Abstract
Sleep is profoundly altered during the course of infectious diseases. The typical response to infection includes an initial increase in nonrapid eye movement sleep (NREMS) followed by an inhibition in NREMS. REMS is inhibited during infections. Bacterial cell wall components, such as peptidoglycan and lipopolysaccharide, macrophage digests of these components, such as muramyl peptides, and viral products, such as viral double-stranded RNA, trigger sleep responses. They do so via pathogen-associated molecular pattern recognition receptors that, in turn, enhance cytokine production. Altered sleep and associated sleep-facilitated fever responses are likely adaptive responses to infection. Normal sleep in physiological conditions may also be influenced by gut microbes because the microbiota is affected by circadian rhythms, stressors, diet, and exercise. Furthermore, sleep loss enhances translocation of viable bacteria from the intestine, which provides another means by which sleep–microbe interactions impact neurobiology.
1. INTRODUCTION: HISTORY
People have likely always been aware of the feelings of sleepiness and excess sleep that accompany many diseases including multiple microbial infections. However, it has only been over the past four decades that direct links between microbes and sleep have been established. This work had its origins in the laboratories of Pappenheimer and Karnovsky in the 1960s and 1970s. At the time they were seeking to isolate and identify a sleep-promoting substance, called Factor S, from cerebral spinal fluid and brains of sleep-deprived animals (Fencl, Koski, & Pappenheimer, 1971; Pappenheimer, Miller, & Goodrich, 1967). By the early 1980s, they had processed very large amounts of brain and, in separate experiments, urine with the goal of chemical identification of the somnogenic agent(s) responsible. By the early 1980s, they had characterized somnogenic muramyl peptides in both urine and brain (Krueger, Bacsik, & García-Arrarás, 1980; Krueger, Karnovsky, et al., 1984; Krueger, Pappenheimer, & Karnovsky, 1982). At the time it was recognized that the somnogenic muramyl peptides may have their origins from intestinal lumen bacterial cell wall peptidoglycan (Krueger et al., 1982; Fig. 1).
Fig. 1.
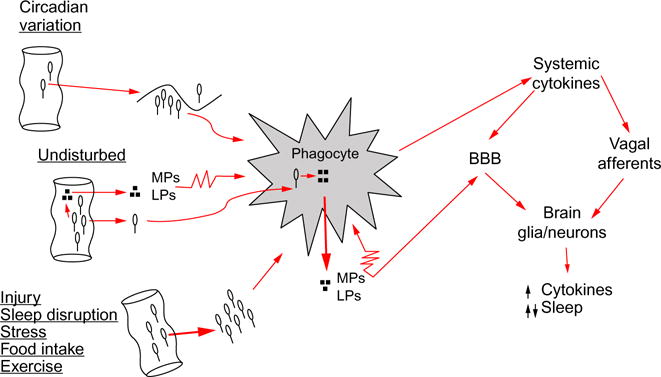
Pathway for intestinal bacteria to affect sleep. From left to right: intestinal bacteria, and/or bacteria cell wall degradation products, such as muramyl peptides (MPs) or lipopolysaccharide (LPS), translocate across the intestinal epithelial barrier. Sleep loss and several conditions that affect sleep, e.g., injury, food intake, stress, circadian rhythm, and exercise, affect bacteria translocation. Bacteria are engulfed by phagocytes, such as macrophages or neutrophils, and digested; digest products (e.g., MPs, LPS) are released into the surrounding intercellular fluid. MPs and LPS in turn activate phagocytes (illustrated by the jagged cell membrane) that then release cytokines such as interleukin-1 and tumor necrosis factor. Systemic cytokines access the brain by at least two routes. Cytokines can signal the brain via vagus nerve afferents whose action potentials induce further cytokine production in the brain by glia and neurons. Cytokines can also cross the blood–brain barrier (BBB) to induce their own and other cytokine productions. Brain cytokines at low concentrations enhance sleep, while at high concentrations fragment sleep. Other microbes, e.g., viruses, and their components also enhance cytokine production via endogenous receptors that recognize pathogen-associated molecular patterns, e.g., Toll-like receptors, to affect sleep (not illustrated).
After the initial identification of somnogenic muramyl peptides, the field expanded rapidly. Muramyl peptides of bacterial origin were tested (Krueger et al., 1987), other bacterial cell wall products such as lipopolysaccharide (LPS) were shown to be somnogenic (Krueger, Kubillus, Shoham, & Davenne, 1986), multiple synthetic muramyl peptides (Krueger, Walter, et al., 1984), and LPS (Cady, Kotani, Shiba, Kusumoto, & Krueger, 1989) derivatives, developed as potential immune adjuvants, were used to define structure-somnogenic activities. Evidence, although remaining insufficient, was obtained showing muramyl peptide components of in normal mammalian tissues (Johannsen & Krueger, 1988; Krysciak, 1980; Zhai & Karnovsky, 1984). Further, the translocation across the intestinal wall of hydrophilic molecules, including muramyl peptides, was demonstrated (Pappenheimer & Zich, 1987). It was known that macrophages could digest peptidoglycan and release muramyl peptides (Vermeulen & Gray, 1984), including somnogenic muramyl peptides (Johannsen, Wecke, Obál, & Krueger, 1991), suggesting that muramyl peptides could be produced in the gut and other tissues. Further, the translocation of bacteria, including lactobacilli, was known in health and disease (Berg & Garlington, 1979). These findings led to the first quantification of sleep changes across the course of bacterial (Toth & Krueger, 1988) and viral (Kimura-Takeuchi, Majde, Toth, & Krueger, 1992a; Toth, Rehg, & Webster, 1995) infections. They also led to investigations of how sleep could affect bacterial translocation across the intestinal wall (Everson, 1993; Everson & Toth, 2000).
This review will focus on bacteria, although an interesting parallel story relating the influence of viruses on sleep is also developing. We will begin by describing how bacterial infections affect sleep and that sleep loss is associated with bacteremia. Then we will describe the profound prolonged effects of cecal ligation an experimental bacteremia model. We describe mechanisms by which bacteria initiate sleep responses including macrophage processing of bacterial cell walls and the subsequent products affect sleep. The effects of other microbes, including viruses, on sleep are described. We end with a summary of how the molecules derived from bacteria and viruses in turn induce cytokine production and the role of cytokines in sleep regulation in health and disease.
2. SLEEP PHYSIOLOGY
The reader is referred to Principles and Practices in Sleep Medicine (Kryger, Roth, & Dement, 2011) for an extensive discussion of sleep physiology and pathology; an abbreviated summary is presented. There are two sleep states, rapid eye movement sleep (REMS) and non-REMS (NREMS). In humans and experimental animals such as rats and mice, NREMS occupies most of the time asleep and there is an oscillation between NREMS and REMS with about a 90-min cycle in humans. In humans, most sleep occurs during the dark phase of the 24-h day. In contrast, mice and rats sleep mostly during the day. NREMS intensity is inferred from the amplitude of electroencephalogram (EEG) slow waves (SWs) (0.5–4 Hz). High-amplitude EEG SWs occur after sleep deprivation (Pappenheimer, Koski, Fencl, Karnovsky, & Krueger, 1975), and EEG SW activity is higher in humans during the initial bout of sleep at night, and in mice and rats during the initial bout of NREMS during the day. Different areas in brain are involved in NREMS and REMS, e.g., the anterior hypothalamus regulates, in part, NREMS, whereas more posterior brain stem nuclei regulate REMS. State oscillations occurring within small neuronal/glial circuits are posited to be a fundamental building block of organism sleep (Krueger & Roy, 2016); e.g., synchronization of cortical column sleep-like states emerges as organism sleep (Krueger, Huang, Rector, & Buysee, 2013; Krueger & Obál, 1993; Rector, Topchiy, Carter, & Rojas, 2005; Roy, Krueger, Rector, & Wan, 2008). The molecules involved in state regulation, whether within small circuits or the entire brain, include multiple cytokines (Churchill et al., 2008; Jewett et al., 2015), hormones (e.g., Chang & Opp, 2001; Obál, Fang, Payne, & Krueger, 1995), neurotransmitters (Hinard et al., 2012), and substances such as ATP (Krueger et al., 2010), adenosine, glutamic acid, GABA, and prostaglandins (reviewed Krueger et al., 2008; Imeri & Opp, 2009). All molecules involved in sleep regulation, including brain cytokines, show enhanced expression or release in response to cell activity, e.g., neuron action potentials, suggesting that, in brain, sleep serves a plasticity function (Krueger & Obál, 1993; Krueger & Tononi, 2011).
Mild infectious challenges or low doses of microbial products enhance duration and intensity of NREMS while simultaneously decreasing duration of REMS (Opp & Krueger, 2015). However, as severe infections progress, or after high doses of bacterial or viral components, sleep becomes fragmented often causing a reduction in duration of sleep. Microbes/microbe components promote sleep via their ability to enhance proinflammatory cytokines whether systemically or centrally. Cytokines in turn act on both individual small neuronal circuits and sleep regulatory centers to affect sleep via effector mechanisms that include NO, adenosine, and glutamate receptor trafficking (Imeri & Opp, 2009; Krueger et al., 2013). Systemic cytokines affect brain function either by stimulating vagal afferents that in turn enhance brain production of cytokines or are transported across the blood–brain barrier (BBB) (Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008). Of intense interest is our hypothesis that under physiological conditions these mechanisms operate at a basal level contributing to normal sleep regulation, while the major changes in sleep occurring during pathology are an amplification of these physiological processes.
3. BACTERIAL CHALLENGE AFFECTS SLEEP
Although Hippocrates made the association between diseases and sleep, the first systematic studies of the effects of a bacterial infection on sleep were not done until about 30 years ago (Toth & Krueger, 1988). In that study, rabbits were inoculated with gram-positive bacteria, Staphylococcus aureus. Within a few hours after challenge, duration of NREMS increased and lasted for about 24 h. During the second day after challenge NREMS was reduced to below baseline control values. The intensity of NREMS, as determined from the amplitude of EEG delta wave (0.5–4 Hz) during NREMS, followed a similar time course, initially increasing followed by prolonged decreases. Initially average individual NREMS bout length increased, but within 20 h of challenge, NREMS bout length decreased to values below baseline. In subsequent studies of the effects on sleep of multiple bacteria and other microbes, a general feature found was that if the induced disease is severe, sleep becomes fragmented in the later stages of the disease. After S. aureus challenge, REMS was inhibited throughout the first 48 h after challenge.
If rabbits were inoculated with the same number of S. aureus that were first heat killed, there was no response to the challenge. However, if a 100-fold higher dose of heat-killed S. aureus was used, changes in sleep occurred, but they were attenuated. Thus, the initial increases in NREMS occurred and were more rapid in onset as occurred after viable S. aureus inoculations, although the subsequent inhibition of sleep was not observed. These results suggested that bacterial replication was required for the full course of the sleep responses. They also suggested that bacteria components could drive initial sleep responses; this is expanded upon below.
The time course of the fever responses to S. aureus challenge was distinct from the sleep response time course. Fevers were initiated during the first phase of the excessive sleep response but then persisted during the time when NREMS was lower than baseline values. These results clearly indicated that the sleep responses are independent of S. aureus-induced fever because temperatures were elevated during both periods of excess NREMS and during periods of NREMS inhibition.
In subsequent studies, other gram-positive (Streptococcus pyogenes) and gram-negative (Escherichia coli, Pasteurella multocida) were also tested with similar results. A notable exception was that after E. coli challenge the onset of NREMS was more rapid in onset and larger in magnitude, but these initial responses only lasted a few hours compared to responses induced by S. aureus (Krueger et al., 1994; Toth & Krueger, 1989). Heat-killed E. coli also elicited sleep responses, again suggesting that bacterial components could trigger sleep responses.
4. SLEEP LOSS PROMOTES INTESTINAL BACTERIAL TRANSLOCATION
Chronic sleep deprivation of rats leads to death after about 19 days (Everson, 1993). The experimental model used to reach this conclusion is called the disc over water model. Rats are placed one on each side of a rotating disc with a physical barrier separating the two rats and causing the rats to either wake up or be knocked into the water below the rotating disc. The disc rotation is turned on when the experimental rat enters sleep as determined from his EEG; the sleeping rat is then knocked into the water and wakes up. In this condition the experimental rat loses most of their sleep for days on end, while the control, yoked, rat maintains most of their sleep because the disc rotation is not timed to its sleep. Under these conditions, rats develop a hypercatabolic state with progressive enhanced food intake and body weight losses. As death approaches, the experimental rat develops a wasted appearance and hypothermia. Although extensive experimentation failed to reveal the cause of death (reviewed Rechtschaffen, Bergmann, Everson, Kushida, & Gilliland, 1989), Everson determined that the experimental sleep-deprived rats became septicemic concluding that host defense failure was a result of sleep loss. In a subsequent study, Everson and Toth (2000) characterized live bacteria in normally sterile body tissues in sleep-deprived rats. Their major conclusion was that mesenteric lymph nodes contained viable bacteria as a result of bacterial translocation from the intestine. Although these studies suggested a relationship between sleep loss, intestinal bacterial translocation, and immune system failure, the disc over water model has been criticized. For example, the sleep-deprived rat lacks control over its environment because when the disc begins to rotate the rat is asleep, while the control rat maintains some degree of control because it knows when the disc will turn due to the inactivity of the experimental rat. Environmental control affects sleep deprivation outcomes (Oonk, Krueger, & Davis, 2016). Thus, the septicemia may result from learned helpless rather than of sleep loss per se.
Sleep is firmly linked to circadian rhythms, food intake, exercise, and stressors; these variables also affect each other compounding their actions on sleep. Feeding rhythms and disruption of circadian rhythms induce time-specific changes in intestinal bacteria (Thaiss et al., 2014; Voigt et al., 2014). Circadian clock disruption also increases permeability of the intestinal epithelial barrier (Summa et al., 2013). Similarly, exercise changes intestinal flora and the clearance of specific bacterial phyla from blood (Shukla et al., 2015; reviewed Bermon et al., 2015). Further, intestinal bacteria influence responses to stressors as they are necessary for stress-induced increases in cytokines such as interleukin-1 (IL1) (Maslanik et al., 2012). IL1, as discussed briefly later, is involved in physiological sleep regulation and in sleep responses to microbes (reviewed Imeri & Opp, 2009). Such findings could indicate a role for gut flora in sleep regulation in health and disease; however, much work is needed to verify and clarify this hypothesis.
5. CECAL LIGATION
Most experimental studies of bacterial infections and sleep have used inoculation of a single pathogen species as the infectious challenge. The gut microbiome, however, is polymicrobial and many infections result from invasion by multiple pathogen species. Such is the case in sepsis, during which polymicrobial infections are routinely the case. The preclinical sepsis model considered to be the gold standard is cecal ligation and puncture (CLP; Nemzek, Hugunin, & Opp, 2008). CLP produces a polymicrobial infection that is considered clinically relevant because of its time course, the dynamic changes in cardiac function, and because there is a progressive release of inflammatory mediators. Sleep is altered during the acute phase of CLP sepsis, which occurs from 1 to 4 days after sepsis induction (Baracchi, Ingiosi, Raymond, & Opp, 2011). During this period, NREMS and REMS of rats increase during the dark period, whereas these sleep phases are reduced during the light period. These changes in sleep coincide with increased cytokine mRNA and protein in brain (Granger, Ratti, Datta, Raymond, & Opp, 2013). Of interest, effects of sepsis on body temperature and activity rhythms of animals that survive persist long after recovery when the subject is no longer at risk of dying (Granger et al., 2013). These observations suggest that sepsis alters brain function, and are in agreement with observations that patients surviving sepsis often suffer severe and debilitating cognitive impairment. Aging and sleep status also impact BBB transport of tumor necrosis factor (TNF). BBB transport of TNF increases during sepsis in young mice, but not in aged (Opp & Krueger, 2015). Interactions among age, sleep status, and BBB function have received little attention, but are likely to be important determinants of outcomes in old and oldest old persons in response to inflammatory insult.
6. BACTERIAL COMPONENTS DRIVING SLEEP RESPONSES
Bacterial walls contain several layers (reviewed Pabst, Beranova, & Krueger, 1999). They have an inner membrane, also called the plasma membrane, which is similar in function and structure to those of other living organisms. Most bacteria also have a cell wall exterior to the plasma membrane that forms a flexible sturdy sheath surrounding the plasma membrane serving to protect the cell from osmotic shock. These cell walls are composed of peptidoglycan, a polymer that gives the bacteria their particular shape. Outside the peptidoglycan layer there is much diversity among bacterial species. For example, in gram-negative bacteria, there is an outer membrane of lipid bilayer of phospholipids and lipoproteins forming the inner leaflet and an outer leaflet composed of lipopolysaccharide (called endotoxin or LPS). Gram-positive bacteria lack an outer membrane but have other components such as teichoic acids anchored to the peptidoglycan layer.
As bacteria divide, grow, or die, the peptidoglycan, LPS, and other components are degraded or altered by bacterial enzymes. Host phagocytic cells like macrophages and neutrophils also can digest peptidoglycan producing muramyl peptides (small glycopeptides). Peptidoglycan, isolated from either gram-positive or gram-negative bacteria, induces sleep responses similar to those induced by whole dead bacteria described earlier. For example, NREMS duration and intensity is enhanced for several hours (Johannsen et al., 1990). If phagocytic mammalian cells are fed bacteria, they release small biologically active muramyl peptides (Johannsen, Wecke, et al., 1991). Some of these muramyl peptides induce sleep responses that mimic those induced by intact peptidoglycan and heat-killed whole bacteria. The somnogenic properties of muramyl peptides are dependent upon precise biochemical structure (reviewed Johannsen et al., 1989; Krueger & Johannsen, 1989). For example, β(1→4)-N-acetylglucosaminyl-β(1→4)-N-acetylmuramyl-L-alanyl-D-iso-glutaminyl-L-lysyl-D-ananyl-D-alanine is somnogenic. Somnogenic activity is retained if either the N-acetylglucosamine or terminal alanines are removed. Further, several naturally occurring muramyl peptides, containing diaminopimelic acid instead of lysine, are somnogenic. In fact, the sleep-promoting material isolated from human urine and rabbit brain contained diaminopimelic acid. It also contained 1,6-anhydro-N-acetylmuramic acid although the anhydro ring could be hydrated without loss of somnogenic activity. Muramyl peptides have multiple activities including induction of fever and are immune adjuvants. The structural requirements for fever induction or for adjuvant activity are distinct from those for sleep promotion, although some muramyl peptides possess all these activities, e.g., N-acetylmuramyl-L-alanyl-D-isoglutamine, also called muramyl dipeptide or MDP (Shoham & Krueger, 1988). MDP was isolated from the killed mycobacteria component of Freund’s complete adjuvant; it is the component responsible for adjuvanticity.
LPS somnogenic activity–LPS structure relationships also were described although to a more limited extent. Thus, for example, lipid A is a biologically active component of LPS; both LPS and lipid A promote NREMS (Krueger et al., 1986). Somnogenic activity of synthetic lipid analogs is dependent on acylation or phosphorylation patterns and backbone structures of these molecules (Cady et al., 1989).
These findings led to the idea that specific muramyl peptides and lipid A molecules are tailor made from peptidoglycan and LPS by mammalian phagocyte enzymes to meet host innate immunity and sleep requirements in health and disease states (Krueger & Johannsen, 1989). Regardless, much investigative work is needed before acceptance of this speculation.
7. SLEEP RESPONSES TO VIRUS CHALLENGE
Viral infections are also associated with changes in sleep although the unfolding of this story is not as extensive as that described for the effects of bacterial infections. Thus, intravenous influenza virus inoculation induces large increases in rabbit sleep time as well as several other facets of the acute-phase response, e.g., fever. These responses occurred despite the inability of influenza virus to undergo complete replication in the rabbit (Kimura-Takeuchi et al., 1992a). Unlike bacteria, heat inactivation of the virus blocked the ability of the virus to induce acute-phase responses. Toth et al. (1995) extended the influenza—sleep work to mice, a species within which influenza can replicate, using a mouse-adapted human influenza virus. In this case, influenza challenge induces large increases in NREMS time in C57BL6 mice but not in Balb/c mice although both strains exhibited increases in NREMS intensity as determined from enhanced EEG delta wave (0.5–4 Hz) amplitudes. Changes in NREMS are most often accompanied by reductions in duration of REMS and hypothermia.
Influenza virus drives sleep responses via viral double-stranded (ds) RNA synthesized in lungs (Majde et al., 1991) and in the olfactory bulb after intranasal challenge in mice (Majde et al., 2007). dsRNA is made by all viruses and thus could serve as a common signal triggering the acute-phase response (Majde et al., 1991). Single-stranded poly I or poly C do not induce sleep responses, whereas if they are annealed together to form dsRNA, it is somnogenic (Kimura-Takeuchi, Majde, Toth, & Krueger, 1992b). Similarly, single-stranded 661 mer or 108 mer containing influenza sequences fail to induce acute-phase responses, whereas if the complement strand of either is annealed to form short dsRNA, the product is somnogenic (Fang, Bredow, Taishi, Majde, & Krueger, 1999). Viral dsRNA is recognized by Toll-like receptors (TLRs) 3 and 7 (Majde & Krueger, 2005) that in turn initiate a cytokine response.
Several mutant mouse models have been used to help decipher the pathways involved in virus-induced sleep responses; of great interest to sleep research are two models. Mice that lack a functional growth hormone-releasing hormone (GHRH) receptor, called lit/lit mice, have poor sleep responses to influenza and higher morbidity and mortality than corresponding wild-type (WT) mice (Alt et al., 2003). Similar weak sleep responses to influenza occur in mice lacking the neuron-specific IL1 receptor accessory protein (Davis et al., 2015). If WT mice are treated with anti-GHRH antibodies, it blocks the sleep responses induced by IL1 (Obál et al., 1995). Further, both GHRH and IL1 induce increases in intracellular Ca2+ hypothalamic neurons (De, Churchill, Obál, Simasko, & Krueger, 2002). IL1 induces GHRH receptor expression in brain (Taishi et al., 2004). These results are exciting because they link two well-known sleep regulatory substances, GHRH and IL1, to virus-induced changes in sleep. Perhaps more important, they demonstrate a brain mechanism involved in host responses to viral challenge that if disturbed leads to impaired outcomes. This mechanism is not yet fully investigated despite its potential for combating viral diseases.
Although much of the work relating viruses to sleep has used influenza virus, other viruses also affect sleep. Thus, humans infected with rhinovirus, or with influenza, exhibit altered sleep (Smith, 1992). Several disorders accompanied by excessive sleepiness and/or fatigue including mononucleosis, chronic fatigue syndrome, and sudden infant death syndrome (Guilleminault & Mondini, 1986; Hoffman, Damus, Hillman, & Krongrad, 1988; Holmes et al., 1988; Komaroff, 1988) are linked to viruses. In contrast, sleep intensity (reduced EEG SWA) and prolonged periods of little or no sleep are reported in mice with fatal experimental rabies infections (Gourmelon, Briet, Clarencom, Court, & Tsiang, 1991).
Humans seropositive for human immunodeficiency virus, but otherwise healthy, have excessive amounts of NREMS. As the disease progresses, sleep becomes disrupted (Norman, Chediak, Kiel, & Chon, 1990; Norman et al., 1988). Preclinical studies demonstrate that HIV envelope glycoproteins alter sleep (Gemma & Opp, 1999; Opp et al., 1996). The effects of HIV on sleep are likely mediated, in part, by cytokines as they upregulate cytokine expression in rat brain (Gemma, Smith, Hughes, & Opp, 2000) and in human plasma (Darko et al., 1995).
8. SLEEP RESPONSES TO OTHER MICROBES
The fungal organism, Candida albicans, live brewer’s yeast, the protozoan Trypanosoma brucei brucei induce sleep responses (Kent, Price, & Satinoff, 1988; Toth & Krueger, 1989, 1995; Toth, Tolley, Broad, Blakelym, & Krueger, 1994). T. brucei brucei’s enhanced sleep is associated with the cyclic parasitemia occurring roughly every 3 weeks, and these increases are superimposed upon a longer-term reduction in sleep caused by the infection in rabbits. This suggests that the changes in sleep are induced by the accompanying immune response to the protozoan antigenic shift (Toth et al., 1994). Finally, pseudomurein derived from Methanobacterium thermoautotrophicum is capable of inducing sleep responses in rabbits (Johannsen, Labischinski, & Krueger, 1991). Pseudomurein is a cell wall component but is chemically distinct from eubacterial peptidoglycan. Pseudomurein has been detected in the rabbit gut but does not induce any known disease.
9. MECHANISMS
There are a variety of pathogen-associated molecular patterns that are recognized by mammalian cells. The recognition receptors include peptidoglycan recognition proteins (PGRP), TLRs, and nucleotide-binding oligomerization domain receptors. Some of these have been linked to sleep (reviewed Majde & Krueger, 2005; Zielinski & Krueger, 2012). Thus, PGRP is constitutively expressed in brain and its hypothalamic and brainstem levels increase after sleep deprivation (Rehman, Taishi, Fang, Majde, & Krueger, 2001). Several TLRs are also linked to sleep (Chen et al., 2015; Hakim et al., 2014; Majde, Kapas, Bohnet, De, & Krueger, 2010; Sartorius et al., 2012; Wisor, Clegern, & Schmidt, 2011). For example, sleep responses to influenza virus are attenuated in TLR-3-deficient mice (Majde et al., 2010). Activation of these pathogen recognition receptors induces proinflammatory and antimicrobial responses by activation of many intracellular pathways (reviewed Zielinski & Krueger, 2012). These actions lead to central and systemic upregulation of many cytokines that are involved in sleep regulation, including IL1 and TNF (reviewed Besedovsky, Lange, & Born, 2012; Imeri & Opp, 2009; Krueger et al., 2008). The sleep-linked cytokines in turn act on brain sleep regulatory centers such as the hypothalamus (Alam et al., 2004; Kubota, Li, Guan, Brown, & Krueger, 2002) and brain stem nuclei (Brambilla, Barajon, Bianchi, Opp, & Imeri, 2010) to promote sleep. They also can act on local neuronal/glial circuits such as cortical columns to affect local state (Churchill et al., 2008; Jewett et al., 2015). Systemic cytokines also can alter sleep either by binding to vagal afferents that in turn upregulate brain cytokine expression (e.g., Zielinski, Dunbrasky, Taishi, Souza, & Krueger, 2013) or by crossing the BBB (reviewed Dantzer et al., 2008).
10. ARE SLEEP RESPONSES TO MICROBES ADAPTIVE?
Caring relatives have likely always advised rest and sleep for loved ones to help recuperate from diseases. Surprisingly there is relatively little evidence that sleep helps recuperate from microbial infections or other diseases. This is a consequence of the almost impossibility of isolating sleep as an independent variable. For instance, when you go to sleep, almost every physiological variable changes, e.g., body temperature, hormone levels, respiratory rate, kidney filtration rates, etc. Thus, it is not possible to know if improved morbidity or mortality rates are due to sleep per se, or to changes in one of the other physiological parameters that change with sleep. Nevertheless, some data suggest that sleep may contribute to, or facilitate, recuperation. First, there is a large and actively growing literature showing that sleep and sleep loss alter many facets of immune function (reviewed Besedovsky et al., 2012; Imeri & Opp, 2009; Majde & Krueger, 2005). There is also evidence that changes in sleep patterns during infection correlate with morbidity and mortality. For example, Toth, Tolley, and Krueger (1993) reported that during experimentally induced infections, long periods of enhanced sleep were associated with lower mortality, reduced morbidity, and less severe clinical symptoms. Conversely, animals that died, or had to be euthanized during the experimental infectious challenge, slept less and had poorer sleep quality than those that survived. These correlative data suggest that sleep may serve as an aid in recuperation.
Although little research has focused directly on the extent to which changes in sleep during microbial infection are adaptive, there is a large literature demonstrating the adaptive value of fever. A comprehensive review of the adaptive nature of fever is beyond the scope of this article, and the interested reader is referred to a classic literature on this topic (Kluger, 1979; Kluger, Kozak, Conn, Leon, & Soszynski, 1996; Kluger, Ringler, & Anver, 1975). Suffice it to say, moderate fevers are a double-edged host defense weapon in that they enhance immune function and make the environment less suitable for microbial replication. As briefly summarized, little evidence suggests that changes in sleep per se contribute to recuperative processes. However, changes in sleep during infection may be adaptive because they facilitate the generation of fever. The most efficient way to raise body temperature is to increase heat production and reduce heat loss. Endothermic animals increase heat production by shivering, yet there are sleep state-specific changes in thermoregulatory effector mechanisms such that during REM sleep shivering does not occur (Glotzbach & Heller, 1976; Parmeggiani, 2003); during infection, REM sleep is essentially abolished. It is unlikely that sleep evolved solely to support the generation of fever during infection, but the changes in sleep during microbial infection are exquisitely designed to fulfill this role (Imeri & Opp, 2009; Opp, 1999).
11. CONCLUSIONS
Thirty-five years ago it was scientific heresy when we first suggested that gut bacteria affect brain state. It should not have been given that it was known that some microbial infections induce coma and that bacteria could translocate across the gut epithelial barrier. Remarkably, a patent was issued 14 years ago entitled “Administering Bacteria to Improve Sleep” (#6.444.203B2). Regardless, in the intervening years, it has become accepted that microbes affect a variety of physiological functions and they are just part of a much larger symbiotic relationship between microbes and mammals. Microbiome analyses are accelerating the progress relating bacteria to physiological sleep, and we anticipate many important interesting findings emanating from that research endeavor.
Acknowledgments
This work was supported by the National Institutes of Health Grant Numbers NS025378 and HD036520 to J.M.K. and AG041827 and AI115706 to M.R.O.
References
- Alam MN, McGinty D, Bashir T, Kumar S, Imeri L, Opp MR, et al. Interleukin-1beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: Role in sleep regulation. The European Journal of Neuroscience. 2004;20:207–216. doi: 10.1111/j.1460-9568.2004.03469.x. [DOI] [PubMed] [Google Scholar]
- Alt J, Obál F, Jr, Traynor TR, Gard J, Majde JA, Krueger JM. Sleep responses to influenza viral infection in GHRH-receptor deficient mice. Journal of Applied Physiology. 2003;95:460–468. doi: 10.1152/japplphysiol.01190.2002. [DOI] [PubMed] [Google Scholar]
- Baracchi F, Ingiosi AM, Raymond RM, Jr, Opp MR. Sepsis-induced alterations in sleep of rats. The American Journal of Physiology. 2011;301:R1467–R1478. doi: 10.1152/ajpregu.00354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infection and Immunity. 1979;23:403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermon S, Petriz B, Kajeniene A, Prestes J, Castell L, Franco OL. The microbiota: An exercise immunology perspective. Exercise Immunology Review. 2015;21:70–79. [PubMed] [Google Scholar]
- Besedovsky L, Lange T, Born J. Sleep and immune function. Pflügers Archiv. 2012;463:121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla D, Barajon I, Bianchi S, Opp MR, Imeri L. Interleukin-1 inhibits putative cholinergic neurons in vitro and REM sleep when microinjected into the rat laterodorsal tegmental nucleus. Sleep. 2010;33:919–929. doi: 10.1093/sleep/33.7.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady AB, Kotani S, Shiba T, Kusumoto S, Krueger JM. Somnogenic activities of synthetic lipid A. Infection and Immunity. 1989;57:396–403. doi: 10.1128/iai.57.2.396-403.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang FC, Opp MR. Corticotropin-releasing hormone (CRH) as a regulator of waking. Neuroscience and Biobehavioral Reviews. 2001;25:445–453. doi: 10.1016/s0149-7634(01)00024-0. [DOI] [PubMed] [Google Scholar]
- Chen YC, Su MC, Liou CW, Liu SF, Chen CJ, Lin HC, et al. Co-upregulation of Toll-like receptors 2 and 6 on peripheral blood cells in patients with obstructive sleep apnea. Sleep & Breathing. 2015;19:873–882. doi: 10.1007/s11325-014-1116-4. [DOI] [PubMed] [Google Scholar]
- Churchill L, Rector DM, Yasuda K, Fix C, Rojas MJ, Yasuda T, et al. Tumor necrosis factor α: Activity dependent expression and promotion of cortical column sleep in rats. Neuroscience. 2008;156:71–80. doi: 10.1016/j.neuroscience.2008.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews. Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darko DF, Miller JC, Gallen C, White J, Koziol J, Brown SJ, et al. Sleep electroencephalogram delta-frequency amplitude, night plasma levels of tumor necrosis factor a, and human immunodeficiency virus infection. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:12080–12084. doi: 10.1073/pnas.92.26.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CJ, Dunbrasky D, Oonk M, Taishi P, Opp MR, Krueger JM. The neuron-specific interleukin-1 receptor accessory protein is required for homeostatic sleep and sleep responses to influenza viral challenge in mice. Brain, Behavior, Immunity. 2015;47:35–43. doi: 10.1016/j.bbi.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De A, Churchill L, Obál F, Jr, Simasko SM, Krueger JM. GHRH and IL1β increase cytoplasmic Ca2+ levels in cultured hypothalamic GABAergic neurons. Brain Research. 2002;949:209–212. doi: 10.1016/s0006-8993(02)03157-8. [DOI] [PubMed] [Google Scholar]
- Everson CA. Sustained sleep deprivation impairs host defense. The American Journal of Physiology. 1993;34:R1148–R1154. doi: 10.1152/ajpregu.1993.265.5.R1148. [DOI] [PubMed] [Google Scholar]
- Everson CA, Toth LA. Systemic bacterial invasion induced by sleep deprivation. The American Journal of Physiology. 2000;278:R905–R916. doi: 10.1152/ajpregu.2000.278.4.R905. [DOI] [PubMed] [Google Scholar]
- Fang J, Bredow S, Taishi P, Majde JA, Krueger JM. Synthetic influenza viral double-stranded RNA induces an acute phase response in rabbits. Journal of Medical Virology. 1999;57:198–203. [PubMed] [Google Scholar]
- Fencl V, Koski G, Pappenheimer JR. Factors in cerebrospinal fluid from goats that affect sleep and activity in rats. The Journal of Physiology. 1971;216:565–589. doi: 10.1113/jphysiol.1971.sp009541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemma C, Opp MR. Human immunodeficiency virus glycoproteins 160 and 41 alter sleep and brain temperature of rats. Journal of Neuroimmunology. 1999;97:94–101. doi: 10.1016/s0165-5728(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Gemma C, Smith EM, Hughes TK, Jr, Opp MR. Human immunodeficiency virus glycoprotein 160 induces cytokine mRNA expression in the rat central nervous system. Cellular and Molecular Neurobiology. 2000;20:419–431. doi: 10.1023/a:1007053129686. [DOI] [PubMed] [Google Scholar]
- Glotzbach SF, Heller HC. Central nervous regulation of body temperature during sleep. Science. 1976;194:537–538. doi: 10.1126/science.973138. [DOI] [PubMed] [Google Scholar]
- Gourmelon P, Briet D, Clarencom D, Court L, Tsiang H. Sleep alterations in experimental street rabies virus infection occur in the absence of major EEG abnormalities. Brain Research. 1991;554:159–165. doi: 10.1016/0006-8993(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Granger JI, Ratti PL, Datta SC, Raymond RM, Opp MR. Sepsisinduced morbidity in mice: Effects on body temperature, body weight, cage activity, social behavior and cytokines in brain. Psychoneuroendocrinology. 2013;38:1047–1057. doi: 10.1016/j.psyneuen.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilleminault C, Mondini S. Mononucleosis and chronic daytime sleepiness: A long-term follow-up study. Archives of Internal Medicine. 1986;146:1333–1335. [PubMed] [Google Scholar]
- Hakim F, Wang Y, Zhang SX, Zheng J, Yolcu ES, Carreras A, et al. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Research. 2014;74:1329–1337. doi: 10.1158/0008-5472.CAN-13-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinard V, Mikhail C, Pradervand S, Curie T, Houtkooper RH, Auwerx J, et al. Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. The Journal of Neuroscience. 2012;32:12506–12517. doi: 10.1523/JNEUROSCI.2306-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HJ, Damus K, Hillman L, Krongrad E. Risk factors for SIDS: Results of the National Instute of Child Health and Human Development SIDS cooperative epidemiological study. Annals of the New York Academy of Sciences. 1988;533:13–30. doi: 10.1111/j.1749-6632.1988.tb37230.x. [DOI] [PubMed] [Google Scholar]
- Holmes GP, Kaplan JE, Gantz NM, Komaroff AL, Schonberger LB, Straus SE, et al. Chronic fatigue syndrome: A working case definition. Annals of Internal Medicine. 1988;108:387–389. doi: 10.7326/0003-4819-108-3-387. [DOI] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nature Reviews. Neuroscience. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett KA, Taishi P, Sengupta P, Roy S, Davis CJ, Krueger JM. Tumor necrosis factor enhances the sleep-like state and electrical stimulation induces a wake-like state in co-cultures of neurons and glia. The European Journal of Neuroscience. 2015;42:2078–2090. doi: 10.1111/ejn.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen L, Krueger JM. Quantitation of diaminopimelic acid in human urine. Advances in the Biosciences. 1988;68:445–449. [Google Scholar]
- Johannsen L, Labischinski H, Krueger JM. Somnogenic activity of pseudomurein in rabbits. Infection and Immunity. 1991;59:2502–2504. doi: 10.1128/iai.59.7.2502-2504.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen L, Rosenthal RS, Martin SA, Cady AB, Obál F, Jr, Guinand M, et al. Somnogenic activity of O-acetylated and dimeric muramyl peptides. Infection and Immunity. 1989;57:2726–2732. doi: 10.1128/iai.57.9.2726-2732.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen L, Toth LA, Rosenthal RS, Opp MR, Obál F, Jr, Cady AB, et al. Somnogenic, pyrogenic, and hematologic effects of bacterial peptidoglycan. The American Journal of Physiology. 1990;259:R182–R186. doi: 10.1152/ajpregu.1990.258.1.R182. [DOI] [PubMed] [Google Scholar]
- Johannsen L, Wecke J, Obál F, Jr, Krueger JM. Macrophages produce somnogenic and pyrogenic muramyl peptides during the digestion of staphylococci. The American Journal of Physiology. 1991;260:R126–R133. doi: 10.1152/ajpregu.1991.260.1.R126. [DOI] [PubMed] [Google Scholar]
- Kent S, Price M, Satinoff E. Fever alters characteristics of sleep in rats. Physiology & Behavior. 1988;44:709–715. doi: 10.1016/0031-9384(88)90051-0. [DOI] [PubMed] [Google Scholar]
- Kimura-Takeuchi M, Majde JA, Toth LA, Krueger JM. Influenza virus-induced changes in rabbit sleep and acute phase responses. The American Journal of Physiology. 1992a;263:R1115–R1121. doi: 10.1152/ajpregu.1992.263.5.R1115. [DOI] [PubMed] [Google Scholar]
- Kimura-Takeuchi M, Majde JA, Toth LA, Krueger JM. The role of double-stranded RNA in the induction of the acute-phase response in an abortive influenza virus infection model. The Journal of Infectious Diseases. 1992b;166:1266–1275. doi: 10.1093/infdis/166.6.1266. [DOI] [PubMed] [Google Scholar]
- Kluger MJ. Fever: It’s biology, evolution and function. Princeton: Princeton University Press; 1979. [Google Scholar]
- Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. The adaptive value of fever. Infectious Disease Clinics of North America. 1996;10:1–21. doi: 10.1016/s0891-5520(05)70282-8. [DOI] [PubMed] [Google Scholar]
- Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science. 1975;188:166–168. [PubMed] [Google Scholar]
- Komaroff AL. Chronic fatigue syndromes: Relationships to chronic viral infections. Journal of Virological Methods. 1988;21:3–10. doi: 10.1016/0166-0934(88)90047-x. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Bacsik J, García-Arrarás J. Sleep-promoting material from human urine and its relation to factor S from brain. The American Journal of Physiology. 1980;238:E116–E123. doi: 10.1152/ajpendo.1980.238.2.E116. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Huang Y, Rector DM, Buysee DJ. Sleep: A synchrony of cell activity-driven small network states. The European Journal of Neuroscience. 2013;38:2199–2209. doi: 10.1111/ejn.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Johannsen L. Bacterial products, cytokines and sleep. The Journal of Rheumatology. Supplement. 1989;16:52–57. [PubMed] [Google Scholar]
- Krueger JM, Karnovsky ML, Martin SL, Pappenheimer JR, Walter J, Biemann K. Peptidoglycans as promoters of slow-wave sleep. II. Somnogenic and pyrogenic activities of some naturally occurring muramyl peptides; correlations with mass spectrometric structure determination. The Journal of Biological Chemistry. 1984;259:12659–12662. [PubMed] [Google Scholar]
- Krueger JM, Kubillus S, Shoham S, Davenne D. Enhancement of slow-wave sleep by endotoxin and lipid A. The American Journal of Physiology. 1986;251:R591–R597. doi: 10.1152/ajpregu.1986.251.3.R591. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Obál F., Jr A neuronal group theory of sleep function. Journal of Sleep Research. 1993;2:63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Pappenheimer JR, Karnovsky ML. The composition of sleep-promoting factor isolated from human urine. The Journal of Biological Chemistry. 1982;257:1664–1669. [PubMed] [Google Scholar]
- Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nature Reviews. Neuroscience. 2008;9:910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Rosenthal RS, Martin SA, Walter J, Davenne D, Shoham S, et al. Bacterial peptidoglycans as modulators of sleep. I. Anhydro forms of muramyl peptides enhance somnogenic potency. Brain Research. 1987;403:249–257. doi: 10.1016/0006-8993(87)90062-x. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Roy S. Sleep’s kernel. The Scientist. 2016:36–41. March 1, 2016 issue. [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Taishi P, De A, Davis C, Winters BD, Clinton J, et al. ATP and the purine type 2 X7 receptor affect sleep. Journal of Applied Physiology. 2010;109:1318–1327. doi: 10.1152/japplphysiol.00586.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Tononi G. Local use-dependent sleep; synthesis of the new paradigm. Current Topics in Medicinal Chemistry. 2011;11:2490–2492. doi: 10.2174/156802611797470330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Toth LA, Floyd R, Fang J, Kapás L, Bredow S, et al. Sleep, microbes and cytokines. Neuroimmunomodulation. 1994;1:100–109. doi: 10.1159/000097142. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Walter J, Karnovsky M, Chedid L, Choay JP, Lefrancier P, et al. Muramyl peptides: Variation of somnogenic activity with structure. The Journal of Experimental Medicine. 1984;159:68–76. doi: 10.1084/jem.159.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryger MH, Roth T, Dement WC. Principles and practice of sleep medicine. 5th. Philadelphia, PA: Elsevier; 2011. [Google Scholar]
- Krysciak J. Diaminopimelate in mammalian urine. Folia Biologica. 1980;28:47–51. [PubMed] [Google Scholar]
- Kubota T, Li N, Guan Z, Brown R, Krueger JM. Intrapreoptic microinjection of TNFα enhances non-REM sleep in rats. Brain Research. 2002;932:37–44. doi: 10.1016/s0006-8993(02)02262-x. [DOI] [PubMed] [Google Scholar]
- Majde JA, Bohnet SG, Ellis GA, Churchill L, Leyva-Grado V, Wu M, et al. Detection of a mouse-adapted human influenza virus in the olfactory bulb of mice within hours after intranasal infection. Journal of Neurovirology. 2007;13:399–409. doi: 10.1080/13550280701427069. [DOI] [PubMed] [Google Scholar]
- Majde JA, Brown RK, Jones MW, Dieffenbach CW, Maitra N, Krueger JM, et al. Detection of toxic viral-associated double-stranded RNA (dsRNA) in influenza-infected lung. Microbial Pathogenesis. 1991;10:105–115. doi: 10.1016/0882-4010(91)90071-h. [DOI] [PubMed] [Google Scholar]
- Majde JA, Kapas L, Bohnet SG, De A, Krueger JM. Attenuation of the influenza virus sickness behavior in mice deficient in Toll-like receptor 3. Brain, Behavior, and Immunity. 2010;24:306–315. doi: 10.1016/j.bbi.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majde JA, Krueger JM. Links between the innate immune system and sleep. The Journal of Allergy and Clinical Immunology. 2005;116:1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Maslanik T, Tannura K, Mahaffey L, Loughridge AB, Benninson L, Ursell L, et al. Commensal bacteria and MMAPs are necessary for stress-induced increases in IL-1β and IL-18 but not IL-6, IL-10 or MCP-1. PLoS One. 2012;7:e50636. doi: 10.1371/journal.pone.0050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemzek JA, Hugunin KM, Opp MR. Modeling sepsis in the laboratory: Merging sound science with animal well-being. Comparative Medicine. 2008;58:120–128. [PMC free article] [PubMed] [Google Scholar]
- Norman SE, Chediak HD, Kiel M, Chon MA. Sleep disturbances in HIV-infected homosexual men. AIDS. 1990;4:775–781. doi: 10.1097/00002030-199008000-00009. [DOI] [PubMed] [Google Scholar]
- Norman SE, Resnik L, Cohn MA, Duara R, Herbst J, Berger JR. Sleep disturbances in HIV-seropositive patients. JAMA. 1988;260:922. [PubMed] [Google Scholar]
- Obál F, Jr, Fang J, Payne LC, Krueger JM. Growth hormone-releasing hormone (GHRH) mediates the sleep promoting activity of interleukin-1 (IL1) in rats. Neuroendocrinology. 1995;61:559–565. doi: 10.1159/000126880. [DOI] [PubMed] [Google Scholar]
- Oonk M, Krueger JM, Davis CJ. Voluntary sleep loss in rats. Sleep. 2016;39:1467–1479. doi: 10.5665/sleep.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opp MR. Fever, body temperature, and levels of arousal. In: Lydic R, Baghdoyan HA, editors. Handbook of behavioral state control: Cellular and molecular mechanisms. Boca Raton: CRC Press; 1999. pp. 623–640. [Google Scholar]
- Opp MR, Krueger JM. Sleep and host defense. In: Kryger M, editor. Principals and practice of sleep medicine. 6th. Philadelphia, PA: Elsevier Science; 2015. [Google Scholar]
- Opp MR, Rady PL, Hughes TK, Jr, Cadet P, Tyring SK, Smith EM. Human immunodeficiency virus envelope glycoprotein 120 alters sleep and induces cytokine mRNA expression in rats. The American Journal of Physiology. 1996;270:R963–R970. doi: 10.1152/ajpregu.1996.270.5.R963. [DOI] [PubMed] [Google Scholar]
- Pabst MJ, Beranova S, Krueger JM. A review of the effects of muramyl peptides on macrophages, monokines and sleep. Neuroimmunomodulation. 1999;6:261–283. doi: 10.1159/000026384. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR, Koski G, Fencl V, Karnovsky ML, Krueger JM. Extraction of sleep-promoting factor S from cerebrospinal fluid and from brains of sleep-deprived animals. Journal of Neurophysiology. 1975;38:1299–1311. doi: 10.1152/jn.1975.38.6.1299. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR, Miller TB, Goodrich CA. Sleep promoting effects of cerebrospinal fluid from sleep-deprived goats. Proceedings of the National Academy of Sciences of the United States of America. 1967;58:513–517. doi: 10.1073/pnas.58.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer JR, Zich KE. Absorption of hydrophilic solutes from the small intestine. The Journal of Physiology. 1987;371:138P. [Google Scholar]
- Parmeggiani PL. Thermoregulation and sleep. Frontiers in Bioscience. 2003;8:s557–s567. doi: 10.2741/1054. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. Sleep deprivation in the rat: X. Integration and discussion of the findings. Sleep. 1989;12:68–87. [PubMed] [Google Scholar]
- Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortical columns. Brain Research. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Rehman A, Taishi P, Fang J, Majde JA, Krueger JM. The cloning of a rat peptidoglycan recognition protein (PGRP) and its induction in brain by sleep deprivation. Cytokine. 2001;13:8–17. doi: 10.1006/cyto.2000.0800. [DOI] [PubMed] [Google Scholar]
- Roy S, Krueger JM, Rector DM, Wan Y. Network models for activity-dependent sleep regulation. Journal of Theoretical Biology. 2008;253:462–468. doi: 10.1016/j.jtbi.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius T, Lutz SZ, Hoene M, Waak J, Peter A, Weigert C, et al. Toll-like receptors 2 and 4 impair insulin-mediated brain activity by interleukin-6 and osteopontin and alter sleep architecture. The FASEB Journal. 2012;26:1799–1809. doi: 10.1096/fj.11-191023. [DOI] [PubMed] [Google Scholar]
- Shoham S, Krueger JM. Muramyl dipeptide-induced sleep and fever: Effects of ambient temperature and time of injections. The American Journal of Physiology. 1988;255:R157–R165. doi: 10.1152/ajpregu.1988.255.1.R157. [DOI] [PubMed] [Google Scholar]
- Shukla SK, Cook D, Meyer J, Vernon SD, Le T, Clevidence D, et al. Changes in gut and plasma microbiome following exercise challenge in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) PLoS One. 2015;10:e0145453. doi: 10.1371/journal.pone.0145453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. Sleep, colds, and performance. In: Broughton RJ, Ogilvie RD, editors. Sleep, arousal and performance. Boston: Birkhauser; 1992. pp. 233–242. [Google Scholar]
- Summa KC, Voight RM, Forsyth CB, Shaikh M, Cavanaugh K, Tang Y, et al. Disruption of the circadian clock in mice increases intestinal permeability and promotes alcohol-induced hepatic pathology and inflammation. PLoS One. 2013;8:e67102. doi: 10.1371/journal.pone.0067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taishi P, De A, Alt J, Gardi J, Obál F, Jr, Krueger JM. Interleukin-1β stimulates GHRH receptor mRNA expression in the rat hypothalamus in vitro and in vivo. Journal of Neuroendocrinology. 2004;16:113–118. doi: 10.1111/j.0953-8194.2004.01138.x. [DOI] [PubMed] [Google Scholar]
- Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Zuez J, Tengeler AC, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–529. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- Toth LA, Krueger JM. Alteration of sleep in rabbits by Staphylococcus aureus infection. Infection and Immunity. 1988;56:1785–1791. doi: 10.1128/iai.56.7.1785-1791.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth LA, Krueger JM. Effects of microbial challenge on sleep in rabbits. The FASEB Journal. 1989;3:2062–2066. doi: 10.1096/fasebj.3.9.2663582. [DOI] [PubMed] [Google Scholar]
- Toth LA, Krueger JM. Lighting conditions alter Candida albicans-induced sleep responses. The American Journal of Physiology. 1995;269:R1441–R1447. doi: 10.1152/ajpregu.1995.269.6.R1441. [DOI] [PubMed] [Google Scholar]
- Toth LA, Rehg JE, Webster RG. Strain differences in sleep and other pathophysiological sequelae of influenza virus infection in naïve and immunized mice. Journal of Neuroimmunology. 1995;58:89–99. doi: 10.1016/0165-5728(94)00193-r. [DOI] [PubMed] [Google Scholar]
- Toth LA, Tolley EA, Broad YR, Blakelym B, Krueger JM. Sleep during experimental trypanosomiasis in rabbits. Proceedings of the Society for Experimental Biology and Medicine. 1994;205:174–181. doi: 10.3181/00379727-205-43694. [DOI] [PubMed] [Google Scholar]
- Toth LA, Tolley EA, Krueger JM. Sleep as a prognostic indicator during infectious disease in rabbits. Proceedings of the Society for Experimental Biology and Medicine. 1993;203:179–192. doi: 10.3181/00379727-203-43590. [DOI] [PubMed] [Google Scholar]
- Vermeulen MW, Gray GR. Processing of Bacillus subtilis peptidoglycan by a mouse macrophage cell line. Infection and Immunity. 1984;46:476–483. doi: 10.1128/iai.46.2.476-483.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt RM, Forsyth CB, Green SJ, Mutlu E, Engen P, Vitaterna MH, et al. Circadian disorganization alters intestinal microbiota. PLoS One. 2014;9:e97500. doi: 10.1371/journal.pone.0097500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor JP, Clegern WC, Schmidt MA. Toll-like receptor 4 is a regulator of monocyte and electroencephalographic responses to sleep loss. Sleep. 2011;34:1335–1345. doi: 10.5665/SLEEP.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S, Karnovsky ML. Qualitative detection of muramic acid in normal mammalian tissues. Infection and Immunity. 1984;43:937–941. doi: 10.1128/iai.43.3.937-941.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski MR, Dunbrasky DL, Taishi P, Souza G, Krueger JM. Vagotomy attenuates brain cytokines and sleep induced by peripherally administered tumor necrosis factor alpha and lipopolysaccharide in mice. Sleep. 2013;36:1227–1238. doi: 10.5665/sleep.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski MR, Krueger JM. Inflammation and sleep. In: Barkoukis TJ, Matheson JK, Ferber R, Doghramji K, editors. Therapy in sleep medicine. Philadelphia, PA: Elsevier; 2012. pp. 607–616. [Google Scholar]


