Abstract
To determine the predictive factors affecting uterine movement during radiotherapy (RT), we quantified interfraction uterine movement using computed tomography (CT) and cone-beam CT (CBCT). A total of 38 patients who underwent definitive RT for cervical cancer were retrospectively analyzed. We compared pre-RT planning CT (n = 38) and intratreatment CBCT (n = 315), measuring cervical and corporal movement in each direction. Correlations between uterine movement and volume changes of the bladder and rectum on all CBCT scans were analyzed using Spearman rank correlation analysis. Relationships between the mean uterine movement and patient factors were analyzed using the Mann–Whitney test. The mean corpus movement was: superior margin (cranio–caudal direction), 7.6 ± 5.9 mm; anterior margin (anteroposterior direction), 8.3 ± 6.3 mm; left margin (lateral direction), 3.3 ± 2.9 mm; and right margin (lateral direction), 3.0 ± 2.3 mm. Generally, the mean values for cervical movement were smaller than those for the corpus. There was a significant, weak correlation between changes in bladder volume and the movement of the superior margin of the corpus (ρ = 0.364, P < 0.001). There was a significant difference in movement of the superior margin of the corpus between the subgroups with and without a history of previous pelvic surgery (P = 0.007). In conclusion, change in bladder volume and a history of previous surgery were significantly related to intrafractional corpus movement; however, our observations suggest that the accurate prediction of uterine movement remains challenging.
Keywords: cervical cancer, radiotherapy, computed tomography, uterus, cone-beam CT
INTRODUCTION
Cervical cancer is highly curable with definitive radiotherapy (RT) [1]. In general, definitive RT consists of whole-pelvic external-beam radiotherapy (WPRT) and intracavitary brachytherapy (ICBT). The conventional, commonly employed techniques for WPRT are the use of two opposing portals for irradiation, or the ‘4-field box technique’. Despite its effectiveness, some patients suffer adverse events from RT [2]. Acute adverse events, such as enterocolitis, could lead to suspension of RT and result in decreasing survival rates. Late adverse events, such as proctitis, intestinal obstruction, and bone fracture, are also critical to decreasing quality of life [1, 3]. The frequency and severity of radiation adverse events depend on the RT dose and volume [4]. Intensity-modulated RT (IMRT) has an advantage in dose distribution over conventional WPRT techniques in that it can spare normal tissue by improving dose conformity to the target volume; this can also be expected to decrease the incidence of adverse events.
There are several studies demonstrating uterine movement, both during the definitive-RT treatment period and within each treatment session, in patients with cervical cancer [5–19]. Some investigators have pointed out that daily changes caused by bladder and rectal filling affect the degree of uterine movement. Movement of the uterus creates the risk of missing the target volume in highly conformal RT techniques such as IMRT.
There are a few studies investigating factors other than volume changes in the bladder and rectum that might affect uterine movement during definitive RT. Patient age and tumor size are reportedly related to uterine position change, while a patient's body habitus has no relation to uterine movement [5, 6].
If uterine movement can be perfectly predicted by factors such as bladder volume or other patient characteristics, this would be valuable information for more accurate, effective planning of definitive RT. These predictions would help to create appropriate internal margin settings for patients with cervical cancer and could also be applicable to IMRT. In this study, we aimed to assess uterine movement during definitive RT for cervical cancer and to verify the predictive factors for uterine movement given in previous publications.
MATERIALS AND METHODS
The Institutional Review Board at our institution (the University of the Ryukyus, Okinawa, Japan) approved this retrospective study; the requirement for written informed consent was waived.
Subjects and clinical course
A total of 59 patients with cervical cancer underwent definitive RT or concurrent chemoradiotherapy (CCRT) at the University of the Ryukyus Hospital between January 2013 and January 2014. All patients underwent pretreatment, diagnostic magnetic resonance imaging (MRI) and computed tomography (CT). This initial CT scan was not for RT simulation. Eighteen patients were excluded from the study because they underwent too few CBCT examinations (n = 14) or their CBCT images were of insufficient quality (n = 4). Three patients were also excluded due to the presence of a large pyometra or hydrometra, which disappeared during RT. A total of 38 patients were considered eligible and were ultimately enrolled in the study. The characteristics of these 38 patients are summarized in Table 1. Twelve patients had a history of previous pelvic surgery: cesarean delivery (n = 5), sterilization (n = 2), appendectomy (n = 2), surgical reduction of intussusception (n = 1), colectomy for sigmoid cancer (n = 1), and salpingo-oophorectomy for either ectopic pregnancy (n = 1) or ovarian tumor (n = 1). One patient underwent multiple pelvic operations.
Table 1.
Patient characteristics
| Patients | Mean ± SD | Range | |
|---|---|---|---|
| Eligible patients | 38 | ||
| Age (years) | 59 ± 15 | 34–85 | |
| Height (cm) | 152.8 ± 6.5 | 141–169.1 | |
| Weight (kg) | 54.3 ± 11.3 | 37.7–92.9 | |
| BMI (kg/m2) | 23.3 ± 4.7 | 16.3–37.1 | |
| History of pelvic surgery | |||
| Yes | 12 | ||
| No | 22 | ||
| Unknown | 4 | ||
| FIGO stage | |||
| IB1 | 9 | ||
| IB2 | 5 | ||
| IIA1 | 1 | ||
| IIB | 12 | ||
| IIIB | 9 | ||
| IVA | 2 |
SD = standard deviation, BMI = body mass index, FIGO = International Federation of Gynecology and Obstetrics.
Each patient underwent CT simulation and then received WPRT in the supine position using a customized vacuum immobilization device (Vac-Lock, Med-Tech). CT simulation used a 4-slice CT scanner with 2.5-mm slice thickness (LightSpeed-RT, GE Healthcare). A total of 38 simulation scans from 38 patients were ultimately obtained. To achieve constant bladder and rectal filling, patients were instructed to urinate and defecate 1 h prior to CT simulation and before each daily WPRT.
Patients received WPRT prior to high-dose-rate ICBT (HDR-ICBT). WPRT was delivered using the 4-field box technique with 10 MV photons. Each patient received a total dose of 50 Gy in 25 fractions (a midline block was inserted after 40 Gy). In HDR-ICBT, 18 Gy in 3 fractions was delivered to point A. Twenty-four patients were treated with CCRT (weekly cisplatin), and 14 patients underwent RT alone. Except for a single patient who had a previous history of breast cancer, an estrogen preparation (Estrana tape, 0.72 mg, or Estriel vaginal tab, 0.5 mg) was prescribed for all patients from the time of CT simulation to the last HDR-ICBT session, in order to increase the extensibility of the vaginal cavity for easy insertion of the HDR-ICBT applicators.
Each of the 38 patients underwent CBCT (2.5-mm slices thickness, 120 kVp, 80 mA, Varian Medical Systems) at least once a week as routine surveillance during RT. CBCT was performed just before each treatment to provide image-guided RT. Although a precise schedule for CBCT was not determined at our institution, the radiation oncologists decided on timing based on the information from the pretreatment CT, pretreatment MRI, and planning CT. A total of 368 CBCT sessions were performed in the 38 patients. Fifty-three were excluded for unacceptable image quality: the images did not demonstrate five or more of the targeted uterine margins described below. Therefore, a total of 315 CBCT scans were eventually collected for this study (5–14 for each patient; median, 11 scans).
Image analysis for patient factors using pretreatment CT and MRI
Three radiation oncologists (HM, TA and TT) evaluated the following factors on T2-weighted pretreatment MRI (MAGNETOM Avanto, 1.5 Tesla, Siemens Healthineers): uterine diameter (length of the major axis of the uterus), uterine position (anteverted vs non-anteverted), tumor size (maximum diameter and anteroposterior diameter) and tumor invasion of the corpus.
Using a commercially available workstation (Vincent, version 4.1.001, Fujifilm), a single radiation oncologist (HM) assessed the following factors on pretreatment CT (LightSpeed VCT, 1.25 mm slice thickness, GE Healthcare, or Aquilion ONE, 1 mm slice thickness, Toshiba Medical Systems): abdominal girth, area of the visceral fat at the level of the navel, and volume of the visceral fat. These three measurements, including the total visceral fat volume, were automatically measured by the workstation.
Image analysis for uterine movement using planning CT and CBCT
CBCT images were merged with the planning CT results to obtain a uterine-movement measurement in each patient, using a RT planning system (Eclipse version 11.0, Varian Medical Systems) with bone-matching technique.
Cervical and corporal margins were evaluated separately. For the uterine corpus, the superior margin (in the cranio–caudal direction), the anterior margin (in the anteroposterior direction), and the right and left margins (in the lateral directions) were targeted and their movements recorded. For the uterine cervix, the anterior and posterior margins (in the anteroposterior direction) and the right and left margins (in the lateral directions) were targeted and their movements recorded.
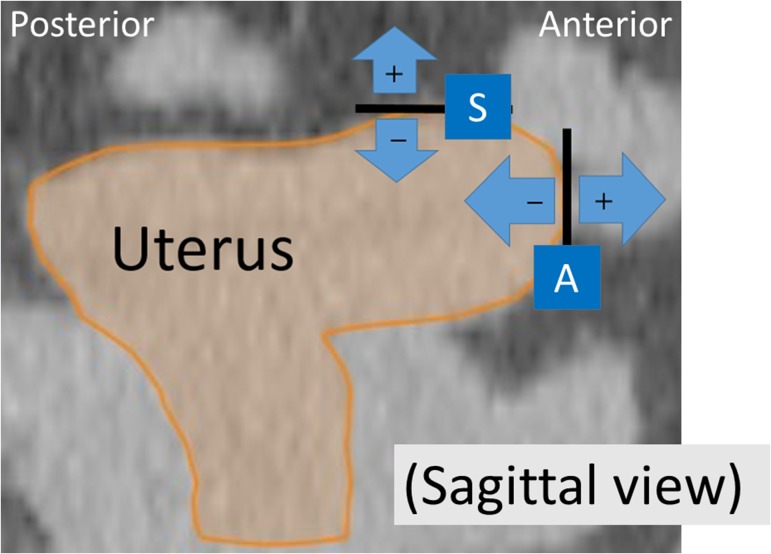
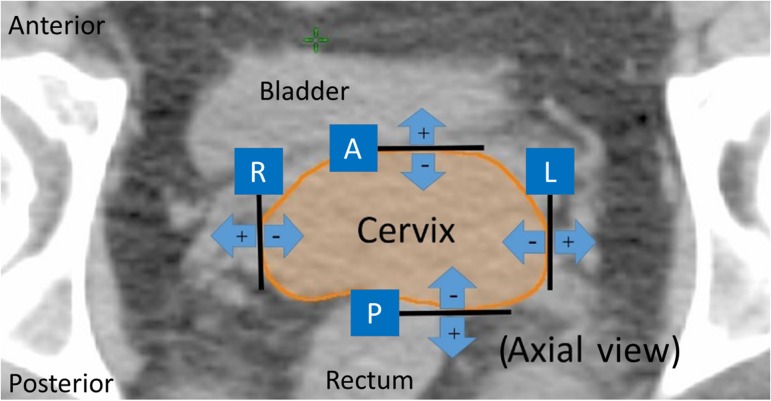
Using the planning CT as a reference, uterine movements in external directions were expressed as positive values (and movements in internal directions were expressed as negative values) for the anteroposterior and lateral directions. For the superior margin of the corpus, cranial movement was defined as positive and caudal movement was defined as negative (Figs 1 and 2). Since uterine movements were measured on multiple CBCT scans (5–14 scans), the mean value of the movement at each uterine margin was also calculated after converting negative values to absolute (positive) values.
Fig. 1.
Definition of corporal movement on planning computed tomography, sagittal image. The superior (S) and anterior (A) margins and their direction of movement are shown (positive movement values are expressed in the plus direction). Movements of the left and right corpus margins (in the lateral directions) were also measured on axial imaging, not shown here.
Fig. 2.
Definition of cervical movement on planning computed tomography, axial image. A = anterior margin, P = posterior margin, L = left margin, R = right margin. Movements in external directions are defined as positive measurements.
The bladder and rectum were contoured on the planning CT images and on all CBCT scans; the difference in volume between the planning CT and each CBCT was calculated. Since it was difficult to determine the upper and lower margins of the rectum, rectal volume was calculated using 10 slices around the center of the cervix (Fig. 3).
Fig. 3.
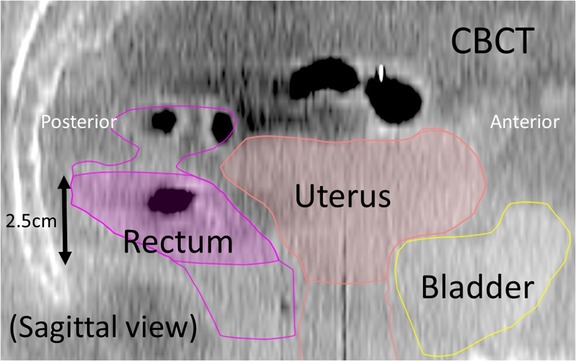
Contoured structures on a cone-beam computed tomography (CBCT), sagittal image. Rectal volume was calculated based on the cross-sectional area (2.5 mm section thickness) on 10 axial images around the center of the cervix.
One radiation oncologist (HM) measured the movement of the uterus and the interfractional volume changes of the bladder and rectum. The uterine corpus and cervix were not always fully contoured on CBCT if the image quality of CBCT was poor and the margins of some corporal/cervical parts were unclear. In case of difficulty determining the margin of the uterus, three radiation oncologists (HM, TA and TT) came to a consensus measurement. However, in some cases, the movement of some uterine margins was not measurable due to poor image quality of CBCT. Thus, the total number of measurable patients differed for measuring the various uterine margins.
Other patient factors
In addition to the patient factors obtained on pretreatment CT and MRI, we collected information on patient age, height, weight, body mass index (BMI), history of previous pelvic surgery, and International Federation of Gynecology and Obstetrics (FIGO) stage (IIA or less vs IIB or greater). We also collected measurements of tumor size, visceral fat, and abdominal girth.
Statistical analysis
Correlations between uterine movements and volume changes in the bladder and rectum on all CBCT scans (n = 315) were assessed using the Spearman rank correlation analysis. Correlations between the mean values of uterine movement at all margins (n = 38 at each margin) and between the continuous variables of patient factors (e.g. age, BMI, area of visceral fat) were also tested using the Spearman test. For analyzing the impact of categorical variables (e.g. history of pelvic surgery, FIGO stage) on uterine movement, patients were divided into two groups based on these factors; the Mann–Whitney test was used to examine the difference in the mean uterine movement at all margins. P-values < 0.05 were considered statistically significant. All statistical analyses were performed using JMP software, version 12.0.1 (SAS Institute).
RESULTS
Table 2 shows patient characteristics assessed by pretreatment MRI and CT. Measurements of corporal and cervical movements are shown in Table 3. There was a large amount of movement at the superior and anterior margins of the corpus. Generally, the mean values of cervical movement were smaller than those of the corpus, but in some cases the movement of the anterior and posterior cervical margins exceeded 10 mm. Lateral movement was relatively small, at both the corpus and the cervix.
Table 2.
Patient and tumor factors assessed by pretreatment MRI and CT
| Patients | Mean ± SD | Range | |
|---|---|---|---|
| Uterus size (major axis length, mm) | 74.1 ± 15.0 | 39.2–103.8 | |
| Uterus position | |||
| Anteverted | 30 | ||
| Retroverted | 3 | ||
| Middle | 5 | ||
| Tumor size (maximum diameter, mm) | 48.5 ± 22.5 | 0–106 | |
| Tumor size (AP, mm) | 38.4 ± 15.0 | 0–68 | |
| Tumor invasion of corpus | |||
| Positive | 15 | ||
| Negative | 23 | ||
| Abdominal girth (cm) | 84.2 ± 9.9 | 63.9–113 | |
| Area of visceral fat (cm2) | 81.2 ± 37.8 | 6.7–143.3 | |
| Volume of visceral fat (cm3) | 2095 ± 1205 | 226–5447 |
MRI = magnetic resonance imaging, CT = computed tomography, SD = standard deviation, AP = anteroposterior.
Table 3.
Movement of the corpus and cervix between planning CT and CBCT
| Mean ± SD(mm) | Range(mm) | Measurable patients(total n = 38) | |
|---|---|---|---|
| Corpus margin (movement direction) | |||
| Superior (CC) | 7.6 ± 5.9 | 0.0–29.0 | 36 |
| Anterior (AP) | 8.3 ± 6.3 | 0.3–26.0 | 37 |
| Left (lateral) | 3.3 ± 2.9 | 0.0–10.6 | 35 |
| Right (lateral) | 3.0 ± 2.3 | 0.0–9.2 | 30 |
| Cervical margin (movement direction) | |||
| Anterior (AP) | 3.7 ± 2.9 | 0.0–11.4 | 36 |
| Posterior (AP) | 3.4 ± 2.5 | 0.0–12.5 | 38 |
| Left (lateral) | 1.7 ± 1.6 | 0.0–9.2 | 38 |
| Right (lateral) | 2.0 ± 1.7 | 0.0–7.3 | 38 |
CT = computed tomography, CBCT = cone-beam computed tomography, SD = standard deviation, CC = cranio–caudal; AP = anteroposterior. ‘Mean’ stands for the mean value of the average corpus and cervical movement in absolute for each patient.
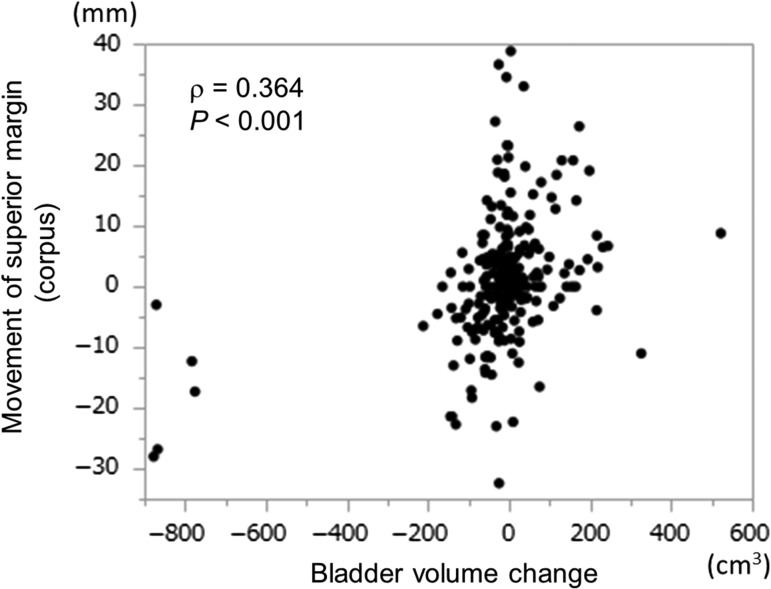
Table 4 shows the correlation between uterine movement and volume change in the bladder and rectum. There was a significant, positive correlation between cranio–caudal movement of the superior corpus margin and bladder-volume change (ρ = 0.364, P < 0.001, Fig. 4). Also, volume change in the rectum showed a very weak, significant correlation with the amount of movement at anterior and posterior margins of the cervix (P < 0.05, Supplementary Figures 1 and 2). However, no significant correlation was found at any other margin of the corpus or cervix. No significant correlation was found between mean uterine movement values and the continuous patient variables (Supplementary Table 1).
Table 4.
Uterine movement and volume change of the bladder and rectum
| Corpus margin | Cervical margin | |||
|---|---|---|---|---|
| superior | anterior | anterior | posterior | |
| ρ (P -value) | ρ (P -value) | ρ (P -value) | ρ (P -value) | |
| Bladder-volume change | 0.364 (<0.001) | −0.046 (NS) | −0.096 (NS) | 0.105 (NS) |
| Rectal-volume change | 0.04 (NS) | −0.08 (NS) | 0.132 (0.03) | −0.125 (0.03) |
NS = not significant (P > 0.05).
Fig. 4.
A scatter plot for the movement of the superior margin (uterine corpus) and the changes in bladder volume.
The differences in uterine movement between the patient subgroups based on categorical factors are shown in Table 5 (corpus) and Supplementary Table 2 (cervix). Only a previous history of pelvic surgery was judged to be a significant indicator of cranio–caudal movement of the superior corpus margin (P = 0.007), suggesting that a history of previous pelvic surgery causes a larger degree of uterine movement during RT. A similar tendency was found for movement of the anterior corpus margin, but this relation to prior surgery was not significant (P = 0.086). No other patient factors were judged to be significant predictors of uterine movement, although some factors, such as tumor invasion of the corpus and the FIGO stage, showed a trend toward an association.
Table 5.
Corpus movement and tumor and patient factors
| Superior margin | Anterior margin | |||||
|---|---|---|---|---|---|---|
| Characteristics | Patients | Mean ± SD (mm) | P -value | Patients | Mean ± SD (mm) | P -value |
| Tumor invasion to the corpus | ||||||
| Positive | 14 | 6.0 ± 5.0 | NS | 15 | 6.8 ± 6.1 | NS |
| Negative | 22 | 8.6 ± 6.3 | 22 | 9.3 ± 6.8 | ||
| FIGO stage | ||||||
| IIA or less | 15 | 8.4 ± 4.4 | NS | 15 | 9.6 ± 6.9 | NS |
| IIB or greater | 21 | 7.0 ± 6.8 | 22 | 7.4 ± 6.4 | ||
| History of pelvic surgery | ||||||
| Yes | 11 | 12.1 ± 7.6 | 0.007 | 11 | 11.4 ± 8.5 | NS |
| No | 22 | 5.4 ± 3.6 | 22 | 6.9 ± 5.3 | ||
| Uterine orientation | ||||||
| Anteverted | 28 | 8.2 ± 6.3 | NS | 29 | 9.0 ± 6.8 | NS |
| Non-anteverted | 8 | 5.3 ± 3.5 | 8 | 5.7 ± 5.2 | ||
SD = standard deviation, NS = not significant (P > 0.05), FIGO = International Federation of Gynecology and Obstetrics. Patients with findings unmeasurable on computed tomography or patients without a definitive clinical history were excluded.
DISCUSSION
We observed that (i) movement of the uterine corpus was greater than that of the cervix, (ii) there was a weak, significant correlation between corporal movement in the cranio–caudal direction and changes in bladder volume, and (iii) a history of pelvic surgery led to greater corporal movement in the cranio–caudal direction. Although some other patient factors trended toward an association with uterine movement, we believe that it is currently still very difficult to predict uterine movement correctly during definitive RT.
There are several studies documenting movement of the uterine corpus and cervix during definitive RT in patients with cervical cancer. Most demonstrate large movement of the corpus and cervix in the anteroposterior and cranio–caudal directions, and small movements in the lateral directions. Less cervical than corporal movement has also been previously reported. Our present results are consistent with those of these previous studies [5, 8, 9].
The relation between bladder filling or rectum filling and uterine movement during RT has also been previously described. Taylor et al. reported that bladder volume alters the cranial margin of the corpus [8] and that bladder-volume changes do not have a significant correlation with cervical movement. They did demonstrate a mild correlation of rectum filling with cervical movement in the anteroposterior direction [8]. Tyagi et al. assessed interfractional motion using CBCT in cervical-cancer patients and reported that there are significant correlations between clinical target volume (CTV) movement and any changes in bladder and rectum volumes; they concluded that consistent interfraction organ volumes would help reduce unexpected uterine movement [9]. In contrast, van de Bunt et al. concluded that the correlation of uterine movement with rectal and bladder volumes is weak, and that it may not be very effective to control the filling status of these organs [10]. Chan et al. maintain that carefully controlled bladder and rectal filling would yield only a small reduction in the interfractional internal target margin movements [5], while Buchali et al. report that bladder volume is related to uterine movement but rectal filling is not [12]. In our study, there was a weak correlation between bladder-volume change and movement at the superior margin of the corpus in the cranio–caudal direction; we also observed a very weak correlation between rectal-volume change and cervical movement.
van de Bunt et al. reported interpatient differences in the relation between bladder filling and uterine movement [10]. Although our patients were instructed to urinate and defecate 1 h before both CT simulation and daily WPRT sessions in order to maintain constant bladder and rectum volumes, there were still large deviations in these volumes in some patients, occasionally resulting in unpredictable uterine movement. These observations suggest that, even if bladder volume and rectal filling are strictly controlled, only a very limited reduction of uterine movement may result. Using other imaging modalities, such as ultrasound, may be an alternative option for checking more precise bladder and rectal volumes immediately before CT simulation or daily RT; however, it remains doubtful whether this would truly minimize uterine movement, since the correlation between bladder and rectum volume and uterine movement itself does not seem to be strong. Based on these observations, we strongly believe that, even when correlations between uterine movements and bladder and rectal volume changes are judged to be statistically significant, the true effect of bladder or rectal volume on uterine movements is very limited and should not be considered to be a decisive factor in determining uterine movement during RT.
Huh et al. investigated spontaneous uterine positional changes by comparing MRI scans before and during the period of RT; they concluded that the positional change of the uterus during RT should be carefully assessed at the time of planning 3D conformal radiotherapy or IMRT, particularly in patients under 60 years of age and in those with a tumor diameter greater than 4 cm [6]. In contrast, we found no significant correlation of uterine movement with patient age or with tumor size. Chan et al. also reported that no obvious relation is observed between patient body habitus and internal-organ motion, similar to our observations [5].
In our study, a previous history of pelvic surgery had a significant influence on uterine movement. Initially, we thought that pelvic surgery would have caused limited uterine movement due to adhesions, but the observed results were opposite to our prediction. It is very difficult to speculate a reason for the presence of greater uterine movement in patients with a previous surgical history than in those without this history.
Although they were judged to be insignificant factors for uterine movement in our study, we noticed a trend toward a relation between some clinical factors and uterine movement. For example, in patients with tumor invasion of the corpus, uterine movement was often restricted, compared with patients without this invasion. This may suggest that corpus invasion itself limits the movement of the entire uterus and that cancer localized to the cervix does not restrict movement of the corpus. Similar tendencies were also observed when dividing patients into groups by FIGO stage: patients with more advanced tumor stages had less uterine movement than patients with lesser stages. These insignificant differences may have turned out to be significant if we had enrolled more patients. Further study is recommended to comprehend the impact of tumor stage and invasion status on uterine movement.
Our observations here, and those of previous publications, imply that it is still very challenging to predict uterine movement during definitive RT based on simple clinical and patient characteristics. We also surmise that it is extremely difficult to create a perfect model to predict uterine movement that can be expanded to calculate necessary margins for definitive RT without the use of daily or regular monitoring using CBCT or other imaging modalities. However, our observations also demonstrate that cervical movement is not as great as that of the corpus. In future, it may be an option to apply IMRT to small and early cervical cancer if the tumor is limited to the cervix and if the fundus or other distant uterine parts are not involved in radical RT.
There are several limitations to our study that should be noted. First, the number of enrolled patients was small. Second, there were a considerable number of CBCT scans that could not be evaluated because of low contrast between pelvic organs. In particular, CBCT image quality was poor in patients with ascites and intestinal gas; this may have led to selection bias. Third, since this was a retrospective study, the total number of CBCT scans differed among enrolled patients. Fourth, time-dependent factors during RT, such as tumor regression and weight loss, were not evaluated in this study. These factors could change the body shape and uterine orientation, possibly influencing uterine movement. Fifth, when calculating the mean uterine movement values, we converted negative values to absolute values. This means that uterine movement in an internal direction due to RT-induced tumor regression (measured as a negative value) would not have been distinguished from uterine movement in an external direction (positive value). Sixth, the plane of the cervix was subjectively determined by a single clinician. Since, in some patients, the CBCT image quality was not good, the adjacent structures of the cervix (such as the upper part of the vagina) may have been included in the apparent ‘cervix’ on CBCT.
In conclusion, uterine corporal movement in the cranio–caudal direction is correlated with bladder volume, and a previous history of pelvic surgery is a predictive factor for corporal movement during definitive RT for cervical cancer. However, it is still difficult to predict the movement of the uterus using only these factors.
SUPPLEMENTARY DATA
Supplementary data are available at the Journal of Radiation Research online.
ACKNOWLEDGEMENTS
The main of observations of this research were presented at the annual meetings of the Japan Radiological Society (JRS), 2016 and the European Society for Radiotherapy and Oncology (ESTRO), 2016.
Supplementary Material
FUNDING
This study was supported by a research grant of the Japan Society for the Promotion of Science (KAKENHI-16K10398).
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- 1. Nakano T, Kato S, Ohno T, et al. Long-term results of high-dose rate intracavitary brachytherapy for squamous cell carcinoma of the uterine cervix. Cancer 2005;103:92–101. [DOI] [PubMed] [Google Scholar]
- 2. Toita T, Kato S, Niibe Y, et al. Prospective multi-institutional study of definitive radiotherapy with high-dose-rate intracavitary brachytherapy in patients with nonbulky (<4-cm) stage I and II uterine cervical cancer (JAROG0401/JROSG04-2). Int J Radiat Oncol Biol Phys 2012;82:e49–56. [DOI] [PubMed] [Google Scholar]
- 3. Tokumaru S, Toita T, Oguchi M, et al. Insufficiency fractures after pelvic radiation therapy for uterine cervical cancer: an analysis of subjects in a prospective multi-institutional trial, and cooperative study of the Japan Radiation Oncology Group (JAROG) and Japanese Radiation Oncology Study Group (JROSG). Int J Radiat Oncol Biol Phys 2012;84:e195–200. [DOI] [PubMed] [Google Scholar]
- 4. Bentzen SM, Constine LS, Deasy JO, et al. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys 2010;76:S3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan P, Dinniwell R, Haider MA, et al. Inter- and intrafractional tumor and organ movement in patients with cervical cancer undergoing radiotherapy: a cinematic-MRI point-of-interest study. Int J Radiat Oncol Biol Phys 2008;70:1507–15. [DOI] [PubMed] [Google Scholar]
- 6. Huh SJ, Park W, Han Y.. Interfractional variation in position of the uterus during radical radiotherapy for cervical cancer. Radiother Oncol 2004;71:73–9. [DOI] [PubMed] [Google Scholar]
- 7. Lee JE, Han Y, Huh SJ, et al. Interfractional variation of uterine position during radical RT: weekly CT evaluation. Gynecol Oncol 2007;104:145–51. [DOI] [PubMed] [Google Scholar]
- 8. Taylor A, Powell ME.. An assessment of interfractional uterine and cervical motion: implications for radiotherapy target volume definition in gynaecological cancer. Radiother Oncol 2008;88:250–7. [DOI] [PubMed] [Google Scholar]
- 9. Tyagi N, Lewis JH, Yashar CM, et al. Daily online cone beam computed tomography to assess interfractional motion in patients with intact cervical cancer. Int J Radiat Oncol Biol Phys 2011;80:273–80. [DOI] [PubMed] [Google Scholar]
- 10. van de Bunt L, Jürgenliemk-Schulz IM, de Kort GA, et al. Motion and deformation of the target volumes during IMRT for cervical cancer: what margins do we need. Radiother Oncol 2008;88:233–40. [DOI] [PubMed] [Google Scholar]
- 11. Haripotepornkul NH, Nath SK, Scanderbeg D, et al. Evaluation of intra- and inter-fraction movement of the cervix during intensity modulated radiation therapy. Radiother Oncol 2011;98:347–51. [DOI] [PubMed] [Google Scholar]
- 12. Buchali A, Koswig S, Dinges S, et al. Impact of the filling status of the bladder and rectum on their integral dose distribution and the movement of the uterus in the treatment planning of gynaecological cancer. Radiother Oncol 1999;52:29–34. [DOI] [PubMed] [Google Scholar]
- 13. Jadon R, Pembroke CA, Hanna CL, et al. A systematic review of organ motion and image-guided strategies in external beam radiotherapy for cervical cancer. Clin Oncol (R Coll Radiol) 2014;26:185–96. [DOI] [PubMed] [Google Scholar]
- 14. Collen C, Engels B, Duchateau M, et al. Volumetric imaging by megavoltage computed tomography for assessment of internal organ motion during radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2010;77:1590–5. [DOI] [PubMed] [Google Scholar]
- 15. Wang X, Yu M, Wang J, et al. An assessment of interfractional bladder, rectum and vagina motion in postoperative cervical cancer based on daily cone-beam computed tomography. Mol Clin Oncol 2016;4:271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris EE, Latifi K, Rusthoven C, et al. Assessment of organ motion in postoperative endometrial and cervical cancer patients treated with intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2011;81:e645–50. [DOI] [PubMed] [Google Scholar]
- 17. Forrest J, Presutti J, Davidson M, et al. A dosimetric planning study comparing intensity-modulated radiotherapy with four-field conformal pelvic radiotherapy for the definitive treatment of cervical carcinoma. Clin Oncol (R Coll Radiol) 2012;24:e63–70. [DOI] [PubMed] [Google Scholar]
- 18. Gaffney D, Mundt A, Schwarz J, et al. Advances in clinical research in gynecologic radiation oncology: an RTOG symposium. Int J Gynecol Cancer 2012;22:667–74. [DOI] [PubMed] [Google Scholar]
- 19. Beadle BM, Jhingran A, Salehpour M, et al. Cervix regression and motion during the course of external beam chemoradiation for cervical cancer. Int J Radiat Oncol Biol Phys 2009;73:235–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.