K.A., Brown J.D., Gerhold R.W., Shearn-Bochsler V.I., Dubovi E.J., Parrish C.R., Holmes E.C. Frequent cross-species transmission of parvoviruses among diverse carnivore hosts. J. Virol. 2013;87:2342–2347. doi: 10.1128/JVI.02428-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCaw D.L., Hoskins J.D. Canine viral enteritis. In: Greene C.E., editor. Infectious Diseases of the Dog and Cat. third ed. ELSEVIER; St. Louis, MO: 2007. [Google Scholar]
- 3.Carman P.S., Povey R.C. Pathogenesis of canine parvovirus-2 in dogs: haematology, serology and virus recovery. Res. Vet. Sci. 1985;38:134–140. [PubMed] [Google Scholar]
- 4.Pollock R.V. Experimental canine parvovirus infection in dogs. Cornell Vet. 1982;72:103–119. [PubMed] [Google Scholar]
- 5.Gordon J.C., Angrick E.J. Canine parvovirus: environmental effects on infectivity. Am. J. Vet. Res. 1986;47:1464–1467. [PubMed] [Google Scholar]
- 6.Truyen U., Platzer G., Parrish C.R. Antigenic type distribution among canine parvoviruses in dogs and cats in Germany. Vet. Rec. 1996;138:365–366. doi: 10.1136/vr.138.15.365. [DOI] [PubMed] [Google Scholar]
- 7.Reed A.P., Jones E.V., Miller T.J. Nucleotide sequence and genome organization of canine parvovirus. J. Virol. 1988;62:266–276. doi: 10.1128/jvi.62.1.266-276.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields B.N., Knipe D.M., Howley P.M. Wolters kluwer/Lippincott Williams & Wilkins; Philadelphia: 2007. Ovid Technologies Inc., Fields' Virology; p. 2. (v. ill) [Google Scholar]
- 9.Hueffer K., Parrish C.R. Parvovirus host range, cell tropism and evolution. Curr. Opin. Microbiol. 2003;6:392–398. doi: 10.1016/s1369-5274(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 10.Nelson C.D., Palermo L.M., Hafenstein S.L., Parrish C.R. Different mechanisms of antibody-mediated neutralization of parvoviruses revealed using the fab fragments of monoclonal antibodies. Virology. 2007;361:283–293. doi: 10.1016/j.virol.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sodhi N.S., Koh L.P., Brook B.W., Ng P.K.L. Southeast Asian biodiversity: an impending disaster. Trends Ecol. Evol. 2004;19:654–660. doi: 10.1016/j.tree.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Bradley C.A., Altizer S. Urbanization and the ecology of wildlife diseases. Trends Ecol. Evol. 2007;22:95–102. doi: 10.1016/j.tree.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chua M.A.H., Lim K.K.P., Low C.H.S. The diversity and status of the civets (Viverridae) of Singapore. Small Carniv. Conserv. 2012;47:1–10. [Google Scholar]
- 14.An D.J., Jeong W., Jeoung H.Y., Yoon S.H., Kim H.J., Park J.Y., Park B.K. Phylogenetic analysis of feline panleukopenia virus (FPLV) strains in Korean cats. Res. Vet. Sci. 2011;90:163–167. doi: 10.1016/j.rvsc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Saxena L., Chaturvedi U., Saxena S., Kumar G.R., Sahoo A.P., Kumar S., Doley J., Rajmani R.S., Singh P.K., Kumar R., Tiwari A.K. Characterization and in vitro expression of non-structural 1 protein of canine parvovirus (CPV-2) in mammalian cell line. Indian J. Exp. Biol. 2011;49:654–659. [PubMed] [Google Scholar]
- 16.Desario C., Decaro N., Campolo M., Cavalli A., Cirone F., Elia G., Martella V., Lorusso E., Camero M., Buonavoglia C. Canine parvovirus infection: which diagnostic test for virus? J. Virol. Methods. 2005;126:179–185. doi: 10.1016/j.jviromet.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., Thierer T., Ashton B., Meintjes P., Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delport W., Poon A.F., Frost S.D., Kosakovsky Pond S.L. Datamonkey 2010: a suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics. 2010;26:2455–2457. doi: 10.1093/bioinformatics/btq429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oshima K., Kikuchi A., Mochizuki S., Arai T., Oishi T., Hanada R. Acute encephalopathy with human parvovirus B19 infection in hereditary spherocytosis. Pediatr. Infect. Dis. J. 2008;27:651–652. doi: 10.1097/INF.0b013e3181694fcf. [DOI] [PubMed] [Google Scholar]
- 20.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ju C., Cheng Y., Ji Y., Wang Y., Sun L., Huang J. Genome sequence of canine parvovirus strain SC02/2011, isolated from a puppy with severe diarrhea in south China. J. Virol. 2012;86:13805. doi: 10.1128/JVI.02532-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maya L., Calleros L., Francia L., Hernandez M., Iraola G., Panzera Y., Sosa K., Perez R. Phylodynamics analysis of canine parvovirus in Uruguay: evidence of two successive invasions by different variants. Arch. Virol. 2013;158:1133–1141. doi: 10.1007/s00705-012-1591-5. [DOI] [PubMed] [Google Scholar]
- 23.Hoelzer K., Shackelton L.A., Parrish C.R., Holmes E.C. Phylogenetic analysis reveals the emergence, evolution and dispersal of carnivore parvoviruses. J. Gen. Virol. 2008;89:2280–2289. doi: 10.1099/vir.0.2008/002055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parrish C.R., Aquadro C.F., Strassheim M.L., Evermann J.F., Sgro J.Y., Mohammed H.O. Rapid antigenic-type replacement and DNA sequence evolution of canine parvovirus. J. Virol. 1991;65:6544–6552. doi: 10.1128/jvi.65.12.6544-6552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buonavoglia C., Martella V., Pratelli A., Tempesta M., Cavalli A., Buonavoglia D., Bozzo G., Elia G., Decaro N., Carmichael L. Evidence for evolution of canine parvovirus type 2 in Italy. J. Gen. Virol. 2001;82:3021–3025. doi: 10.1099/0022-1317-82-12-3021. [DOI] [PubMed] [Google Scholar]
- 26.Chang S.F., Sgro J.Y., Parrish C.R. Multiple amino acids in the capsid structure of canine parvovirus coordinately determine the canine host range and specific antigenic and hemagglutination properties. J. Virol. 1992;66:6858–6867. doi: 10.1128/jvi.66.12.6858-6867.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allison A.B., Kohler D.J., Ortega A., Hoover E.A., Grove D.M., Holmes E.C., Parrish C.R. Host-specific parvovirus evolution in nature is recapitulated by in vitro adaptation to different carnivore species. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stucker K.M., Pagan I., Cifuente J.O., Kaelber J.T., Lillie T.D., Hafenstein S., Holmes E.C., Parrish C.R. The role of evolutionary intermediates in the host adaptation of canine parvovirus. J. Virol. 2012;86:1514–1521. doi: 10.1128/JVI.06222-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker J.S., Murphy W.J., Wang D., O'Brien S.J., Parrish C.R. Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells. J. Virol. 2001;75:3896–3902. doi: 10.1128/JVI.75.8.3896-3902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Graaf M., Fouchier R.A. Role of receptor binding specificity in influenza a virus transmission and pathogenesis. EMBO J. 2014;33:823–841. doi: 10.1002/embj.201387442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Y., Wu Y., Zhang W., Qi J., Gao G.F. Enabling the 'host jump': structural determinants of receptor-binding specificity in influenza a viruses. Nat. Rev. Microbiol. 2014;12:822–831. doi: 10.1038/nrmicro3362. [DOI] [PubMed] [Google Scholar]
- 32.Gutierrez S., Michalakis Y., Blanc S. Virus population bottlenecks during within-host progression and host-to-host transmission. Curr. Opin. Virol. 2012;2:546–555. doi: 10.1016/j.coviro.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Parrish C.R., Holmes E.C., Morens D.M., Park E.C., Burke D.S., Calisher C.H., Laughlin C.A., Saif L.J., Daszak P. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol. Mol. Biol. Rev. 2008;72:457–470. doi: 10.1128/MMBR.00004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duffy S., Shackelton L.A., Holmes E.C. Rates of evolutionary change in viruses: patterns and determinants. Nat. Rev. Genet. 2008;9:267–276. doi: 10.1038/nrg2323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Primer list for CPV genome sequencing.
Synonymous and non-synonymous mutations detected in the civet cat parvovirus genome.